Abstract
Systemic sclerosis (SSc) is an autoimmune disease in which the tyrosine kinases platelet derived growth factor receptor (PDGFR) and Abl are hypothesized to contribute to the fibrosis and vasculopathy of the skin and internal organs. We describe two patients with early diffuse SSc who experienced reductions in cutaneous sclerosis in response to therapy with the tyrosine kinase inhibitor, imatinib mesylate. Immunohistochemical analyses of skin biopsies demonstrated reductions of phosphorylated PDGFRβ and Abl with imatinib therapy. Gene expression profiling identified an imatinib-responsive signature specific to diffuse SSc (P<10−8, chi-square). Our results suggest that PDGFRβ and Abl synergistically play critical roles in the pathogenesis of SSc, and that imatinib targets a gene expression program frequently dysregulated in diffuse SSc.
Systemic sclerosis (SSc) is an autoimmune disease characterized by fibrosis of the skin and internal organs and widespread vasculopathy. Current therapies for SSc focus on treating specific symptoms, but disease-modifying agents targeting the underlying pathogenesis are lacking.
The pathogenesis of SSc involves activation of profibrotic pathways, with over-expression of the cytokines transforming growth factor (TGF)-β and platelet derived growth factor (PDGF). A recent report showed that SSc patients have autoantibodies against the PDGF receptor, which stimulate the production of reactive oxygen species and type I collagen expression(1). PDGF receptors are upregulated in the skin and bronchoalveolar lavage fluid of patients with SSc, and when activated, lead to fibroblast and myofibroblast proliferation(2, 3). PDGF participates in smooth muscle cell recruitment and mitogenic signaling that underlie the vasculopathy associated with pulmonary arterial hypertension (PAH), a complication of SSc associated with high mortality(4). In addition, stimulation of the TGF-β profibrotic pathway involves activation of c-Abl(2). Thus, the PDGF and TGF-β pathways are thought to contribute to the fibrotic and vascular complications in SSc.
Imatinib mesylate (Gleevec, Novartis, East Hanover, New Jersey) is a small molecule that antagonizes specific tyrosine kinases that mediate fibrotic pathways, including c-Abl, a downstream mediator of TGF-β(2) and PDGF receptors(5). Imatinib has been shown to inhibit lung and dermal fibrosis in bleomycin-induced mouse models(6, 7), and the proliferation of synovial fibroblasts derived from patients with rheumatoid arthritis(8). Imatinib has also been reported to provide benefit in the treatment of refractory idiopathic PAH through its effects on vascular remodeling(9). We now describe two patients with early diffuse SSc who experienced clinical improvement in response to imatinib therapy and provide evidence that both c-Abl and PDGFR are targets of imatinib in scleroderma skin. Finally, we show that an imatinib-responsive gene signature is present in most cases of diffuse SSc.
CASE REPORTS
Patient 1
A 24-year old female with a 3-year history of diffuse SSc presented with increasing tightness of her skin and shortness of breath. The patient had a history of severe Raynaud’s phenomenon and digital ulcerations (Figure 1A) despite bilateral sympathectomies and treatment with multiple vasodilators. She suffered from arthritis requiring chronic prednisone at 10 mg daily. The patient had noticed increasing dyspnea on exertion and a high resolution computed tomography (HRCT) of the chest showed bibasilar ground glass opacities (Figure 1C) consistent with interstitial lung disease (ILD). Pulmonary function tests showed a forced vital capacity (FVC) of 48% predicted and a diffusion capacity of carbon monoxide (DLCO) of 62% predicted. A transthoracic echocardiogram revealed a small pericardial effusion, but normal right ventricular systolic pressure (RVSP). The patient was intolerant to intravenous immunoglobulins and mycophenolate mofetil. She declined cyclophosphamide therapy and was referred to our center for a trial of imatinib.
