Abstract
Cognitive strategies used in volitional emotion regulation include self-distraction and reappraisal (reinterpretation). There is debate as to what the psychological and neurobiological mechanisms underlying these strategies are. For example, it is unclear whether self-distraction and reappraisal, although distinct at a phenomenological level, are also mediated by distinct neural processes. This is partly because imaging studies on reappraisal and self-distraction have been performed in different emotional contexts and are difficult to compare. We have therefore investigated the neural correlates of self-distraction, as indexed by a thought suppression task, in an anticipatory anxiety paradigm previously employed by us to study reappraisal. Brain activity was measured by functional magnetic resonance imaging. We show that self-distraction recruits the left lateral prefrontal cortex. Based on a review of the existing data, we develop a process model of cognitive emotion regulation. The model posits that both self-distraction and reappraisal attenuate emotional reactions through replacement of emotional by neutral mental contents but achieve replacement in different ways. This is associated with a dependence of self-distraction on a left prefrontal production function, whereas reappraisal depends on a right prefrontal higher order monitoring process.
INTRODUCTION
Emotion regulation comprises a heterogeneous set of processes by which “individuals influence which emotions they have, when they have them, and how they experience and express these emotions” (Gross, 1999). Understanding how emotion regulation works is of potential interest for the therapy of affective and mood disorders where emotional reactions or states are dys-regulated. Volitional regulation of emotions, which is the deliberate effort to regulate one’s emotions, is used as a therapeutic tool in cognitive therapeutic approaches. Gross (2002) has described two cognitive strategies used in volitional emotion regulation: self-distraction and reappraisal. Whereas self-distraction refers to the effort to selectively attend to nonemotional (or emotionally less disturbing) aspects of a situation, reappraisal consists in deliberately interpreting or reinterpreting emotional stimuli or an emotional situation in nonemotional (or emotionally less disturbing) terms. These phenomenological differences suggest distinct neurobiological mechanisms underlie self-distraction and reappraisal. However, it has also been asked whether reappraisal is not simply a form of self-distraction (McRae, Ochsner, Gross, & Gabrieli, 2002).
Neuroimaging can help answer these questions by delineating the neural correlates of self-distraction and reappraisal. We previously reported that reappraisal of anticipatory anxiety for impending pain (Kalisch et al., 2005) was associated with activation in the right anterolateral prefrontal cortex (PFC), around Brodmann’s areas 10/46. Similar activations in or close to the right anterolateral PFC have been reported during reappraisal of other types of emotional situations (Phan et al., 2005; Levesque, Joanette, et al., 2004; Ochsner, Ray, et al., 2004; Levesque, Eugene, et al., 2003; Schaefer et al., 2003; Beauregard, Levesque, & Bourgouin, 2001), whereas left-sided areas (Levesque, Joanette, et al., 2004; Ochsner, Bunge, Gross, & Gabrieli, 2002) seem to be relatively less implicated. On this basis, there is strong evidence that activation of the right anterolateral PFC mediates components of reappraisal.
The imaging studies most relevant to self-distraction are those where subjects are asked not to think particular thoughts while no explicit alternative mental contents are provided by the experimenter, as opposed to studies using salient external distractors or engaging subjects in a demanding task. In these thought suppression tasks (Wegner, 1994), alternative foci of attentional deployment have to be self-generated or chosen from incidental external (e.g., scanner noise) or internal (e.g., spontaneous thoughts, bodily sensations, etc.) stimuli. Such a design assures best that subjects do not simply passively follow proposed alternative stimulation but have to actively engage in selective attentional deployment, which is the essential component of self-distraction. Performance in thought suppression is therefore an adequate index of self-distraction efforts. One study (Wyland, Kelley, Macrae, Gordon, & Heatherton, 2003) described activation in the dorsal anterior cingulate cortex (ACC) while subjects suppressed personally salient but not explicitly emotional thoughts (such as “study for an exam” or “a phone call with a distant girlfriend”). A similar activation focus was found by Frankenstein, Richter, McIntyre, and Rémy (2001) while subjects generated neutral words during tonic pain. Anderson et al. (2004) reported lateral PFC (LPFC), ACC, and premotor activation during suppression of cued retrieval of recently formed, emotionally neutral memories. These studies indicate that a prefrontal network may be implicated in keeping unwanted information “out of the mind” (see also Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001). They do not provide information on self-distraction from anxiety.
In the present study, we therefore used a thought suppression paradigm to have subjects self-distract from anticipatory anxiety for pain while we recorded physiological (heart rate) and brain activity (by using functional magnetic resonance imaging [fMRI]). Specifically, subjects were instructed to quickly think of something else as soon as a thought or feeling of anxiety or about the potential pain came up in the mind (self-distraction condition). We did not tell subjects what to think of when suppressing anxiety thoughts/feelings. In a control condition (no self-distraction), subjects were free to think whatever they wanted under the constraint that they were disallowed from using the suppression technique. The anticipatory anxiety paradigm was identical to the one employed in Kalisch et al. (2005) (see Figure 1) and thus allows for qualitative comparison with their results.1 The study involved a 2 × 2 factorial design, with factors Anticipation (No anticipation vs. Anticipation) and Self-distraction (No self-distraction vs. Self-distraction) and the four conditions no anticipation/no self-distraction, no anticipation/self-distraction, anticipation/no self-distraction, anticipation/self-distraction.
Figure 1.
