The case: A previously healthy 37-year-old woman (gravida 3, para 1 with 2 first-trimester miscarriages) had an unremarkable pregnancy until the 35th week. Her blood pressure readings during pregnancy were between 100/70 mm Hg and 110/80 mm Hg. She had no previous seizure disorders or hypertension. At week 28, gestational diabetes was diagnosed by means of an oral glucose tolerance test and subsequently controlled by diet.
At 35 weeks, mild pitting edema developed in both of the patient's feet, but she had no other symptoms of eclampsia (Box 1). Her blood pressure was 149/89 mm Hg, and her patellar deep tendon reflexes were normal. A urine dipstick test revealed a protein level of about 0.3 g/L. The patient's complete blood count and international normalized ratio were normal, as were her levels of bilirubin and liver transaminases. A nonstress test found normal reactivity. The patient was monitored closely. Her blood pressure remained marginally high (131/83 mm Hg to 141/84 mm Hg) over the next week. The results of repeat laboratory tests and nonstress tests were normal.
Box 1.

At 36 weeks' gestation, the patient had premature rupture of membranes followed by preterm onset of labour. On admission to hospital, her blood pressure was 131/83 mm Hg, the edema in her feet was unchanged, and a urine dipstick test showed no proteinuria. During the 49-hour labour, the patient's blood pressure fluctuated between 120/70 mm Hg and 130/80 mm Hg. Because of failure to progress, she had labour augmentation with oxytocin, and delivery was assisted by the use of midforceps. A healthy boy (3.023 kg) was delivered. The patient was discharged 1 day after delivery with blood pressure readings between 120/80 mm Hg and 135/85 mm Hg.
On postpartum day 5, the patient presented to the emergency department with a 1-day history of a gradual-onset throbbing occipital headache that was associated with photophobia and 3 episodes of vomiting. Her blood pressure on presentation was 205/105 mm Hg. Her other vital signs were unremarkable. She was given prochlorperazine maleate (10 mg administered intravenously) as an antiemetic. On assessment by the emergency department physician, her blood pressure was 172/82 mm Hg, and she had moderate pitting edema in both feet. She had no meningism, and her neurologic examination showed only increased patellar deep tendon reflexes. At the end of the assessment (about 2 hours and 10 minutes after she arrived at the emergency department), she had a generalized seizure that lasted for 2 minutes. The seizure was terminated by diazepam (2 mg administered intravenously). Subsequently she was in a postictal state for 20 minutes. Five minutes after the seizure, her blood pressure was 130/70 mm Hg.
Initial laboratory investigations showed mild leukocytosis (leukocyte count 14.8 × 109) and mild anemia with a hemoglobin level of 122 (normal 125–155) g/L. She had slightly elevated levels of aspartate transaminase (91 [normal < 37] U/L) and alkaline phosphatase (150 [normal < 104] U/L). Her platelet count and international normalized ratio were normal, as were her levels of serum bilirubin, urea, creatinine, electrolytes, calcium, magnesium and phosphate. A dipstick urinalysis showed a trace amount of protein (< 0.3 g/L). The results of a computed tomography (CT) scan of her head performed without contrast and subsequent lumbar puncture were normal.
Two hours after the initial seizure, the patient reported having no headache, and her mental status was clear. Her blood pressure was 104/49 mm Hg. The results of a neurologic examination were unremarkable except for very exaggerated patellar deep tendon reflexes. Minutes later, the patient had a second generalized seizure that was terminated with lorazepam (2 mg administered intravenously). An intravenous magnesium sulfate drip was started as per protocol (4 g loading dose followed by 2 g per hour). The patient was transferred to the intensive care unit, where she continued to receive intravenous magnesium sulfate. She was also given intravenous phenytoin, labetalol (200 mg orally twice a day) and hydralazine (10 mg intravenously every 6 hours and hourly as required for systolic blood pressure over 160 mm Hg or diastolic blood pressure over 110 mm Hg). In the intensive care unit, her highest blood pressure was 187/102 mm Hg, which was normalized with antihypertensive therapy.
On the day after admission, magnetic resonance imaging (MRI) and magnetic resonance venography showed bilateral, marked subcortical white signal changes predominantly in the occipital lobes. There were small patchy areas of involvement in the frontal lobes and the posterior temporal lobes (Figure 1). These changes were in keeping with changes seen in eclampsia.
