SUMMARY
Reactive oxygen species (ROS) have been implicated in the pathogenesis of a variety of diseases, and antioxidant treatment is currently being investigated as a potential therapy to attenuate the detrimental effects of ROS-mediated oxidative stress. Melatonin is a potent naturally produced antioxidant, which acts through various mechanisms to ameliorate the toxic effects of ROS. However, little is known about the mechanisms or signaling pathways through which melatonin acts to reverse the effects of ROS. In the present study, the effect of melatonin treatment on the hydrogen peroxide (H2O2)-induced activation of the mitogen-activated protein kinase (MAPK) and mammalian target of rapamycin (mTOR) signaling pathways was assessed in H4IIE hepatoma cells. It was found that melatonin strongly attenuated H2O2-induced activation of the ERK1/2 and p38 MAP kinases, as well as several of their downstream targets. Melatonin also attenuated the H2O2-induced phosphorylation of Akt and the Akt substrate mTOR, as well as a downstream target of mTOR action, 4E-BP1. Upregulation of ERK1/2, p38, and Akt signaling by H2O2 was accompanied by activation of Ras, an effect that was blocked by melatonin. Overall, the results suggest that melatonin acts to prevent many of the H2O2-induced alterations in the MAPK and mTOR signaling pathways through inhibition of Ras, at least in H4IIE hepatoma cells.
Keywords: p38 MAP kinase, ERK1/2, Akt, mTOR, reactive oxygen species
INTRODUCTION
Reactive oxygen species (ROS) are a natural by-product of oxidative energy metabolism and are thought to be an important physiological modulator of a number of intracellular signaling pathways [1]. For example, ROS are generated in cells following treatment with cytokines [e.g. tumor necrosis factor (TNF)α] [2, 3] or growth factors [e.g. platelet-derived growth factor (PDGF)] [2, 4, 5]. However, although generation of ROS is important in normal cellular function, their overproduction can be deleterious to cell survival. Depending upon the severity of the oxidative insult, the consequence of these modifications can vary from altered cell function to cell death. Thus, oxidative stress has been implicated as a causative factor in the etiology of diseases as diverse as Alzheimer’s [6], diabetic neuropathy [7], sepsis [8], and cancer [9, 10].
Normally, the production of oxidant molecules like ROS is counterbalanced by antioxidants such as glutathione and vitamins C and E as well as by enzymes such as catalase, superoxide dismutase, and glutathione peroxidase that convert ROS to less damaging molecules [reviewed in 7]. Indeed, dietary supplementation with antioxidants such as vitamins C and E has been suggested as a potential therapy for diseases such as diabetic neuropathy [7]. Melatonin (N-acetyl-5-methoxytryptamine) is a particularly effective endogenous antioxidant that is produced mainly by the pineal gland [11], and has an important role in controlling circadian rhythms [12]. However, a variety of studies have shown that the indoleamine is also a potent antioxidant and counteracts the deleterious effects of oxidants such as ROS and reactive nitrogen species [reviewed in 13]. In the clinic, melatonin has been used to counteract the oxidative damage associated with oxygen delivery to preterm newborns [14, 15].
One reason melatonin is such an effective antioxidant is that it does not act through a single mechanism, but instead functions in a multifactorial manner to counteract oxidative stress. For example, melatonin acts as a direct scavenger of free radicals, it induces enzymes involved in the metabolism of free radicals, and it attenuates free radical production by mitochondria [13, 16-19]. Melatonin also has been shown to protect cells from oxidative stress and apoptosis induced by mitochondrial DNA deletion [20]. In addition, several melatonin metabolites (e.g. cyclic 3-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine and N-acetyl-5-methoxy-kynuramine) also act as reducing agents [21-23]. Therefore, many of the antioxidant properties of melatonin are preserved during its metabolism. However, not all of the antioxidant functions of melatonin are observed at physiological concentrations. Indeed, pharmacological doses of the hormone are often required to produce a maximal antioxidant effect and to ameliorate the damage induced by oxidative stress [24].