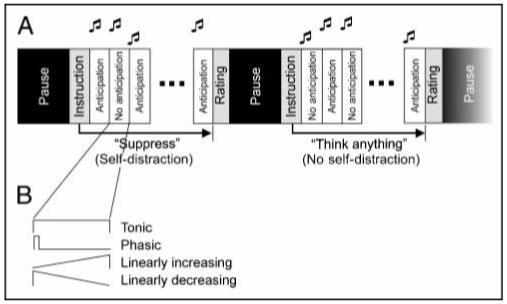
Design and analysis. (A) The study involves a 2 × 2-factorial design, with factors Anticipation (No anticipation vs. Anticipation) and Self-distraction (No self-distraction vs. Self-distraction).Anticipatory anxiety was induced by forewarning subjects with a high-pitch double-beep that they might receive a painful electric stimulus to the hand at a probability of 25% during the following 15.6-sec epoch. During a control condition (No anticipation) of the same length, announced by a low-pitch double-beep, subjects knew they would not be stimulated. The Self-distraction factor was operationalized in” megablocks” that spanned eight blocks of anticipation/no anticipation. During Self-distraction (announced by “Suppress”) subjects had to suppress thoughts or feelings of anxiety or about the upcoming potential pain; during No self-distraction (“Think anything”) subjects were allowed to think what they wanted but not to use the thought suppression strategy (see Methods). (B) Neural activations during blocks of anticipation/no anticipation were modeled as tonic, phasic, and linearly increasing and decreasing responses (see Methods).
We did not train subjects in thought suppression prior to scanning. We therefore had no predictions as to whether thought suppression would be successful in reducing anxiety. Rather, we aimed at showing the neural networks subserving the attempt to suppress anxious thoughts.
In addition to reporting whole-brain data, we used a region-of-interest-based approach to ask whether self-distraction activated the same right anterolateral PFC region as in the case of reappraisal in Kalisch et al. (2005), and whether effects akin to those previously reported (a reduction of anticipatory anxiety-related activity) would be evident in medial PFC/ACC (MPFC/ACC; Kalisch et al., 2005).
METHODS
Subjects
Fifteen right-handed healthy normal subjects (mean age 26 years, range 22-38 years, 8 women) participated in the experiment. The subjects were preassessed to exclude those with a prior history of neurological or psychiatric illness, including anxiety disorders. Subjects’ scores for trait and state anxiety (obtained prior to scanning using questionnaires STAI-S [Spielberger state-trait inventory-trait version] and STAI-T [Spielberger state-trait inventory-state version], Mind Garden, Redwood, CA, USA) were 31.8 ± 1.4 (mean ± SEM) and 34.5 ± 1.6, respectively, and thus deviated less than one standard deviation from a normal working adult population (Spielberger, 1983). Scores on a social desirability questionnaire indicating the tendency to give desirable responses (Crowne & Marlowe, 1960) were 16.9 ± 1.0 and likewise deviated less than one standard deviation from a general population.
All subjects gave informed consent, and the study was approved by the Joint Ethics Committee of the National Hospital for Neurology and Neurosurgery.
Self-distraction Strategy
Self-distraction (Self-distraction condition) was indexed through a thought suppression task. Subjects were instructed to “suppress any thought or feeling of anxiety or about the shock.” This was specified further: “Whenever you realize an anxious thought or feeling coming up in your mind or whenever you realize that you think about the shock, quickly think of something else.” Subjects were free to choose what “else” to think of. In a control condition (No self-distraction), they were allowed to think whatever they liked but were explicitly told not to use the suppression strategy.
Anxiety Induction
Anxiety was induced by forewarning subjects they might receive an electric painful stimulus at a probability of 25% at any time during an epoch of 15.6 sec (Anticipation condition). In a control condition (No anticipation), subjects knew they would not be stimulated. Pain stimuli were applied to the back of the left or right hand (counterbalanced between subjects) using a custom-built electrical stimulator delivering 20- or 100-Hz trains of electrical pulses (4-msec monopolar square waveform pulses, 1-sec duration, 0.1 to 6 mA) through a silver chloride electrode. In an independent sample of n = 9 subjects, this method selectively induced anxiety (7.3 ± 0.5 on a 1 to 10 scale) as opposed to anger (1.7 ± 0.6), eagerness (4.8 ± 0.9), sadness (1.0 ± 0.2), and surprise (3.3 ± 0.9). The difference between anxiety and eagerness was significant (t test,p = .012, one tailed).
A current calibration procedure prior to scanning assured that current levels and stimulation frequencies were chosen that induced intermediate subjective anxiety. To achieve this, subjects verbally rated their anxiety experienced during a 16-to-0 countdown during which they knew they might receive a previously experienced painful stimulus at any time at 25% probability. The countdowns were repeated with different current levels, starting at low levels, until an anxiety level between 30 to 60 (on a 100-point scale) was reached. Current levels were adjusted between experimental runs if subjective ratings in the Anticipation minus No anticipation comparison during the No self-distraction condition differed markedly from the previously calibrated value.
Paradigm
Prior to scanning, all subjects were familiarized with the paradigm and scanner noise by completing a short practice run without pain stimulation.
During experimental scans, subjects remained eyes closed, instructions were given over headphones. The experiment was split into three runs of 9-, 12-, and 9-min duration. Runs consisted of three, four, and three megablocks of approximately 2.5 min each (separated by pauses of 30 sec) during which subjects either had to employ the self-distraction strategy (Self-distraction condition, five megablocks, announced by “Suppress”) or the control strategy (No self-distraction condition, five megablocks, announced by “Think anything”). The sequence of these conditions was randomized. Suppress or Think anything instructions were followed by eight pseudorandomized 15.6-sec blocks of either Anticipation (announced by a high-pitch double beep) or No anticipation (announced by a low-pitch double beep). Thus, there were four experimental conditions: no anticipation/no self-distraction (n = 17), no anticipation/self-distraction (n = 17), anticipation/no self-distraction (n = 23, six pain stimuli), anticipation/self-distraction (n = 23, six painful stimuli). At the end of a megablock, subjects indicated by a button press how much of the time after the instruction they had spent thinking about anxiety or pain or feeling anxiety, separately for the No anticipation (Question: “How many anxious thoughts during safety?”) and the Anticipation blocks (Question: “How many anxious thoughts during danger?”). Time for responses was 6 sec each. Possible ratings were 0 (not at all), 1 (roughly one third of the time), 2 (roughly two thirds of the time), and 3 (the whole time).
