Abstract
Acute kidney injury (AKI) is associated with high morbidity and mortality. The lack of sensitive and specific injury biomarkers has greatly impeded the development of therapeutic strategies to improve outcomes of AKI.
The unique objective of this study was to evaluate the diagnostic performance of nine urinary biomarkers of AKI—kidney injury molecule‐1 (KIM‐1), neutrophil gelatinase associated lipocalin (NGAL), interleukin‐18 (IL‐18), hepatocyte growth factor (HGF), cystatin C (Cys), N‐acetyl‐β‐D‐glucosaminidase (NAG), vascular endothelial growth factor (VEGF), chemokine interferon‐inducible protein 10 (IP‐10; CXCL10), and total protein—in a cross‐sectional comparison of 204 patients with or without AKI.
Median urinary concentrations of each biomarker were significantly higher in patients with AKI than in those without AKI (p < 0.001). The area under the receiver operating characteristics curve (AUC‐ROC) for the combination of biomarkers using a logic regression model [risk score of 2.93*(NGAL > 5.72 and HGF > 0.17) + 2.93*(PROTEIN > 0.22) −2*(KIM < 0.58)] was greater (0.94) than individual biomarker AUC‐ROCs. Age‐adjusted levels of urinary KIM‐1, NAG, HGF, VEGF, and total protein were significantly higher in patients who died or required renal replacement therapy (RRT) when compared to those who survived and did not require RRT.
Our results demonstrate the comparative value of multiple biomarkers in the diagnosis and prognosis of AKI.
Keywords: biomarkers, acute kidney injury, kidney injury molecule‐1, neutrophil gelatinase associated lipocalin, hepatocyte growth factor
Introduction
Acute kidney injury (AKI) is a common and devastating problem with in‐hospital mortality of 40% to 80% in the intensive care setting. 1 The traditional blood (creatinine, blood urea nitrogen) and urine markers of kidney injury (casts, fractional excretion of sodium, urinary concentrating ability) that have been used for decades in clinical studies for diagnosis and prognosis of AKI are insensitive and nonspecific and do not directly reflect injury to kidney cells. Most of these markers are functional consequences of the injury 2 Easily quantifiable and sensitive injury biomarkers can be influential in every phase of clinical decision making as well as drug development from drug discovery and preclinical evaluation through each phase of clinical trials and into post‐marketing studies. Identification and qualification of biomarkers of drug safety is a central theme of the U.S. Food and Drug Administration (FDA) critical path initiative and an important topic for the European Medicines Agency (EMEA). 3 A single biomarker may not be adequate to define AKI given inherent renal structural heterogeneity and the disparate settings under whichkidney injury occurs. 4 Prior studies have focused on one or two biomarkers and hence have not provided a context to compare relative performance of urinary biomarkers nor evaluate if predictive algorithms can be developed using multiple biomarkers. 5
The primary objective of this study was to evaluate the sensitivity, specificity, and prognostic ability of urinary biomarkers for AKI and AKI‐associated mortality either individually or in combination. Specifically, our aims were to (1) compare the diagnostic performance of nine promising novel urinary biomarkers for AKI: KIM‐1, NGAL, IL‐18, NAG, HGF, Cystatin C, VEGF, IP‐10, and total protein in a cross‐sectional study involving 102 patients with clinically established AKI, and 102 patients with no AKI; (2) apply logic regression methodology to construct Boolean combinations of binary coded biomarkers and compare the sensitivity and specificity of these biomarker combinations to individual biomarkers in the diagnosis of AKI. This initial evaluation of relative performance of potential biomarkers of injury is best done when the end point, AKI, is well established because ambiguity in the endpoint of injury would introduce great uncertainty into the evaluative process. A secondary aim was to develop and evaluate a microbead‐based assay for multiple biomarker quantitation in the same aliquot of urine sample thereby increasing the analyses throughput. 6
Methods
Selection of participants
Patients with documented AKI of at least the “Risk” category of the RIFLE criterion 7 (peakSCr > 50% increase over admission value or known baseline) were recruited from the inpatient nephrology consultation service. Causes of AKI were obtained by detailed chart review including the treating nephrologist's consultation note and evaluation of laboratory data by a co‐author not involved in the patients' care (S.S.W.). Individuals without AKI were selected from three distinct populations: healthy volunteers, patients undergoing cardiac catheterization, and patients admitted to the intensive care unit. Healthy volunteers were excluded if they reported a recent hospitalization, diagnosis of chronic kidney disease, or treatment with nephrotoxic medications (nonsteroidal anti‐inflammatory drugs were allowed). Patients undergoing cardiac catheterization and those admitted to the intensive care unit were included in the non‐AKI cohort if they had normal urine output (>0.5 mL/kg/hr), stable SCr during hospitalization (<0.3 mg/dL change from baseline), and an estimated GFR > 50 mL/min. Urine samples from cardiac catheterization patients were taken before administration of intravenous contrast. All participants were patients or employees (healthy volunteers) of Brigham and Women's Hospital, a tertiary care teaching hospital. The Institutional Review Board approved the protocols for recruitment and sample collection.
Urine samples
Urine was collected from spontaneous voids or from indwelling Foley catheters. Urine dipstick analysis was performed (Multistix 8 SG, Bayer Corporation, Tarrytown, NY), followed by centrifugation and microscopic examination of the urine sediment (Olympus Microscope, Nashua, NH). The urine supernatant was aliquoted into 1.8 mL eppendorf tubes and frozen within 2 hours of collection at −80°C. At the time of assay samples were thawed, vortexed, and centrifuged at 14,000 rpm at 4°C and 30–100 μL of supernatant was pipetted for biomarker measurement. Assays were performed within 3 months of urine collection after a maximum of three freeze‐thaw cycles. Urine samples from patients with established AKI were collected close to the time of initial consultation.