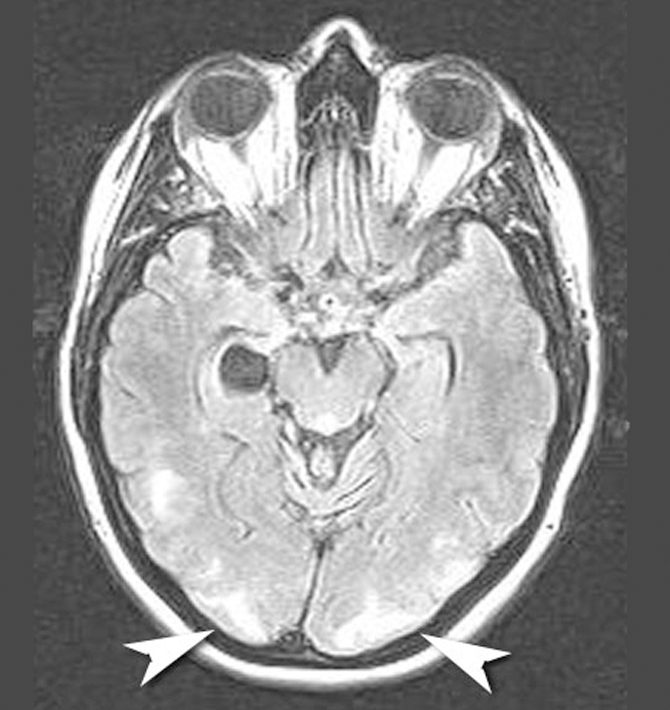
Figure 1: Magnetic resonance image showing bilateral marked changes in the subcortical white matter, predominantly in the occipital lobes (arrowheads).
Two days later, she was discharged home. She was prescribed labetolol (200 mg, taken orally twice a day). An MRI scan taken 3 months showed normal findings.
Our patient had late postpartum seizure and hypertension that occurred without true pre-existing pre-eclampsia. Eclampsia is a serious complication of pregnancy and is characterized by tonic-clonic seizure. Usually eclampsia occurs after the onset of pre-eclampsia, although sometimes no pre-eclamptic symptoms are observed. Pre-eclampsia is defined as hypertension (pre-existing or gestational onset after 20 weeks' gestation) with a diastolic blood pressure of 90 mm Hg or greater at 2 or more visits using the same arm and either proteinuria (0.3 g/d in a 24-hour urine collection or 30 mg/mmol creatinine in a random urine sample) or 1 or more of the adverse conditions listed in Box 1. Late postpartum eclampsia is eclampsia that develops more than 2 days after delivery.1,2
Eclampsia affects less than 0.2% of pregnancies, and it accounts for 1.5 pregnancy-related maternal deaths per 100 000 live births.3–5 Eclampsia is the second leading cause of maternal death (19.6%) in the United States, after pulmonary embolism.5,6
Between 14% and 33% of cases of eclampsia occur after delivery.7 Late postpartum eclampsia affects between 4% and 26% of patients with eclampsia and between 28% and 79% of patients with postpartum eclampsia.7–10 Despite a decline in the overall incidence of eclampsia because of the early diagnosis and treatment of pre-eclampsia, the incidence of late postpartum eclampsia remained unchanged between 1931 and 1991.11 Thus, the relative incidence of late postpartum eclampsia is increasing.4
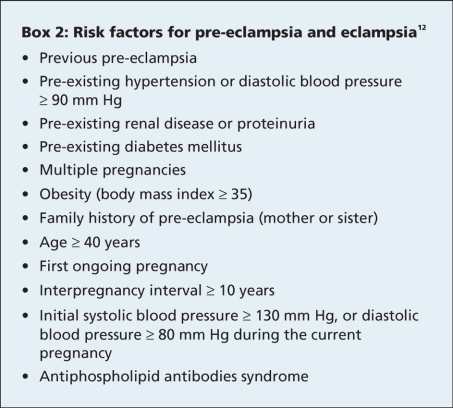
The cause of pre-eclampsia and its progression to eclampsia or late postpartum eclampsia is unknown. The widely accepted theory is based on a mismatch between uteroplacental supply and fetal demand that results in endothelial cell injury and widespread vasospasm that culminates with clinical symptoms due to multiorgan ischemia.7,9,11,12 There are several recognizable factors that increase the risk of eclampsia (Box 2).
Box 2.
Clinical characteristics
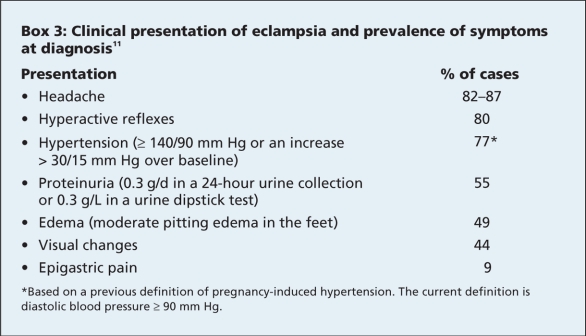
The clinical characteristics of pre-eclampsia and eclampsia are presented in Box 3. Hypertension may develop for the first time after delivery, with a peak blood pressure 3–6 days after delivery. This corresponds with the mobilization of extracellular fluid accumulated during pregnancy.12 Thus, blood pressure measurement during the first week after delivery is key, even for women with previously normal blood pressure.
