Abstract
Background
Although insulin analogues are commonly prescribed for the management of diabetes mellitus, there is uncertainty regarding their optimal use. We conducted meta-analyses to compare the outcomes of insulin analogues with conventional insulins in the treatment of type 1, type 2 and gestational diabetes.
Methods
We updated 2 earlier systematic reviews of the efficacy and safety of rapid-and long-acting insulin analogues. We searched electronic databases, conference proceedings and “grey literature” up to April 2007 to identify randomized controlled trials that compared insulin analogues with conventional insulins. Study populations of interest were people with type 1 and type 2 diabetes (adult and pediatric) and women with gestational diabetes.
Results
We included 68 randomized controlled trials in the analysis of rapid-acting insulin analogues and 49 in the analysis of long-acting insulin analogues. Most of the studies were of short to medium duration and of low quality. In terms of hemoglobin A1c, we found minimal differences between rapid-acting insulin analogues and regular human insulin in adults with type 1 diabetes (weighted mean difference for insulin lispro: –0.09%, 95% confidence interval [CI] –0.16% to –0.02%; for insulin aspart: –0.13%, 95% CI –0.20% to –0.07%). We observed similar outcomes among patients with type 2 diabetes (weighted mean difference for insulin lispro: –0.03%, 95% CI –0.12% to –0.06%; for insulin aspart: –0.09%, 95% CI –0.21% to 0.04%). Differences between long-acting insulin analogues and neutral protamine Hagedorn insulin in terms of hemoglobin A1c were marginal among adults with type 1 diabetes (weighted mean difference for insulin glargine: –0.11%, 95% CI –0.21% to –0.02%; for insulin detemir: –0.06%, 95% CI –0.13% to 0.02%) and among adults with type 2 diabetes (weighted mean difference for insulin glargine: –0.05%, 95% CI –0.13% to 0.04%; for insulin detemir: 0.13%, 95% CI 0.03% to 0.22%). Benefits in terms of reduced hypoglycemia were inconsistent. There were insufficient data to determine whether insulin analogues are better than conventional insulins in reducing long-term diabetes-related complications or death.
Interpretation
Rapid-and long-acting insulin analogues offer little benefit relative to conventional insulins in terms of glycemic control or reduced hypoglycemia. Long-term, high-quality studies are needed to determine whether insulin analogues reduce the risk of long-term complications of diabetes.
Diabetes mellitus is associated with serious long-term complications and premature death.1 Data from the Health Canada National Diabetes Surveillance System indicate that, in 2004/05, diabetes was diagnosed in about 5.5% (1.8 million) of Canadians aged 20 years and older.2 Because the disease goes undetected in many cases, the true prevalence may approach 1.9 million.3
Tight glycemic control, to maintain a hemoglobin A1c concentration of 7.0% or less, is recommended for all patients with diabetes to reduce the risk of long-term complications such as cardiovascular-related death, retinopathy and nephropathy.4 Insulin is indicated for all patients with type 1 diabetes and for patients with type 2 diabetes if adequate glycemic control cannot be achieved through exercise, diet or oral antidiabetic therapy.4
Conventional insulins include regular human insulin and intermediate-acting neutral protamine Hagedorn insulin. However, these agents do not replicate the pattern of basal and postprandial endogenous secretion of insulin. Insulin analogues are modified human insulins developed to address this limitation.5 The rapid-acting insulin analogues insulin lispro, insulin aspart and insulin glulisine are marketed in Canada as bolus insulins; the long-acting agents insulin glargine and insulin detemir are marketed as basal insulins.6
Systematic reviews of the insulin analogues have been published previously.7–10 However, through our comprehensive search of the literature, we did not identify any reviews of long-acting insulin analogues in the management of type 1 diabetes or gestational diabetes. In this article, we provide an up-to-date, comprehensive systematic review and meta-analysis of outcomes associated with the use of rapid-and long-acting insulin analogues in type 1 and type 2 diabetes (adult and pediatric patients) and gestational diabetes. Detailed methods and complete results are reported elsewhere.11,12
Methods
We based our current study on 2 health technology assessments of the insulin analogues from the Canadian Agency for Drugs and Technologies in Health (CADTH).13,14 Such reports from the agency consist of a systematic review of the available clinical and economic evidence regarding specific drugs or health technologies. We updated the 2 reports to include recently published studies, additional outcomes of interest, and intraclass comparisons of the rapid-and long-acting insulin analogues.
Literature search
We updated the original search strategy used for the health technology assessments to include studies published up to April 2007 (Appendix 1, available at www.cmaj.ca/cgi/content/full/180/4/385/DC2). We developed supplemental searches to include studies that addressed additional comparisons and outcomes of interest (Appendices 2 and 3, available at www.cmaj.ca/cgi/content/full/180/4/385/DC2).
We searched the following databases: MEDLINE (1966 to April 2007), MEDLINE In-Process and Other Non-Indexed Citations, MEDLINE Daily Update, EMBASE (1980 to April 2007), BIOSIS Previews (1989 to April 2007) and the Cochrane Library (Issue 3, 2007). We constructed the search terms using controlled vocabulary, such as the National Library of Medicine's MeSH (Medical Subject Headings), and key words.
The main search concepts were diabetes, long-acting insulin analogues and rapid-acting insulin analogues. We limited our search to randomized controlled trials. We identified “grey literature” by searching the websites of agencies that conduct health technology assessments and other related agencies, as well as endocrine and diabetes associations and their associated conference sites (Appendix 4, available at www.cmaj.ca/cgi/content/full/180/4/385/DC2). Stakeholders, including manufacturers of the agents under review, were given an opportunity to provide additional evidence.
Outcomes of interest
In this article, we present results for hemoglobin A1c concentration, hypoglycemia, quality-of-life, patient satisfaction, complications of diabetes (including death) and adverse effects. We analyzed data on hypoglycemia using the relative risk of experiencing 1 or more episodes of hypoglycemia during the study period; we used the rate ratio for the frequency of episodes (i.e., number of episodes per patient per unit time).15
Results for other outcomes of interest (i.e., fasting plasma glucose level, 2-hour postprandial glucose level, body weight, cholesterol level and blood pressure) are presented elsewhere.11,12
Selection criteria
We included randomized controlled trials published in English if they reported data for one of the following comparisons in patients with type 1, type 2 or gestational diabetes: rapid-acting insulin analogue versus regular human insulin; one rapid-acting insulin analogue versus another; premixed (i.e., biphasic) rapid-acting insulin analogue versus another premixed insulin (either rapid-acting insulin analogue or human insulin); long-acting insulin analogue versus neutral protamine Hagedorn insulin or another intermediate-acting conventional insulin; or one long-acting insulin analogue versus another. We excluded studies of insulin glulisine from the systematic review because this agent had not been marketed in Canada at the time of our analysis.
Quality assessment
Using a modified Jadad scale,16 2 reviewers (F.A. and A.L.) independently assessed the methodologic quality of the included studies of rapid-acting insulin analogues; 2 others (S.R.S and C.Y.) assessed the included studies of long-acting analogues. Specifically, they evaluated the extent of allocation concealment, blinding of assessors and reporting of intention-to-treat analysis.17 Disagreements were resolved by consensus or a third reviewer.
Data extraction and analysis
Each of the reviewers independently extracted data from the articles included in the analysis using a predesigned form. Disagreements were resolved in the same manner as for the quality assessment. Data extraction at the study level was not repeated for studies contained in the 2 original health technology assessments from the Canadian Agency for Drugs and Technologies in Health.13,14
We combined data using a random-effects model.15 We conducted separate analyses for gestational diabetes, pediatric type 1 diabetes, adult type 1 diabetes, pediatric type 2 diabetes and adult type 2 diabetes. We performed subgroup analyses for (a) administration method of rapid-acting insulin analogues (i.e., multiple daily injections or continuous subcutaneous insulin infusion); (b) type of bolus insulin (i.e., regular human insulin or rapid-acting insulin analogue) administered with long-acting analogues; and (c) type of oral antidiabetic agent administered with long-acting insulin analogues in the management of type 2 diabetes. We included the results from studies of premixed insulins in the same meta-analyses with results from studies of bolus insulins. In the absence of reported carryover effects, we combined data from crossover and parallel trials in the same meta-analysis.
We conducted sensitivity analyses to determine whether inclusion of studies deemed to be of low methodologic quality affected the results. We assessed the potential for publication bias, in meta-analyses that included more than 5 studies, using funnel plots.15
We determined heterogeneity using the I2 statistic, which describes the proportion of unexplained variability in effect estimates across studies in a meta-analysis.18 An I2 of 50% represents moderate heterogeneity.18 For analyses above this threshold, we explored possible causes of systematic variability through comparison of population, methodologic and treatment characteristics across included studies.
Results
Study selection
For rapid-acting insulin analogues, we identified 765 citations, of which we reviewed 26 and selected 5 trials19–23 for inclusion in our analysis. We thus had a total of 68 randomized controlled trials19–89 for the current meta-analysis, including 63 trials24–89 from the original health technology assessment13 (Figure 1).