Measurement of urinary biomarkers
Urinary total protein (Sigma, St. Louis, MO) and NAG (Roche Diagnostics, Basel Switzerland) were measured spectrophotometrically according to the manufacturers' protocols. Urinary Cystatin C was measured as reported previously 8 with the N latex Cystatin C kit (Dade Behring, Marburg, Germany) using a BN II nephelometer. Briefly, sample was diluted 1:5 with phosphate buffer. Reagent buffer (195 μL) and diluted sample (50 μL) were then pipetted into the cuvette and preincubated at 37 °C for 5 min. The reaction was initiated by the addition of particles coated with polyclonal anti‐cystatin C antibodies (15 μL) to form a complex. The scatter signal from the antigen‐antibody reaction is dependent on the size of the complex and the cystatin C content of the sample. The rate of change of the scatter signal was calculated, and the concentration in the sample was interpolated from the calibration curve. KIM‐1, NGAL, IL‐18, HGF, IP‐10, and VEGF were measured using microbead based assays described below.
Development and evaluation of microbead‐based assay for urinary biomarker quantitation
Coupling of the beads to respective capture antibodies.
The microbead‐based assays for KIM‐1 and NGAL were developed and evaluated in this study using an amine coupling kit from Bio‐Rad (Hercules, CA) whereas microbead assays for HGF, IL‐18, VEGF, and IP‐10 were commercially available from Bio‐Rad Laboratories.
Evaluation of the assay.
The performance characteristics of the microbead‐based assay were evaluated in the same way that we evaluated the Kim‐1 ELISA 9 by measuring the sensitivity, assay range, specificity, reproducibility, recovery, and interference ( Table 1 ). The sensitivity or the lowest limit of detection (LLD) was determined by diluting the respective standard in sample diluent; the concentration which is two standard deviations above the background (sample diluent alone) was determined to be the LLD. The analytical recovery in control and diseased urines was determined by adding a known amount (low, medium, and high concentrations) of respective recombinant proteins into urine of control/healthy volunteers or diseased urine samples and quantitating the levels of respective antigens prior to and subsequent to the addition. This was done to verify that there were no interfering substances in the urine of patients with AKI. 10 Dilutional linearity was evaluated in normal and diseased urines to justify sample dilution, which was needed to eliminate the interference in antigen recovery for KIM‐1, NGAL, IL‐18, HGF, VEGF, and IP‐10 assays. Sample dilution was required for NAG, total protein, and cystatin C assays to fit the concentrations of respective antigens in the linear range of the standard curve. Diseased urine samples containing low, medium, and high concentrations of respective antigens (as measured by the microbead‐based assay) were diluted 1:2, 1:10, 1:20, 1:100, 1:500 using sample diluent.
Table 1.
Evaluation of assays to measure biomarkers for acute kidney injury.
| Parameters | KIM‐1 | NGAL | IL‐18 | HGF | VEGF | IP‐10 | Cystatin C | NAG | Protein |
|---|---|---|---|---|---|---|---|---|---|
| Assay principle | Microbead‐based sandwich ELISA | Microbead‐based sandwich ELISA | Microbead‐based sandwich ELISA | Microbead‐based sandwich ELISA | Microbead‐based sandwich ELISA | Microbead‐based sandwich ELISA | Latex bead‐based turbidimetry | Enzyme‐substrate‐based colorimetry | Absorbance shift‐based colorimetry |
| LLD | 4.4 pg/mL | 0.53 ng/mL | 0.125 pg/mL | 0.709 pg/mL | lOpg/mL | 32 pg/mL | 0.043 mg/L | 0.2 U/L | 0.011 |
| Assay range | 40–160,000 pg/mL | 0.49–1,000 ng/mL | 0.12–2,000 pg/mL | 0.7–1,446 pg/mL | 7.8–31,982 pg/mL | 25–10,000 pg/mL | 0.043–27.2 mg/L | 0.2–52.9 U/L | 0.01–2 mg/mL |
| Intra assay | <15% | <15% | <10% | <10% | <10% | <10% | <5% | <2% | <10% |
| Inter assay | <20% | <20% | <20% | <20% | <20% | <20% | <5% | <2% | <10% |
| Recovery | 85–100% | 85–100% | 85–110% | 85–110% | 85–110% | 85–110% | 90–100% | 90–100% | 90–100% |
| Linearity | Linear over dilutions: 1:2, 1:10, 1:20 | Linear over dilutions: 1:10, 1:100, 1:500 | Linear over dilutions: 1:10, 1:20, 1:40 | Linear over dilutions: 1:2, 1:10, 1:20 | Linear over dilutions: 1:5, 1:10, 1:20 | Linear over dilutions: 1:5, 1:10, 1:20 | Linear over dilutions: 1:20, 1:100, 1:400 | Linear over dilutions: 1:2, 1:10, 1:20 | Linear over dilutions: 1:2, 1:5 |
| Interference | No interference when tested for albumin, bilirubin, creatinine, creatine, glucose, hemoglobin, urea. unknown interference does exist with the human KIm‐1 and nGal assay but dilution of the sample with diluent results in 85–100% recovery. | ||||||||
Statistics
Continuous variables were expressed as means ± SD or medians, and compared using the Student's t‐test or Kruskal‐Wallis test, as appropriate. Categorical variables were expressed as proportions and compared with the χ2 test. Urinary creatinine concentration was used to normalize biomarker measurements to account for the influence of urinary dilution on biomarker concentrations. Scatterplots were used to graphically display log‐transformed normalized biomarker levels in the four groups of subjects. Diagnostic performance (i.e., the ability of a urinary biomarker to identify AKI) was assessed by evaluating sensitivity and specificity using the receiver operating characteristics (ROC) curve. The area under the ROC curve (AUC) and 95% confidence interval (CI) were calculated using the nonparametric method of Hanley and McNeil. 11 The AUC for a diagnostic test ranges from 0.5 (no better than chance alone) to 1.0 (perfect test, equivalent to the gold standard). Logic regression 12 was used to construct Boolean combinations of binary coded biomarkers to allow for high‐order interactions between the biomarker outcomes. To apply this methodology, we created indicator variables for the biomarkers, using their 33rd and 67th percentiles from the non‐AKI subjects. Cross‐validation was used to select the optimal number of logic trees and total leaves (i.e., complexity) in the model. Software for this approach was downloaded from http://bear.fhcrc.org/~ingor/logic/. The resulting score was then used to construct an ROC curve. The bootstrap percentile method (2,000 replications) was used to obtain a 95% confidence interval for the AUC. A similar approach was taken by Janes et al. 13 in an analysis of screening for colorectal cancer. The ability of urinary biomarkers to predict in‐hospital mortality and identify the need for renal replacement therapy in patients with established AKI was tested using logistic regression analysis, adjusting for age. Two‐tailed p‐value of <0.05 was considered statistically significant.