Box 3.
One-third of women with late postpartum eclampsia have no prior history of hypertension, proteinuria or edema.3,10 Two retrospective reviews showed that, for 44%–79% of patients with late postpartum eclampsia, pre-eclampsia was not diagnosed before the onset of seizures.7,9 Pulmonary edema, hepatic failure, hemolysis, elevated liver enzyme levels, low platelet count and disseminated intravascular coagulation are several well-recognized complications of eclampsia.8 Abnormalities may be present in the patient's complete blood count, blood film, electrolytes, international normalized ratio and partial thromboplastin time. There may also be abnormalities in the patient's levels of aspartate transaminase, alanine transaminase, lactate dehydrogenase, bilirubin, albumin, creatinine, urea, uric acid, glucose, fibrinogen and fibrin degradation products.12
Diagnostic imaging is not essential for diagnosing eclampsia. However, imaging can support the diagnosis by showing edema in the posterior regions of the cerebral hemispheres on an otherwise normal CT or MRI scan.8,13,14
Management
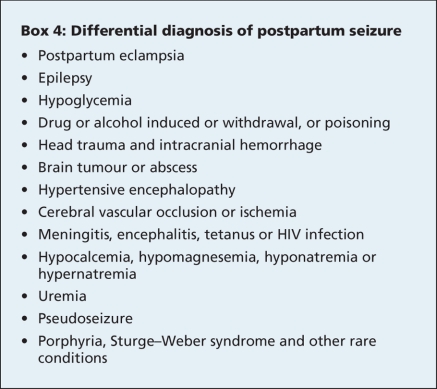
Early recognition of the signs of eclampsia (Box 3) in women who are pregnant or who have recently given birth may allow for the early use of anticonvulsant drugs, such as magnesium sulfate. The differential diagnosis of a postpartum seizure is shown in Box 4.
Box 4.
Although delivery of the newborn usually corrects the signs and symptoms of pre-eclampsia and eclampsia, this does not occur in the case of late postpartum eclampsia.
Several studies have examined therapeutic protocols for the management of eclampsia. However, these protocols have not been evaluated in late postpartum eclampsia.15 Because late postpartum eclampsia is considered a subtype of eclampsia, the same therapeutic approaches can be used. Intravenous magnesium sulfate therapy (bolus of 2–4 g delivered over 10–30 minutes, followed by an infusion of 1–2 g per hour for 24–48 hours) has been found to be superior to other anticonvulsive therapies.8,12,15 However, excessive levels of magnesium sulfate can lead to hypotension, loss of reflexes and respiratory arrest.
One may question whether the use of prochlorperazine may have helped to precipitate our patient's seizure. There is a reported low incidence of seizures being precipitated by the use of dopamine antagonists. We feel that the use of prochlorperazine was unlikely to have had a role in our patient's seizure. She had clear clinical findings of pre-eclampsia (headache, severe hypertension and increased deep tendon reflexes). The results of an MRI were also consistent with eclampsia.
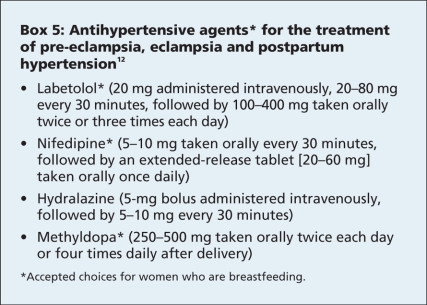
Several agents can be used to treat hypertension in patients with pre-eclampsia, eclampsia and postpartum hypertension (Box 5). Diuretics should be avoided, especially in combination with other antihypertensive agents, except in cases of life-threatening fluid overload.8,12 This is because, although one might expect patients with eclampsia to have increased total body fluids, they actually have a depletion of intravascular volume, and the use of diuretics can precipitate severe hypotension. The guidelines of the Society of Obstetricians and Gynaecologists of Canada state that, on average, longer durations of antihypertensive therapy are needed for women who have pre-eclampsia (about 2 weeks postpartum) compared with those who have gestational hypertension without proteinuria (1 week post partum). The blood pressure treatment target is 130–155 mm Hg systolic and 80–105 mm Hg diastolic for those without comorbid conditions and 130–139 mm Hg systolic and 80–89 mm Hg diastolic for those with comorbid conditions. Nonsteroidal anti-inflammatory drugs should not be given after delivery if the patient's hypertension is difficult to control. Gestational hypertension usually resolves within 6 weeks after delivery; however, women with severe pre-eclampsia may have hypertension for several months after delivery.