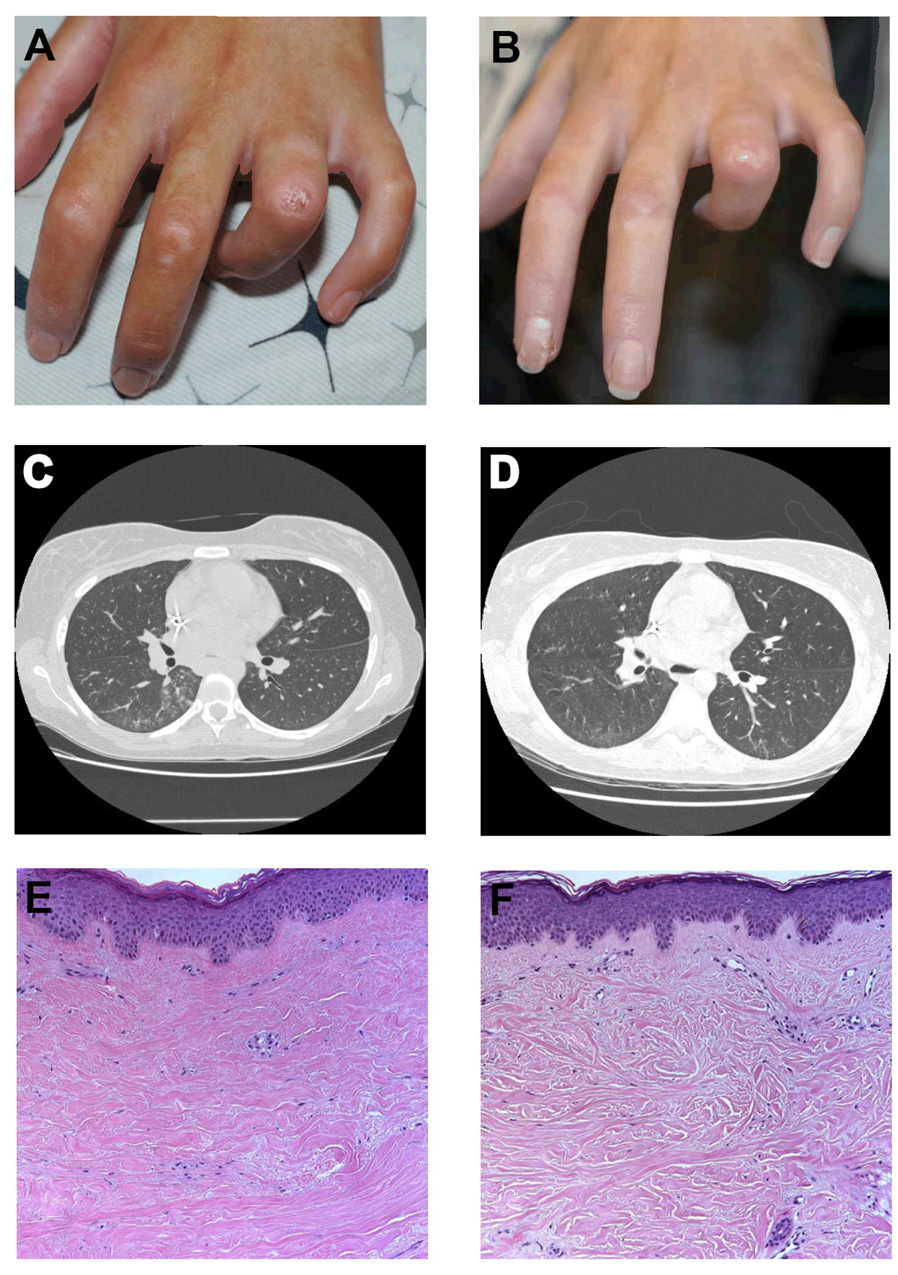
Figure 1. Effect of imatinib on digital ulcers, interstitial lung disease, and collagen architecture in a patient with SSc.
(A) Digital ulcer located over the left fourth proximal interphalangeal joint prior to imatinib therapy. (B) Healing of digital ulcer after 3 months of imatinib therpy. (C) HRCT of the chest prior to imatinib therapy demonstrates patchy infiltrates associated with ground glass opacities in the bilateral lower lobes. (D) HRCT after 3 months of imatinib therapy shows resolution of ground glass opacities. (E) Hematoxylin and eosin stained skin biopsy from the right arm taken prior to imatinib therapy shows dense, eosinophilic, tightly packed collagen bundles of the papillary and reticular dermis with an average dermal thickness of 2.81 mm (Magnification 100×). (F) Skin biopsy after 3 months of imatinib taken within 1 cm of initial biopsy shows normalization of collagen architecture, with loose spacing and thinning of collagen bundles and an average dermal thickness of 2.31 mm.