Besides exhibiting antioxidant properties, melatonin in several studies has been shown to play an important role in maintaining cellular homeostasis in response to a variety of cellular stresses. For example, melatonin inhibits apoptosis in immune cells [25] and prevents neuronal cell death [26]. In addition, in experimental colitis melatonin reduces TNFα expression and suppresses the phosphorylation of transcription factors, such as NF-κB, Jun, and Fas [27]. In other studies, melatonin has been shown to reduce cell proliferation [28, 29] and to exhibit an anti-proliferative effect in cancer [30, 31]. Whether or not such effects are due to the antioxidant properties of the hormone is unknown.
Although a number of studies have investigated the beneficial effects of melatonin on cell growth and survival, few have examined the mechanism(s) through which the indoleamine acts to attenuate oxidative stress-induced alterations in intracellular signaling pathways. Therefore, in the present study, the effect of melatonin treatment on the hydrogen peroxide (H2O2)-induced activation of the mitogen activated protein kinases (MAPK), extracellular signal regulated protein kinases (ERK)1/2 and p38 kinase, and the mammalian target of rapamycin (mTOR signaling pathway was examined. The results show that in H4IIE hepatoma cells, H2O2 treatment induces a rapid increase in phosphorylation of both the p38 and ERK1/2 MAP kinases as well as Akt. Pretreatment with melatonin dramatically attenuates the H2O2-induced phosphorylation of p38, Akt, and ERK2, but not ERK1. Melatonin also represses the H2O2-induced phosphorylation of downstream targets of p38 and ERK1/2 action such as p90RSK, eukaryotic initiation factor (eIF)4E, and the ribosomal protein (rp)S6 kinase S6K1, as well as downstream targets of Akt action including mTOR and the mTOR substrate, eIF4E binding protein 1 (4E-BP1). All of theses changes occur concomitantly with alterations in Ras activation. Overall, the results suggest that H2O2 activates the MAPK and mTOR signaling pathways through activation of Ras, and that melatonin attenuates H2O2-induced changes in these signaling pathways by preventing Ras activation.
MATERIAL AND METHODS
Melatonin was purchased from Sigma Chemical Co. (St. Louis, MO). Hydrogen peroxide was purchased from VWR International (West Chester, PA). Dulbecco’s modified Eagles’s medium (DMEM) was purchased from Gibco/Invitrogen (Carlsbad, CA) and the EZ-Detect Ras Activation Kit was from Pierce Biotechnology (Rockford, IL). PD98059 was obtained from Calbiochem/EMD Biosciences (La Jolla, CA) and UO126 from Promega Corp. (Madison, WI). Antibodies recognizing phospho-p38(Thr-180/Tyr-182), total p38, phospho-ERK1/2(Thr-202/Tyr-204), total ERK1/2, phospho-eIF4E(Ser-209), total eIF4E, phospho-p90RSK(Ser-380), total p90RSK, phospho-mTOR(Ser2448), phospho-rpS6(Ser-240/244), and phospho-S6K1(Thr-421/Ser-424), were purchased from Cell Signaling Technology (Beverly, MA). Anti-mTOR antibody was obtained from Bethyl Laboratories (Montgomery, TX). Horseradish peroxidase-labeled goat anti-rabbit-IgG and enhanced chemiluminescence (ECL) reagents were purchased from GE HealthCare Life Sciences (Fairfield, CT).
Cell culture
H4IIE rat hepatoma cells were grown in DMEM with l-glutamine containing 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) in a humidified atmosphere of 5% CO2 in air at 37°C. Cells were seeded in 6 well plates at 1 × 106 cells/well, a concentration that allowed them to reach 90% confluence within 48 hours. The plates were divided into 4 groups (each group had three replicates). On the day of the study, the first two groups received fresh DMEM without melatonin and the second two groups received DMEM containing melatonin at various concentrations as described in the figure legends. One hour after changing the culture medium, H2O2 was added to the second and third groups at various concentrations as described in the figure legends, and cells were harvested 1 hour later.