Results
Urinary biomarkers in individuals with and without acute kidney injury
Quantitation of biomarkers.
The microbead‐based assays for KIM‐1 and NGAL were developed and evaluated in this study whereas all other biomarker assays were commercially available. The sensitivity, specificity, precision profile, recovery, interference, and dilutional linearity for each assay were extensively evaluated and were within the acceptable range ( Table 1 ). Urinary biomarker values were calculated using a 12 to 14 point five‐parametric logarithmic standard curve ( Figure 1 ).
Figure 1.
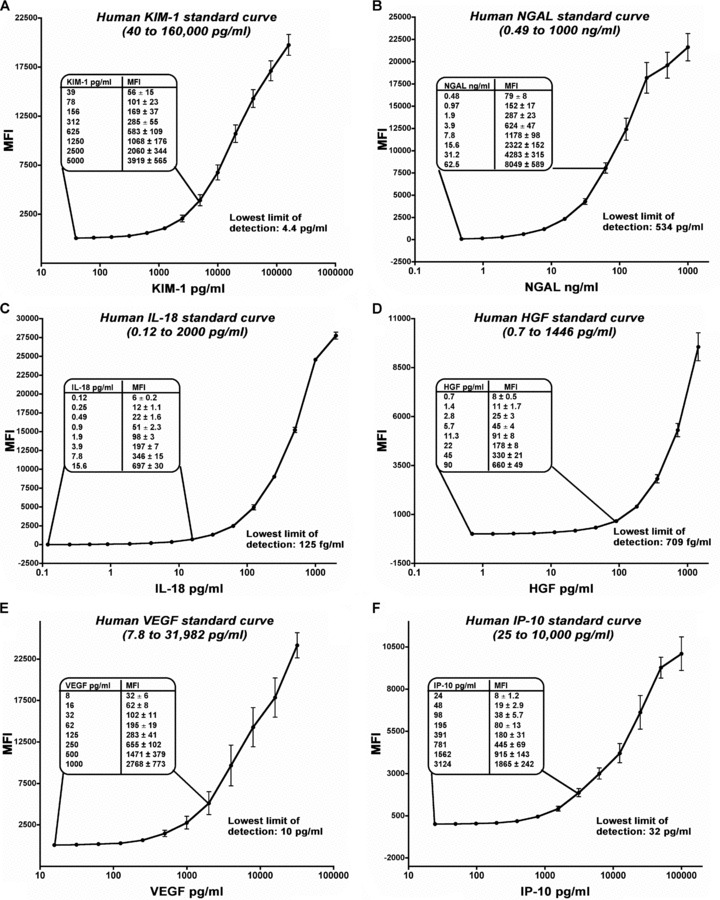
Evaluation of microbead‐based assay for quantitation of human urinary KIM‐1, NGAL, IL‐18, HGF, VEGF, and IP‐10. (A) Standard curve for human KIM‐1 was obtained using purified recombinant human KIM‐1 ectodomain fusion protein. It demonstrated linearity over five orders of magnitude from 40 pg/mL to 160,000 pg/mL with the lowest limit of detection (LLD) to be 4.4 pg/mL (B) Standard curve for human NGAL was obtained using a commercially available purified NGAL protein. The NGAL standard curve was also linear over five orders of magnitude from 0.49 to 1,000 ng/mL with the LLD of 534 pg/mL The standard curves for IL‐18 (C) and HGF (D) ranged from 0.12 to 2,000 pg/mL and 0.7 to 1,446 pg/mL with the LLD of 125 fg/mL and 709 fg/mL respectively. Similarly, the standard curves for VEGF (E) and IP‐10 (F) ranged from 7.8 to 31,982 pg/mL and 25 to 10,000 pg/mL with the LLD of 10 pg/mL and 32 pg/mL, respectively. The standard curves were plotted as five parameter logistic curves and repeated eight times on different sets of samples on different days using different sets of beads coupled with different batches of primary antibody. The inset in each panel documents the linearity of the maximum fluorescence intensity at lower concentrations.
Human subjects.
Urinary biomarkers were measured in 102 patients with established AKI from a variety of causes and in 102 individuals without a clinical diagnosis of AKI: 39 patients undergoing cardiac catheterization, 13 patients admitted to the intensive care unit, and 50 healthy volunteers. Demographic and clinical information are shown in Table 2 .
Table 2.
Demographic and clinical characteristics of human subjects.
| Established acute kidney injury (N= 102) | Cardiac catheterization (N= 39) | Intensive care unit (N= 13) | Healthy volunteers (N= 50) | |
|---|---|---|---|---|
| Mean age, years, ±SD* | 61.2 ± 17.2 | 69.1 ± 14.1 | 67.7 ± 13.2 | 35.7 ± 10.6 |
| Female** | 45% | 36% | 31% | 76% |
| Black§ | 11% | 10% | 0% | 10% |
| Cause of AKI or reason for ICU admission† | Sepsis (34%), ischemia (18%), nephrotoxin exposure (15%), post‐cardiac surgery (13%), radiocontrast administration (11%), pre‐renal azotemia (10%), other (25%) | – | Postoperative complications (54%), trauma (32%), sepsis (14%) | – |
| Serum creatinine | Peak: range 1.7–10.0 mg/dL | Median 1.0 mg/dL | Median 0.7 mg/dL | –‡ |
| Required renal replacement therapy: 46% | Range 0.6 to 1.4 mg/dL | Range 0.4 to 1.0 mg/dL |
*Statistically significant pairwise comparisons between AKI versus cardiac catheterization (p= 0.01), AKI versus healthy volunteers (p < 0.001), and AKI versus non‐AKI (p= 0.001).