After each run, subjects verbally rated their anxiety during the four conditions on the 100-point scale. They were reminded not to rate their affective responses to the actual receipt of pain.
At the end of the experiment, subjects were asked about their thought contents during each of the four conditions. Their answers were consistent with increased anxiety during the Anticipation conditions and attempted and partly successful thought suppression during the Self-distraction conditions. Subjects finally had to rate on a scale from 0 to 10 how difficult they found it and how much effort they had made to suppress unwanted thoughts during the two Self-distraction conditions.
During the experimental runs, heart rate (HR) was monitored using a pulse oximeter (Nonin 8600FO, Non-in Medical, Plymouth, MN); the pulse probe was placed on the index finger of the hand without an electrode.
Imaging
A 3-Tesla Siemens Allegra MRI scanner was used to acquire gradient-echo T2*-weighted echo-planar images (EPI) images with blood oxygenation level-dependent (BOLD) contrast (TE = 30 msec, TR = 65 msec, flip angle = 90°). Each volume comprised 44 tilted slices of 2-mm thickness and 3 × 3 mm2 in-plane resolution with a slice distance of 1 mm. A total of 645 volumes, distributed over three runs (194, 257, 194 volumes), were acquired continuously every 2.86 sec. These parameters produced EPI images in which signal dropout from susceptibility artifact was restricted to the far caudal orbitofrontal cortex (OFC), leaving the remaining sectors of the OFC intact (Deichmann, Gottfried, Hutton, & Turner, 2003). Subjects were placed in a light head restraint within the scanner to limit head movement during acquisition. A T1-weighted structural image was also acquired (Deichmann, Schwarzbauer, & Turner, 2004).
Data Analysis
The subjective anxiety ratings obtained at the end of each run were weighted by the number of Self-distraction or No self-distraction megablocks within that run before averaging across the whole experiment. Raw HR waveforms were visually inspected and excluded where automatic pulse detection was inaccurate (four subjects). Blockwise averaged HR levels (HRLs) were normalized to the last 20 sec of the pause at the beginning of each megablock; initial phasic heart rate responses (HRRs) were taken from 1 to 6 sec after start of block and normalized identically. Blocks where subjects actually received pain stimuli were excluded from the analysis. HRRs to receipt of pain were calculated as average heart rate during the 1-sec poststimulus time window that showed maximum physiological activation in the group data (from 1000 to 2000 msec), normalized to the second prior to stimulus. To account for HRR delays of around 3 sec during Self-distraction (see Results), the above HRR to pain values from the anticipation/ no self-distraction condition were also compared to responses in a time window from 4000 to 5000 msec in the anticipation/self-distraction condition. Statistical inference was based on one-way analysis of variance (ANOVA) with repeated measures and Student’s t test within SPSS 11 (SPSS Inc., Chicago, IL).
Imaging data were analyzed using SPM2 (www.fil.ion.ucl.ac.uk/spm/spm2). The four initial images of each run were discarded. Images were realigned to the fifth volume of the first run, spatially normalized to a standard T2 * template, spatially smoothed using a Gaussian kernel with a full width at half maximum (FWHM) of 6 mm, temporally high-pass filtered (cutoff 128 sec) and corrected for temporal autocorrelations. Statistical analysis was carried out by applying a random effects analysis using the general linear model across the 15 subjects. Each of the four experimental conditions was modeled using three different temporal profiles of neuronal response during the 15.6-sec block: a phasic response occurring at the beginning of the block, a tonic neuronal response lasting the whole duration of the block, and a linearly increasing response across the block (Figure 1B). Multiplication of the linearly increasing regressor by - 1 in the definition of contrasts (see below) allowed assessment of linearly decreasing effects. Receipt of pain was modeled as distinct events. Blocks during which subjects actually received pain stimuli, instructions at the beginning of the megablocks, and ratings were modeled as boxcar regressors. To retain degrees of freedom, the regressors for the three runs were concatenated. Residual motion effects were corrected for by including the six estimated motion parameters for each subject as regressors in the model. Each regressor was convolved with the canonical hemodynamic response function. Calculation of voxelwise within-subject effects of linear combinations of the regressors yielded contrast images that were spatially smoothed (FWHM 10 mm), resulting in an estimated smoothness of 10-11 mm, and compared between subjects using one-sample t tests. One subject had considerable signal dropout in the anteroventral PFC. However, the results of the random effects analysis in this area were not changed by excluding this subject.
Clusters with >5 voxels activated at a statistical threshold of p < .001 are reported. Clear white matter activations are not reported. Because behavioral data indicated a general attenuation or delay in emotional processing due to the main effect of Self-distraction, we also investigated negative BOLD signals in this contrast. However, there were no significant deactivations. Correction for multiple comparisons following Gaussian random field theory was limited to spherical regions of interest (radius 10 mm) in the MPFC/ACC (-2/45/27, mean coordinates from Kalisch et al. [2005]) and the right anterolateral PFC as well as its contralateral counterpart (±42/48/19, Kalisch et al., 2005). To illustrate group effect sizes in selected voxels, mean parameter estimates from the main effect of a second-level ANOVA over the four experimental conditions were used. To test whether activation patterns in voxels showing a significant interaction were consistent with a reduction of anxiety-related activity by self-distraction (i.e., whether they showed a significant simple main effect of Anticipation [anticipation/no self-distraction > no anticipation/no self-distraction] and also a significant simple main effect of Self-distraction under Anticipation [anticipation/no self-distraction > anticipation/self-distraction]), post hoc t tests for the relevant contrasts were calculated within SPSS11 using single-subject parameter estimates from the ANOVA (threshold p = .05, one tailed). Anatomical localization was carried out with reference to the atlas of Duvernoy (1999). Coordinates follow MNI conventions.