Western blot analysis
Cells were harvested by scraping in 1X SDS sample buffer and prepared for Western blot analysis by boiling for 5 min followed by centrifugation at 10,000 x g for 5 min. Total cell lysate containing 150 μg protein was resolved by electrophoresis on a 12.5% SDS polyacrylamide gel for p38, eIF4E, rpS6, ERK1/2, and Ras or a 7.5% SDS polyacrylamide gel for S6K1 and p90RSK. Phosphorylation was assessed using antibodies that specifically recognize the phosphorylated forms of the individual proteins as described previously [32]. Values for phosphorylated p38, eIF4E, rpS6, ERK1/2, mTOR, and p90RSK were expressed relative to the total amount of the respective protein.
Measurement of Ras activation
Ras activation was assessed using a Pierce EZ-Detect Ras Activation Kit according to the manufacturer’s instructions. Briefly, H4IIE cells were lysed by scraping in lysis buffer. Cell lysate containing 0.75 mg of protein was incubated in a spin column with 80 μg of GST-Raf1-RBD (the Ras binding domain of Raf1 coupled to GST) and SwellGel Immobilized Glutathione for 1 hr at 4 °C. The column was centrifuged at 7200 x g for 30 sec and the gel was washed 3 times with 400 μl lysis buffer by resuspension followed by centrifugation. After the last centrifugation, 50 μl of SDS sample buffer was added to the resin and the column was boiled for three min. The column was centrifuged a final time and the eluate was collected and resolved by electrophoresis on a 12.5% SDS polyacrylamide gel followed by Western blot analysis as described above.
Statistical Analysis
Within each study, three dishes of cells were individually analyzed and each study was repeated at least three times. Statistical differences among treatments were assessed using one way ANOVA with Tukey’s Multiple Comparison Test (Instat, GraphPad Software, San Diego, CA, USA). Statistical significance was set at p < 0.05.
RESULTS
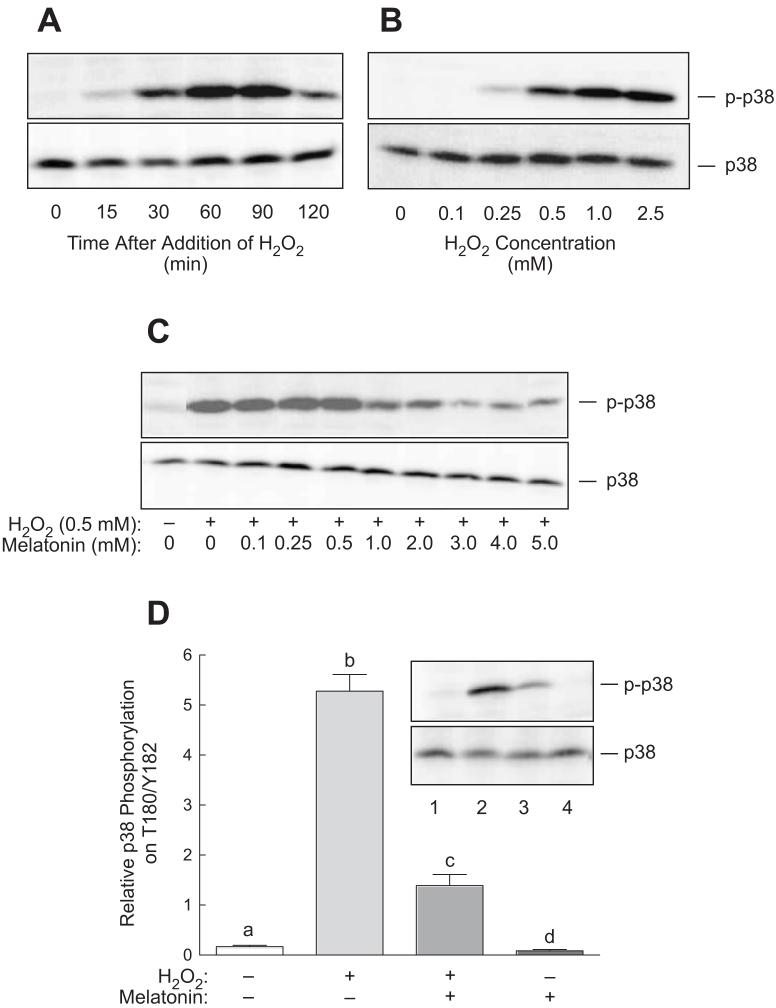