Prior to initiating therapy, the patient’s modified Rodnan skin thickness score (MRSS) was 36 (scale 0–51) and she had nine digital ulcers. Her complete blood count, comprehensive metabolic panel, creatine kinase, and urinalysis were within normal limits. C-reactive protein (CRP) level was 2.8 mg/dL (normal < 0.5 mg/dL). A skin biopsy demonstrated thickened, closely packed collagen bundles with an average dermal thickness of 2.81 mm (Figure 1E).
After three months of imatinib at 100 mg orally twice daily, the patient reported softening of her skin, increased joint mobility, and decreased shortness of breath. Physical examination revealed a MRSS of 21 and four digital ulcers (Figure 1B). CRP had normalized to 0.2 mg/dL and the patient had been able to taper her prednisone to 5 mg daily. HRCT showed resolution of the interstitial changes (Figure 1D) and a repeat TTE showed no evidence of a pericardial effusion. Repeat PFTs showed a slight improvement in her FVC to 52% predicted, but a decline in DLCO to 54% predicted. A repeat skin biopsy showed more widely spaced, thinner collagen bundles with an average dermal thickness of 2.31 mm (Figure 1F).
Patient 2
A 62-year old female with newly diagnosed diffuse SSc presented to our clinic with progressive cutaneous sclerosis. The patient had a 2-year history of Raynaud’s phenomenon and noted increasing tightening of her skin over the previous 6 months. Initial therapies included benazepril for her Raynaud’s and moderate doses of prednisone and methotrexate (12.5 mg/week) for her skin disease. The patient did not tolerate corticosteroid therapy and was referred to our center for investigational treatment with imatinib.
At initial evaluation, the patient had prominent capillary dilation and drop-out on nailfold capillaroscopy and her skin examination revealed a MRSS of 36. Her complete blood count, comprehensive metabolic panel, creatine kinase, urinalysis, and sedimentation rate were within normal limits. She had no evidence of ILD on HRCT of the chest and her PFTs were unremarkable. A baseline TTE showed a normal ejection fraction and an RVSP of 35 mmHg with a small pericardial effusion.
After 6 months of imatinib at 200 mg orally daily, the patient had noticed improvement in her skin tightening. Her Raynaud’s worsened in severity during the winter season, but she did not develop any digital ulcers. On physical examination, her MRSS had improved to 20. Her PFTs and HRCT remained stable, and her TTE showed an RVSP of 23 mmHg and resolution of the pericardial effusion.
METHODS
Patients
Adult patients with progressive SSc refractory to at least one immunomodulatory agent were treated with imatinib. Lesional skin biopsies of the upper extremities (upper arm or forearm) were obtained at baseline and during therapy (at three months for Patient 1 and one month for Patient 2) for histologic, immunohistochemical, and microarray analyses. The protocol was approved by the institutional review board at Stanford University School of Medicine, and all patients provided written informed consent.
Immunohistochemistry
Tissue from skin biopsies was fixed in formalin and paraffin embedded. Sections were stained with antibodies specific for the phosphorylated (activated) states of the tyrosine kinases PDGFRβ and c-Abl.
Global transcriptional analysis of skin using oligonucleotide microarrays
Total RNA was extracted from snap frozen skin biopsies (taken adjacent to those processed for paraffin embedding) before and after imatinib treatment using Qiagen RNeasy fibrous tissue kit. RNA was amplified using the Ambion Amino Allyl MessageAmp II aRNA kit. Amplified skin RNA (labeled with Cy5) and amplified Stratagene Human Universal Reference RNA (labeled with Cy3) were competitively hybridized to human exon evidence-based oligonucleotide (HEEBO) microarrays in duplicate as described (http://www.microarray.org/sfgf/heebo.do).