**p < 0.001.
§p= 0.75.
†Sum exceeds 100% in AKI due to multiple diagnoses in individual patients.
‡Healthy volunteers were excluded if they reported a diagnosis of chronic kidney disease; serum creatinine was not measured.
Diagnostic ability of urinary biomarkers.
Median urinary concentrations of cystatin C, HGF, IL‐18, IP‐10, KIM‐1, NAG, NGAL, total protein, and VEGF were each significantly higher in patients with AKI than in those without AKI (p < 0.001). A scatterplot of the distribution of biomarkers levels is shown in Figure 2 . Each of the nine urinary biomarkers was able to differentiate between the established AKI and non‐AKI groups (p < 0.001). The diagnostic performances were best when defining the non‐AKI group as healthy volunteers, but remained high for most biomarkers when comparing AKI versus all non‐AKI (i.e., including cardiac catheterization and intensive care unit patients without a clinical diagnosis of AKI). NAG had nearly perfect diagnostic ability (AUC‐ROC1.00) when comparing AKI to healthy individuals, but had substantially lower diagnostic performance when all non‐AKI individuals (AUC‐ROC 0.83) were included. The same phenomenon was observed for VEGF (AUC‐ROC 0.90 versus 0.73). By contrast, the diagnostic performance characteristics of cystatin C, HGF, IL‐18, IP‐10, KIM‐1, NGAL, and total protein were comparable (i.e., overlapping 95% CI for AUC‐ROC) irrespective of the non‐AKI groups with which the AKI group was compared ( Table 3).
Figure 2.
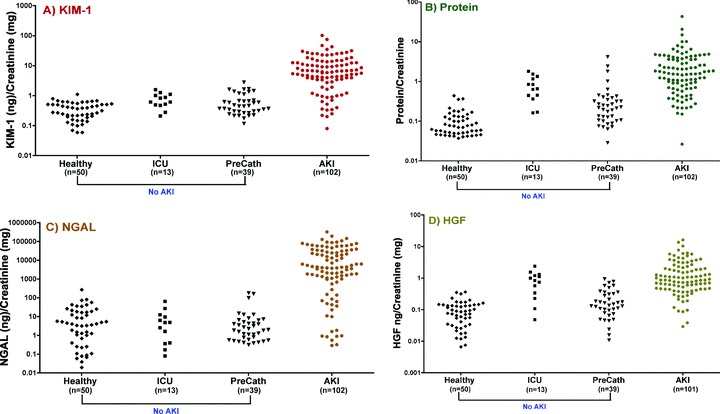
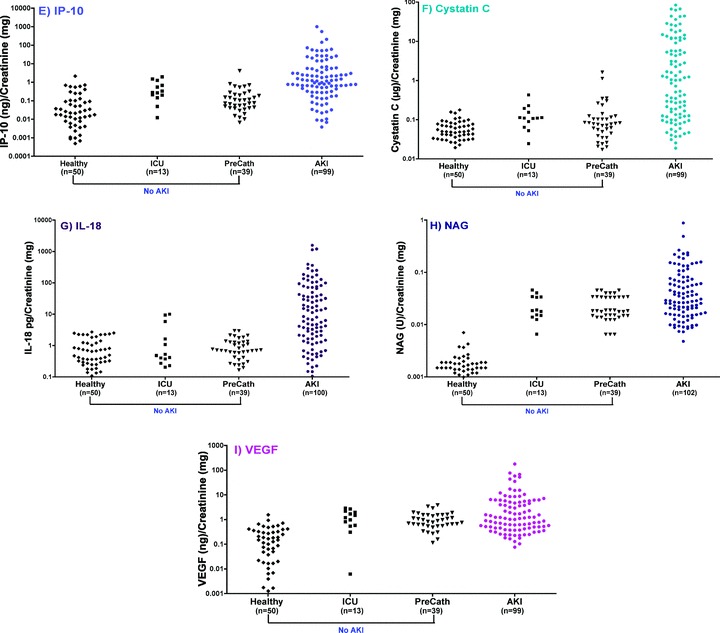
Scatterplot of human urinary (A) KIM‐1, (B) protein, (C) NGAL, (D) HGF, (E) IP‐10, (F) Cystatin C, (G) IL‐18, (H) NAG, and (I) VEGF in patients with and without a clinical diagnosis of acute kidney injury. Urinary biomarker measurements were normalized to urine creatinine and were plotted on a logarithmic Y‐axis. The number of patients in each group is indicated below each category on the X‐axis.
Table 3.
Comparative diagnostic performance characteristics of urinary biomarkers for the identification of established AKI using the area under the receiver operating characteristics curve (AUC‐ROC).