Activation of p38 is a characteristic response to oxidative stress observed in many studies [33]. Therefore, phosphorylation of p38 on Thr189/Tyr182 was used as an indicator of oxidative stress in preliminary experiments designed to establish the optimal exposure time and concentrations of H2O2 and melatonin to be used in the remainder of the studies. As shown in Fig. 1A, p38 phosphorylation was detectable as early as 15 min and was maximal 60 min after addition of H2O2 to the culture medium. Moreover, p38 phosphorylation was increased in cells exposed to 0.25 mM H2O2, and maximally increased at 0.5-1.0 mM (Fig. 1B). To establish the optimal concentration of melatonin needed to reverse H2O2-induced oxidative stress, cells were exposed to 0.5 mM H2O2 in the absence or presence of various concentrations of the indoleamine and p38 phosphorylation was assessed. As shown in Fig. 1C, H2O2-induced p38 phosphorylation was maximally suppressed by 3 mM melatonin. Therefore, in the remainder of the studies, cells were exposed to either 0.5 mM H2O2, 3 mM melatonin, or both for 60 min. Using this protocol, p38 phosphorylation was reproducibly increased over 25-fold, an effect that was severely attenuated in the presence of melatonin (Fig. 1D).
Fig. 1.
Phosphorylation of p38 at Thr180/Tyr182 in response to H2O2 in H4IIE cells. (A) H4IIE cells were incubated with 1 mM H2O2 for the indicated time periods and phosphorylation of p38 was assessed by Western blot analysis as described under “Materials and Methods”. (B) Cells were stimulated for 60 min with H2O2 at the concentrations indicated in the figure and p38 phosphorylation was assessed by Western blot analysis. (C) Cells were pre-incubated with 3 mM melatonin at the indicated concentrations. Sixty minutes later, the cells were stimulated with 0.5 mM H2O2 for 60 min in the continued presence of melatonin, and p38 phosphorylation was assessed by Western blot analysis. (D) Cells were incubated for 60 min in the presence or absence of 3 mM melatonin and H2O2 was then added to some of the dishes as indicated in the figure. Sixty min later, cells were harvested for determination of p38 phosphorylation. A representative Western blot is shown in the inset. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. In the graph in panel D, bars not sharing the same letter are significantly different, p<0.05.