RESULTS
Behavior
Main Effect of Anticipation
Self-report showed that subjective anxiety (Figure 2A) was increased during anticipation of pain [main effect of Anticipation: F(1,14) = 146.1, p < .001] as was time spent thinking about anxiety or pain [main effect of Anticipation: F(1,14) = 151.1,p < .001 (Figure 2B)]. As in our previous study, Anticipation did not change tonic HRLs (not shown) but induced an initial phasic HRR (see time courses in Figure 2C) with onset of an anticipatory period. However, unlike in our previous study, restricting analysis to the first 6 sec did not yield a significant main effect of Anticipation. Anticipation increased the perceived difficulty of the thought suppression task (6.1 ± 0.4, mean ± SEM, in anticipation/self-distraction vs. 3.0 ± 0.6 in no anticipation/self-distraction, on a scale from 0 to 10, p < .001, two-tailed t test), and subjects reported making a greater effort to suppress anxiety thoughts during anticipation/self-distraction relative to no anticipation/self-distraction (7.1 ± 0.4 vs. 4.1 ± 0.5, p < .001). Taken together, self-report and physiological data indicate that anticipation of pain successfully induced anxiety.
Figure 2.
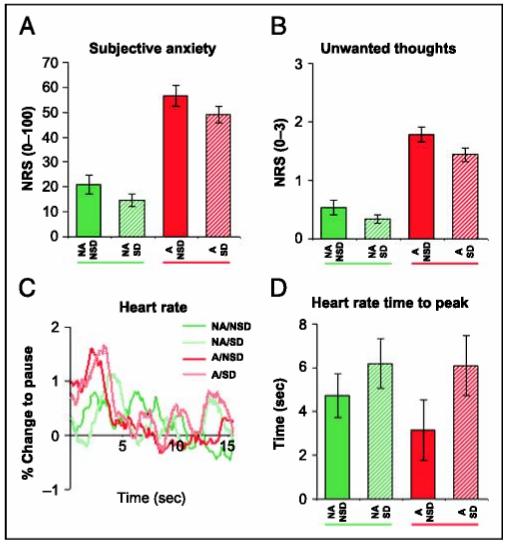
Behavior. (A) Subjective anxiety was increased by anticipation of pain (anticipation condition). Self-distraction operationalized through thought suppression did not reduce anticipatory anxiety. (B) The time spent thinking thoughts of anxiety or pain or feeling anxiety was increased by anticipation and reduced by self-distraction. However, there was no interaction; that is, self-distraction did not reduce unwanted thoughts during the anticipation condition. (C) Heart rate showed initial accelerations to the cues signaling blocks of anticipation of pain (Anticipation) or safety (No anticipation), but differences between the conditions were not significant. (D) Maximum HRRs were significantly delayed during Self-distraction. Solid green: no anticipation/no self-distraction (NA/NSD); hatched green: no anticipation/self-distraction (NA/SD); solid red: anticipation/no self-distraction (A/NSD); hatched red:anticipation/self-distraction (A/SD). NRS = numerical rating scale. Values are mean ± SEM.
Main Effect of Self-distraction (Thought Suppression)
The self-reported time spent thinking of anxiety/pain was significantly reduced during Self-distraction [main effect of Self-distraction: F(1,14) = 4.7, p = .049 (Figure 2B)]. This suggests thought suppression did not lead to paradoxical increases in unwanted thoughts (Wenzlaff & Wegner, 2000). Reduced thinking about anxiety/pain was associated with a trend level tendency for the Self-distraction conditions to be perceived as less anxiogenic than the No self-distraction conditions [main effect of Self-distraction on subjective anxiety ratings: F(1,14) = 3.4,p = .085 (Figure 2A)]. Both HRLs and HRRs were unaffected by Self-distraction. However, time courses suggested that the time to peak of HRRs was increased relative to the No self-distraction condition (see Figure 2C). When comparing times to peak between the four conditions, there was a significant main effect of Self-distraction, F(1,10) = 7.7, p = .02 (Figure 2D). The data thus show distraction effects on anxiety-related mental representations and on processing of sensory information (delayed HRR peaks to the auditory cues signaling the onsets of the different conditions). In conjunction with a reported increased difficulty and effort for thought suppression during Anticipation (see above)—an indirect indicator of self-distraction attempts—and the consistent debriefing responses (see Methods), these data indicate subjects indeed attempted to perform thought suppression.
Interactions: Modulation of Anticipatory Anxiety by Self-distraction
Self-distraction did not reduce subjective anxiety over and above a global reduction observed in the main effect above; that is, there was no interaction (p = .757; Figure 2A). Likewise, HRLs, HRRs, and times to peak showed no interactions. Anticipatory anxiety has previously been reported as causing increased reactivity to noxious stimuli (Kalisch et al., 2005; Ploghaus et al., 2001; Epstein & Clarke, 1970), and this metric can be used as a physiological indicator of anxiety. We found a significant difference (reduction) in the phasic HRR to pain stimuli between the anticipation/no self-distraction and anticipation/self-distraction conditions (p = .02 1, one-tailed t test). Because HRR times to peak after anticipation onset were delayed by about 3 sec during Self-distraction (see above), we also compared the phasic HRRs to pain stimuli in the anticipation/no self-distraction condition to later responses in the anticipation/self-distraction conditions (see Methods). In this comparison, there was only a trend level significance (p = .082, one tailed), suggesting the delayed response onsets in the Self-distraction condition at least partly accounted for attenuation of pain reactivity.