| AKI (N= 102) versus healthy individuals (N= 50) | AKI (N= 102) versus all non‐AKI controls (N= 102) | |||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker* | AUC‐ROC | Cutoff | Sensitivity | Specificity | AUC‐ROC | Cutoff | Sensitivity | Specificity |
| (95% Cl) | (95% Cl) | |||||||
| Urine creatinine (mg) | 0.78 | 62 | 67% | 76% | 0.72 | 37 | 45% | 92% |
| (0.70–0.84) | (0.65–0.78) | |||||||
| Cystatin C (ug/mg) | 0.90 | 0.11 | 78% | 94% | 0.85 | 0.12 | 78% | 83% |
| (0.84–0.94) | (0.80–0.90) | |||||||
| HGF (ng/mg) | 0.96 | 0.23 | 91% | 94% | 0.89 | 0.37 | 84% | 84% |
| (0.92–0.99) | (0.84–0.93) | |||||||
| IL‐18 (pg/mg) | 0.85 | 2.30 | 69% | 92% | 0.83 | 2.74 | 68% | 95% |
| (0.78–0.90) | (0.77–0.88) | |||||||
| IP‐10 (ng/mg) | 0.89 | 0.13 | 85% | 80% | 0.84 | 0.62 | 69% | 89% |
| (0.83–0.93) | (0.79–0.89) | |||||||
| KIM‐1 (ng/mg) | 0.95 | 0.70 | 90% | 96% | 0.93 | 1.73 | 80% | 99% |
| (0.90–0.98) | (0.88–0.96) | |||||||
| NAG (U/mg) | 1.00 | 0.007 | 99% | 100% | 0.83 | 0.015 | 80% | 65% |
| (0.98–1.00) | (0.77–0.88) | |||||||
| NGAL (ng/mg) | 0.89 | 83.0 | 80% | 98% | 0.89 | 82.7 | 80% | 96% |
| (0.83–0.94) | (0.84–0.93) | |||||||
| Protein (mg/mg) | 0.98 | 0.22 | 96% | 94% | 0.91 | 0.46 | 81% | 87% |
| (0.94–1.00) | (0.87–0.95) | |||||||
| VEGF (ng/mg) | 0.90 | 0.43 | 77% | 84% | 0.73 | 0.64 | 62% | 62% |
| (0.84–0.94) | (0.66–0.79) | |||||||
Cross‐validation of logic regression models that included up to three trees with up to seven total leaves resulted in a model with three trees and four leaves as optimal. This model corresponds to a risk score of 2.93*(NGAL > 5.72 and HGF > 0.17) + 2.93*(PROTEIN > 0.22) − 2*(KIM < 0.58) and was derived comparing AKI versus all non‐AKI combined. We then constructed the ROC curve that reflected sensitivity and specificity for every threshold value for this derived risk score. The AUC for this combination of biomarkers is 0.94 (95% bootstrap percentile confidence interval (CI): 0.901, 0.969).
Prognostic ability of urinary biomarkers.
Individuals with established AKI had an in‐hospital mortality rate of 36%, and 46% required renal replacement therapy (RRT); 60% had the composite outcome of death or RRT. Table 4 shows median biomarker values in patients with established AKI according to clinical outcome. In age‐adjusted analyses using log‐transformed biomarker values, the following were significant predictors of outcome: HGF (composite of death/RRT); KIM‐1 (mortality); total protein (RRT and composite mortality/RRT); NAG (mortality, RRT, and composite of mortality/RRT); and VEGF (composite of mortality/RRT). Peak SCr was associated inversely with mortality (age‐adjusted odds ratio, 0.78, 95% CI 0.62–0.99) but not with RRT or the composite of mortality/RRT. SCr at the time of sample collection was not significantly associated with mortality and/or RRT.
Table 4.
Median normalized biomarker levels in patients with established AKI, according to clinical outcome.
| In hospital mortality (36%) | Renal replacement therapy (46%) | Mortality or renal replacement therapy (60%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Died | Survived | p‐value | Yes | No | p‐value | Yes | No | p‐value | |
| Cystatin C (ug/mg) | 1.19 | 0.72 | 0.63 | 1.21 | 0.69 | 0.87 | 1.03 | 0.85 | 0.60 |
| HGF (ng/mg) | 1.23 | 0.77 | 0.07 | 1.13 | 0.76 | 0.24 | 1.15 | 0.74 | 0.03 |
| IL‐18 (pg/mg) | 16.89 | 6.12 | 0.27 | 16.22 | 5.90 | 0.29 | 15.19 | 4.93 | 0.29 |
| IP‐10 (ng/mg) | 1.21 | 0.97 | 0.74 | 1.25 | 0.92 | 0.66 | 1.38 | 0.85 | 0.29 |
| KIM‐1 (ng/mg) | 10.17 | 5.19 | 0.008 | 7.24 | 5.19 | 0.37 | 6.84 | 4.80 | 0.10 |
| Protein (mg/mg) | 2.20 | 1.51 | 0.13 | 2.21 | 1.14 | 0.02 | 2.20 | 1.13 | 0.02 |
| NGAL (ng/mg) | 5,384.4 | 3,113.2 | 0.94 | 12,883.3 | 2,063.0 | 0.14 | 6,389.1 | 2,044.3 | 0.40 |
| NAG (U/mg) | 0.05 | 0.03 | 0.02 | 0.06 | 0.02 | 0.003 | 0.06 | 0.02 | <0.001 |
| VEGF (ng/mg) | 1.63 | 0.91 | 0.07 | 1.24 | 0.95 | 0.11 | 1.55 | 0.75 | 0.008 |
All biomarker values normalized to urinary creatinine. p‐value represents results from logistic regression analysis using log‐transformed biomarker levels, adjusting for age.
Urinary biomarkers in AKI of different causes.
Urinary biomarkers were compared across‐diagnostic categories of AKI ( Table 5), after establishing for each patient a single most likely diagnosis based on chart review (ATN (including postcardiac surgery, ischemia, and pigment nephropathy), N= 33; sepsis, N= 32; contrast nephropathy, N= 6; nephrotoxin administration, N= 6; and other, N= 24). Statistically significant differences in at least one diagnostic category compared to others were observed for HGF (p= 0.03), KIM‐1 (p < 0.007), and NAG (p < 0.007); generally, higher levels were seen in ATN and sepsis than in the other causes of AKI.
Table 5.
