Abstract
Purpose
To document the tibiofemoral (TF) compression forces produced during clinical initial graft tension protocols.
Methods
An image analysis system was used to track the position of the tibia relative to the femur in 11 cadaver knees. TF compression forces were quantified using thin-film pressure sensors. Prior to performing ACL reconstructions with patellar tendon grafts, measurements of TF compression force were obtained from the ACL-intact knee with knee flexion. ACL reconstructions were then performed using “force-based” and “laxity-based” graft tension approaches. Within each approach, high- and low-tension conditions were compared to the ACL-intact condition over the range of knee flexion angles.
Results
The TF compression forces for all initial graft tension conditions were significantly greater than that of the normal knee when the knee was in full extension (0°). The TF compression forces when using the laxity-based approach were greater than those produced with the force-based approach. However the laxity-based approach was necessary to restore normal laxity at the time of surgery.
Conclusions
The initial graft tension conditions produce different TF compressive force profiles at the time of surgery. A compromise must be made between restoring knee laxity or TF compressive forces when reconstructing the ACL with patellar tendon graft.
Clinical Relevance
The TF compression forces were greater in the ACL-reconstructed knee for all the initial graft tension conditions when compared to the ACL-intact knee, and that clinically relevant initial graft tension conditions produce different TF compressive forces.
Keywords: ACL, ligament, reconstruction, tension, contact force, biomechanics
INTRODUCTION
Although the anterior cruciate ligament (ACL) is the most commonly reconstructed ligament in the body, there is considerable debate regarding the optimal surgical technique. While ACL reconstruction improves knee joint function in most patients, increasing evidence suggests that many patients will develop post-traumatic osteoarthritis (OA) following surgical treatment.1 Knee joint contact mechanics are not fully restored with surgery; this may contribute to OA progression within the ACL-reconstructed patient population.2
One surgical parameter that is known to control the joint contact mechanics is the amount of tension applied to the graft at the time of fixation (initial graft tension).3, 4 Initial graft tensioning is commonly performed using a “force-based” approach, where a discrete tension level is applied to the graft at the time of surgical fixation, though there is little agreement as to which tension value is best.5–9 Furthermore, the tension required to restore normal anterior-posterior (AP) laxity in one patient may differ from that of another. Since one of the functions of the ACL is to oppose anterior translation of the tibia relative to the femur, “laxity-based” initial graft tension protocols that adjust the tension to restore the normal AP laxity of the knee at the time of surgery have also been recommended.3 Others recommend over-constraining joint laxity, since the initial graft tension can decrease with initial flexion-extension cycling of the joint after fixation.10 Non-optimal graft tensioning may not only influence AP laxity but has the potential for long-term damage to the knee articular cartilage. If the initial graft tension is too great, abnormal tibiofemoral (TF) compression forces may develop across the joint, hindering knee motion and subjecting the articular surface to increased contact stresses. However, if the initial tension is too low, the graft may not restore knee kinematics, and increased joint laxity could result. Either high TF contact stress or increased sliding between the joint surfaces could induce cartilage degradation.2
The joint contact mechanics of the ACL-reconstructed knee under clinically relevant initial graft tension protocols have not been established. It has been determined that the amount of initial graft tension, and the knee flexion angle at which it is applied, modulates the magnitude of the TF compression forces.4 A low graft tension (1-15N) best replicated normal TF contact forces when the knee was in extension; however, this tension range was less than that used clinically. The purpose of the present study was to document the TF compression forces of clinically relevant laxity- and force-based initial graft tension protocols, and to determine how the resulting TF compression forces for each protocol change during passive flexion-extension motion of the knee. We hypothesized that the laxity-based initial graft tension approach that most closely approximated normal AP laxity at the time of surgery would best restore the TF compression forces with passive knee extension after graft fixation.
METHODS
Specimens
Eleven fresh frozen human cadaver knees (mean age=59 (±4.0) years; 3 male/8 female) with no clinical signs of a ligament injury were dissected to the joint capsule and potted for biomechanical testing.4
Custom Test Fixture
A test fixture was designed to secure the femur in the horizontal plane while permitting the tibia to be locked at any desired knee flexion angle (Fig. 1).4 The remaining five degrees of freedom of joint motion were left unconstrained via a sliding universal joint that was attached to the distal tibia. Thus, the natural motion of the tibia was permitted as the knee was passively flexed and extended by the arm of the test fixture while protecting the specimen from out of plane loads by the examiner. It should be noted that a gravitational load on the tibia was present.
Fig. 1.