Pain reactivity as an indirect indicator of anxiety can also be measured in evoked neural responses to receipt of pain where pain responses are modeled as distinct events. Pain-evoked neural activity was attenuated in anticipation/self-distraction relative to anticipation/no self-distraction in a single cluster in the right supramarginal gyrus (6/-52/76, z = 3.25,p = .001, 20 voxels). We note considerably more widespread attenuations, encompassing a prominent MPFC cluster, produced by successful reappraisal of anxiety in Kalisch et al. (2005). Regions in or near the somatomotor cortex are involved in sensory-discriminative processing of pain, whereas MPFC supports affective responses to pain (Rainville, Duncan, Price, Carrier, & Bushnell, 1997). Therefore, we suggest the most likely interpretation of the observed supramarginal attenuation is that it reflects reduced attention to sensory stimulation (in accordance with the delayed HRRs to auditory cues reported above) rather than reductions in the affective pain processing.
Overall, the behavioral and neural data offer only tentative evidence for a reduction of anticipatory anxiety by Distraction (nor the opposite).
Neural Responses during Anticipation
Main Effect of Anticipation
Activation due to the main effect of Anticipation was seen in a wide network including cingulate, anterior insula, PFC, cerebellum, and brainstem (Supplementary Figure 1). In particular, an MPFC/ACC region responsive to anticipatory anxiety and attenuated by reappraisal in our previous study (Kalisch et al., 2005; see Methods) was (tonically) activated (2/40/34, z = 3.73, p < .001, p = .005 corrected for search volume 42, voxels). The activation was part of a larger cluster extending far posteriorly into the dorsal MPFC/ACC (peak activations 0/14/60, 0/30/58, and 4/38/36, 2781 voxels). The response pattern is additional evidence for successful anxiety induction through anticipation of pain.2
Main Effect of Self-distraction
Activation due to Self-distraction was found in the left LPFC (tonic response), right parahippocampal gyrus, and left subgenual ACC (linearly decreasing response; Table 1, Figure 3). There was no activation in the right anterolateral PFC region seen for reappraisal (Kalisch et al., 2005) nor its left-sided counterpart at bothp < .05 corrected for the search volume and p < .001 uncorrected (for definition of region of interest, see Methods). Only at a liberal threshold of p < .01 uncorrected was a left-hemispheric cluster found for the tonic response that was part of the extended left LPFC main effect reported above and whose overall maximum was identical with the main effect maximum (situated outside the search volume). Because PFC activation as a function of Anticipation may cancel a main effect of Self-distraction, we also searched for anterolateral PFC activations in the simple main effect of Self-distraction (no anticipation/self-distraction > no anticipation/no self-distraction). At a liberal threshold of p < .01 uncorrected, there was again a left-sided activation as part of a more posteriorly situated cluster (tonic response) and no right-sided activation. At this threshold, a (bilateral) phasic response was also evident (not shown), which, however, is an unlikely candidate for sustaining a self-distraction strategy. Taken together, there was little evidence for self-distraction-related activation akin to reappraisal-related activation observed in our previous study.
Table 1.
Neuroimaging: Main Effect of Self-distraction (Thought Suppression)
| Region | MNI Coordinates | Z Score | Cluster Size |
|---|---|---|---|
| Tonic response Left LPFC |
-56/30/22 | 3.46 | 42 |
| Linearly decreasing response | |||
| Right parahippocampal gyrus | 26/-26/-30 | 3.29 | 14 |
| Left subgenual ACC | -6/26/- 14 | 3.27 | 10 |
Statistical threshold: p < .001 uncorrected.
Figure 3.
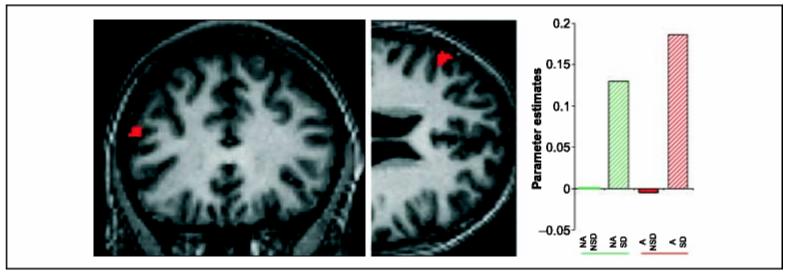
Neuroimaging: main effect of Self-distraction (thought suppression). Thought suppression tonically activated the left LPFC (-56/30/22). Threshold: p <.001 uncorrected.SPM results are superimposed on an individual subject’s normalized structural scan. Further activations are given in Table 1. Parameter estimates are relative to no anticipation/no self-distraction. Solid green: no anticipation/no self-distraction (NA/NSD); hatched green: no anticipation/self-distraction (NA/SD); solid red: anticipation/no self-distraction (A/NSD); hatched red: anticipation/self-distraction (A/SD).
Interactions: Modulation of Self-distraction by Anticipatory Anxiety
There were no significant interactions of the type (Self-distraction > No self_distraction)Anticipation > (Self-distraction > No self-distraction)No anticipation for tonic, phasic, and linearly decreasing responses. Interactions for increasing responses (Supplementary Figure 2, http://www.fil.ion.ucl.ac.uk/Publications/Kalisch/Kalisch_suppl fig2.tif), mathematically identical to their complementary interactions (below) for decreasing responses, showed activation patterns across the four conditions that were not consistent with increased thought suppression activity during anticipation of pain. This indicates that the neural implementation of self-distraction under conditions of anticipatory anxiety did not differ (qualitatively or quantitatively) from distraction under nonanxiety conditions. Thus, although suppression was perceived as more difficult and effortful during anticipation (see Behavior), implementation of self-distraction under more demanding contexts was not associated with any augmentation in activation.