Genes selected for analysis had fluorescent hybridization signal at least 1.5-fold over local background in either Cy5 or Cy3 channel and had technically adequate data in at least 75% of experiments. Genes were analyzed by mean value centering within the dataset for each patient. Imatinib-responsive genes were identified using Significance Analysis of Microarrays with false discovery rate (FDR)<0.001. Samples were scored for their similarity to the transcriptional response of fibroblasts to serum as described (10). The database of 75 SSc and control gene expression profiles will be described elsewhere (11), and include 75 microarray analyses on 61 skin biopsies from 34 subjects, including samples from 18 patients with diffuse SSc, 7 with limited SSc, 3 with morphea, and 6 healthy controls. 817 of 1050 imatinib-responsive genes were successfully mapped in the SSc database using EntrezGene ID, and their pattern of expression was analyzed by unsupervised hierarchical clustering, revealing two distinct clusters. The imatinib-responsive gene expression pattern was similar to one of the clusters, which was highly enriched for diffuse SSc samples (29 of the 31 gene expression profiles in this cluster were derived from diffuse SSc, P<10−8, chi-square). All data is publically available at Stanford Microarray Database (http://genome-www5.stanford.edu) and Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession # GSE11130), and the imatinib responsive gene signature is provided in Supplemental Table 1.
DISCUSSION
IMATINIB DECREASES THE LEVELS OF ACTIVATED PDGFRβ AND ABL
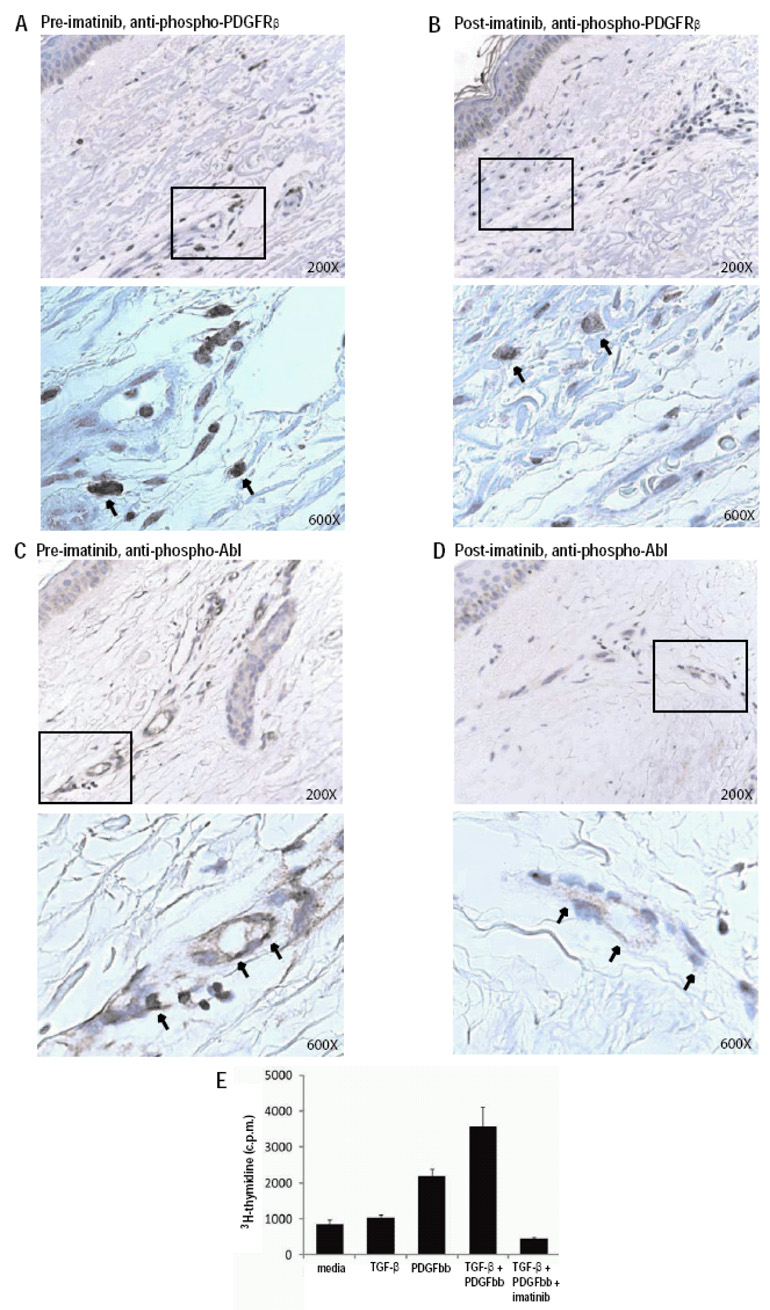
We performed immunohistochemical analysis on serial skin biopsies obtained pre-treatment and 1+ months following initiation of imatinib therapy. An anti-phospho-PDGFRβ antibody strongly stained dermal cells with fibroblast-like morphology in the pre-treatment sample (Figure 2A), and there was a significant decrease in staining 1 month following initiation of imatinib therapy (Figure 2B). Anti-phospho-Abl antibodies stained dermal vessels in the pre-treatment samples (Figure 2C), and there was a significant decrease in staining 1 month following initiation of therapy (Figure 2D).