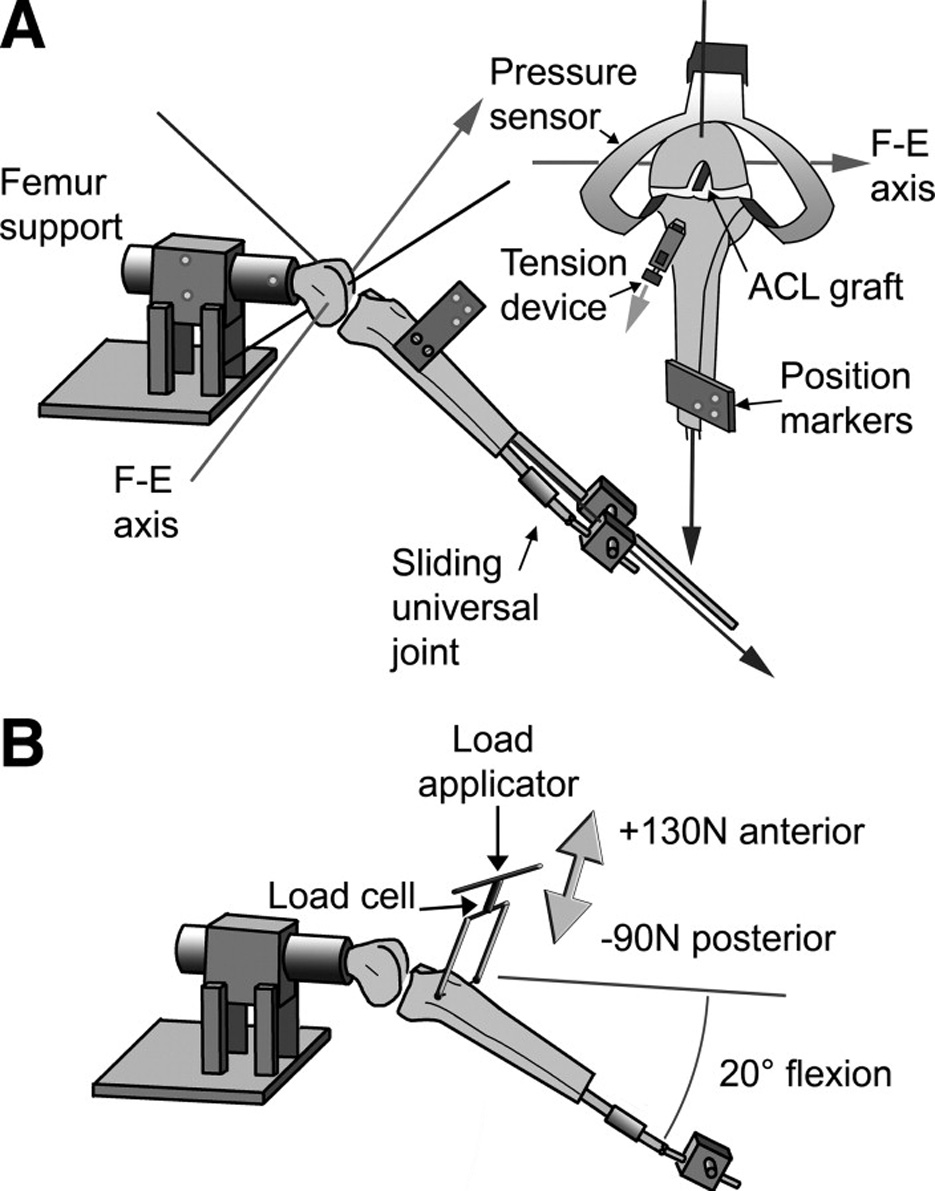
A. Schematic showing the test fixture used to support the knee throughout the range of motion4. B. AP laxity tests were performed with the knee within the same fixture with the knee locked at 20° of flexion. A load applicator was used to apply the shear loads to the proximal tibia relative to the femur.
Joint Position Measurements
The Optotrak Motion Analysis System (Northern Digital; Waterloo, Ontario) was used to record the positions of the tibia relative to the femur. The system utilizes infrared light-emitting diodes (LEDs), which were attached to the tibia and femur.4 A joint coordinate system was constructed based on bony landmarks commonly used to describe clinical motion.11 A line directed through the epicondyles was assigned as the flexion-extension axis. The internal-external tibial rotation axis was defined by a line originating from the center of the distal tibia and directed normal to the flexion-extension axis. Euler angles and translation vectors for the tibia were calculated using the Optotrak Image Analysis System software.
TF Pressure Sensors
The K-Scan thin-film pressure sensor (4000/2594T1/1500; Tekscan, Inc., South Boston, MA) was used to measure compression forces in the medial and lateral TF compartments of each knee (Fig. 1). The pressure in each compartment was multiplied by the contact area to get the average force in each compartment at the respective knee flexion angles. The forces measured in each compartment were summed to obtain the total TF compressive force. The K-scan output was calibrated using a cadaver specimen on which known loads were applied through each condyle with a MTS 810 Material Test System as previously described (Prairie Eden, MN).4
Laxity Testing
When performing laxity-based tensioning protocols, AP laxity was measured by applying AP-directed shear loads to the proximal tibia relative to the distal femur using a custom hand-held load applicator (Fig. 1). The load applicator utilizes a 100lb load cell (Sensotec; Model 31/F210-01-01, Columbus OH) to measure the AP shear loads applied to the knee. The test was performed between the posterior shear load limit of −90N and an anterior shear load limit of +130N. During the laxity testing, only the knee flexion angle (20° of flexion) was constrained. The resulting AP displacement was measured using the Optotrak system. The axis of AP translation was relative to the tibia and was defined as the line normal to both the flexion-extension axis and the internal-external tibial rotation axis. All laxity tests were performed after five knee flexion-extension cycles for preconditioning.
Initial Graft Tension Protocols
Laxity- and force-based initial graft tension approaches were investigated (Fig. 2). With the “laxity-based” approach, two initial graft tension conditions were compared: 1) a graft tension level that restored the AP laxity of the reconstructed knee to that of the normal knee (the “LaxNorm” condition), and 2) a graft tension level that reduced the AP laxity by 2mm in comparison to the normal knee (the “LaxTight” condition). For the “LaxNorm” condition, the tension was applied to the graft with the knee at 0° flexion, a position in which the ACL supports a high load or strain.12 After an initial tension level was selected, the knee was flexed to 20° and the AP laxity test was repeated and compared to that of the ACL-intact knee. The tension was adjusted iteratively until the AP laxity of the reconstructed knee matched that of the ACL-intact knee. For the “LaxTight” condition, the tension was applied with the knee at 30° of flexion, a position in which the ACL carries very little load.12 The tension was adjusted until the AP laxity value at 20° of flexion was 2mm less than the ACL-intact knee. The tension values applied during the laxity-based tensioning procedures were not recorded.