Interactions: Modulation of Anticipatory Anxiety by Self-distraction
Interactions of the type (Anticipation > No anticipation)No self-distraction> (Anticipation > No anticipation)Self-distraction, potentially signifying attenuation of anticipatory anxiety-related activity through self-distraction, are reported in Supplementary Figure 2. Of note, these interactions were not accompanied by parallel interactions in behavior. Significant effects were mainly observed for the phasic and linearly decreasing responses, including a phasic interaction in the MPFC/ACC region of interest (see Methods). This implies that the amplitude of transient neural responses to the onset of an anticipatory period was influenced by both anticipatory anxiety and self-distraction. However, the pattern of activations in the MPFC/ACC, although exhibiting a significant simple main effect of Anticipation (anticipation/no self-distraction > no anticipation/no self-distraction), did not show a significant reduction of anticipatory anxiety-related activation during self-distraction (anticipation/self-distraction < anticipation/no self-distraction). That is, the MPFC/ACC interaction was not consistent with attenuation of anticipatory anxiety-related activity, in accordance with behavioral data.
DISCUSSION
The main finding of this study is that self-distraction from anticipatory anxiety for pain tonically activated a left LPFC region (approximately Brodmann’s area 46). We found no activation in a right anterolateral region of interest active during reappraisal of anxiety (Kalisch et al., 2005). A preliminary conclusion is that self-distraction-related PFC activity is distinct from reappraisal-related PFC activity. This conclusion comes under the caveat that a negative result (here, in the anterolateral PFC) can be due to insufficient sensitivity. Furthermore, the self-distraction and reappraisal studies differed in a number of aspects, first of all, in success of anxiety reduction. The result therefore warrants further testing, ideally by directly comparing self-distraction against reappraisal within the same study.
We note that Anderson et al. (2004), in a study of suppression of cued retrieval of emotionally neutral memories, found a similar left LPFC area (their Figure 2). By contrast, neither Wyland et al. (2003) nor Frankenstein et al. (2001) reported LPFC during self-distraction from painful stimulation and suppression of personally salient thoughts, respectively. None of the factors self-report demand (possibly implying increased self-monitoring), nature of the to-be-suppressed material, or success in self-distraction consistently distinguish Anderson and colleagues’ and our study from the latter two, suggesting insufficient sensitivity is the most likely explanation for the negative result in Wyland et al. and Frankenstein et al.
Self-distraction in our study, although reducing unwanted thoughts, was not successful in attenuating anxiety. This was paralleled by a paucity of corresponding interactions in the imaging data. In particular, there was no interaction consistent with an attenuation of anxiety-related activity in the MPFC/ACC, a region that was successfully down-regulated by reappraisal (Kalisch et al., 2005). Given the delayed transient HRRs to onset of an anticipatory period during Self-distraction (see Behavior), the other phasic interactions in the imaging data may partly reflect delayed serial processing under Self-distraction. Alternatively, they may indicate authentic reductions in anxiety processing that were, however, not pronounced enough to reduce anxiety. The most likely explanation for these weak effects is insufficient training. It is also possible that suppressing thinking about anxiety/pain reduced the frequency of spontaneous reappraising thoughts (which attenuate anxiety) along with the frequency of spontaneous catastrophizing thoughts (which maintain/augment anxiety). Thus, the effects of thought suppression on anxiety may have canceled each other out. We note that distraction studies that employ a no-distraction control condition that excludes reappraisal do find anxiolytic effects of distraction (e.g., Johnstone & Page, 2004).
A process model of self-distraction and reappraisal can explain the observed neural effects and serve as a theoretical foundation for further studies on cognitive emotion regulation processes. The model we propose is based on an information processing view of anxiety (Dalgleish, 2004; Foa & Kozak, 1986; Lang, 1985; Bower, 1981) that assumes that anxiety-related memory elements are organized in relatively cohesive associative networks. That is, associations between anxiety-related elements are strong compared to associations between elements representing nonanxious material (Lang, 1985), which can explain the often persistent and infectious nature of anxious mental states. We propose that emotion regulation essentially consists in the replacement (Bower, 1981) in working memory of anxiety-related mental contents by nonanxious material (in the following “safe” or “safety” material), resulting in deactivation of anxiety networks. Because—in addition to thoughts, feelings, and sensory-type representations—response programs are a further type of memory element thought to be part of the associative network structure (Anderson, 1993; Lang, 1985), a general anxiety network deactivation through replacement can also explain the often observed parallel attenuation of autonomic responses or action tendencies during emotion regulation (Kalisch et al., 2005; Gross, 2002; Jackson, Malmstadt, Larson, & Davidson, 2000).
More recent variants of information processing models are hybrid models that assume that some aspects of the information contained in emotional stimuli (such as valence, arousal, or value) are processed by a parallel, presumably limbic, system that is directly linked to emotional response output (e.g., Reisenzein, 2001; Siegle, 1999; LeDoux, 1996). Under the assumption that mental contents (thoughts, feelings, sensory-type representations) are, like actual sensory stimuli, evaluated by this system, a general anxiety network deactivation by replacement would lead to attenuation of autonomic responses or action tendencies. Our viewpoint is explicitly not based on the idea that the LPFC directly (or indirectly, via medial or orbital PFC connections) inhibits limbic structures. Notably, it explains observations of an inverse relation between LPFC and amygdala activations in a more parsimonious fashion.
Within this general framework, it is important to identify the executive processes by which replacement of anxiety-related by safe material can be successfully achieved. An influential view holds that voluntary production of working memory contents (through retrieval of long-term memories or generation of new thoughts and maintenance of these in working memory) involves a supervisory attentional control mechanism (the “production” function) located in the left PFC (e.g., Cabeza, Locantore, & Anderson, 2003; Gabrieli, Poldrack, & Desmond, 1998; Shallice & Burgess, 1996). It can be assumed that this production function is required in both self-distraction and reappraisal. However, self-distraction and reappraisal may be distinguished in two aspects. Whereas self-distraction, by definition, produces working memory contents that are unrelated to the to-be-suppressed anxiety-related material, reappraisal, also by definition, produces working memory contents that reinterpret anxiety-related material and which—albeit “safe”—are anxiety related themselves. Thus, anxious thoughts come to serve as cues for the retrieval of reappraisal thoughts. This implies that production (replacement) in reappraisal may ultimately become automatized, especially where subjects are well experienced in a particular reappraisal strategy (see Kalisch et al., 2005). As a consequence, reappraisal may depend less on left-hemispheric PFC. By contrast, intrusion of unwanted thoughts during self-distraction can only be answered by increased replacement efforts leading to a constant need of a production function in this type of strategy.