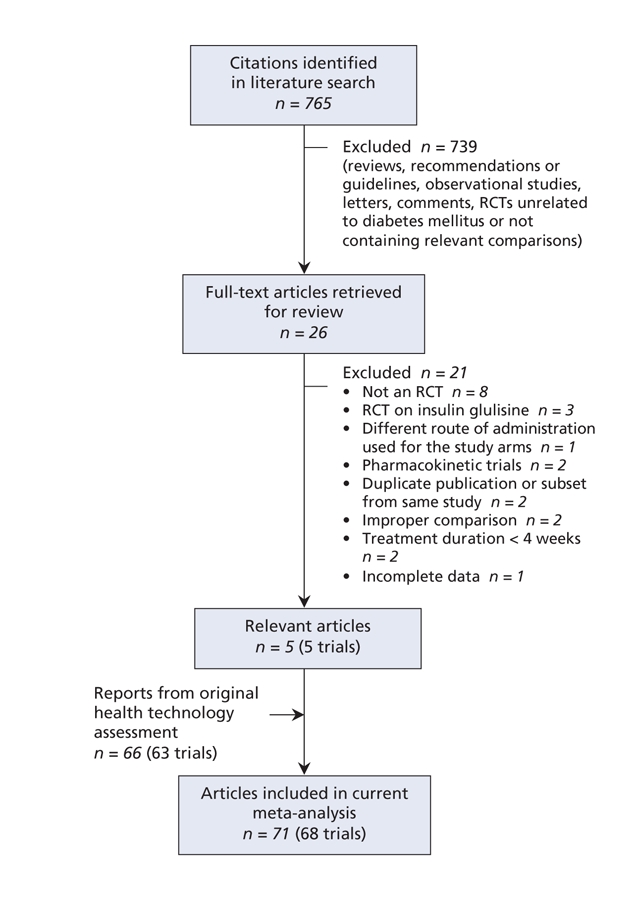
Figure 1: Selection of randomized controlled trials (RCTs) of rapid-acting insulin analogues for inclusion in the meta-analysis.
For long-acting insulin analogues, we identified 940 citations, of which we reviewed 55 and selected 20.90–109 A further trial110 was identified by stakeholders. We thus had a total of 49 randomized controlled trials for the analysis,90–102,104–142 including 28 trials111–140,143–146 from the original health technology assessment14 (Figure 2).
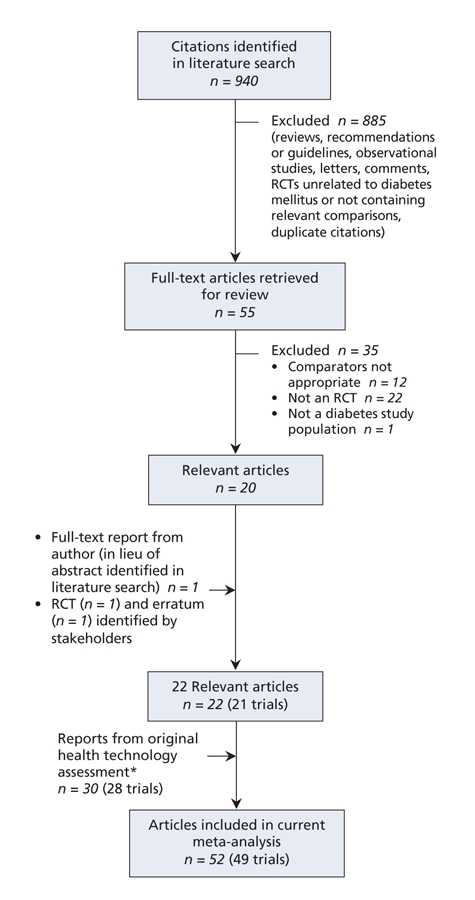
Figure 2: Selection of randomized controlled trials (RCTs) of long-acting insulin analogues for inclusion in the meta-analysis. *Thirty-four studies were included in the original health technology assessment;14 however, 4 abstracts143–146 were replaced by full-text publications identified during the update.
We identified no studies of insulin analogues in pediatric type 2 diabetes, or of long-acting insulin analogues in pregnant women with diabetes. Also, we found few studies evaluating the insulin analogues in specific ethnic groups, and none in First Nations populations.
Study characteristics and methodologic quality
Most of the trials included in the current meta-analysis were multinational and sponsored by industry. The number of patients in each study ranged from 7 to 1008. Of the 48 crossover studies,19,20,24,25,30,32,33,35,37–39,42–45,47,48,51,55,56,58,62–67,69–74,78,79,81–83,85,87,89,93,96,100–102,121 most lacked or did not mention a washout period. All studies were of open-label design. Trial duration ranged from 4 weeks to 30 months. Within each population and comparison, we found no major differences across trials in terms of patient characteristics (e.g., sex, degree of obesity, and severity or duration of diabetes).
The methodologic quality of most of the trials was rated as poor (Jadad score 2 or 3). No study was double-blinded, and allocation concealment was rarely described. Detailed ratings of study quality are reported in Appendices 5, 6 and 7 for rapid-acting insulin analogues and in Appendices 8 and 9 for long-acting insulin analogues (appendices are available at www.cmaj.ca/cgi/content/full/180/4/385/DC2).
Efficacy and safety in type 1 diabetes
Adults
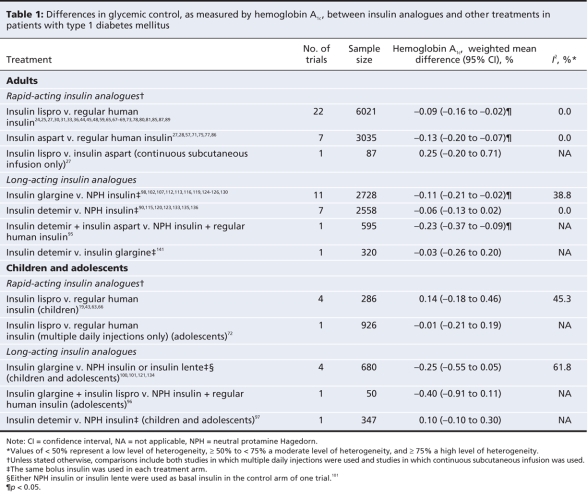
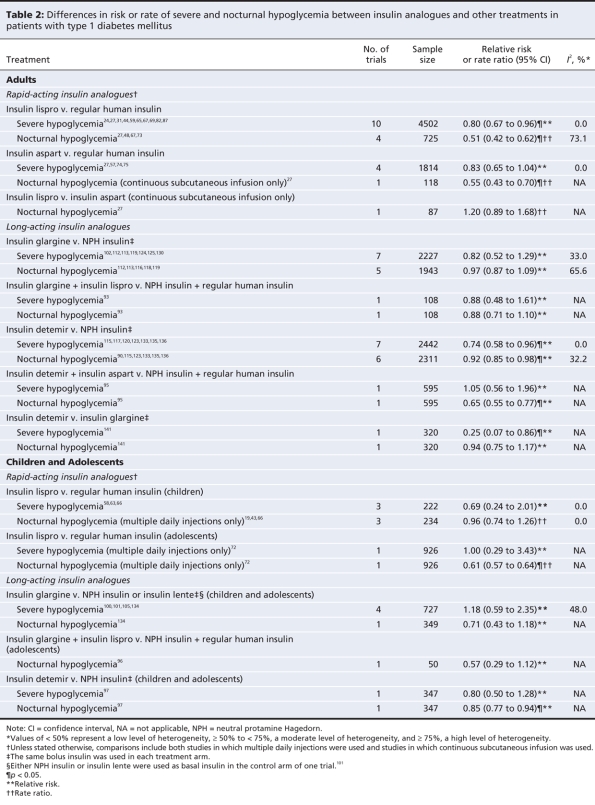
Differences between treatments in terms of glycemic control in adults with type 1 diabetes are presented in Table 1 and Appendix 10 (available at www.cmaj.ca/cgi/content/full/180/4/385/DC2). Differences between treatments in terms of severe and nocturnal hypoglycemia in this patient group are presented in Table 2.
Table 1
Table 2
Compared with regular human insulin, use of insulin lispro resulted in a marginally lower hemoglobin A1c concentration (weighted mean difference –0.09%, 95% CI –0.16% to –0.02%), a lower risk of severe hypoglycemia (relative risk 0.80, 95% CI 0.67 to 0.96) and a lower rate of nocturnal hypoglycemia (rate ratio 0.51, 95% CI 0.42 to 0.62). There was a high degree of heterogeneity that was not explained by differences in patient characteristics or treatments across the studies that reported rates of nocturnal hypoglycemia in the overall analysis (I2 = 73.1%). For overall hypoglycemia, the rate was similar between the groups receiving insulin lispro and those receiving regular human insulin (data not shown). Subgroup analyses by method of administration did not reveal substantial differences in treatment effects between patients using multiple daily injections and those using continuous subcutaneous infusion (data not shown). In the group using continuous subcutaneous infusion, fewer of the patients given regular human insulin than of those given insulin lispro experienced severe hypoglycemia; however, the difference in risk between treatment groups was statistically nonsignificant.
For insulin aspart, the mean hemoglobin A1c concentration was slightly lower than the concentration with regular human insulin (weighted mean difference –0.13%, 95% CI –0.20% to –0.07%). There were no significant differences between treatments in the risk of severe hypoglycemia or the rate of overall hypoglycemia (data not shown). In the only study reporting data on nocturnal hypoglycemia, the rate among patients given insulin aspart through continuous subcutaneous infusion was significantly lower than the rate among those given regular human insulin (rate ratio 0.55, 95% CI 0.43 to 0.70).27 Subgroup analyses did not reveal important differences in treatment effects between patients using multiple daily injections and those using continuous subcutaneous infusion (data not shown).
Patients generally preferred rapid-acting insulin analogues over regular human insulin because of flexibility in dosing relative to mealtimes.25,39,42,73,83 Some studies that assessed quality of life and patient satisfaction reported statistically significant improvements with the use of rapid-acting insulin analogues compared with regular human insulin, whereas others found no differences between treatments (data not shown).25,29,39,42,57,62,65,67,73,79–83,89
The single study comparing insulin lispro with insulin aspart administered through continuous subcutaneous infusion reported nonsignificant differences in hemoglobin A1c (weighted mean difference 0.25%, 95% CI –0.20% to 0.71%) and rate of nocturnal hypoglycemia (rate ratio 1.20, 95% CI 0.89 to 1.68). However, the rate of overall hypoglycemia significantly favoured insulin aspart (rate ratio for insulin lispro v. insulin aspart 1.49, 95% CI 1.37 to 1.63).
Relative to neutral protamine Hagedorn insulin, insulin glargine provided a small but statistically significant improvement in hemoglobin A1c (weighted mean difference –0.11%, 95% CI –0.21% to –0.02%). There were no significant differences in the risk or rate of any type of hypoglycemia when the same bolus insulin was used in each treatment arm. The relative risk estimate for nocturnal hypoglycemia demonstrated a high degree of heterogeneity (I2 = 65.6%), which was substantially reduced when the study of shortest duration (4 weeks)112 was removed from the meta-analysis. This study demonstrated the largest risk reduction in favour of insulin glargine (relative risk 0.64, 95% CI 0.47 to 0.87).