Fig. 2.
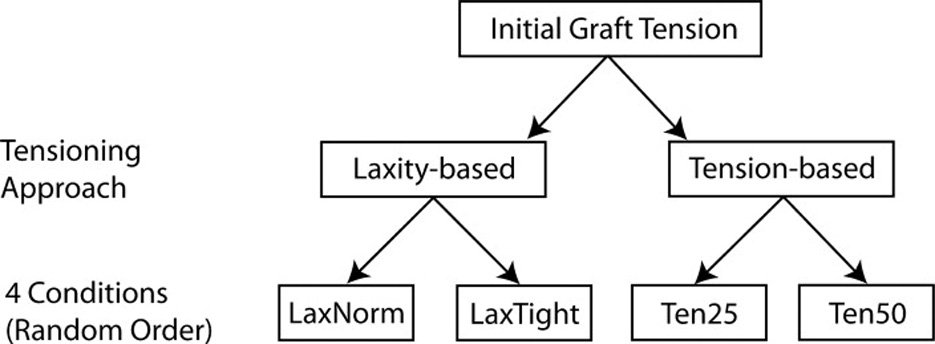
Overview of the experimental design. LaxNorm = Normal Laxity; LaxTight = laxity constrained by 2mm; Ten25 = 25N tension; Ten50 = 50N tension. The four test conditions were performed in a random order.
When using the “force-based” approach, two initial graft tension conditions were compared: 1) 25N (the “Ten25” condition) and 2) 50N (the “Ten50” condition). These values were selected because they were utilized in a previous clinical study evaluating the effects of initial graft tension using patellar tendon autografts.6 In this study, the forces were applied to the distal end of the graft using the tensioning device while the knee was at full extension (0°).
Experimental Protocol
First, the ACL-intact cadaver specimens were mounted to the testing jig with the Optotrak LEDs attached. Each knee underwent five pre-conditioning flexion-extension cycles. The TF kinematics of the normal knee were measured as the knee was passively flexed from full extension to 110° of flexion (quasi-static increments of 0°, 20°, 40°, 60°, 90°, and 110°). The AP laxity of the ACL-intact knee (“ACLint”) was then measured with the knee at 20° of flexion. It should be noted that it was not possible to implant the pressure sensors to measure the TF compressive loads generated in the ACL-intact, capsule-intact state.
The bone-tendon-bone graft was then harvested (bone blocks: 10mm × 25mm) from the central third of the patellar tendon, and the remaining quadriceps tendon, patella, and patellar tendon were removed to facilitate subsequent pressure sensor insertion. The AP laxity measurement protocol was repeated for the ACL-intact, capsule-open state (“Open”).
Anterior and posterior incisions were made beneath the rim of both menisci to allow the pressure sensors to be inserted.4 The AP laxity of the TF joint in the ACL-intact, capsule-open, sensor-implanted (“Open+Tek”) state was then evaluated to ensure that the sensor did not significantly change the joint laxity response. The quasi-static flexion-extension joint positions and TF compressive loads in the “Open+Tek” state were then recorded as described above.
Next, the ACL was excised and surgically reconstructed using the 10mm bone-patellar tendon-bone graft. A cannulated endoscopic drill guide system (Arthrex; Naples, FL) was used to standardize anatomic graft tunnel placement. All reconstructions were performed with the capsule open, permitting direct visualization of the femoral over-the-top position from the anterior perspective into the notch. A 12mm tibial tunnel was created by drilling obliquely across the anteromedial tibial metaphysic entering the joint at the center of the anatomic ACL attachment. An 11mm femoral tunnel was formed at the center of the anatomic ACL attachment by drilling through the intercondylar notch via the anterior capsulotomy while the knee was hyperflexed. The tibial tunnel diameter was made 1mm greater to minimize friction with the ACL bone-tendon-bone graft during the graft tensioning procedure.4 Once the graft was passed through the joint, the proximal bone block was fixed within the femoral bone tunnel using a 9×20mm titanium interference screw (Arthrex; AR-1390T, Naples, FL). Care was taken to ensure that the tendinous portion of the bone block was pressed against the posterior aspect of the femoral tunnel, and that no axial twist was introduced into the graft. The distal end of the graft was positioned within the tibial tunnel and secured via #2 FiberWire (Arthrex; Naples, FL) to a custom tensioning device rigidly mounted on the anterior-medial aspect of the tibia.13 The tensioning device was aligned and attached to the anteromedial tibia at the entrance site of the tibial tunnel using 1mm k-wires. The graft tension approaches and conditions were randomized using a random number generator (Fig. 2). The TF compressive forces were measured for each of the four initial graft tension conditions (LaxNorm, LaxTight, Ten25, Ten50) when the knee was positioned at 0°, 20°, 40°, 60°, 90°, and 110° of flexion.
Statistical Analysis
A two-way repeated measures ANOVA was employed to compare the TF compressive forces of the ACL-intact knee (Open+Tek) with those of the reconstructed conditions, and to determine the difference between tension conditions. The ANOVA was designed to evaluate TF compression loads as a function of knee joint status and flexion angle within specimens. Pair-wise comparisons between treatment groups at specific knee flexion angles were examined using the Holm-Sidak method (SigmaStat 3.5; Systat Software, Inc., San Jose CA).