A second distinctive quality of reappraisal is that it can produce relatively paradoxical or “nonstandard” solutions that go against powerful schemata that guide first-pass appraisal of anxiogenic stimuli and compete with them. For instance, reinterpreting the tears of an old woman standing in front of a church as signifying her joy about her daughter’s wedding (Ochsner, Bunge, et al., 2002) or denying the personal relevance of a potential upcoming electric shock (Kalisch et al., 2005) runs contrary to habitual attributions. As a consequence, reappraisal may require a process of monitoring the produced solutions in terms of whether they are compatible with reality and whether interference from habitual appraisals has been successfully resolved. Given that produced safe working memory contents in reappraisal are associatively linked to anxiety-related elements, reappraisal may also require increased monitoring to prevent retrograde reactivation of anxiety memories. Such a monitoring process, which operates on the contents of working memory rather than directly changing or maintaining them (sometimes referred to as ‘postretrieval’ monitoring), has been proposed to be localized in the right LPFC (e.g., Cabeza et al., 2003; Allan, Dolan, Fletcher, & Rugg, 2000; Henson, Shallice, & Dolan, 1999; Schacter, Curran, Galluccio, Milberg, & Bates, 1996; Shallice & Burgess, 1996).
The above theoretical account can explain why reappraisal in a well-learned subject activated the right anterolateral PFC (corresponding to the monitoring function) but not the left LPFC (corresponding to the production function) and why self-distraction showed the opposite pattern. As stated earlier, this pattern will have to be reproduced in further studies directly comparing self-distraction and reappraisal. The model also generates the prediction that right anterolateral lesions interfere with reappraisal but not self-distraction. Furthermore, it can be hypothesized that during each individual (successful) reappraisal effort, the left-hemispheric production function should show a decrease in activity, whereas the right-hemispheric monitoring function should exhibit increasing or tonic activity. We note that in our reappraisal study (Kalisch et al., 2005), reappraisal-specific activity in the right anterolateral PFC increased during the 16-sec epochs, whereas activity related to the main effect of regulation (the retrieval of a relaxed and detached mental state independent of whether the external situation was anxiogenic or not) showed a linearly decreasing profile in a left PFC region close to the self-distraction region found here (-54/24/32; see Supplementary Table 1 in Kalisch et al., 2005). However, EEG studies may be better suited to test this hypothesis. Finally, we note a potential relationship between our results and reported left-lateralized frontal base-line activity in repressors that may habitually use self-distraction for emotional self-regulation (Tomarken & Davidson, 1994).
Alternative accounts of prefrontal activation patterns in cognitive emotion regulation have been proposed. Ochsner, Ray, et al. (2004) reported that the right LPFC is activated when negative emotion is down- as opposed to up-regulated and suggested this reflects involvement of the right LPFC in behavioral inhibition and interference resolution (Bunge et al., 2001; Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998). Conversely, left LPFC activation was observed during both up- and down-regulation that was interpreted as a recruitment of general working memory and cognitive control functions needed to generate and maintain any form of regulation strategy (Ochsner, Ray, et al., 2004). Our production-monitoring hypothesis is similar to this account. The left lateral production function generating and maintaining neutral or positive working memory contents that replace negative contents during down-regulation of negative emotion would also be needed to actively maintain negative working memory contents during up-regulation of negative emotion. On the other hand, attempted down-regulation, but not up-regulation, presumably induces interference between to-be replaced negative and the replacing neutral or positive working memory contents. This, we suggest, results in an increased need to monitor working memory contents, thus activating the right anterolateral PFC. Obviously, monitoring is only useful where its products can be employed in the service of performance adjustment (i.e., interference resolution).
To summarize, neuroimaging studies of self-distraction and reappraisal suggest these regulation strategies are subserved by distinct neural networks and therefore rely on distinct psychological mechanisms. Specifically, we propose reappraisal is different from self-distraction in that it uses anxiety-related mental contents as cues for the retrieval of safety memories, resulting in a decreased dependence on production relative to higher order monitoring functions. It is worth adding that by building our model on general supervisory attentional control functions, we implicitly predict these mechanisms to work in the replacement of other types of thoughts as well. In particular, it is conceivable that the left lateral production function helps suppress any type of thought, as suggested by activation of the left LPFC during suppression of emotionally neutral memories (Anderson et al., 2004).
Supplementary Material
Acknowledgments
We thank P. Allen, P. Aston, O. Josephs, and E. Featherstone for help with the setup, and L. Otten for helpful comments on the manuscript. The authors also thank two anonymous reviewers for their useful comments and suggestions. This work was supported by grants from the Marie Curie Programme (R. K.), Deutsche Forschungsgemeinschaft (K. W.) and the Wellcome Trust (R.J. D.).
Footnotes
In Kalisch et al. (2005), subjects reappraised emotional reactions associated with pain anticipation as not relevant to themselves by explicitly adopting an uninvolved observer perspective. This was compared to a condition in which the habitual appraisal of the emotional reaction as self-relevant remained unchallenged. Reappraisal reduced subjective and physiological measures of anxiety, attenuated anxiety-related MPFC/ACC activation, and recruited the right anterolateral PFC.