Figure 2. Imatinib reduces PDGFRβ and Abl activation in SSc skin and function in SSc fibroblasts.
(A–D) Immunohistochemical staining of serial skin biopsy samples obtained pretreatment (A,C) and one month following the initiation of imatinib treatment (B,D) with anti-phospho-PDGFRβ (A,B) and anti-phospho-Abl (C,D) antibodies. Boxed areas of upper panels (200× magnification), are presented at higher magnification in their corresponding lower panels (600×). Results are representative of those obtained from multiple sections from two independent patients. Phospho-PDGFRβ was observed in interstitial fibroblasts as well as perivascular spindle-like cells and some cells resembling mast cells. Phospho-Abl was observed in endothelial cells in small vessels and in scattered dermal fibroblasts. (E) Stimulation of a SSc fibroblast line with PDGF (10 ng/ml), TGF-β (0.5 ng/ml), PDGF + TGF-β, or PDGF + TGF-β+ imatinib (1 µM). Proliferation was quantitated after 48 hours by 3H-thymidine incorporation (Y axis). Results are representative of experiments performed on two independent SSc fibroblast lines, and similar results were obtained with normal fibroblast lines.
IMATINIB INHIBITS PDGF AND TGF-β INDUCED SSc FIBROBLAST PROLIFERATION
To assess the ability of imatinib to inhibit PDGF and TGF-β induced fibroblast proliferation, titration curves for TGF-β and PDGF stimulation of SSc fibroblast proliferation were generated (data not shown). Concentrations of TGF-β (0.5 ng/ml) and PDGF (10 ng/ml) that submaximally stimulated SSc fibroblast proliferation were selected and used alone, in combination, or in