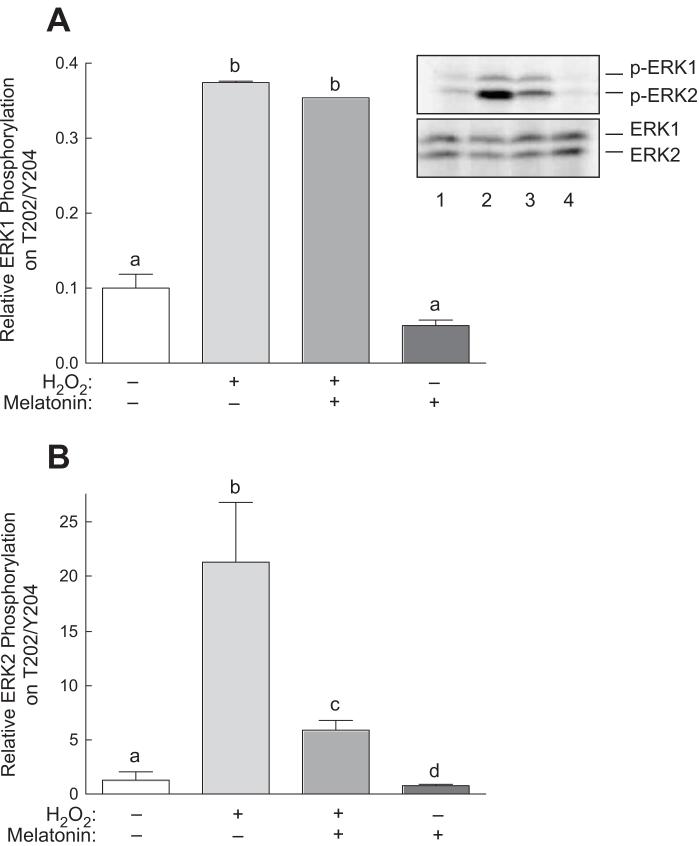
In addition to activating p38, previous studies have shown that oxidative stress promotes activation of the ERK1/2 signaling pathway [33]. As shown in Fig. 2, treatment with H2O2 resulted in a 3.5-fold increase in ERK1 phosphorylation and a 20-fold increase in ERK2 phosphorylation. Melatonin alone had no significant effect on phosphorylation of either ERK1 or ERK2 and had no detectable effect on H2O2-induced ERK1 phosphorylation. However, melatonin dramatically repressed the increase in ERK2 phosphorylation observed in cells treated with H2O2.
Fig. 2.
Phosphorylation of ERK1/2 at Thr202/Tyr204 in response to H2O2 and/or melatonin. H4IIE cells were incubated with or without 3 mM melatonin for 60 min, and one-half of the dishes then received 0.5 mM H2O2. Sixty min later, phosphorylation of (A) ERK1 and (B) ERK2 was assessed by Western blot analysis as described under “Materials and Methods”. Representative blots are shown in panel A. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. Bars not sharing the same letter are significantly different, p<0.05.
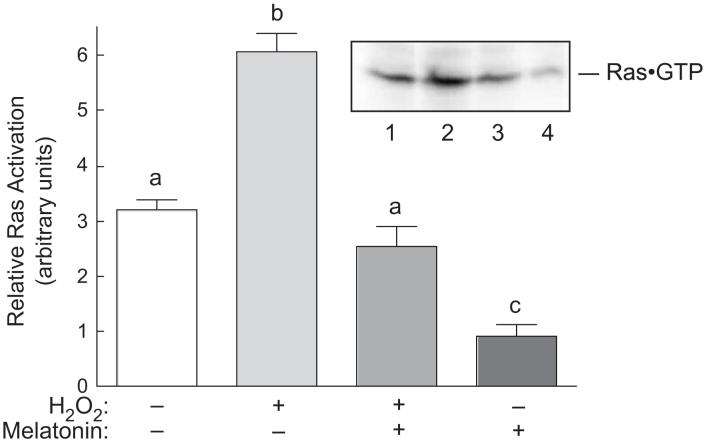
A previous report [34] showed that oxidative stress promotes assembly of a glutathione·Ras complex through formation of a disulfide bond between the peptide and the protein. Assembly of the complex results in Ras activation, which subsequently leads to upregulated signaling through MAPK pathways. In the present study, Ras activation was assessed by measuring the proportion of the protein present in the active, GTP-bound form. It was found that approximately twice as much Ras was associated with GTP in cells exposed to H2O2 as in control cells (Fig. 3). Melatonin alone reduced GTP association with Ras, and prevented completely the H2O2-induced increase in Ras·GTP association. Thus, changes in GTP binding to Ras correlate well with alterations in p38 and ERK2, but not ERK1, phosphorylation.