In the pooled analysis of trials comparing insulin detemir and neutral protamine Hagedorn insulin, we found no significant difference in hemoglobin A1c (weighted mean difference –0.06%, 95% CI –0.13% to 0.02%). We found slight reductions in the risk of severe hypoglycemia (relative risk 0.74, 95% CI 0.58 to 0.96) and nocturnal hypoglycemia (relative risk 0.92, 95% CI 0.85 to 0.98) in favour of insulin detemir, but not overall hypoglycemia (data not shown). Also, we found statistically significant reductions in the rates of nocturnal and overall hypoglycemia in favour of insulin detemir (data not shown).
For insulin glargine and insulin detemir, each compared with neutral protamine Hagedorn insulin, we found that the effect estimates for hemoglobin A1c and hypoglycemia did not differ substantially according to the type of bolus insulin used (data not shown).
A single study reported that insulin glargine was not significantly different from neutral protamine Hagedorn insulin in terms of quality of life; however, it did show significantly greater patient satisfaction with insulin glargine (data not shown).137 No study reported data on quality of life or patient satisfaction with insulin detemir.
We found no significant difference in hemoglobin A1c between insulin detemir and insulin glargine in the single trial that compared the 2 agents (weighted mean difference –0.03%, 95% CI –0.26% to 0.20%). The risk of severe hypoglycemia (relative risk 0.25, 95% CI 0.07 to 0.86) and the rate ratios for severe and nocturnal hypoglycemia (data not shown) were statistically significant in favour of insulin detemir.
There were insufficient data available to compare insulin analogues and conventional insulins in terms of diabetic complications or death.
Children and adolescents
Differences between treatments in terms of glycemic control in children and adolescents with type 1 diabetes are presented in Table 1. Differences in the risk of hypoglycemia are presented in Table 2.
The only trial that compared insulin lispro with regular human insulin in adolescents with type 1 diabetes showed no significant difference in hemoglobin A1c (weighted mean difference –0.01%, 95% CI –0.21% to 0.19%) or risk of severe hypoglycemia (relative risk 1.00, 95% CI 0.29 to 3.43). The rate ratios for nocturnal hypoglycemia (rate ratio 0.61, 95% CI 0.57 to 0.64) and overall hypoglycemia (data not shown) significantly favoured insulin lispro.
In the pooled analysis of trials comparing insulin lispro and regular human insulin in preadolescent patients with type 1 diabetes, we found no significant difference in hemoglobin A1c (weighted mean difference 0.14%, 95% CI –0.18% to 0.46%), risk of severe hypoglycemia (relative risk 0.69, 95% CI 0.24 to 2.01) or rates of nocturnal hypoglycemia (rate ratio 0.96, 95% CI 0.74 to 1.26) and overall hypoglycemia (data not shown).
The only study that compared insulin aspart and regular human insulin in preadolescent patients with type 1 diabetes showed no significant difference in hemoglobin A1c or risk of overall hypoglycemia between treatment groups (data not shown).62 A second study that compared insulin aspart with regular human insulin and insulin lispro in 378 patients aged 6–18 years also reported no significant differences between treatments in terms of hemoglobin A1c or hypoglycemia (data not shown).26
We did not observe statistically significant differences between insulin glargine and conventional intermediate-acting insulins (mostly neutral protamine Hagedorn insulin) in children and adolescents with type 1 diabetes in terms of hemoglobin A1c (weighted mean difference –0.25%, 95% CI –0.55% to 0.05%) or any type of hypoglycemia. We observed a large degree of heterogeneity in the hemoglobin A1c estimate (I2 = 61.8%). This was due, at least in part, to the trial that reported the largest mean difference in hemoglobin A1c in favour of insulin glargine (–0.70%, 95% CI –1.12% to –0.28%).121 This study differed from the others in 2 ways: it involved Japanese patients as old as 21 years, and insulin aspart was used as the bolus insulin in both treatment arms.
The only trial that compared insulin detemir with neutral protamine Hagedorn insulin in children and adolescents with type 1 diabetes showed no significant differences between treatments in hemoglobin A1c (weighted mean difference 0.10%, 95% CI –0.10% to 0.30%) or severe hypoglycemia (relative risk 0.80, 95% CI 0.50 to 1.28). The relative risk of nocturnal hypoglycemia (0.85, 95% CI 0.77 to 0.94) and the rate ratios for nocturnal and overall hypoglycemia (data not shown) demonstrated small, statistically significant benefits in favour of insulin detemir.
No data on quality of life, patient satisfaction, diabetes-related complications or death were reported in any of the studies comparing insulin analogues with conventional insulins in children and adolescents. Also, we found no intraclass comparisons for either the rapid-acting or the long-acting insulin analogues.
Efficacy and safety in type 2 diabetes
Adults
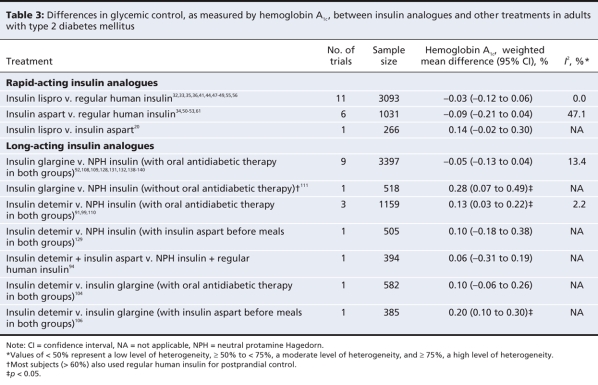
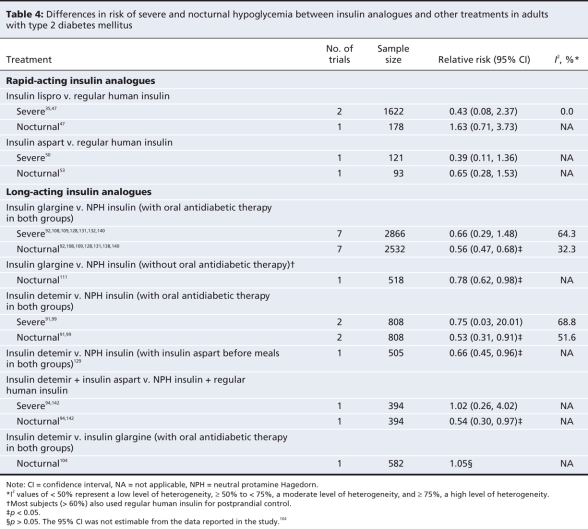
Differences between treatments in terms of glycemic control in adults with type 2 diabetes are presented in Table 3 and Appendix 11 (available at www.cmaj.ca/cgi/content/full/180/4/385/DC2). Differences in terms of hypoglycemia are presented in Table 4. In the pooled analysis of trials comparing insulin lispro and regular human insulin, we observed no significant differences in hemoglobin A1c (weighted mean difference –0.03%, 95% CI –0.12% to 0.06%) or in the risk of severe hypoglycemia (relative risk 0.43, 95% CI 0.08 to 2.37), nocturnal hypoglycemia (relative risk 1.63, 95% CI 0.71 to 3.73) or overall hypoglycemia (data not shown). However, the rate ratio for nocturnal, but not severe, hypoglycemia, was statistically significant in favour of insulin lispro (data not shown).
Table 3
Table 4
The pooled analysis of trials comparing insulin aspart and regular human insulin showed no significant differences in hemoglobin A1c (weighted mean difference –0.09%, 95% CI –0.21% to 0.04%) or in the risk of any type of hypoglycemia between the treatment groups. The patients given insulin aspart had significantly fewer events of overall hypoglycemia than did those given regular human insulin (data not shown).
Two studies comparing insulin lispro with regular human insulin reported data on quality of life and patient satisfaction.39,41 They found no significant differences between treatment groups except that “worry related to diabetes” was significantly improved with insulin lispro in one of the studies (data not shown).41 None of the studies of insulin aspart in type 2 diabetes reported data on quality of life or patient satisfaction.
A single study comparing biphasic insulin lispro and biphasic insulin aspart reported no significant difference in hemoglobin A1c (mean difference 0.14%, 95% CI –0.02% to 0.30%) or overall hypoglycemia (data not shown) in adults with type 2 diabetes.
Combined therapy with oral antidiabetic agents was allowed in most trials that compared insulin glargine with neutral protamine Hagedorn insulin in adults with type 2 diabetes. Only one study compared insulin glargine with neutral protamine Hagedorn insulin in combination with a bolus insulin (i.e., without combined therapy with oral antidiabetic agents). Glycemic control was no better in the insulin glargine group regardless of the type of combined therapy (weighted mean difference in hemoglobin A1c –0.05%, 95% CI –0.13% to 0.04%, for insulin glargine with oral antidiabetic therapy; 0.28%, 95% CI 0.07% to 0.49%, for insulin glargine with bolus insulin).
There was no significant difference in the risk of severe hypoglycemia in the studies that used oral antidiabetic therapy (relative risk 0.66, 95% CI 0.29 to 1.48). However, the rate ratio was statistically significant in favour of insulin glargine (data not shown). Both the relative risk (I2 = 64%) and rate ratio (I2 = 83%) estimates demonstrated a high degree of heterogeneity that was due at least in part to opposite effects in the studies combining insulins with sulfonylureas,92,108,109,128 versus those combining insulins with various oral antidiabetic therapies.131,132,140 The risk and rate for severe hypoglycemia were significantly lower among patients given insulin glargine in the sulfonylurea subgroup, but not in the subgroup in which various oral antidiabetic therapies were used (data not shown).