For the laxity-based approach, AP laxity values with the knee flexed at 20° were also compared between initial graft tension conditions and the ACL-intact conditions (ACLint, Open, Open+Tek) using a one-way repeated measures ANOVA. Pair-wise comparisons between treatment groups were made using the Holm-Sidak method. This analysis enabled comparisons between the laxity values of the two ACL reconstruction treatments and the ACL-intact condition (ACLint), and determined if differences in AP laxity values resulted between the ACLint, Open, and Open+Tek conditions, ensuring that the sensor had minimal impact on the laxity measurements.
A power analysis was performed a priori to determine the sample size required to detect a clinically relevant difference between treatment groups using an estimate of the contact force. The variability (standard deviation) in contact force was assumed to be 22N across specimens from the TF contact force measurements performed by Singerman et al.14 It is likely that the variation within a specimen would be less than that value. Thus, it was possible to detect a difference of 20N between experimental conditions with a sample size of 11 specimens. The expected difference of 20N seemed reasonable, considering that a difference in initial graft tension of approximately 25N is required to produce a 2mm difference in AP laxity.15 This would correspond to a difference in compression force greater than 24N when the knee is in full extension.16
RESULTS
TF Compression Forces
The TF compressive forces for all of the initial graft tension conditions followed a repeatable pattern that was consistent with that of the normal ACL during passive extension motion of the joint (Fig. 3). On average, the TF compressive forces were less than 20N from 110° to approximately 20° of flexion, and they rapidly increased as the knee was brought into full extension (0°).
Fig. 3.
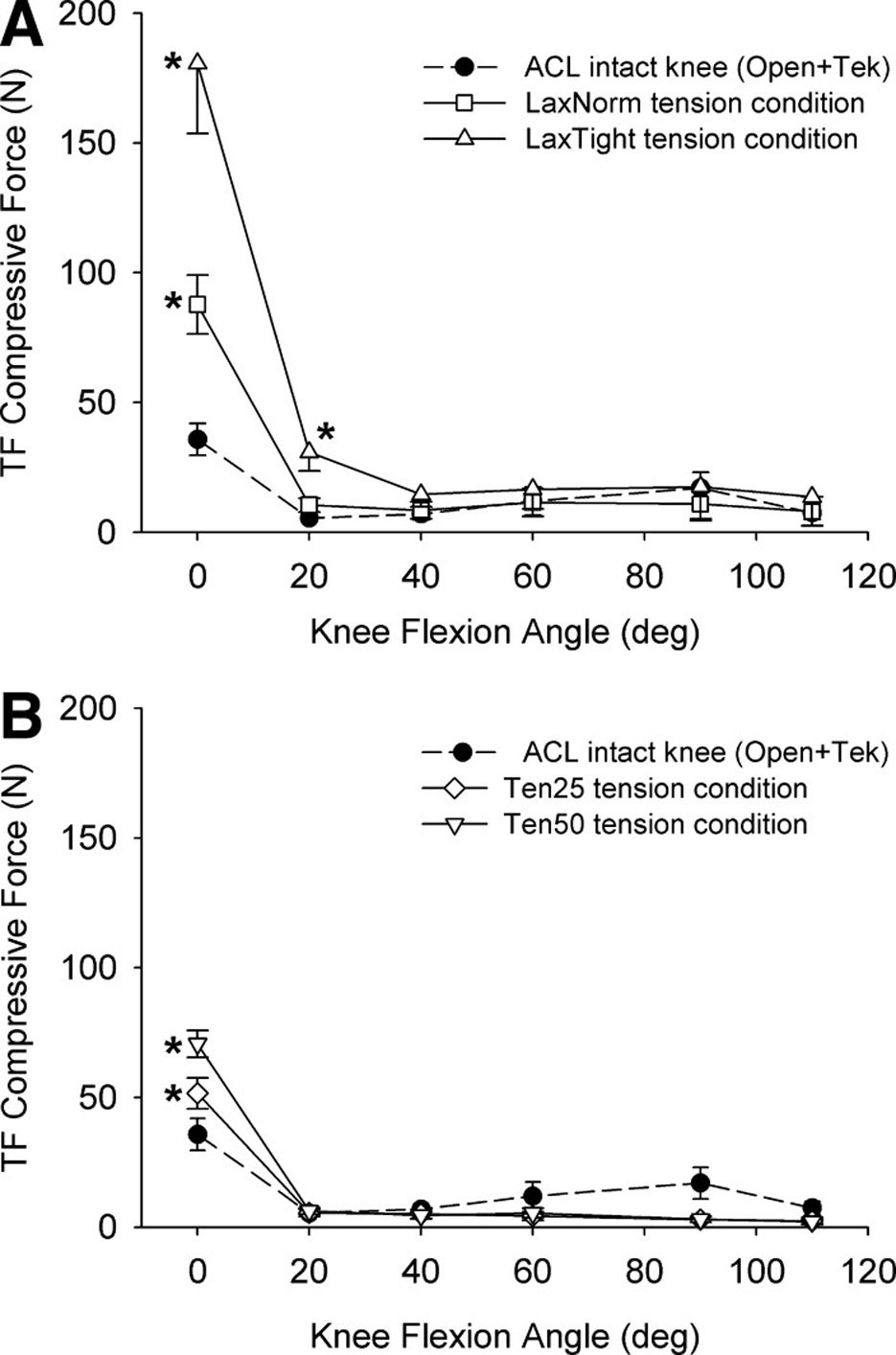
When the knee was at full extension (0°), all of the initial graft tension conditions produced significantly greater TF compressive forces than the ACL-intact knee. At 20°, only the laxity-based LaxTight condition was significantly greater than normal (A). On average the force-based approach more closely restored the TF compressive forces across the joint (B). The error bars represent 1 standard error. * indicates a significant difference (p<0.03).