Fig. 3.
Ras activation in response to H2O2 and/or melatonin in H4IIE cells. Cells were treated as described in the legend to Fig. 2, lysed, and ras·GTP complexes were isolated from cell homogenates as described under “Materials and Methods”. The amount of ras present in the isolates was measured by Western blot analysis. A representative blot is shown in the inset. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. Bars not sharing the same letter are significantly different, p<0.05.
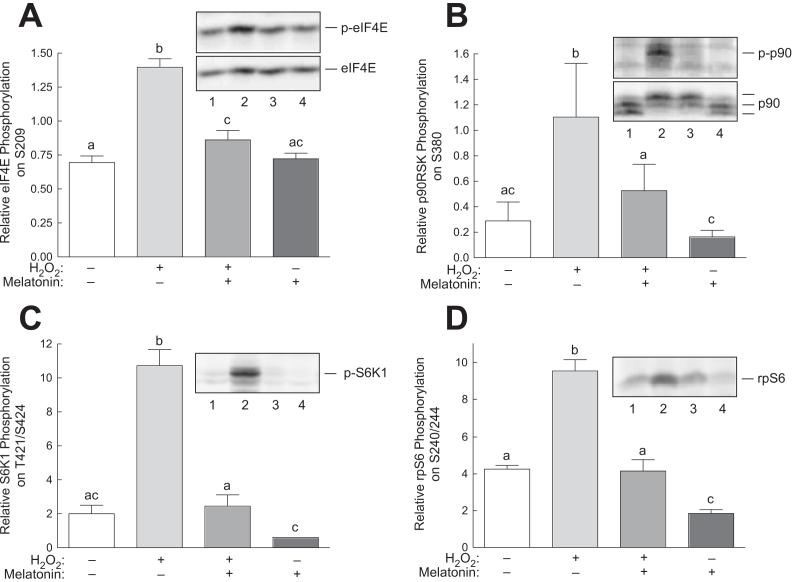
To assess potential consequences of H2O2-induced p38 and ERK activation, changes in phosphorylation eIF4E, p90RSK, and S6K1, previously identified downstream targets of one or both of the kinases, were examined. As shown in Fig. 4A changes in phosphorylation of eIF4E, a downstream target of both p38 and ERK [33], was increased in cells incubated with H2O2 and melatonin blocked the H2O2-induced increase. Moreover, phosphorylation of the ERK substrates, p90RSK and S6K1, paralleled H2O2- and melatonin-induced changes in ERK2 phosphorylation (Fig. 4B and 4C, respectively). To confirm that changes in S6K1 phosphorylation resulted in altered kinase activity, phosphorylation of the S6K1 substrate, rpS6 on Ser240/244 was assessed. As shown in Fig. 4D, H2O2- and melatonin-induced changes in rpS6 phosphorylation closely mirrored alterations in S6K1 phosphorylation.
Fig. 4.
Phosphorylation of downstream targets of ERK1/2 and p38 in response to H2O2 in H4IIE cells. H4IIE cells were incubated with or without 3 mM melatonin for 60 min, and one-half of the dishes then received 0.5 mM H2O2. Sixty min later, phosphorylation of (A) eIF4E, (B) p90RSK, (C) S6K1, and (D) rpS6 was assessed by Western blot analysis as described under “Materials and Methods”. Representative blots are shown as insets to the panels. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. Bars not sharing the same letter are significantly different, p<0.05.