We note that the amygdala is rarely found in imaging studies of anticipatory anxiety. Negative findings are reported by Ploghaus et al. (1999), Chua et al. (1999), Simpson et al. (2001), Naliboff et al. (2001), Porro et al. (2002), Boshuisen et al. (2002), Jensen et al. (2003), and Kalisch et al. (2005). Positive findings are reported by Phelps et al. (2001) and Wager et al. (2004) only. This may reflect rapid habituation within or between trials (which are usually extended over several seconds in the employed paradigms), although explicitly modeling habituation did not yield amygdala activation in Kalisch et al. (2005). Alternatively, the amygdala may be relatively less implicated in the expression of anxiety than in (preexperimental) contingency learning.
The data reported in this experiment have been deposited with the fMRI Data Center (www.fmridc.org). The accession number is 2-2006-120WD.
REFERENCES
- Allan K, Dolan RJ, Fletcher PC, Rugg MD. The role of the right anterior prefrontal cortex in episodic retrieval. Neuroimage. 2000;11:2 17–227. doi: 10.1006/nimg.2000.0531. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Rules of the mind. Erlbaum; Hillsdale, NJ: 1993. [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Neural systems underlying the suppression of unwanted memories. Science. 2004;303:232–23 5. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshuisen ML, Ter Horst GJ, Paans AM, Reinders AA, den Boer JA. rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biological Psychiatry. 2002;52:126–135. doi: 10.1016/s0006-3223(02)01355-0. [DOI] [PubMed] [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: Evidence for the production-monitoring hypothesis. Journal of Cognitive Neuroscience. 2003;15:249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Chua P, Krams M, Toni I, Passingham R, Dolan R. A functional anatomy of anticipatory anxiety. Neuroimage. 1999;9:563–571. doi: 10.1006/nimg.1999.0407. [DOI] [PubMed] [Google Scholar]
- Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology. 1960;24:349–3 54. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- Dalgleish T. Cognitive approaches to posttraumatic stress disorder: The evolution of multirepresentational theorizing. Psychological Bulletin. 2004;130:228–260. doi: 10.1037/0033-2909.130.2.228. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: Technical implications at 1.5 and 3 T. Neuroimage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: Surface, blood supply, and three-dimensional sectional anatomy. 2nd ed. Springer; Vienna: 1999. [Google Scholar]
- Epstein S, Clarke S. Heart rate and skin conductance during experimentally induced anxiety: Effects of anticipated intensity of noxious stimulation and experience. Journal of Experimental Psychology. 1970;84:105–112. doi: 10.1037/h0028929. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–3 5. [PubMed] [Google Scholar]
- Frankenstein UN, Richter W, McIntyre MC, Rémy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14:827–836. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Past, present, future. Cognition and Emotion. 1999;13:55 1–573. [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–29 1. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999;122:1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–1257. doi: 10.1016/s0896-6273(03)00724-4. [DOI] [PubMed] [Google Scholar]
- Johnstone KA, Page AC. Attention to phobic stimuli during exposure: The effect of distraction on anxiety reduction, self-efficacy and perceived control. Behaviour Research and Therapy. 2004;42:249–275. doi: 10.1016/S0005-7967(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Seymour B, O’Doherty JP, Oakley DA, Allen P, Dolan RJ. Anxiety reduction through detachment: Subjective, physiological and neural effects. Journal of Cognitive Neuroscience. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The cognitive psychophysiology of emotion: Fear and anxiety. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Erlbaum; Hillsdale, NJ: 1985. pp. 131–170. [Google Scholar]
- LeDoux . The emotional brain. Simon & Schuster; New York: 1996. [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beaurogard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Gross JJ, Gabrieli JD. Exploring the neural bases of emotion regulation: Comparing the effects of cognitive reappraisal and working memory load; Proceedings of the 31st Annual Meeting of the Society for Neuroscience; Orlando: FL. 2002. [Google Scholar]
- Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosomatic Medicine. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. Journal of Neuroscience. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, Nichelli P. Does anticipation of pain affect cortical nociceptive systems? Journal ofNeuroscience. 2002;22:3206–3214. doi: 10.1523/JNEUROSCI.22-08-03206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Reisenzein R. Appraisal processes conceptualized from a schema-theoretic perspective. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, methods, research. Oxford University Press; New York: 2001. pp. 187–201. [Google Scholar]
- Schacter DL, Curran T, Galluccio L, Milberg WP, Bates JF. False recognition and the right frontal lobe: A case study. Neuropsychologia. 1996;34:793–808. doi: 10.1016/0028-3932(95)00165-4. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Collette F, Philippot P, van der LM, Laureys S, Delfiore G, Degueldre C, Maquet P, Luxen A, Salmon E. Neural correlates of “hot” and “cold” emotional processing: A multilevel approach to the functional anatomy of emotion. Neuroimage. 2003;18:938–949. doi: 10.1016/s1053-8119(03)00009-0. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1996;351:1405–14 11. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Siegle GJ. A neural network model of attention biases in depression. Progress in Brain Research. 1999;121:415–441. doi: 10.1016/s0079-6123(08)63085-x. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr., Drevets WC, Synder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences, U.S.A. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anxiety inventory (Form Y) Mindgarden; Redwood City, CA: 1983. [Google Scholar]
- Tomarken AJ, Davidson RJ. Frontal brain activation in repressors and nonrepressors. Journal of Abnormal Psychology. 1994;103:149–153. doi: 10.1037//0021-843x.103.2.339. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wegner DM. Ironic processes of mental control. Psychological Review. 1994;101:34–52. doi: 10.1037/0033-295x.101.1.34. [DOI] [PubMed] [Google Scholar]
- Wenzlaff RM, Wegner DM. Thought suppression. Annual Review of Psychology. 2000;51:59–9 1. doi: 10.1146/annurev.psych.51.1.59. [DOI] [PubMed] [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, Heatherton TF. Neural correlates of thought suppression. Neuropsychologia. 2003;41:1863–1867. doi: 10.1016/j.neuropsychologia.2003.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