The TF compressive forces produced by the different initial graft tension protocols were knee flexion angle dependent (interaction p<0.001). The laxity-based tension conditions produced the highest TF compressive forces (Fig. 3a). On average, the TF compressive forces for the LaxTight and LaxNorm conditions were 429% (p<0.001) and 159% (p<0.001) more than that of the ACL-intact knee, respectively, when the knee was in extension (Table 1). The force-based conditions produced forces that were significantly elevated relative to the ACL-intact knee (Ten50 = 105% greater; p=0.01) and Ten25 = 50% greater; p=0.03) when the knee was in extension (Fig. 3b). With the knee at 20° of flexion, only the LaxTight condition was significantly greater than normal (p=0.002). No differences were found between any tension condition and the normal knee with the knee at 40°, 60°, 90° or 110° (p>0.64). A post-hoc power analysis based on the values obtained in this study found that it was 99% powered to detect the differences in TF compressive loads that were measured.
TABLE 1.
The total TF compressive forces for the ACL-intact (open+tek) and the four initial graft tension conditions for each knee specimen.
| Specimen | Open+Tek | LaxNorm | LaxTight | Ten25 | Ten50 |
|---|---|---|---|---|---|
| 872R | 28 | 68 | 104 | 35 | 53 |
| 873R | 11 | 18 | 29 | 35 | 67 |
| 873L | 12 | 74 | 137 | 35 | 57 |
| 874L | 9 | 58 | 126 | 43 | 77 |
| 934L | 39 | 92 | 212 | 63 | 52 |
| 936R | 30 | 122 | 235 | 72 | 97 |
| 936L | 50 | 149 | 313 | 72 | 95 |
| 937R | 58 | 118 | 217 | 73 | 78 |
| 937L | 70 | 112 | 300 | 63 | 73 |
| 939R | 18 | 100 | 222 | missing | 82 |
| 939L | 53 | 53 | 92 | 25 | 45 |
| x | 34.8 | 87.7 | 180.7 | 51.5 | 70.6 |
| sd | 21.16 | 37.65 | 89.75 | 18.82 | 17.23 |
| p-value* | - | p<0.001 | p<0.001 | p=0.02 | p<0.001 |
The p-values denote significant differences from the ACL-intact (open+tek) condition with the sensor implanted (via the Holm-Sidak Test).
AP Laxity
The laxity-based tension conditions produced significantly different AP laxity values (p<0.001). The LaxNorm condition resulted in a joint with 2.8±2.6mm (mean±standard deviation) greater AP laxity than that of the LaxTight condition (Fig. 4).
Fig. 4.
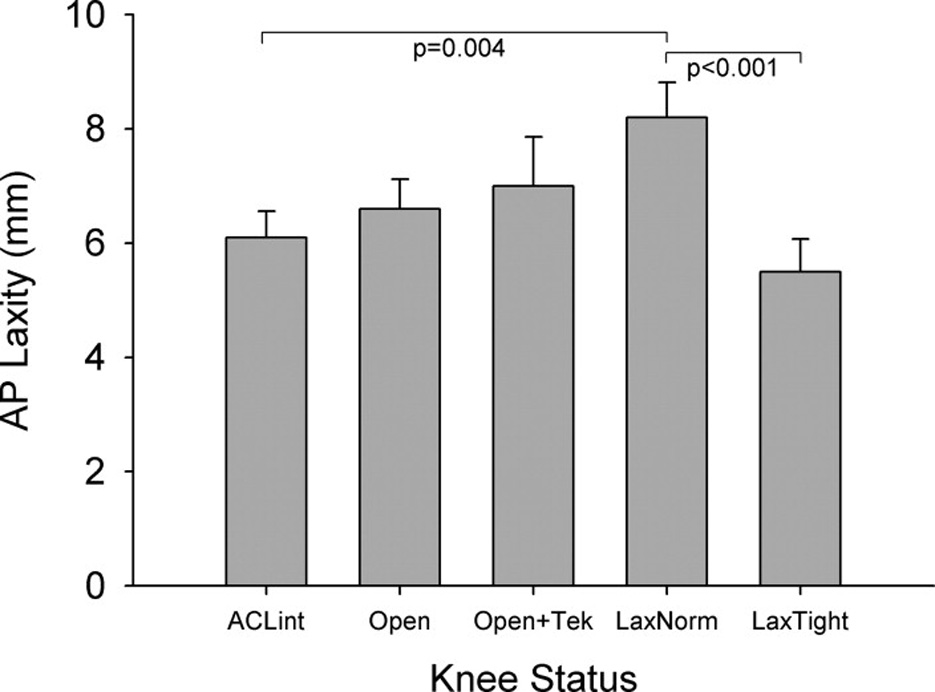
A significant difference was found between the two laxity-based tension conditions. No differences were seen between the ACL-intact (ACLint), ACL-intact with capsule-open (Open), or ACL-intact with capsule open and tekscan inserted (Open+Tek).
On average, the AP laxity values of the LaxTight condition were 11% lower than that of the ACLint joint, though this was not a significant decrease (p=0.37). The LaxTight condition produced a joint that was 0.6±1.9mm less than the ACLint joint (Fig. 4).
The AP laxity values of the LaxNorm condition were 35% greater than that of the ACLint joint (p=0.004). The LaxNorm condition produced a joint that was 2.1±2.5mm greater than the ACLint joint (Fig. 4).
No significant differences were found between the three ACL-intact conditions (ACLint, Open, Open+Tek)(Fig. 4). There was an 8% increase in laxity when the capsule was opened relative to the ACLint state (p=0.48). When the menisci were violated to insert the pressure sensors, there was an additional 5% increase (p=0.60). No significant difference was found between the Open+Tek and ACLint states (p=0.22).