Median values (10th and 90th percentiles) of normalized biomarkers in patients with established AKI, according to most likely single diagnosis.
| ATN | Sepsis | Contrast | Nephrotoxin | Other* | p‐value** | |
|---|---|---|---|---|---|---|
| (N= 33) | (N= 32) | (N= 6) | (N= 6) | (N= 24) | ||
| Cystatin C (ug/mg) | 0.36 | 1.31 | 0.38 | 5.87 | 0.69 | 0.51 |
| (0.05–18.71) | (0.06–44.01) | (0.03–15.59) | (0.38–66.38) | (0.08–14.83) | ||
| HGF (ng/mg) | 1.05 | 1.26 | 0.93 | 0.70 | 0.48 | 0.03 |
| (0.21–4.31) | (0.06–44.01) | (0.64–2.25) | (0.21–12.53) | (0.28–1.50) | ||
| IL‐18 (Pg/mg) | 5.90 | 32.40 | 2.06 | 15.82 | 6.34 | 0.20 |
| (0.61–169.58) | (0.39–283.56) | (0.05–160.80) | (0.30–141.55) | (0.39–103.84) | ||
| IP‐10 (ng/mg) | 2.21 | 1.16 | 0.71 | 1.39 | 0.97 | 0.33 |
| (0.39–42.24) | (0.08–54.67) | (0.01–5.56) | (0.01–186.90) | (0.03–26.13) | ||
| KIM‐1 (ng/mg) | 9.90 | 6.78 | 4.53 | 3.92 | 3.45 | 0.007 |
| (3.08–30.56) | (0.36–28.10) | (0.01–5.56) | (0.45–25.58) | (0.52–12.53) | ||
| NAG (U/mg) | 0.04 | 0.06 | 0.02 | 0.05 | 0.02 | 0.007 |
| (0.01–0.19) | (0.02–0.22) | (0.01–0.15) | (0.01–0.22) | (0.01–0.04) | ||
| NGAL (ng/mg) | 5,346.0 | 18,005.7 | 1,486.0 | 1,714.0 | 1,756.7 | 0.06 |
| (0.5–64,834.5) | (98.6–97,036.6) | (69.0–74,134.3) | (972.3–172,795.0) | (22.9–30,325.1) | ||
| Protein (mg/mg) | 1.60 | 1.82 | 1.47 | 1.16 | 1.54 | 0.50 |
| (0.36–10.09) | (0.59–6.75) | (0.52–3.28) | (0.04–5.30) | (0.22–4.61) | ||
| VEGF (ng/mg) | 1.23 | 1.74 | 0.64 | 0.63 | 0.67 | 0.15 |
| (0.29–55.12) | (0.33—11.88) | (0.10–1.56) | (0.26–61.42) | (0.24–8.59) |
*Other diagnoses included: pre‐renal azotemia (N= 8), acute interstitial nephritis (N= 4), acute on chronic kidney disease without single precipitant (N= 3), acute glomerulonephritis (N= 2), myeloma (N= 2), tumor lysis syndrome (N= 1), urate nephropathy (N= 1), scleroderma renal crisis (N= 1), obstructive uropathy (N= 1), veno‐occlusive disease (N= 1).
**Kruskal–Wallis test.
Discussion
The diagnostic approach to AKI has stagnated and rests today upon the same “legacy” biomarkers—BUN, creatinine, and urine output—that do not directly reflect cell injury but rather delayed functional consequences of the injury. This has greatly impeded therapeutic innovation. A first step in the validation of novel biomarkers of AKI is the demonstration that established AKI can be distinguished from non‐AKI controls. Previous studies from our group and others have identified several promising candidate biomarkers—including urinary NGAL, KIM‐1, IL‐18, and NAG that readily differentiate between individuals with and without AKI (reviewed in Refs. 5 and 14 ).
We have cloned KIM‐1 (also known as TIM‐1 or HAVCR‐1), a type I cell membrane glycoprotein, that is upregulated approximately 50–100 fold in the kidney and the ectodomain of KIM‐1 is shed into the urine in rodents 15 , 16 and humans 17 after proximal tubular kidney injury. There have been studies suggesting that urinary NGAL levels increased 10–100‐fold in rodents after cisplatin‐induced nephrotoxicity and in patients with ischemic and septic AKI 18 and that high levels of urinary NGAL predicted the onset of AKI 2 hours after cardiopulmonary bypass in children undergoing cardiac surgery, 2–4 days before AKI was identified by changes in serum creatinine. 19 Urinary Cys‐C levels have been found to be elevated in individuals with known tubular dysfunction. 8 , 20 Herget‐Rosenthal et al. reported that elevated urinary Cys‐C levels were highly predictive of poor outcome (requirement for RRT) in a heterogeneous group of patients with initially nonoliguric AKI. 21 Urinary IL‐18 levels are elevated in patients with AKI and delayed graft function compared with normal subjects. Elevation of urinary IL‐18 could predict AKI one day before creatinine in 138 patients with adult respiratory distress syndrome (ARDS) and IL‐18 levels were independent predictors of mortality at the time of mechanical ventilation. 22 A marked increase in urinary HGF levels was observed in patients with AKI and was correlated with the disease severity 23 HGF values declined to control values in patients recovering from AKI. A number of recent studies have also demonstrated that the expression of the CXC chemokine interferon‐inducible protein 10 (IP‐10; CXCL10) and vascular endothelial growth factor (VEGF) in urine are significantly elevated during kidney allograft rejection 24 , 26 and diabetic nephropathy 27 , 28
We have found that these nine urinary biomarkers all identify AKI in a cross‐sectional study of individuals with and without AKI. As expected, the diagnostic performance characteristics were best when comparing AKI with healthy individuals; biomarker levels were higher in hospitalized individuals without evidence of AKI than in healthy individuals, accounting for the lower AUC‐ROC when including all non‐AKI controls. This may relate to the insensitivity and nonspecificity of changes in serum creatinine to reflect acute tubular injury. The selection of subjects in the nondisease group in studies of diagnostic performance is critical. Because urinary biomarkers of AKI will be tested in hospitalized patients at risk of AKI, we chose to include patients admitted to the intensive care unit (ICU) and those undergoing cardiac catheterization (before receiving radiocontrast dye) without clinical evidence of AKI. There is a difference in age of the “healthy volunteers” and cardiac catheterization and ICU controls. We do not feel that the age differential explains differences we find when comparing AKI patients to non‐AKI patients since when studied in animal models, age is only associated with increase in urinary markers if there is an associated age‐related incidence of renal disease. 29 It is possible that some non‐AKI patients in fact had subclinical AKI that was correctly identified by the biomarkers but missed when relying on changes by SCr, leading to an apparent but incorrect reduction in specificity. Larger studies comparing long‐term outcomes after episodes of AKI will be needed to determine the best urinary biomarkers for the diagnosis and prognosis of AKI. The non‐AKI subjects in our study were also selected not to have severe CKD, which could also affect the diagnostic performance characteristics of novel biomarkers. Several urinary biomarkers may be expected to be increased chronically in CKD due to ongoing tubular injury. For an AKI biomarker to retain diagnostic ability in patients with CKD one would expect levels to increase over baseline after AKI; a cross‐sectional study cannot address that issue, and for that reason subjects with estimated GFR less than 50 mL/min were excluded from the non‐AKI control group in this study. The performance of total urinary protein was excellent in this cross‐sectional study. However, total urinary protein was higher in patients undergoing cardiac catheterization and ICU controls than in healthy volunteers, and some overlap with AKI patients was evident, raising the possibility of nonspecificity ( Figure 2 ). Whether total urinary protein (or albuminuria, which we did not test) retains prognostic and diagnostic ability in prospective studies remains to be determined.