The relative risk for nocturnal hypoglycemia significantly favoured insulin glargine in both the bolus insulin study (relative risk 0.78, 95% CI 0.62 to 0.98) and the studies that allowed oral antidiabetic therapy (relative risk 0.56, 95% CI 0.47 to 0.68). Rate ratio results were similar to those for relative risk in the studies allowing oral antidiabetic therapy, and not estimable in the bolus insulin study. There was a small, statistically significant reduction in risk of overall hypoglycemia in favour of insulin glargine in the studies allowing oral antidiabetic therapy but not in the bolus insulin study (data not shown).
Four studies compared insulin detemir with neutral protamine Hagedorn insulin in adults with type 2 diabetes. Three allowed the use of oral antidiabetic therapy, and 1 study used bolus insulin (insulin aspart) before meals. In the study that used bolus insulin, there was no significant difference between treatment groups in terms of hemoglobin A1c (weighted mean difference 0.10%, 95% CI –0.18% to 0.38%) or risk of overall hypoglycemia (data not shown). The risk of nocturnal hypoglycemia was lower in the insulin detemir group (relative risk 0.66, 95% CI 0.45 to 0.96). The pooled analysis of results from the studies that allowed oral antidiabetic therapy showed a small but statistically significant difference in hemoglobin A1c in favour of neutral protamine Hagedorn insulin (weighted mean difference 0.13%, 95% CI 0.03% to 0.22%). The relative risk for severe hypoglycemia was not statistically significant, although the relative risks for nocturnal hypoglycemia (relative risk 0.53, 95% CI 0.31 to 0.91) and overall hypoglycemia (data not shown) significantly favoured insulin detemir. All 3 relative risk estimates, obtained by pooling data across 2 studies,91,99 demonstrated a high degree of heterogeneity. This may have been due to the fact that one study administered insulin detemir and neutral protamine Hagedorn insulin once daily99 and the other study administered both agents twice daily.91 The study with doses given once daily reported larger reductions in risk of hypoglycemia in favour of insulin detemir than did the study with doses given twice daily. Rate ratios for all types of hypoglycemia were statistically significant in favour of insulin detemir (data not shown).
In terms of patient satisfaction with long-acting insulin analogue treatment, one study92 found a small yet statistically significant benefit in favour of insulin glargine over neutral protamine Hagedorn insulin (data not shown). No studies of long-acting insulin analogues reported data on quality of life.
Two studies compared insulin detemir with insulin glargine in patients with type 2 diabetes. One of the studies allowed the use of oral antidiabetic therapy; it showed no significant difference in terms of hemoglobin A1c (weighted mean difference 0.10%, 95% CI –0.06% to 0.26%) or nocturnal hypoglycemia. The other study used bolus insulin (insulin aspart); it reported a slightly higher hemoglobin A1c with insulin detemir (weighted mean difference 0.20%, 95% CI 0.10% to 0.30%). Neither study reported a difference in risk of overall hypoglycemia (data not shown).
There were insufficient data available for comparisons between insulin analogues and conventional insulins in terms of diabetes-related complications or death.
Efficacy and safety in pregnant women with diabetes
In the pooled analysis of results from studies comparing insulin lispro and regular human insulin in pregnant women, we observed no significant differences in hemoglobin A1c (weighted mean difference 0.20%, 95% CI –1.03% to 1.43%) or risk of severe hypoglycemia (relative risk 0.21, 95% CI 0.01 to 4.10) among women with type 1 diabetes.40,46,84 We also observed no significant difference in hemoglobin A1c among women with gestational diabetes (weighted mean difference 0.06%, 95% CI –0.11% to 0.23%).40,46,84
Results from a single trial comparing insulin aspart with regular human insulin in pregnant women with type 1 diabetes were similar to those for insulin lispro in terms of hemoglobin A1c (weighted mean difference –0.08%, 95% CI –0.28% to 0.12%), risk of severe hypoglycemia (relative risk 1.14, 95% CI 0.76 to 1.71) and risk of overall hypoglycemia (relative risk 1.04, 95% CI 0.98 to 1.11).22
We did not identify randomized controlled trials of long-acting insulin analogues in pregnant women.
Adverse events
Adverse events other than hypoglycemia that were reported in the included studies are presented for rapid-acting and long-acting insulin analogues in type 1 and type 2 diabetes (Appendices 12 to 15; available at www.cmaj.ca/cgi/content/full/180/4/385/DC2). The most commonly reported adverse events were infections of the upper respiratory tract, reactions at the injection site and weight gain. The incidence of adverse events was similar between insulin analogues and conventional insulins. Serious adverse events were uncommon.
Interpretation
Our results suggest that differences between conventional insulins and insulin analogues are minimal in the management of type 1, type 2 and gestational diabetes. Compared with the original health technology assessments,13,14 we included studies published up to April 2007. As well, we assessed more outcomes and conducted intraclass comparisons for both the rapid-and long-acting insulin analogues.
We found that most estimates of differences in hemoglobin A1c between treatment groups were not statistically significant. Where they were statistically significant in favour of insulin analogues, the differences were smaller than minimal clinically important differences described in the literature.10,147
We found statistically significant benefits of insulin analogues over conventional insulins in terms of hypoglycemia for some comparisons, populations and hypoglycemia types. However, we did not consistently observe a major clinical advantage in terms of hypoglycemia for either the rapid-acting or the long-acting insulin analogues over conventional insulins. In particular, no relative risk or rate ratio estimates for hypoglycemia were statistically significant for insulin glargine versus neutral protamine Hagedorn insulin in type 1 diabetes, for insulin aspart versus regular human insulin in pediatric type 1 diabetes or for rapid-acting insulin analogues versus regular human insulin in gestational diabetes. Furthermore, several trials excluded subjects with a history of recurrent major hypoglycemia; therefore, the benefits of insulin analogues in such patients remain uncertain. This was particularly the case for trials that compared insulin detemir with neutral protamine Hagedorn insulin.
Few of the studies reported on patient satisfaction with treatment or quality of life. This suggests that these outcomes are rarely measured or are selectively reported. When data for these outcomes were available, substantial heterogeneity in methods across studies precluded pooling of the results. Some studies reported insulin analogues to be statistically significantly superior to conventional insulins in terms of quality of life; however, results were inconsistent, and differences often appeared to be small and of uncertain clinical significance.
All of the head-to-head comparisons between insulin analogues of the same class showed little or no differences in glycemic control or risk of hypoglycemia.
Studies of insulin analogues were not sufficiently powered or of adequate duration to measure differences in long-term diabetes-related complications or death.
Our results regarding the effects of rapid-acting insulin analogues in type 1, type 2 and gestational diabetes and of long-acting insulin analogues in type 2 diabetes are similar to those reported by others.7–10 In a recent systematic review, biphasic insulin analogues were found to be similar to biphasic human insulin in terms of glycemic control and hypoglycemia rates among patients with type 2 diabetes.148 In our analysis, such trials were pooled with data from trials comparing rapid-acting insulin analogues and regular human insulin. However, subgroup analysis of trials of biphasic insulin did not yield substantially different results from the overall analysis (data not shown). Consistent with our findings, previous reviews observed that most trials of insulin analogues had methodologic limitations.7,10 For example, allocation concealment was adequate in only 10 of 117 trials included in our review (Appendices 5 to 9, available online at www.cmaj.ca/cgi/content/full/180/4/385/DC2). Thus, the potential for ascertainment bias is heightened,149 especially for subjective outcomes such as patient-reported hypoglycemia and quality of life.
Limitations
Like all systematic reviews, our analysis has limitations. First, we restricted our search to trials published in English; therefore, we may have missed articles published in other languages. However, empirical evidence suggests that exclusion of non-English trials has minimal impact on the results of systematic reviews and meta-analyses.150–152 Furthermore, additional trials published in languages other than English were not identified by stakeholders.
Second, there was heterogeneity across the trial results, as indicated by high I2 values. The degree of heterogeneity was particularly high for hypoglycemia outcomes. Although we could not always identify reasons for heterogeneity, we observed that studies reporting outlying estimates of effect differed in terms of patient characteristics or treatment strategies in some analyses. In most cases, results were qualitatively similar across studies in terms of direction of effect, even in the presence of large I2 values.
Third, we pooled data for hemoglobin A1c and hypoglycemia separately. Investigators instituting more aggressive glycemic control may have been less likely to find differences in hemoglobin A1c between treatment groups but more likely to observe benefits in hypoglycemia. By pooling studies without accounting for this correlation, we may have underestimated the benefit of the insulin analogues. However, the results of a recent metaregression analysis of insulin glargine using patient-level data suggest that adjustment for hemoglobin A1c does not greatly affect estimates of relative risk for hypoglycemia.153
The remaining limitations pertain to the available evidence on insulin analogues. More studies are needed to understand better the impact of insulin analogues on long-term diabetes-related complications, death, quality of life and patient satisfaction. Improvements in methodologic quality of trials are also necessary to produce valid assessments of the efficacy and safety of these agents. Furthermore, the relative safety and effectiveness of insulin analogues versus conventional insulins requires study in patients with a prior history of significant hypoglycemia, children with type 2 diabetes and, for the long-acting insulin analogues, pregnant women.
Conclusion
Our results indicate that insulin analogues offer few clinical advantages over conventional insulins in the management of most patients with type 1, type 2 or gestational diabetes. Although the evidence supporting the benefit of insulin analogues in terms of hypoglycemia is weak, these agents may be an option for patients with problematic hypoglycemia despite optimization of conventional insulin therapy. In a companion paper (see page 369 of this issue),154 we report on the cost-effectiveness of insulin analogues in the management of type 1 and type 2 diabetes in adults. The results of the cost-effectiveness analysis serve to clarify further the optimal place of insulin analogues relative to conventional insulins in the management of diabetes in the Canadian health care system.
@@ See related commentary by Siebenhofer-Kroitzsch and colleagues, page 369
Supplementary Material
Acknowledgments
We thank Greg Bak and Michelle Fiander for developing and implementing the literature search strategies, and Samantha Verbrugghe for assistance with data management.