combination with imatinib (1 µM) to stimulate SSc fibroblast lines (Figure 2E). As compared to the low level proliferation induced by PDGF or TGF-β alone, co-stimulation with PDGF and TGF-β synergistically induced SSc fibroblast proliferation (Figure 2E; the increase in proliferation of the co-stimulated fibroblasts was two-times higher than the sum of the increases in proliferation observed with the individual stimuli). Imatinib completely abrogated SSc fibroblast proliferation induced by PDGF and TGF-β.
IDENTIFICATION OF AN IMATINIB-RESPONSIVE GENE SIGNATURE
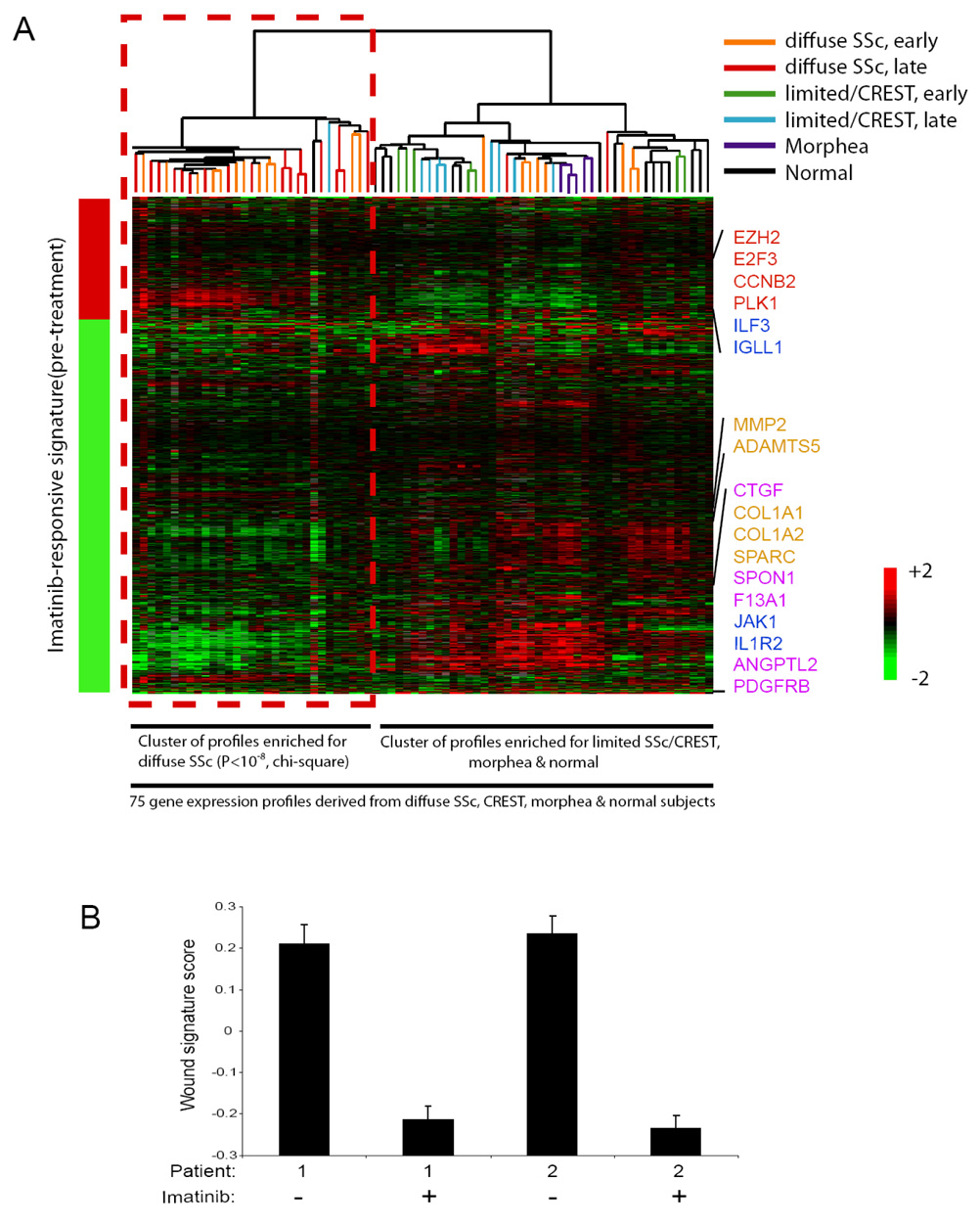
To gain further insights into the molecular mechanisms of imatinib action, we determined the global gene expression profiles of lesional skin before and after imatinib treatment. Comparison of gene expression patterns in the two patients before and after imatinib revealed a consistent set of 1050 genes that were changed by imatinib in both patients (FDR<0.001). To test whether the gene targets of imatinib in SSc, as defined in these two patients, may be generalizable to other patients with SSc or other fibrotic diseases, we interrogated the pattern of activation of the imatinib-responsive signature in a database of 75 gene expression profiles of SSc and control samples (11). We found that both early and late (≤ or > 3 years in duration, respectively) diffuse SSc tended to express the imatinib-responsive signature, whereas most samples of normal skin, morphea, and limited SSc/CREST did not (Figure 3A; P<10−8, chi-square).
Figure 3. An imatinib-responsive signature is present in most diffuse SSc.