DISCUSSION
We evaluated two laxity-based and two force-based protocols that are used clinically for the reconstruction of the ACL with bone-patellar tendon-bone graft. We hypothesized that the laxity-based protocol that restored normal joint kinematics would best approximate the normal TF compressive forces over the range of passive flexion-extension motion. However, the data did not support our hypothesis. The laxity-based initial graft tension protocols produced the highest TF compressive forces with the knee near extension as compared to the two force-based protocols. All initial graft tension conditions, except for the 25N initial graft tension condition (Ten25), were significantly greater than normal. In the flexed knee (from 40° to 110°), there were no differences between the four initial graft tension conditions and the ACL-intact knee (Open+Tek). Although we did not measure the laxity values for the force-based initial graft tension protocols, the laxity values would be greater than those of the two laxity-based tension conditions.13 This raises an unresolved conflict: Should surgeons attempt to restore normal AP laxity, or to restore normal TF contact conditions when performing ACL reconstruction?
There are several surgical parameters, including graft placement and graft tension, which are known to influence the AP laxity3, 13, 17–20 and TF compressive forces of the joint4 at the time of surgery. The long-term effects of graft placement on surgical outcome have also been assessed, though the mixed findings are possibly due to the sensitivity of the outcome measures or the interactions of confounding factors.21–24 Nonetheless, cadaver studies have shown that graft placement influences the graft tension pattern during passive flexion-extension, while an increase in graft tension will not alter the shape of the pattern but only shifts the loads higher.3, 13 Because these two parameters interact with each other, we attempted to control graft position so we could independently look at the effects of graft tension. A commercially available trans-tibial drill guide system was used to create the femoral tunnels in all specimens. The procedure was performed with the joint opened anteriorly so that we were able to visualize the native insertion of the ACL. A femoral drill guide with a 6 mm offset aimed at the 10:30 or 1:30 positions was used. The resulting TF compressive force patterns of the ACL-reconstructed knees were consistent with one another (Fig. 3) and thus the changes observed were primarily due to the initial graft tension conditions. Our finding that the TF compressive force in the extended knee for the LaxNorm condition was greater than that of the ACL-intact knee may suggest that small errors in graft placement, at least for single bundle ACL reconstruction, may have an impact on TF compressive forces.
Little is known about the effects of initial graft tension on TF compressive forces.4 Due to orientation of the ACL relative to the tibia and femur, it is clear that an increase in initial graft tension will increase the TF compressive forces while decreasing the laxity of the joint.16 Results from the present study support this hypothesis. A previous study determined that a low initial graft tension (1-15N with the knee at extension) was sufficient to restore the TF compressive load when the knee was in full extension.4 Based on our present study, this value would be insufficient to restore the AP laxity of the joint. For the LaxNorm condition, an initial graft tension of 87±38N when applied at 0° of flexion was needed which is significantly greater that those produced for the two force-based initial graft tension protocols (Fig. 3).
In this study, the relationship between initial graft tension, the TF compressive force and AP laxity were evaluated at the time of surgery. However, it is well known that the grafts undergo stress relaxation after fixation due to the viscoelastic response of the tissue. 25–27 Cicconi et al has shown that the tension and stiffness of hamstring tendon grafts will to approximately 50% and 80% following stress relaxation which in turn would result in an increase in knee laxity. Likewise, cyclic creep can occur with initial cycling of the knee 10. These viscoelastic changes will result in an increase in joint laxity. Preconditioning was performed in this study to minimize these changes, though increases in laxity were still observed. The implications of this from a biological perspective are not known. A graft that is over-tensioned will produce higher forces on the articular cartilage. The graft may also be subject to fiber failure during the initial healing stage as part of the healing process. A graft that is under-tensioned may permit excessive sliding between the articular surfaces. In a graft that is loose, it is possible that myofibroblast could improve laxity in the long term.28 Clinical studies are needed to resolve these questions.
There have been four prospective randomized clinical trials investigating the effects of force-based initial graft tension protocols on AP laxity and clinical outcome following ACL-reconstruction.6–9 Caution should be used when comparing the Time 0 results of a cadaver study to those of a clinical study in which healing is present. Nonetheless, two found significant differences in AP laxity after two years of healing when comparing high and low initial graft tensions conditions,8, 9 while two did not.6, 7 It should be noted that in those studies in which no differences were reported after healing, the differences in initial graft tension conditions were relatively small (20N to 25N),6, 7 and no differences in AP laxity values were observed soon after graft fixation.6 A clinical study which evaluates long-term cartilage changes following different initial graft tension conditions is warranted to settle which initial graft tensioning technique and condition provides the best profile in terms of knee joint function and cartilage degeneration.
There are several limitations that should be considered when interpreting our data. In a cadaver model, the TF compressive forces are relevant to those produced at the time of surgery. viscoelastic changes occur after fixation. 10, 15, 25–27 We attempted to minimize this effect by preconditioning the joint by flexing and extending the joint and interatively resetting the tension until the graft load did not drop off.4 However, increases in laxity were still observed. The order of testing between the initial tension conditions was also randomized to minimize these biases.
Cadaver models do not consider the healing response of the graft and the subsequent changes induced in the cartilage. However, it is important to characterize the time zero response so that clinical studies can be performed to evaluate these long-term healing effects.
The TF compressive forces were measured during passive extension motion of the joint without simulating the muscle forces. However, TF motion is guided primarily by the cruciate linkage when the knee is passively flexed and extended.29 Therefore, passive extension motion provides a relevant indicator of graft function and baseline data for more complicated in vivo loading situations.