Footnotes
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/180/4/385/DC1
Funding: This research was supported through a financial contribution from Health Canada to COMPUS.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the study. Sumeet Singh, Fida Ahmad, Avtar Lal, Changhua Yu and Zemin Bai extracted data from primary studies and analyzed and interpreted the results. Heather Bennett provided oversight for the extraction, analysis and interpretation of the data. Sumeet Singh, with the help of Avtar Lal, Changhua Yu and Heather Bennett, drafted the manuscript. All of the authors critically reviewed the manuscript. Zemin Bai ensured that the analysis was conducted appropriately and verified the accuracy of results presented in the manuscript. All of the authors approved the final version submitted for publication.
This systematic review was conducted by researchers at the Canadian Optimal Medication Prescribing and Utilization Service (COMPUS), a directorate of the Canadian Agency for Drugs and Technologies in Health (CADTH). COMPUS identifies optimal drug therapy, develops intervention tools and provides services to promote and encourage the use of evidence-based clinical and cost-effectiveness information in decision-making by health care providers and patients. COMPUS is a collaborative, pan-Canadian service funded by Health Canada. For more information, visit www.cadth.ca.
Competing interests: None declared.
Correspondence to: Mr. Sumeet Singh, Canadian Agency for Drugs and Technologies in Health, 600–865 Carling Ave., Ottawa ON K1S 5S8; fax 613 226-5392; sumeets@cadth.ca
REFERENCES
- 1.Diabetes: facts and figures. Ottawa (ON): Public Health Agency of Canada; 2003. Available: www.phac-aspc.gc.ca/ccdpc-cpcmc/diabetes-diabete/english/facts/index.html (accessed 2008 Dec. 2).
- 2.Public Health Agency of Canada Diabetes in Canada. Highlights from the National Diabetes Surveillance System 2004–2005. Ottawa (ON):The Agency; 2008. Available: www.phac-aspc.gc.ca/publicat/2008/dicndss-dacsnsd-04-05/pdf/dicndss-04-05-eng.pdf (accessed 2008 Dec. 2).
- 3.Dawson KG, Gomes D, Gerstein H, et al. The economic cost of diabetes in Canada, 1998. Diabetes Care 2002;25:1303-7. Available: http://care.diabetesjournals.org/cgi/reprint/25/8/1303.pdf (accessed 2008 Dec. 2). [DOI] [PubMed]
- 4.Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes (Suppl 2) 2003;27:i-S140. Available: www.diabetes.ca/cpg2003/downloads/cpgcomplete.pdf (accessed 2008 Dec. 2).
- 5.Hirsch IB. Insulin analogues. N Engl J Med 2005;352:174-83. [DOI] [PubMed]
- 6.Compendium of pharmaceuticals and specialties, online version (e-CPS). Ottawa (ON): Canadian Pharmacists Association; 2006.
- 7.Siebenhofer A, Plank J, Berghold A, et al. Short acting insulin analogues versus regular human insulin in patients with diabetes mellitus. Cochrane Database of Syst Rev 2006;19;(2):CD003287. [DOI] [PubMed]
- 8.Plank J, Siebenhofer A, Berghold A, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005;165:1337-44. [DOI] [PubMed]
- 9.Institute for Quality and Efficiency in Health Care. Rapid-acting insulin analogues in the treatment of diabetes mellitus type 1. Cologne (Germany): The Institute; 2007. Available: www.iqwig.de/download/A05-02_Executive_Summary_Rapid-acting_insulin_analogues_in_the_treatment_of_diabetes_mellitus_type_1.pdf (accessed 2008 Dec. 2). [PubMed]
- 10.Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007;(2):CD005613. [DOI] [PubMed]
- 11.Canadian Agency for Drugs and Technologies in Health. Rapid-acting insulin analogues for the treatment of diabetes mellitus: meta-analyses of clinical outcomes. Ottawa (ON): The Agency; 2008. Available: http://cadth.ca/media/compus/reports/compus_Rapid-Acting-Insulin-Analogues-Report_Clinical=Outcomes.pdf (accessed 2008 Dec. 2).
- 12.Canadian Agency for Drugs and Technologies in Health. Long-acting insulin analogues for the treatment of diabetes mellitus: meta-analyses of clinical outcomes. Ottawa (ON): The Agency; 2008. Available: http://cadth.ca/media/compus/reports/compus_Long-Acting-Insulin-Analogs-Report_Clinical-Outcomes.pdf (accessed 2008 Dec. 2).
- 13.Banerjee S, Tran K, Li H, et al. Short-acting insulin analogues for diabetes mellitus: meta-analysis of clinical outcomes and assessment of cost-effectiveness [report]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2007. Available: www.cadth.ca/media/pdf/341A_Insulin_tr_e.pdf (accessed 2008 Dec. 2).
- 14.Tran K, Banerjee S, Li H, et al. Long-acting insulin analogues for diabetes mellitus: meta-analysis of clinical outcomes and assessment of cost-effectiveness [report]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2007. Available: www.cadth.ca/media/pdf/341b_Long-acting-insulin_tr_e.pdf (accessed 2008 Dec. 2).
- 15.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions 4.2.6. Chichester (UK): John Wiley & Sons, Ltd.; 2006. Available: www.cochrane.org/resources/handbook/Handbook4.2.6Sep2006.pdf (accessed 2008 Dec. 2).
- 16.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed]
- 17.Schulz KF, Chalmers I, Hayes RJ, et al. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. [DOI] [PubMed]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed]
- 19.Fairchild JM, Ambler GR, Genoud-Lawton CH, et al. Insulin lispro versus regular insulin in children with type 1 diabetes on twice daily insulin. Pediatr Diabetes 2000;1:135-41. [DOI] [PubMed]
- 20.Niskanen L, Jensen LE, Rastam J, et al. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther 2004;26:531-40. [DOI] [PubMed]
- 21.Ampudia-Blasco FJ, Girbes J, Sanz J, et al. Regular insulin is as effective as rapid-acting insulin analogs in combination with glargine insulin in type 1 diabetic patients. Proceedings from the 41st Annual Meeting of the European Association for the Study of Diabetes; 2005 Sept. 10; Athens (Greece).
- 22.Mathiesen ER, Kinsley B, Amiel SA, et al. Maternal glycemic control and hypoglycemia in type 1 diabetic pregnancy: a randomized trial of insulin aspart versus human insulin in 322 pregnant women. Diabetes Care 2007;30:771-6. Available: http://care.diabetesjournals.org/cgi/reprint/30/4/771 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 23.Gallagher A, Butler TJ, Home PD. The effect of the optimal use of rapid-acting insulin analogues on insulin secretion in type 2 diabetes. Diabetes Res Clin Pract 2007;76:327-34. [DOI] [PubMed]
- 24.Anderson JH, Brunelle RL, Koivisto VA, et al. Reduction of postprandial hyperglycemia and frequency of hypoglycemia in IDDM patients on insulin-analog treatment. Diabetes 1997;46:265-70. [DOI] [PubMed]
- 25.Annuzzi G, Del Prato S, Arcari R, et al. Preprandial combination of lispro and NPH insulin improves overall blood glucose control in type 1 diabetic patients: a multicenter randomized crossover trial. Nutr Metab Cardiovasc Dis 2001;11:168-75. [PubMed]
- 26.Arslanian S, Foster C, Wright NM, et al. Insulin aspart compared to regular insulin and insulin lispro in basal bolus therapy with NPH to treat pediatric patients with type 1 diabetes mellitus. Proceedings from the 41st Annual Meeting of the European Association for the Study of Diabetes; 2005 Sep 10; Athens (Greece).