(A) The imatinib-responsive signature was determined by applying Significance Analysis of Microarrays (SAM) to identify mRNA that exhibited statistically significant changes in their levels in pre-treatment as compared to post-treatment skin biopsy samples derived from the two imatinib-treated SSc patients. SAM identified 1050 genes that were changed by imatinib therapy in both patients (FDR<0.001), and this imatinib responsive signature is represented by the bar to the left of the heatmap image (red represents an increase, and green a decrease, in mRNA expression post-treatment; the genes comprising the imatinib responsive signature are presented in Supplemental Table 1). The imatinib-responsive genes were then used to organize via unsupervised hierarchical clustering the 75 gene expression profiles derived from skin biopsies from SSc, limited SSc/CREST, morphea and health control patients contained in a database. The results of the hierarchical clustering are presented as a heatmap, with each column representing the mRNA profile of a sample, and rows representing the genes present in the imatinib responsive signature. Unsupervised hierarchical clustering revealed two distinct clusters, with the imatinib-responsive gene expression pattern being similar to one of the clusters, and this cluster being highly enriched for diffuse SSc samples (29 out of the 31 gene expression profiles contained in this cluster are from diffuse SSc, P<10−8, chi-square). This cluster of gene expression profiles derived from most of the diffuse SSc samples exhibited a pattern of gene activation and repression concordant with the imatinib-responsive signature, including alterations in the expression of genes involved in cell proliferation (red), immune signaling (blue), matrix remodeling (tan), and growth factor signaling (pink) (indicated to the right of the heatmap). The other cluster contained most of the profiles derived from limited/CREST, morphea and normal subjects, and the gene expression profiles from these patients did not exhibit the imatinib-responsive signature (this cluster contains 44 gene expression profiles, including 14 from normal skin, 15 from limited SSc/CREST, 5 from morphea, and 10 from diffuse SSc). (B) Reduction in the wound signature by imatinib in two patients with SSc. Replicate array analysis was performed for each sample; mean ± standard deviation is shown.
To determine which cell types may be contributing to the gene expression changes associated with imatinib therapy, using previously published data and methodology (12) we compared the imatinib-response signature to the gene expression profiles of 11 individual cell types that are likely to be present in skin. These 11 comparison cell types include normal and SSc fibroblasts, myofibroblasts, T and B cells, epithelial cells, and endothelial cells. This analysis suggests that about half of the expression changes can be attributed to one of three single cell types, including fibroblasts, endothelia cells and B cells, while the rest are likely expressed in multiple cell types (Supplemental Figure 1).
We report two patients with diffuse SSc who experienced clinical improvement with imatinib therapy. Similar to the patient reported by Sfikakis et al. (13), both of our patients experienced improvement in cutaneous sclerosis and resolution of inflammatory manifestations of their disease (ie. ILD, arthritis, pericardial inflammation). Despite the concern that wound healing may be attenuated by PDGFR blockade (14), neither patient developed new digital ulcers, and Patient 1 experienced healing of several ulcers while receiving imatinib therapy. Thus, although PDGFR blockade by imatinib may slow wound healing, it did not prevent healing in our patients. The patients tolerated imatinib well, with no evidence of bone marrow or liver toxicity. Both patients experienced gastrointestinal side effects, but these were mild and tolerable at a dose of 200 mg/day. Immediately following her 6-month evaluation, Patient 2 suffered from bronchitis requiring oral antibiotic therapy, but there were no other infectious complications.
Immunohistochemistry demonstrated high levels of phospho-PDGFRβ in dermal fibroblasts and phospho-Abl in vascular structures in pre-treatment skin biopsy samples, and reductions in phospho-PDGFRβ and phospho-Abl following initiation of imatinib therapy (Figure 2A–D). Imatinib binds to the ATP-binding pockets to inhibit phosphorylation of the tyrosine kinases PDGFRβ and Abl, and these results suggest that imatinib-mediated inhibition of the activation of PDGFRβ and Abl is associated with the clinical benefit observed.
We demonstrated that PDGF and TGF-β each stimulate proliferation of SSc fibroblasts, while co-stimulation with PDGF + TGF-β synergistically induced proliferation. Addition of 1 µM imatinib, a concentration achieved in human dosing, inhibited the proliferation induced by PDGF + TGF-β (Figure 2E). These data provide further evidence suggesting that aberrant activation of PDGFRβ and Abl contribute to the pathogenesis of SSc, and that imatinib could provide benefit by inhibiting activation of these tyrosine kinases. Fibroblasts from patients with SSc have recently been shown to express increased levels of c-Kit (15), another tyrosine kinase potently inhibited by imatinib and that could play a significant role in the pathogenesis of SSc. The ability of imatinib to simultaneously inhibit multiple tyrosine kinase pathways involved in the pathogenesis of SSc likely contributes to the clinical benefit observed. Further, the effects in SSc were observed with lower doses of imatinib relative to those typically used to treat cancers. This may be due to the involvement of wild-type kinases in the pathogenesis of systemic sclerosis that are effectively inhibited at low doses of imatinib, while higher doses are needed to inhibit cancer cell growth mediated by mutated and aberrantly overexpressed kinases.
