Abstract
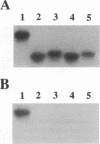
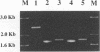
Three new isolates of porcine respiratory coronavirus (PRCV) were isolated and partially characterized. These PRCV isolates showed a selective tropism for respiratory tissue and were antigenically related to transmissible gastroenteritis virus. PCR amplification of the 5' half of the spike (S) genes of the three PRCV isolates indicated that a large deletion, characteristic of PRCV, was present. By using cDNA probes specific for the transmissible gastroenteritis virus S gene, the PCR products were shown to be specific in a Southern blot. The three new PRCV isolates were shown to vary in S gene deletion size. In a separate study, these isolates have also been shown to vary in pathogenicity. These new PRCV isolates should serve as important tools in gaining a better understanding of the pathogenesis of coronavirus infections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton P., Mawditt K. L., Page K. W. The cloning and sequencing of the virion protein genes from a British isolate of porcine respiratory coronavirus: comparison with transmissible gastroenteritis virus genes. Virus Res. 1991 Nov;21(3):181–198. doi: 10.1016/0168-1702(91)90032-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton P., Page K. W. Sequence of the S gene from a virulent British field isolate of transmissible gastroenteritis virus. Virus Res. 1990 Dec;18(1):71–80. doi: 10.1016/0168-1702(90)90090-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut P., Pensaert M. B., Hooyberghs J. A competitive inhibition ELISA for the differentiation of serum antibodies from pigs infected with transmissible gastroenteritis virus (TGEV) or with the TGEV-related porcine respiratory coronavirus. Vet Microbiol. 1989 May;20(1):9–19. doi: 10.1016/0378-1135(89)90003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E., Hooyberghs J., Pensaert M. B. Sites of replication of a porcine respiratory coronavirus related to transmissible gastroenteritis virus. Res Vet Sci. 1990 Mar;48(2):165–169. doi: 10.1016/S0034-5288(18)30984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E., Pensaert M. B., Callebaut P., van Deun K. Intestinal replication of a porcine respiratory coronavirus closely related antigenically to the enteric transmissible gastroenteritis virus. Vet Microbiol. 1990 Jun;23(1-4):237–243. doi: 10.1016/0378-1135(90)90154-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Stewart F., Cartwright S. F., Brown I. Differentiation of porcine coronavirus from transmissible gastroenteritis virus. Vet Rec. 1988 Jan 23;122(4):86–87. doi: 10.1136/vr.122.4.86. [DOI] [PubMed] [Google Scholar]
- Halbur P. G., Paul P. S., Vaughn E. M., Andrews J. J. Experimental reproduction of pneumonia in gnotobiotic pigs with porcine respiratory coronavirus isolate AR310. J Vet Diagn Invest. 1993 Apr;5(2):184–188. doi: 10.1177/104063879300500207. [DOI] [PubMed] [Google Scholar]
- Laude H., Van Reeth K., Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Vet Res. 1993;24(2):125–150. [PubMed] [Google Scholar]
- O'Toole D., Brown I., Bridges A., Cartwright S. F. Pathogenicity of experimental infection with 'pneumotropic' porcine coronavirus. Res Vet Sci. 1989 Jul;47(1):23–29. doi: 10.1016/S0034-5288(18)31226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. E., Gallagher T. M., Buchmeier M. J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989 Dec;173(2):664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M., Callebaut P., Vergote J. Isolation of a porcine respiratory, non-enteric coronavirus related to transmissible gastroenteritis. Vet Q. 1986 Jul;8(3):257–261. doi: 10.1080/01652176.1986.9694050. [DOI] [PubMed] [Google Scholar]
- Pensaert M., Cox E., van Deun K., Callebaut P. A sero-epizootiological study of porcine respiratory coronavirus in Belgian swine. Vet Q. 1993 Mar;15(1):16–20. doi: 10.1080/01652176.1993.9694361. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Duarte M., Laude H. Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J Gen Virol. 1990 Nov;71(Pt 11):2599–2607. doi: 10.1099/0022-1317-71-11-2599. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jul;68(Pt 7):1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Simkins R. A., Weilnau P. A., Van Cott J., Brim T. A., Saif L. J. Competition ELISA, using monoclonal antibodies to the transmissible gastroenteritis virus (TGEV) S protein, for serologic differentiation of pigs infected with TGEV or porcine respiratory coronavirus. Am J Vet Res. 1993 Feb;54(2):254–259. [PubMed] [Google Scholar]
- Sánchez C. M., Gebauer F., Suñ C., Mendez A., Dopazo J., Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992 Sep;190(1):92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn E. M., Paul P. S. Antigenic and biological diversity among transmissible gastroenteritis virus isolates of swine. Vet Microbiol. 1993 Sep;36(3-4):333–347. doi: 10.1016/0378-1135(93)90099-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Cheung A. K. Genetic analysis of porcine respiratory coronavirus, an attenuated variant of transmissible gastroenteritis virus. J Virol. 1991 Jun;65(6):3369–3373. doi: 10.1128/jvi.65.6.3369-3373.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Cheung A. K. Genetic basis for the pathogenesis of transmissible gastroenteritis virus. J Virol. 1990 Oct;64(10):4761–4766. doi: 10.1128/jvi.64.10.4761-4766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Hill H. T., Biwer J. D. Evidence for a porcine respiratory coronavirus, antigenically similar to transmissible gastroenteritis virus, in the United States. J Vet Diagn Invest. 1990 Oct;2(4):312–317. doi: 10.1177/104063879000200411. [DOI] [PubMed] [Google Scholar]
- Zhu X. L., Paul P. S., Vaughn E., Morales A. Characterization and reactivity of monoclonal antibodies to the Miller strain of transmissible gastroenteritis virus of swine. Am J Vet Res. 1990 Feb;51(2):232–238. [PubMed] [Google Scholar]
- van Nieuwstadt A. P., Boonstra J. Comparison of the antibody response to transmissible gastroenteritis virus and porcine respiratory coronavirus, using monoclonal antibodies to antigenic sites A and X of the S glycoprotein. Am J Vet Res. 1992 Feb;53(2):184–190. [PubMed] [Google Scholar]
- van Nieuwstadt A. P., Pol J. M. Isolation of a TGE virus-related respiratory coronavirus causing fatal pneumonia in pigs. Vet Rec. 1989 Jan 14;124(2):43–44. doi: 10.1136/vr.124.2.43. [DOI] [PubMed] [Google Scholar]