- 27.Bode B, Weinstein R, Bell D, et al. Comparison of insulin aspart with buffered regular insulin and insulin lispro in continuous subcutaneous insulin infusion: a randomized study in type 1 diabetes. Diabetes Care 2002;25:439-44. Available: http://care.diabetesjournals.org/cgi/content/full/25/3/439 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 28.Bode BW, Strange P. Efficacy, safety, and pump compatibility of insulin aspart used in continuous subcutaneous insulin infusion therapy in patients with type 1 diabetes. Diabetes Care 2001;24:69-72. Available: http://care.diabetesjournals.org/cgi/reprint/24/1/69 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 29.Bott U, Ebrahim S, Hirschberger S, et al. Effect of the rapid-acting insulin analogue insulin aspart on quality of life and treatment satisfaction in patients with type 1 diabetes. Diabet Med 2003;20:626-34. [DOI] [PubMed]
- 30.Caixàs A, Pérez A, Payés A, et al. Effects of a short-acting insulin analog (insulin lispro) versus regular insulin on lipid metabolism in insulin-dependent diabetes mellitus. Metabolism 1998;47:371-6. [DOI] [PubMed]
- 31.Ciofetta M, Lalli C, Del Sindaco P, et al. Contribution of postprandial versus interprandial blood glucose to HbA1c in type 1 diabetes on physiologic intensive therapy with lispro insulin at mealtime. Diabetes Care 1999;22:795-800. Available: http://care.diabetesjournals.org/cgi/reprint/22/5/795 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 32.Schernthaner G, Kopp HP, Ristic S, et al. Metabolic control in patients with type 2 diabetes using Humalog Mix50T injected three times daily: crossover comparison with human insulin 30/70. Horm Metab Res 2004;36:188-93. [DOI] [PubMed]
- 33.Chan WB, Chow CC, Yeung VT, et al. Effect of insulin lispro on glycaemic control in Chinese diabetic patients receiving twice-daily regimens of insulin. Chin Med J (Engl) 2004;117:1404-7. [PubMed]
- 34.Bretzel RG, Arnolds S, Medding J, et al. A direct efficacy and safety comparison of insulin aspart, human soluble insulin, and human premix insulin (70/30) in patients with type 2 diabetes. Diabetes Care 2004;27:1023-7. Available: http://care.diabetesjournals.org/cgi/reprint/27/5/1023 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 35.Anderson JH, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patients with non-insulin-dependent diabetes mellitus. Arch Intern Med 1997;157:1249-55. [PubMed]
- 36.Anderson JH, Brunelle RL, Koivisto VA, et al. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clin Ther 1997;19:62-72. [DOI] [PubMed]
- 37.Herz M, Profozic V, Arora V, et al. Effects of a fixed mixture of 25% insulin lispro and 75% NPL on plasma glucose during and after moderate physical exercise in patients with type 2 diabetes. Curr Med Res Opin 2002;18:188-93. [DOI] [PubMed]
- 38.Herz M, Arora V, Campaigne BN, et al. Humalog Mix25 improves 24-hour plasma glucose profiles compared with the human insulin mixture 30/70 in patients with type 2 diabetes mellitus. S Afr Med J 2003;93:219-23. [PubMed]
- 39.Kotsanos JG, Vignati L, Huster W, et al. Health-related quality-of-life results from multinational clinical trials of insulin lispro: assessing benefits of a new diabetes therapy. Diabetes Care 1997;20:948-58. [DOI] [PubMed]
- 40.Mecacci F, Carignani L, Cioni R, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol 2003;111:19-24. [DOI] [PubMed]
- 41.Ross SA, Zinman B, Campos RV, et al.; Canadian Lispro Study Group. A comparative study of insulin lispro and human regular insulin in patients with type 2 diabetes mellitus and secondary failure of oral hypoglycemic agents. Clin Invest Med 2001;24:292-8. [PubMed]
- 42.Schmauss S, König A, Landgraf R. Human insulin analogue [LYS(B28), PRO(B29)]: The ideal pump insulin? Diabet Med 1998;15:247-9. [DOI] [PubMed]
- 43.Tupola S, Komulainen J, Jääskeläinen J, et al. Post-prandial insulin lispro vs. human regular insulin in prepubertal children with type 1 diabetes mellitus. Diabet Med 2001;18:654-8. [DOI] [PubMed]
- 44.Vignati L, Anderson JH, Iversen PW; Multicenter Insulin Lispro Study Group. Efficacy of insulin lispro in combination with NPH human insulin twice per day in patients with insulin-dependent or non-insulin-dependent diabetes mellitus. Clin Ther 1997;19:1408-21. [DOI] [PubMed]
- 45.Zinman B, Tildesley H, Chiasson JL, et al. Insulin lispro in CSII: results of a double-blind crossover study. Diabetes 1997;46:440-3. [DOI] [PubMed]
- 46.Jovanovic L, Ilic S, Pettitt DJ, et al. Metabolic and immunologic effects of insulin lispro in gestational diabetes. Diabetes Care 1999;22:1422-7. Available: http://care.diabetesjournals.org/cgi/reprint/22/9/1422 (accessed 2008 Dec 4). [DOI] [PubMed]
- 47.Roach P, Yue L, Arora V; Humalog Mix25 Study Group. Improved postprandial glycemic control during treatment with Humalog Mix25, a novel protamine-based insulin lispro formulation. Diabetes Care 1999;22:1258-61. Available: http://care.diabetesjournals.org/cgi/reprint/22/8/1258 (accessed 2008 Dec 4). [DOI] [PubMed]
- 48.Roach P, Trautmann M, Arora V, et al.; Mix50 Study Group. Improved postprandial blood glucose control and reduced nocturnal hypoglycemia during treatment with two novel insulin lispro-protamine formulations, insulin lispro mix25 and insulin lispro mix50. Clin Ther 1999;21:523-34. [DOI] [PubMed]
- 49.Altuntas Y, Ozen B, Ozturk B, et al. Comparison of additional metformin or NPH insulin to mealtime insulin lispro therapy with mealtime human insulin therapy in secondary OAD failure. Diabetes Obes Metab 2003;5:371-8. [DOI] [PubMed]
- 50.Boehm BO, Vaz JA. Brøndsted L, Home PD. Long-term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. Eur J Intern Med 2004;15:496-502. [DOI] [PubMed]
- 51.Gallagher A, Home PD. The effect of improved post-prandial blood glucose control on post-prandial metabolism and markers of vascular risk in people with type 2 diabetes. Diabetes Res Clin Pract 2005;67:196-203. [DOI] [PubMed]
- 52.Iwamoto Y. A randomised, multicentre trial of biphasic insulin aspart versus biphasic human insulin in type 2 diabetes [abstract]. Diabetologia 2003;46(2 Suppl): A270.
- 53.Kilo C, Mezitis N, Jain R, et al. Starting patients with type 2 diabetes on insulin therapy using once-daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. J Diabetes Complications 2003;17:307-13. [DOI] [PubMed]
- 54.Kokic S, Bukovic D, Radman M, et al. Lispro insulin and metformin versus other combination in the diabetes mellitus type 2 management after secondary oral antidiabetic drug failure. Coll Antropol 2003;27:181-7. [PubMed]
- 55.Laube H, Heller M, Liersch J, et al. Erfahrungen mit lispro -insulin bei intensivierter Behandlung von Typ-i-und Typ-ii-Diabetikern [Experience with lispro insulin in the intensified therapy of IDDM and NIDDM patients]. Diabetes Stoffwechsel 1996;5:273-6.
- 56.Lourens W, Bonnici F, Matthias H, et al. Improved glycaemic control with humalog mix25 compared with human insulin 30/70 in patients with type 2 diabetes. Jemdsa 2000;5:87-92.
- 57.Tamás G, Marre M, Astorga R, et al. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res Clin Pract 2001;54:105-14. [DOI] [PubMed]
- 58.Tubiana-Rufi N, Coutant R, Bloch J, et al. Special management of insulin lispro in continuous subcutaneous insulin infusion in young diabetic children: a randomized cross-over study. Horm Res 2004;62:265-71. [DOI] [PubMed]
- 59.Valle D, Santoro D, Bates P, et al. Italian multicentre study of intensive therapy with insulin lispro in 1184 patients with type 1 diabetes. Diabetes Nutr Metab 2001;14:126-32. [PubMed]
- 60.Ilic S, Jovanovic L, Mezic J, et al. Health related quality of life is associated with insulin lispro use in gestational diabetes mellitus (GDM) [abstract]. Diabetologia 1999;42(Suppl 1):A260.
- 61.Raskin P, McGill J, Kilo C, et al. Human insulin analog (insulin aspart, IAsp) is comparable to human insulin (HI) in type 2 diabetes [abstract]. Diabetes 1999;48(Suppl 1):A355.
- 62.Danne T, Odendahl R, Naeke A, et al. Postprandial insulin aspart is preferred to preprandial human insulin by parents of preschool children with type 1 diabetes [poster]. Proceedings of the 65th Annual Scientific Sessions; 2005 June 10; San Diego (CA).
- 63.Deeb LC, Holcombe JH, Brunelle R, et al. Insulin lispro lowers postprandial glucose in prepubertal children with diabetes. Pediatrics 2001;108:1175-9. Available: http://pediatrics.aappublications.org/cgi/reprint/108/5/1175 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 64.Del Sindaco P, Ciofetta M, Lalli C, et al. Use of the short-acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time-interval injection-meal. Diabet Med 1998;15:592-600. [DOI] [PubMed]
- 65.Ferguson SC, Strachan MW, Janes JM, et al. Severe hypoglycaemia in patients with type 1 diabetes and impaired awareness of hypoglycaemia: a comparative study of insulin lispro and regular human insulin. Diabetes Metab Res Rev 2001;17:285-91. [DOI] [PubMed]
- 66.Ford-Adams ME, Murphy NP, Moore EJ, et al. Insulin lispro: a potential role in preventing nocturnal hypoglycaemia in young children with diabetes mellitus. Diabet Med 2003;20:656-60. [DOI] [PubMed]
- 67.Gale EA; UK Trial Group. A randomized, controlled trial comparing insulin lispro with human soluble insulin in patients with type 1 diabetes on intensified insulin therapy. Diabet Med 2000;17:209-14. [DOI] [PubMed]
- 68.Garg SK, Carmain JA, Braddy KC, et al. Pre-meal insulin analogue insulin lispro vs Humulin R insulin treatment in young subjects with type 1 diabetes. Diabet Med 1996;13:47-52. [DOI] [PubMed]
- 69.Hedman CA, Orre-Pettersson AC, Lindström T, et al. Treatment with insulin lispro changes the insulin profile but does not affect the plasma concentrations of IGF-I and IGFBP-1 in type 1 diabetes. Clin Endocrinol (Oxf) 2001;55:107-12. [DOI] [PubMed]
- 70.Heller SR, Amiel SA, Mansell P; U.K. Lispro Study Group. Effect of the fast-acting insulin analog lispro on the risk of nocturnal hypoglycemia during intensified insulin therapy. Diabetes Care 1999;22:1607-11. Available: http://care.diabetesjournals.org/cgi/reprint/22/10/1607 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 71.Heller SR, Colagiuri S, Vaaler S, et al. Hypoglycaemia with insulin aspart: a double-blind, randomised, crossover trial in subjects with type 1 diabetes. Diabet Med 2004;21:769-75. [DOI] [PubMed]
- 72.Holcombe JH, Zalani S, Arora VK, et al.; Lispro in Adolescents Study Group. Comparison of insulin lispro with regular human insulin for the treatment of type 1 diabetes in adolescents. Clin Ther 2002;24:629-38. [DOI] [PubMed]
- 73.Holleman F, Schmitt H, Rottiers R, et al. Reduced frequency of severe hypoglycemia and coma in well-controlled IDDM patients treated with insulin lispro. Diabetes Care 1997;20:1827-32. [DOI] [PubMed]
- 74.Home PD, Lindholm A, Hylleberg B, et al.; UK Insulin Aspart Study Group. Improved glycemic control with insulin aspart: a multicenter randomized double-blind crossover trial in type 1 diabetic patients. Diabetes Care 1998;21:1904-9. Available: http://care.diabetesjournals.org/cgi/reprint/21/11/1904 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 75.Home PD, Lindholm A, Riis A. the European Insulin Aspart Study Group. Insulin aspart vs. human insulin in the management of long-term blood glucose control in type 1 diabetes mellitus: a randomized controlled trial. Diabet Med 2000;17:762-70. [DOI] [PubMed]
- 76.Home PD, Hallgren P, Usadel KH, et al. Pre-meal insulin aspart compared with pre-meal soluble human insulin in type 1 diabetes. Diabetes Res Clin Pract 2006;71:131-9. [DOI] [PubMed]
- 77.Iwamoto Y, Akanuma Y, Niimi H, et al. Comparison between insulin aspart and soluble human insulin in type 1 diabetes (IDDM) patients treated with basal– bolus insulin therapy — phase III clinical trial in Japan [article in Japanese]. J Japan Diabet Soc 2001;44:799-811.