Because our study included patients with established AKI at varying stages, it would be inappropriate to rank the biomarkers tested according to AUC‐ROC. For example, a perfectly sensitive and specific biomarker that increases early after AKI and declines to normal values shortly thereafter would appear to have poor diagnostic ability in our study. Just as troponin, CK‐MB, and myoglobin vary in their rate and duration of rise after myocardial infarction, urinary biomarkers may have different kinetics following AKI. Our results cannot establish the temporal pattern of excretion of urinary biomarkers, which will be important for early diagnosis before a rise in SCr. It should also be emphasized that urine samples were obtained well after the diagnosis of AKI was made, and therefore this study does not address the issue of early diagnosis prior to a rise in SCr. Prospective studies—in which urine is obtained serially, for example, before cardiopulmonary bypass and then at various time points thereafter—are ongoing and will be able to assess the temporal pattern of excretion. The biomarkers we have studied here are well suited for such investigations.
Another important role for AKI biomarkers is to provide information about prognosis. We found that age‐adjusted levels of KIM‐1, NAG, HGF, total protein, and VEGF predicted death and/or RRT. Our findings corroborate those of our collaborators Liangos et al., who found that KIM‐1 and NAG were independent predictors of the composite outcome of death or dialysis in a separate cohort of 201 individuals with established AKI. 30 We did not compare urinary biomarkers to generic disease severity scores because of the heterogeneity of the established AKI population in this cohort. We found an inverse correlation between peak SCr and mortality. In other words, patients with higher peak SCr had a lower risk of in‐hospital mortality. A paradoxical improvement in outcome with higher SCr was also observed in a previous study of 134 patients with severe AKI requiring RRT. 31 Similar findings have been established in the setting of end stage renal disease and likely relate to the confounding effect of muscle mass and nutritional status. 32
Using the microbead technology we were able to measure KIM‐1 and NGAL or KIM‐1 and HGF or HGF and IL‐18 in the same aliquot of urine sample at the same time. This is important because a single biornarker is rarely adequate to clearly define a particular pathologic state. 33 , 34 An assay that is capable of measuring multiple biomarkers in the same aliquot of biological sample at the same time is extremely useful.
The sensitivity and specificity for diagnosis of AKI was significantly greater by combining the urinary levels of KIM‐1, NGAL, HGF, and total protein using the logic regression model of 2.93*(NGAL > 5.72 and HGF > 0.17) + 2.93*(PROTEIN > 0.22) − 2*(KIM < 0.58) than individual biomarkers. Our application of logic regression for combination of the multiple biomarkers yielded an AUC of 0.94, exceeding all of the AUC's for the individual biomarkers (for comparison vs. all non‐AKI controls). Furthermore, the combination of biomarkers confers the advantage of a slightly narrower confidence interval for the AUC, and thus more precise estimation.
Conclusion
Thus in our clinical cross‐sectional study we report that all nine urinary biomarkers performed well in differentiating between patients with and without AKI with AUC‐ROCs each greater than 0.83. Using logic regression analysis the four best performers individually and in combination were KIM‐1, NGAL, HGF, and total protein. Confirmation of the utility of this combinatorial approach in prospective studies will be very useful in moving kidney injury biomarkers closer to routine clinical use.
Competing Interests
Dr. Bonventre is co‐inventor on patents for KIM‐1.
Acknowledgments
Part of this work was presented at the American Society of Nephrology meeting in San Francisco, CA, October 31 to November 1, 2007. We acknowledge Dr. Mary Lou Gantzer from Dade Behring Inc. (now Siemens Healthcare Diagnostics Inc.) for kindly providing reagents to perform Cystatin C measurements. This work was supported by scientist development grant (0535492T) by AHA and NIH pathway to independence grant (K99/R00 ES16723) to V.S.V.; NIH career development grant DK 075941 to S.S.W.; NKF research fellowship to M.A.F., and NIH grants DK39773, DK72831, and DK74099 to J.V.B.
V.S.V. and S.S.W contributed equally to this article.