We characterized the global gene expression profiles in SSc skin before and after imatinib treatment (Figure 3). Because the post-treatment sample from patient 2 was obtained one month into imatinib treatment and before obvious clinical improvement, this gene expression signature may reflect the primary response of SSc to imatinib, rather than secondary changes associated with disease resolution. We identified an imatinib-responsive signature with genes involved in multiple functional pathways, including genes involved in cell proliferation, matrix and vascular remodeling, immune signaling, and growth factor signaling. The imatinib-responsive gene expression program was also specifically and frequently dysregulated in both early and late diffuse SSc. Importantly, consistent with the hypothesis that PDGF signaling may be activated in SSc, a gene signature of the transcriptional response of fibroblasts to serum (termed the wound signature (10)), a principle component of which is PDGF, was induced in both SSc samples and substantially reduced by imatinib treatment (Figure 3B; P<0.01, Student’s t-test).
The effects of imatinib on the fibrotic and vascular complications of SSc warrant further investigation, and results from randomized clinical trials are needed. While case reports can highlight new disease entities or treatment options, they are traditionally limited by the uncertainty of general applicability. Here we have used genomic profiling to bridge this gap. We have identified a specific gene signature of imatinib response from our SSc patients undergoing experimental therapy with imatinib. By comparison with a larger database of gene profiles from patients with fibrosing disorders, we found that the majority of patients with diffuse SSc, but not limited SSc or morphea, also exhibit the same transcriptional signature. These data raise the possibility that diffuse SSc patients who express this signature may benefit clinically from imatinib.
Supplementary Material
Footnotes
ClinicalTrials.gov Registration Number: NCT00506831
REFERENCES
- 1.Baroni SS, Santillo M, Bevilacqua F, Luchetti M, Spadoni T, Mancini M, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354(25):2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 2.Ludwicka A, Ohba T, Trojanowska M, Yamakage A, Strange C, Smith EA, et al. Elevated levels of platelet derived growth factor and transforming growth factor-beta 1 in bronchoalveolar lavage fluid from patients with scleroderma. J Rheumatol. 1995;22(10):1876–1883. [PubMed] [Google Scholar]
- 3.Yamakage A, Kikuchi K, Smith EA, LeRoy EC, Trojanowska M. Selective upregulation of platelet-derived growth factor alpha receptors by transforming growth factor beta in scleroderma fibroblasts. J Exp Med. 1992;175(5):1227–1234. doi: 10.1084/jem.175.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soria A, Cario-Andre M, Lepreux S, Rezvani HR, Pasquet JM, Pain C, et al. The effect of imatinib (Glivec) on scleroderma and normal dermal fibroblasts: a preclinical study. Dermatology. 2008;216(2):109–117. doi: 10.1159/000111507. [DOI] [PubMed] [Google Scholar]
- 6.Distler JHW, Jüngel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56(1):311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 7.Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114(9):1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paniagua RT, Sharpe O, Ho PP, Chan SM, Chang A, Higgins JP, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116(10):2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza R, Sitbon O, Parent F, Simonneau G, Humbert M. Long term imatinib treatment in pulmonary arterial hypertension. Thorax. 2006;61(8):736–736. doi: 10.1136/thx.2006.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang HY, Nuyten DSA, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102(10):3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS ONE. 2008;3(7):e2696. doi: 10.1371/journal.pone.0002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitfield ML, Finlay DR, Murray JI, Troyanskaya OG, Chi JT, Pergamenschikov A, et al. Systemic and cell type-specific gene expression patterns in scleroderma skin. Proc Natl Acad Sci U S A. 2003;100(21):12319–12324. doi: 10.1073/pnas.1635114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sfikakis PP, Gorgoulis VG, Katsiari CG, Evangelou K, Kostopoulos C, Black CM. Imatinib for the treatment of refractory, diffuse systemic sclerosis. Rheumatology. 2008;47(5):735–737. doi: 10.1093/rheumatology/ken104. [DOI] [PubMed] [Google Scholar]
- 14.Rajkumar VS, Shiwen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169(6):2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Papa N, Quirici N, Corti L, Graziani D, Fasoli E, Maglione W, et al. Clinical and molecular evidence for c-kit receptor as a therapeutic target in systemic sclerosis (SSc) Arthritis Rheum. 2007;56(9):S816. (Abstract). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.