- 78.Jacobs MA, Keulen ET, Kanc K, et al. Metabolic efficacy of preprandial administration of Lys(B28), Pro(B29) human insulin analog in IDDM patients: a comparison with human regular insulin during a three-meal test period. Diabetes Care 1997;20:1279-86. [DOI] [PubMed]
- 79.Janes JM, Bradley C, Rees A. Preferences for, and improvements in aspects of quality of life (QoL) with, insulin lispro in a multiple injection regime [abstract]. Diabetologia 1997;40(Suppl 1):A353.
- 80.Jansson PA, Ebeling P, Smith U, et al. Improved glycemic control can be better maintained with insulin lispro than with human regular insulin. Diabetes Nutr Metab 1998;11:194-9.
- 81.Johansson UB, Adamson UC, Lins PE, et al. Improved blood glucose variability, HbA1c insuman Infusat and less insulin requirement in IDDM patients using insulin lispro in CSII. The Swedish Multicenter Lispro Insulin Study. Diabetes Metab 2000;26:192-6. [PubMed]
- 82.Linkeschova R, Spraul M, Jatzkowski E, et al. Quality of life, treatment satisfaction and diabetes control on insulin lispro and regular human insulin during CSII: a randomised double-blind crossover study. Diabetes Metab 2003;29.
- 83.Melki V, Renard E, Lassmann-Vague V, et al. Improvement of HbA1c and blood glucose stability in IDDM patients treated with lispro insulin analog in external pumps. Diabetes Care 1998;21:977-82. Available: http://care.diabetesjournals.org/cgi/reprint/21/6/977 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 84.Persson B, Swahn ML, Hjertberg R, et al. Insulin lispro therapy in pregnancies complicated by type 1 diabetes mellitus. Diabetes Res Clin Pract 2002;58:115-21. [DOI] [PubMed]
- 85.Provenzano C, Vero R, Oliva A, et al. Lispro insulin in type 1 diabetic patients on a Mediterranean or normal diet: a randomized, cross-over comparative study with regular insulin. Diabetes Nutr Metab 2001;14:133-9. [PubMed]
- 86.Raskin P, Guthrie RA, Leiter L, et al. Use of insulin aspart, a fast-acting insulin analog, as the mealtime insulin in the management of patients with type 1 diabetes. Diabetes Care 2000;23:583-8. Available: http://care.diabetesjournals.org/cgi/reprint/23/5/583 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 87.Raskin P, Holcombe JH, Tamborlane WV, et al. A comparison of insulin lispro and buffered regular human insulin administered via continuous subcutaneous insulin infusion pump. J Diabetes Complications 2001;15:295-300. [DOI] [PubMed]
- 88.Recasens M, Aguilera E, Morínigo R, et al. Insulin lispro is as effective as regular insulin in optimising metabolic control and preserving b-cell function at onset of type 1 diabetes mellitus. Diabetes Res Clin Pract 2003;60:153-9. [DOI] [PubMed]
- 89.Renner R, Pfutzner A, Trautmann M, et al. Use of insulin lispro in continuous subcutaneous insulin infusion treatment. Results of a multicenter trial. Diabetes Care 1999;22:784-8. Available: http://care.diabetesjournals.org/cgi/reprint/22/5/784 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 90.Kolendorf K, Ross GP, Pavlic-Renar I, et al. Insulin detemir lowers the risk of hypoglycaemia and provides more consistent plasma glucose levels compared with NPH insulin in type 1 diabetes. Diabet Med 2006;23:729-35. [DOI] [PubMed]
- 91.Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269-74. Available: http://care.diabetesjournals.org/cgi/reprint/29/6/1269 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 92.Eliaschewitz FG, Calvo C, Valbuena H, et al. Therapy in type 2 diabetes: insulin glargine vs. NPH insulin both in combination with glimepiride. Arch Med Res 2006;37:495-501. [DOI] [PubMed]
- 93.Ashwell SG, Amiel SA, Bilous RW, et al. Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with type 1 diabetes. Diabet Med 2006;23:285-92. [DOI] [PubMed]
- 94.Raölová K, Bogoev M, Raz I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 2004;66:193-201. [DOI] [PubMed]
- 95.Hermansen K, Fontaine P, Kukolja KK, et al. Insulin analogues (insulin detemir and insulin aspart) versus traditional human insulins (NPH insulin and regular human insulin) in basal-bolus therapy for patients with type 1 diabetes. Diabetologia 2004;47:622-9. [DOI] [PubMed]
- 96.Murphy NP, Keane SM, Ong KK, et al. Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with type 1 diabetes on intensive insulin regimens. Diabetes Care 2003;26:799-804. Available: http://care.diabetesjournals.org/cgi/reprint/26/3/799 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 97.Robertson KJ, Schoenle E, Gucev Z, et al. Insulin detemir compared with NPH insulin in children and adolescents with type 1 diabetes. Diabet Med 2007;24:27-34. [DOI] [PubMed]
- 98.Bolli G, Songini M, Trovati M, et al. Transfer of patients with type 1 diabetes from NPH insulin to insulin glargine as basal insulin: a multicentre, randomised, parallel-group, open-label study Diabetologia 2006;49(Suppl 1):607.16450091
- 99.Philis-Tsimikas A, Charpentier G, Clauson P, et al. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 2006;28:1569-81. [DOI] [PubMed]
- 100.Mianowska B, Szadkowska A, Czerniawska E, et al. A randomized cross-over trial comparing glargine and NPH insulin in preadolescent type 1 diabetic children. Diabetologia 2006;49(Suppl 1):559.
- 101.Chase HP, Arslanian S, White N, et al. Insulin glargine (GLAR) vs intermediate-acting insulin in adolescents with type 1 diabetes (T1DM) using multiple daily injection (MDI) therapy. Diabetologia 2006;49(Suppl 1):559-60.
- 102.Davies MJ, Chatterjee S, Rengarajan T, Lawrence IG, McNally PG. Glargine vs insulatard: efficacy in comparison with insulin aspart in a basal bolus regimen in type 1 diabetes — the Glargine and Aspart Study (GLASS) [abstract]. Diabetologia 2005; 48(Suppl 1):A329. [DOI] [PubMed]
- 103.Pieber TR, Treichel HC, Robertson LI, et al. Insulin detemir plus insulin aspart is associated with less risk of major as well as nocturnal hypoglycaemia than insulin glargine plus insulin aspart at comparable levels of glycaemic control in type 1 diabetes [abstract]. Proceedings from the 41st Annual Meeting of the European Association for the Study of Diabetes; 2005 Sept. 10; Athens (Greece).
- 104.Rosenstock J, Davies M, Home PD, et al. Insulin detemir added to oral anti-diabetic drugs in type 2 diabetes provides glycemic control comparable to insulin glargine with less weight gain. Proceedings of the American Diabetes Association 66th Scientific Sessions; 2006 June 9; Washington (DC).
- 105.White NH, Tamborlane W, Usiskin K. Less variability in blood glucose (BG) values with insulin glargine (GLAR) vs intermediate-acting insulin (NPH or Lente) in adolescents with type 1 diabetes. Proceedings of the American Diabetes Association 66th Scientific Sessions; 2006 June 9; Washington (DC).
- 106.Raskin P, Chaykin L, Mak C, et al. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a treat-to-target study in people with type 2 diabetes. Proceedings of the 19th World Diabetes Congress; 2006 Dec. 3; Cape Town (South Africa).
- 107.Pesic M, Radenkovic S, Zivic S, et al. Comparison between NPH insulin and insulin glargine in intensive insulin therapy in type 1 diabetes mellitus. Endocrine Abstracts 2006;11:(P328). Available: www.endocrine-abstracts.org/ea/0011/ea0011p328.htm (accessed 2008 Dec. 4).
- 108.Pan CY, Sinnassamy P, Chung KD, et al.; LEAD Study Investigators Group. Insulin glargine versus NPH insulin therapy in Asian type 2 diabetes patients. Diabetes Res Clin Pract 2007;76:111-8. [DOI] [PubMed]
- 109.Wang XL, Lu JM, Pan CY, et al. Evaluation of the superiority of insulin glargine as basal insulin replacement by continuous glucose monitoring system. Diabetes Res Clin Pract 2007;76:30-6. [DOI] [PubMed]
- 110.Tajima N, Iwamoto Y, Kaku K, et al. Once-daily insulin detemir added to oral antidiabetic drugs results in less weight gain and a trend for reduced hypoglycaemia in comparison to NPH insulin in Japanese patients with type 2 diabetes. Diabetologia 2006;49(Suppl 1):609.
- 111.Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631-6. Available: http://care.diabetesjournals.org/cgi/reprint/24/4/631 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 112.Pieber TR, Eugene-Jolchine I, Derobert E; The European Study Group of HOE 901 in type 1 diabetes. Efficacy and safety of HOE 901 versus NPH insulin in patients with type 1 diabetes. Diabetes Care 2000;23:157-62. Available: http://care.diabetesjournals.org/cgi/reprint/23/2/157 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 113.Raskin P, Klaff L, Bergenstal R, et al. A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 2000;23:1666-71. Available: http://care.diabetesjournals.org/cgi/reprint/23/11/1666 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 114.Garg S, Gerard L, Pennington M, et al. Efficacy of the new long acting insulin analog (HOE901) on fasting glucose values in IDDM. Diabetes 1998;47(Suppl 1):A359.