References
- 1. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005; 16: 3365–3370. [DOI] [PubMed] [Google Scholar]
- 2. Star RA. Treatment of acute renal failure. Kidney Int. 1998; 54: 1817–1831. [DOI] [PubMed] [Google Scholar]
- 3. Woosley RL, Cossman J. Drug development and the FDAs Critical Path Initiative. Gin Pharmacol Ther. 2007; 81: 129–133. [DOI] [PubMed] [Google Scholar]
- 4. Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004; 15: 1677–1689. [DOI] [PubMed] [Google Scholar]
- 5. Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008; 48: 463–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carson RT, Vignali DA. Simultaneous quantitation of 15 cytokines using a multiplexed flow cytometric assay. J Immunol Methods. 1999; 227: 41–52. [DOI] [PubMed] [Google Scholar]
- 7. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004; 8: R204‐R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conti M, Moutereau S, Zater M, Lallali K, Durrbach A, Manivet P, Eschwege P, Loric S. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med. 2006; 44: 288–291. [DOI] [PubMed] [Google Scholar]
- 9. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule‐1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physio! Renal Physiol. 2006; 290: F517‐F529. [DOI] [PubMed] [Google Scholar]
- 10. Oda H, Shiina Y, Seiki K, Sato N, Eguchi N, Urade Y. Development and evaluation of a practical EUSAfor human urinary lipocalin‐type prostaglandin D synthase. Clin Chem. 2002; 48: 1445–1453. [PubMed] [Google Scholar]
- 11. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983; 148: 839–843. [DOI] [PubMed] [Google Scholar]
- 12. Ruczinski I, Kooperberg C, Leblanc M. Logic regression. J Comput Graph Stat. 2003; 12: 475–511. [Google Scholar]
- 13. Janes H, Pepe M, Kooperberg C, Newcomb P. Identifying target populations for screening or not screening using logic regression. Stat Med. 2005; 24: 1321–1338. [DOI] [PubMed] [Google Scholar]
- 14. Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Curr Opin Nephrol Hypertens. 2007; 16: 557–564. [DOI] [PubMed] [Google Scholar]
- 15. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule‐1 (KIM‐1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up‐regulated in renal cells after injury. J Biol Chem. 1998; 273: 4135–4142. [DOI] [PubMed] [Google Scholar]
- 16. Vaidya VS, Bonventre JV. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin DrugMetab Toxicol. 2006; 2: 697–713. [DOI] [PubMed] [Google Scholar]
- 17. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule‐1 (KIM‐1): a novel biomarkerfor human renal proximal tubule injury. Kidney Int. 2002; 62: 237–244. [DOI] [PubMed] [Google Scholar]
- 18. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt‐Ott KM, Chen X, Li JY, Weiss S, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J. Endocytic delivery of lipocalin‐siderophore‐iron complex rescues the kidney from ischemia‐reperfusion injury. J Clin Invest. 2005; 115: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase‐associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005; 365: 1231–1238. [DOI] [PubMed] [Google Scholar]
- 20. Uchida K, Gotoh A. Measurement of cystatin‐C and creatinine in urine. Clin Chim Ada. 2002; 323: 121–128. [DOI] [PubMed] [Google Scholar]
- 21. Herget‐Rosenthal S, Poppen D, Husing J, Marggraf G, Pietruck F, Jakob HG, Philipp T, Kribben A. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin Chem. 2004; 50: 552–558. [DOI] [PubMed] [Google Scholar]
- 22. Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL‐18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005; 16: 3046–3052. [DOI] [PubMed] [Google Scholar]
- 23. Taman M, Liu Y, Tolbert E, Dworkin LD. Increase urinary hepatocyte growth factor excretion in human acute renal failure. Clin Nephrol. 1997; 48: 241–245. [PubMed] [Google Scholar]
- 24. Matz M, Beyer J, Wunsch D, Mashreghi MF, Seller M, Pratschke J, Babel N, Volk HD, Reinke P, Kotsch K. Early post‐transplant urinary IP‐10 expression after kidney transplantation is predictive of short‐and long‐term graft function. Kidney Int. 2006; 69: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 25. Peng W, Chen J, Jiang Y, Shou Z, Chen Y, Wang H. Non‐invasive detection of acute renal allograft rejection by measurement of vascular endothelial growth factor in urine. J Int Med Res. 2007; 35: 442–449. [DOI] [PubMed] [Google Scholar]
- 26. Tatapudi RR, Muthukumar T, Dadhania D, Ding R, Li B, Sharma VK, Lozada‐Pastorio E, Seetharamu N, Hartono C, Serur D, Seshan SV, Kapur S, Hancock WW, Suthanthiran M. Moninvasive detection of renal allograft inflammation by measurements of mRNA for IP‐10 and CXCR3 in urine. Kidney Int. 2004; 65: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 27. Kim NH, Oh JH, Seo JA, Lee KW, Kim SG, Choi KM, Baik SH, Choi DS, Kang YS, Han SY, Han KH, Ji YH, Cha DR. Vascular endothelial growth factor (VEGF) and soluble VEGF receptor FLT‐1 in diabetic nephropathy. Kidney Int. 2005; 67: 167–177 [DOI] [PubMed] [Google Scholar]
- 28. Ruster C, Wolf G. The Role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008; 13: 944–955. [DOI] [PubMed] [Google Scholar]
- 29. Chen G, Bridenbaugh EA, Akintola AD, Catania JM, Vaidya VS, Bonventre JV, Dearman AC, Sampson HW, Zawieja DC, Burghardt RC, Parrish AR. Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol. 2007; 293: F1272‐F1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, Bonventre JV, Jaber BL. Urinary N‐acetyl‐beta‐(D)‐glucosaminidase activity and kidney injury molecule‐1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007; 18: 904–912. [DOI] [PubMed] [Google Scholar]
- 31. Cerda J, Cerda M, Kilcullen P, Prendergast J. In severe acute kidney injury, a higher serum creatinine is paradoxically associated with better patient survival. Nephrol Dial Transplant. 2007; 22: 2781–2784. [DOI] [PubMed] [Google Scholar]
- 32. Owen WF, Jr , Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAm Med Assoc. 1998; 280: 1764–1768. [DOI] [PubMed] [Google Scholar]
- 33. Fliser D, Novak J, Thongboonkerd V, Argiles A, Jankowski V, Girolami MA, Jankowski J, Mischak H. Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol. 2007; 18: 1057–1071. [DOI] [PubMed] [Google Scholar]
- 34. Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006; 24: 971–983. [DOI] [PubMed] [Google Scholar]