Box 5.
With the recent trend of discharging new mothers soon after delivery (often within 1 day), patients with eclampsia may present to primary care physicians and emergency departments with early signs and symptoms of eclampsia. The early recognition of the signs and symptoms of postpartum eclampsia may lead to early treatment and fewer complications.
Key points.
Late postpartum eclampsia is eclampsia that develops more than 2 days after delivery.
Maternal blood pressure should be monitored at the time of peak postpartum blood pressure (days 3–6 after delivery) to detect eclampsia before symptoms develop.
Late postpartum eclampsia can occur up to 23 days after delivery.
A history of diagnosed pre-eclampsia is not essential for the development (or diagnosis) of late postpartum eclampsia.
Severe headache is the most common presenting symptom, followed by edema, visual changes and epigastric pain.
Brisk deep tendon reflexes and hypertension are the most common clinical signs.
An eclampsia-induced seizure can occur in a patient with normal blood pressure.
Magnesium sulfate is the best available treatment for eclampsia-induced seizure.
Footnotes
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a brief summary (100 words) outlining the case and its relevance to a general audience. The case presentation follows (500 words maximum) as well as a discussion of the underlying condition (1000 words maximum). Generally, up to 5 references are permitted and visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Written consent from patients for publication of their story is a necessity and should accompany submissions. See information for authors at www.cmaj.ca.
This article has been peer reviewed.
Competing interests: None declared.
REFERENCES
- 1.Graeber B, Vanderwal T, Stiller RJ, et al. Late postpartum eclampsia as an obstetric complication seen in the ED. Am J Emerg Med 2005;23:168-70. [DOI] [PubMed]
- 2.Raps EC, Galetta SL, Broderick M, et al. Delayed peripartum vasculopathy: cerebral eclampsia revisited. Ann Neurol 1993;33:222-5. [DOI] [PubMed]
- 3.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ 1994;309:1395-400. [DOI] [PMC free article] [PubMed]
- 4.Leitch CR, Cameron AD, Walker JJ. The changing pattern of eclampsia over a 60-year period. Br J Obstet Gynaecol 1997;104:917-22. [DOI] [PubMed]
- 5.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet Gynecol 2001;97:533-8. [DOI] [PubMed]
- 6.Matthys LA, Coppage KH, Lambers DS, et al. Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol 2004;190:1464-6. [DOI] [PubMed]
- 7.Chames MC, Livingston JC, Ivester TS, et al. Late postpartum eclampsia: A preventable disease? Am J Obstet Gynecol 2002;186:1174-7. [DOI] [PubMed]
- 8.Hirshfeld-Cytron J, Lam C, Karumanchi SA, et al. Late postpartum eclampsia: examples and review. Obstet Gynecol Surv 2006;61:471-80. [DOI] [PubMed]
- 9.Lubarsky SL, Barton JR, Friedman SA, et al. Late postpartum eclampsia revisited. Obstet Gynecol 1994;83:502-5. [DOI] [PubMed]
- 10.Sibai BM, Schneider JM, Morrison JC, et al. The late postpartum eclampsia controversy. Obstet Gynecol 1980;55:74-8. [PubMed]
- 11.Martin J, Sidman R. Late postpartum eclampsia: a common presentation of an uncommon diagnosis. J Emerg Med 2003;25:387-90. [DOI] [PubMed]
- 12.Magee LA, Helewa M, Moutquin J-M, et al. Diagnosis, evaluation and management of the hypertensive disorders of pregnancy J Obstet Gynaecol Can 2008;30(Suppl 3):S1-S48. Available: www.sogc.org/guidelines/documents/gui206CPG0803.pdf (accessed 2009 Jan. 9). [DOI] [PubMed]
- 13.Dziewas R, Stogbauer F, Freund M, et al. Late onset postpartum eclampsia: a rare and difficult diagnosis. J Neurol 2002;249:1287-91. [DOI] [PubMed]
- 14.Munjuluri N, Lipman M, Valentine A, et al. Postpartum eclampsia of late onset. BMJ 2005;331:1070-1. [DOI] [PMC free article] [PubMed]
- 15.Duley L. Magnesium sulphate in eclampsia. Eclampsia Trial Collaborative Group. Lancet 1998;352:67-8. [DOI] [PubMed]