The TF compressive forces were directly measured using thin-film pressure sensor technology. Although the sensor elements have a thin profile for easy insertion between the surfaces of the medial and lateral compartments (thickness = 0.1mm), it is necessary to make incisions in the anterior and posterior capsule partially detaching the meniscus coronary ligament attachment from the tibia in order to slide them into place. Because the tibial plateau is not flat, it is possible that slight bending or sensors could occur which could introduce artifact. This problem was minimized however since the calibrations were performed on a knee specimen under similar conditions. Likewise, comparisons were made between treatments within a specimen. To ensure that these anterior and posterior incisions had minimal impact, laxity measurements were performed in the ACL-intact knee with and without the sensors in place. Although there was a slight increase in joint laxity when the incisions were made, the increase was not significant. The calibration of these sensors can also be difficult because their output is dependent on several factors including the stiffness and geometry of the contacting surfaces.30–32 To minimize these effects, calibrations were performed in a human knee where the forces could be directly applied and measured using a material testing protocol.4
Finally, graft fixation was different than the clinical situation. Although the proximal bone block of the graft was secured with an interference screw, the distal bone block was attached via sutures to the tensioning device rigidly mounted to the anterior aspect of the tibia. FiberWire was selected since it is relatively non-compliant. Nonetheless, suture compliance could artificially increase the laxity values and hence reduce the TF compressive force values. Other sources of increased laxity could be toggling of the 10 mm bone block within the 12 mm tibial bone tunnel or differences in the linear stiffness of the ACL and patellar tendon graft. Clinically, firm tension on the graft with the knee at 30° produces a knee that is 2mm less than that of the contralateral (ACL intact) knee.33 When firmly tensioning it in extension, AP laxity is restored at the time of surgery.34 In this study, a 2mm difference in laxity was present but the LaxTight condition was closer to normal and the LaxNorm condition was 2mm greater than normal. This could be due to the invasiveness of the pressure measurement technique and/or viscoelastic changes in the graft-suture construct.10, 15, 25 Since the TF compressive forces were greater than the normal knee, this potential concern is minimized.
CONCLUSIONS
The results of this study demonstrate that initial graft tension conditions influence TF compressive forces at the time of surgery, and that clinically relevant initial graft tension conditions produce different TF compressive forces.
Acknowledgments
Supported by: Arthroscopy Association of North America and the National Institutes of Health (AR047910).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63:269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming BC, Hulstyn MJ, Oksendahl HL, Fadale PD. Ligament injury, reconstruction, and osteoarthritis. Curr Opin Orthop. 2005;16:354–362. doi: 10.1097/01.bco.0000176423.07865.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning, and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6:S2–S12. doi: 10.1007/s001670050215. [DOI] [PubMed] [Google Scholar]
- 4.Brady MF, Fleming BC, Bradley MP, Banerjee R, Fadale PD, Hulstyn MJ. Effects of initial graft tension on the tibiofemoral compressive forces and joint position following ACL reconstruction. Am J Sports Med. 2007;35:395–403. doi: 10.1177/0363546506294363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burks RT, Leland R. Determination of graft tension before fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1988;4:260–266. doi: 10.1016/s0749-8063(88)80041-0. [DOI] [PubMed] [Google Scholar]
- 6.Yoshiya S, Kurosaka M, Ouchi K, Kuroda R, Mizuno K. Graft tension and knee stability after anterior cruciate ligament reconstruction. Clin Orthop. 2002;394:154–160. doi: 10.1097/00003086-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 7.van Kampen A, Wymenga AB, van der Heide HJL. The effect of different graft tensioning in anterior cruciate ligament reconstruction: A prospective randomized study. Arthroscopy. 1998;14:845–850. doi: 10.1016/s0749-8063(98)70022-2. [DOI] [PubMed] [Google Scholar]
- 8.Yasuda K, Tsujino J, Tanabe Y, Kaneda K. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction - Autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25:99–106. doi: 10.1177/036354659702500120. [DOI] [PubMed] [Google Scholar]
- 9.Nicholas SJ, D'Amato MJ, Mullaney MJ, Tyler TF, Kolstad K, McHugh MP. A prospectively randomized double-blind study on the effect of initial graft tension on knee stability after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:1881–1886. doi: 10.1177/0363546504265924. [DOI] [PubMed] [Google Scholar]
- 10.Arnold MP, Lie DT, Verdonschot N, de Graaf R, Amis AA, Van Kampen A. The remains of anterior cruciate ligament graft tension after cyclic knee motion. Am J Sports Med. 2005;33:536–542. doi: 10.1177/0363546504269938. [DOI] [PubMed] [Google Scholar]
- 11.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three dimensional motions: Application to the knee. J Biomech Engin. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 12.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. J Bone Joint Surg. 1990;72:557–567. [PubMed] [Google Scholar]
- 13.Fleming BC, Beynnon BD, McLeod WD, Howe JG, Pope MH. Effect of tension and placement of a prosthetic anterior cruciate ligament on the anteroposterior laxity of the knee. J Orthop Res. 1992;10:177–186. doi: 10.1002/jor.1100100204. [DOI] [PubMed] [Google Scholar]