- 115.De Leeuw I, Vague P, Selam JL, et al. Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab 2005;7:73-82. [DOI] [PubMed]
- 116.Fulcher GR, Gilbert RE, Yue DK. Glargine is superior to neutral protamine Hagedorn for improving glycated haemoglobin and fasting blood glucose levels during intensive insulin therapy. Intern Med J 2005;35:536-42. [DOI] [PubMed]
- 117.Hermansen K, Madsbad S, Perrild H, et al. Comparison of the soluble basal insulin analog insulin detemir with NPH insulin: a randomized open crossover trial in type 1 diabetic subjects on basal-bolus therapy. Diabetes Care 2001;24:296-301. Available: http://care.diabetesjournals.org/cgi/reprint/24/2/296.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 118.Hershon KS, Blevins TC, Mayo CA, et al. Once-daily insulin glargine compared with twice-daily NPH insulin in patients with type 1 diabetes. Endocr Pract 2004;10:10-7. [DOI] [PubMed]
- 119.Home PD, Rosskamp R, Forjanic-Klapproth J, et al. A randomized multicentre trial of insulin glargine compared with NPH insulin in people with type 1 diabetes. Diabetes Metab Res Rev 2005;21:545-53. [DOI] [PubMed]
- 120.Home P, Bartley P, Russell-Jones D, et al. Insulin detemir offers improved glycemic control compared with NPH insulin in people with type 1 diabetes: a randomized clinical trial. Diabetes Care 2004;27:1081-7. Available: http://care.diabetesjournals.org/cgi/reprint/27/5/1081 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 121.Kawamura T, Higashide T, Hirose M, et al. Prospective, randomized, crossover study using insulin glargine and aspart compared with basal-bolus using NPH in Japanese children and adolescents with type 1 diabetes [poster]. Proceedings of the 65th Annual Scientific Sessions; 2005 June 10; San Diego (CA).
- 122.Kudva YC, Basu A, Jenkins GD, et al. Randomized controlled clinical trial of glargine versus ultralente insulin in the treatment of type 1 diabetes. Diabetes Care 2005;28:10-4. Available: http://care.diabetesjournals.org/cgi/reprint/28/1/10 (accessed 2008 Dec. 4). [DOI] [PubMed]
- 123.Pieber TR, Draeger E, Kristensen A, et al. Comparison of three multiple injection regimens for Type 1 diabetes: morning plus dinner or bedtime administration of insulin detemir vs. morning plus bedtime NPH insulin. Diabet Med 2005;22:850-7. [DOI] [PubMed]
- 124.Porcellati F, Rossetti P, Pampanelli S, et al. Better long-term glycaemic control with the basal insulin glargine as compared with NPH in patients with Type 1 diabetes mellitus given meal-time lispro insulin. Diabet Med 2004;21:1213-20. [DOI] [PubMed]
- 125.Ratner RE, Hirsch IB, Neifing JL, et al. Less hypoglycemia with insulin glargine in intensive insulin therapy for type 1 diabetes. Diabetes Care 2000;23:639-43. Available: http://care.diabetesjournals.org/cgi/reprint/23/5/639.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 126.Rosenstock J, Park G, Zimmerman J. U.S. Insulin Glargine (HOE 901) Type 1 Diabetes Investigator Group. Basal insulin glargine (HOE 901) versus NPH insulin in patients with type 1 diabetes on multiple daily insulin regimens. Diabetes Care 2000; 23:1137-42. Available: http://care.diabetesjournals.org/cgi/reprint/23/8/1137.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 127.Fonseca V, Bell DS, Berger S, et al. A comparison of bedtime insulin glargine with bedtime neutral protamine Hagedorn insulin in patients with type 2 diabetes: subgroup analysis of patients taking once-daily insulin in a multicenter, randomized, parallel group study. Am J Med Sci 2004;328:274-80. [DOI] [PubMed]
- 128.Fritsche A, Schweitzer MA, Häring HU; 4001 Study Group. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 2003;138:952-9. [DOI] [PubMed]
- 129.Haak T, Tiengo A, Draeger E, et al. Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 2005;7:56-64. [DOI] [PubMed]
- 130.Rossetti P, Pampanelli S, Fanelli C, et al. Intensive replacement of basal insulin in patients with type 1 diabetes given rapid-acting insulin analog at mealtime: a 3-month comparison between administration of NPH insulin four times daily and glargine insulin at dinner or bedtime. Diabetes Care 2003;26:1490-6. Available: http://care.diabetesjournals.org/cgi/reprint/26/5/1490 (accessed 2008 Dec.4). [DOI] [PubMed]
- 131.Massi Benedetti M, Humburg E, Dressler A, et al.; 3002 Study Group. A one-year, randomised, multicentre trial comparing insulin glargine with NPH insulin in combination with oral agents in patients with type 2 diabetes. Horm Metab Res 2003;35:189-96. [DOI] [PubMed]
- 132.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080-6. Available: http://care.diabetesjournals.org/cgi/reprint/26/11/3080 (accessed Dec.4). [DOI] [PubMed]
- 133.Russell-Jones D, Simpson R, Hylleberg B, et al. Effects of QD insulin detemir or neutral protamine Hagedorn on blood glucose control in patients with type I diabetes mellitus using a basal-bolus regimen. Clin Ther 2004;26:724-36. [DOI] [PubMed]
- 134.Schober E, Schoenle E, Van Dyk J, et al.; the Pediatric Study Group on Insulin Glargine. Comparative trial between insulin glargine and NPH insulin in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2002;15:369-76. [DOI] [PubMed]
- 135.Standl E, Lang H, Roberts A. The 12-month efficacy and safety of insulin detemir and NPH insulin in basal-bolus therapy for the treatment of type 1 diabetes. Diabetes Technol Ther 2004;6:579-88. [DOI] [PubMed]
- 136.Vague P, Selam JL, Skeie S, et al. Insulin detemir is associated with more predictable glycemic control and reduced risk of hypoglycemia than NPH insulin in patients with type 1 diabetes on a basal-bolus regimen with premeal insulin aspart. Diabetes Care 2003;26:590-6. Available: http://care.diabetesjournals.org/cgi/reprint/26/3/590.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 137.Witthaus E, Stewart J, Bradley C. Treatment satisfaction and psychological well-being with insulin glargine compared with NPH in patients with type 1 diabetes. Diabet Med 2001;18:619-25. [DOI] [PubMed]
- 138.Yki-Järvinen H, Dressler A, Ziemen M; HOE 901/3002 Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. Diabetes Care 2000;23:1130-6. Available: http://care.diabetesjournals.org/cgi/reprint/23/8/1130.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 139.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M, et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 2006;49:442-51. [DOI] [PubMed]
- 140.HOE 901/2004 Study Investigators Group. Safety and efficacy of insulin glargine (HOE 901) versus NPH insulin in combination with oral treatment in Type 2 diabetic patients. Diabet Med 2003;20:545-51. [DOI] [PubMed]
- 141.Pieber TR, Treichel HC, Hompesch B, et al. Comparison of insulin detemir and insulin glargine in subjects with Type 1 diabetes using intensive insulin therapy. Diabet Med 2007;24:635-42. [DOI] [PubMed]
- 142.Raölová K, Bogoev M, Raz I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes [correction of Diabetes Res Clin Pract 2004;66:193-201]. Diabetes Res Clin Pract 2006;72:112. [DOI] [PubMed]
- 143.Kølendorf K, Prasek M, Santeusanio F, et al. Insulin detemir is associated with lower risk of hypoglycaemia compared to NPH insulin in people with type 1 diabetes [abstract]. Diabetologia 2004;47(Suppl 1):913.
- 144.Robertson K, Schönle E, Gucev Z, et al. Benefits of insulin detemir over NPH insulin in children and adolescents with Type 1 diabetes: lower and more predictable fasting plasma glucose and lower risk of nocturnal hypoglycemia [abstract]. Diabetes 2004;53(Suppl 2):A144.
- 145.Hermansen K, Derezinski T, Kim H, et al. Treatment with insulin detemir in combination with oral agents is associated with less risk of hypoglycaemia and less weight gain than NPH insulin at comparable levels of glycaemic improvement in people with type 2 diabetes [abstract]. Diabetologia 2004;47(Suppl 1):A273.
- 146.Kolendorf K, Kim H. Lower risk of hypoglycemia at each level of glycemic control when insulin detemir is added to oral agents compared to NPH insulin in patients with type 2 diabetes [abstract]. Diabetes 2005;54(Suppl 1):A121.
- 147.Bowker SL, Majumdar SR, Johnson JA. Systematic review of Indicators and measurements used in controlled studies of quality improvement for type-2 diabetes. Can J Diabetes 2005;29:230-8. Available: www.diabetes.ca/files/Johnson_Systematic_Review-pages%20230-238.pdf (accessed 2008 Dec. 4).
- 148.Qayyum R, Bolen S, Maruthur N, et al. Systematic review: comparative effectiveness and safety of premixed insulin analogues in type 2 diabetes. Ann Intern Med 2008;149:549-59. [DOI] [PMC free article] [PubMed]
- 149.Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed]
- 150.Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol 2000;53:964-72. [DOI] [PubMed]
- 151.Moher D, Pham B, Lawson ML, et al. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess 2003;7:1-90. Available: www.ncchta.org/fullmono/mon741.pdf (accessed 2008 Dec. 4). [DOI] [PubMed]
- 152.Juni P, Holenstein F, Sterne J, et al. Direction and impact of language bias in meta-analyses of controlled trials: empirical study. Int J Epidemiol 2002;31:115-23. [DOI] [PubMed]
- 153.Mullins P, Sharplin P, Yki-Järvinen H, et al. Negative binomial meta-regression analysis of combined glycosylated hemoglobin and hypoglycemia outcomes across eleven Phase III and IV studies of insulin glargine compared with neutral protamine Hagedorn insulin in type 1 and type 2 diabetes mellitus. Clin Ther 2007;29:1607-19. [DOI] [PubMed]
- 154.Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. CMAJ 2009;180:400-7. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.