- 14.Singerman R, Berilla J, Archdeacon M, Peyser A. In vitro forces in the normal and cruciate-deficient knee during simulated squatting motion. J Biomech Engin. 1999;121:234–242. doi: 10.1115/1.2835109. [DOI] [PubMed] [Google Scholar]
- 15.Boylan D, Greis PE, West JR, Bachus KN, Burks RT. Effects of initial graft tension on knee stability after anterior cruciate ligament reconstruction using hamstring tendons: A cadaver study. Arthroscopy. 2003;19:700–705. doi: 10.1016/s0749-8063(03)00400-6. [DOI] [PubMed] [Google Scholar]
- 16.Zavatsky AB, O'Connor JJ. A model of human knee ligaments in the sagittal plane. Part I.: Response to passive flexion. Eng Med. 1992;206:125–134. doi: 10.1243/PIME_PROC_1992_206_280_02. [DOI] [PubMed] [Google Scholar]
- 17.Zavras TD, Race A, Amis AA. The effect of femoral attachment location on anterior cruciate ligament reconstruction: graft tension patterns and restoration of normal anterior-posterior laxity patterns. Knee Surg Sports Traumatol Arthrosc. 2005;13:92–100. doi: 10.1007/s00167-004-0541-5. [DOI] [PubMed] [Google Scholar]
- 18.Markolf KL, Burchfield DM, Shapiro MM, DAvis RR, Finerman GAM, Slauterbeck JL. Biomechanical consequences of replacement of the anterior cruciate ligament with a patellar ligament allograft. 1. Insertion of the graft and anterior-posterior testing. J Bone Joint Surg. 1996;78:1720–1727. doi: 10.2106/00004623-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Fleming BC, Beynnon BD, Johnson RJ, McLeod WD, Pope MH. Isometric versus tension measurements: A comparison for the reconstruction of the anterior cruciate ligament. Am J Sports Med. 1993;21:82–86. doi: 10.1177/036354659302100115. [DOI] [PubMed] [Google Scholar]
- 20.Bylski-Austrow DI, Grood ES, Hefzy MS, Holden JP, Butler DL. Anterior cruciate ligament replacements: A mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522–531. doi: 10.1002/jor.1100080408. [DOI] [PubMed] [Google Scholar]
- 21.Beynnon BD, Uh BS, Johnson RJ, Fleming BC, Renstrom PA, Nichols CE. The elongation behavior of the anterior cruciate ligament graft in vivo - A long-term follow-up study. Am J Sports Med. 2001;29:161–166. doi: 10.1177/03635465010290020801. [DOI] [PubMed] [Google Scholar]
- 22.Khalfayan EE, Sharkey PF, Alexander AH, Bruckner JD, Bynum EB. The relationship between tunnel placement and clinical results after anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:335–341. doi: 10.1177/036354659602400315. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda H, Muneta T, Niga S, Hoshino A, Asahina S, Yamamoto H. The long-term effects of tibial drill hole position on the outcome of anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:287–291. doi: 10.1016/s0749-8063(99)70036-8. [DOI] [PubMed] [Google Scholar]
- 24.Panni AS, Milano G, Tartarone M, Demontis A, Fabbriciani C. Clinical and radiographic results of ACL reconstruction: a 5-to 7-year follow-up study of outside-in versus inside-out reconstruction techniques. Knee Surg Sports Traumatol Arthrosc. 2001;9:77–85. doi: 10.1007/s001670000171. [DOI] [PubMed] [Google Scholar]
- 25.Ciccone WJ, Bratton DR, Weinstein DM, Elias JJ. Viscoelasticity and Temperature Variations Decrease Tension and Stiffness of Hamstring Tendon Grafts Following Anterior Cruciate Ligament Reconstruction. J Bone Joint Surg - Am. 2006;88:1071–1107. doi: 10.2106/JBJS.E.00576. [DOI] [PubMed] [Google Scholar]
- 26.Thornton GM, Shrive NG, Frank CB. Healing ligaments have decreased cyclic modulus compared to normal ligaments and immobilization further compromises healing ligament response to cyclic loading. J Orthop Res. 2003;21:716–722. doi: 10.1016/S0736-0266(03)00051-2. [DOI] [PubMed] [Google Scholar]
- 27.Boorman RS, Thornton GM, Shrive NG, Frank CB. Ligament grafts become more susceptible to creep within days after surgery - Evidence for early enzymatic degradation of a ligament graft in a rabbit model. Acta Orthop Scand. 2002:568–574. doi: 10.1080/000164702321022866. [DOI] [PubMed] [Google Scholar]
- 28.Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008 doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 29.Imran A, O'Connor JJ. Theoretical estimates of cruciate ligament forces: effects of tibial surface geometry and ligament orientations. Eng Med. 1997;211:425–439. doi: 10.1243/0954411981534556. [DOI] [PubMed] [Google Scholar]
- 30.Otto JK, Brown TD, Callaghan JJ. Static and dynamic response of a multiplexed-array piezoresistive contact sensor. Exp Mechanics. 1999;39:317–323. [Google Scholar]
- 31.Drewniak EI, Crisco JJ, Spenciner DB, Fleming BC. Accuracy of circular contact area measurements with thin-film pressure sensors. J Biomech. 2007;40:2569–2572. doi: 10.1016/j.jbiomech.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Bachus KN, DeMarco AL, Judd TA, Horwitz DS, Brodke DS. Measuring contact area, force, and pressure for bioengineering applications: Using Fuji Film and TekScan systems. Med Engin Phys. 2005;28:483–488. doi: 10.1016/j.medengphy.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, et al. Anterior cruciate ligament reconstruction using bone-patellar tendon-bone versus two-stranded hamstring grafts: A prospective, randomized study. J Bone Joint Surg. 2002;84:1503–1513. doi: 10.2106/00004623-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Beynnon BD, Uh BS, Fleming BC, Renstrom P, Roos H, Poole RA, et al. Rehabilitation following anterior cruciate ligament reconstruction; A prospective, randomized, double-blind comparison of accelerated versus non-accelerated rehabilitation. Am J Sports Med. 2005;33:347–359. doi: 10.1177/0363546504268406. [DOI] [PubMed] [Google Scholar]