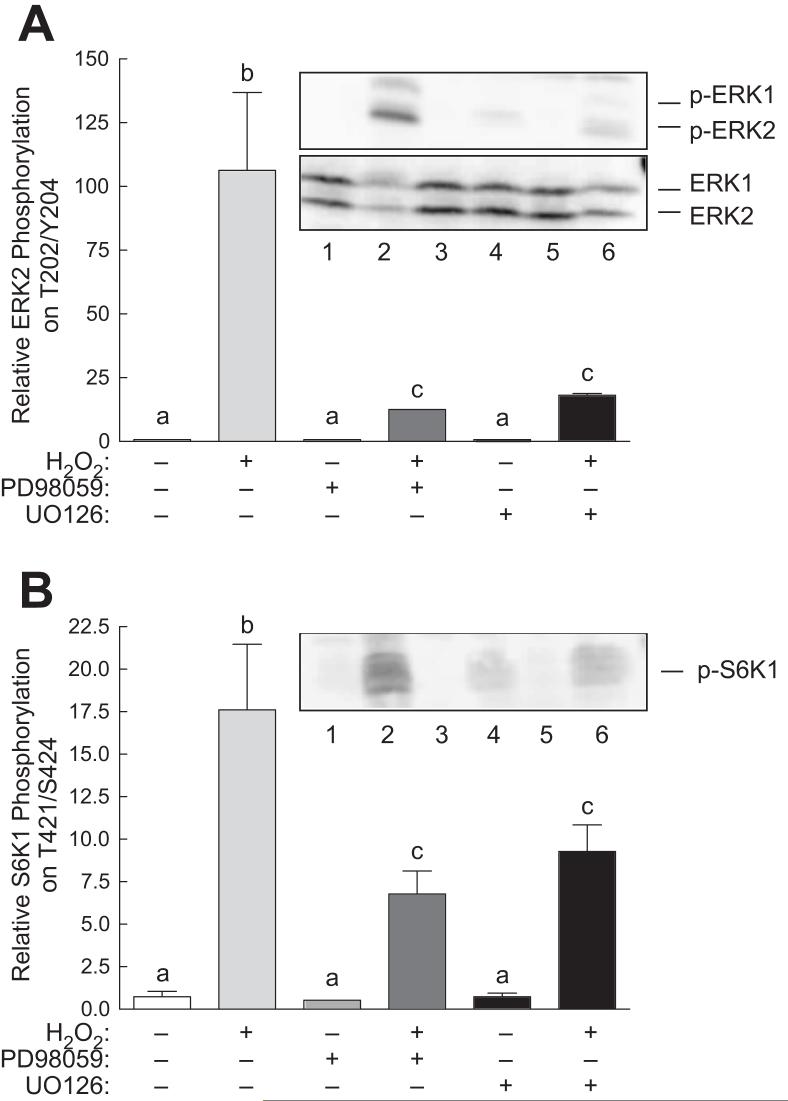
Previous studies have reported that phosphorylation of S6K1 on Thr421/Ser424 is inhibited by PD98059 [35], suggesting that phosphorylation of the residues can occur through an ERK-dependent mechanism. To define the role of the ERK1/2 signaling pathway in the H2O2-induced changes in S6K1 phosphorylation, cells were treated with two structurally distinct MEK inhibitors, PD98059 and U0126, prior to exposure to H2O2. Because ERK2 phosphorylation was already low, neither PD98059 nor U0126 had any detectable effect on its phosphorylation in control cells (Fig. 5A). Similarly, neither inhibitor had any significant effect on S6K1 phosphorylation in control cells (Fig. 5B). However, both inhibitors significantly attenuated the increase in ERK2 and S6K1 phosphorylation associated with H2O2 treatment.
Fig. 5.
Inhibitors of ERK1/2 attenuate H2O2-induced ERK2 and S6K1 phosphorylation in H4IIE cells. Cells were pre-treated with 10 μM PD98059 or 10 μM UO126 for 15 min before addition of H2O2. Sixty min later, the cells were lysed and phosphorylation of (A) ERK2 and (B) S6K1 was assessed by Western blot analysis as described under “Materials and Methods”. Representative blots are shown as insets to each panel. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. Bars not sharing the same letter are significantly different, p<0.05.
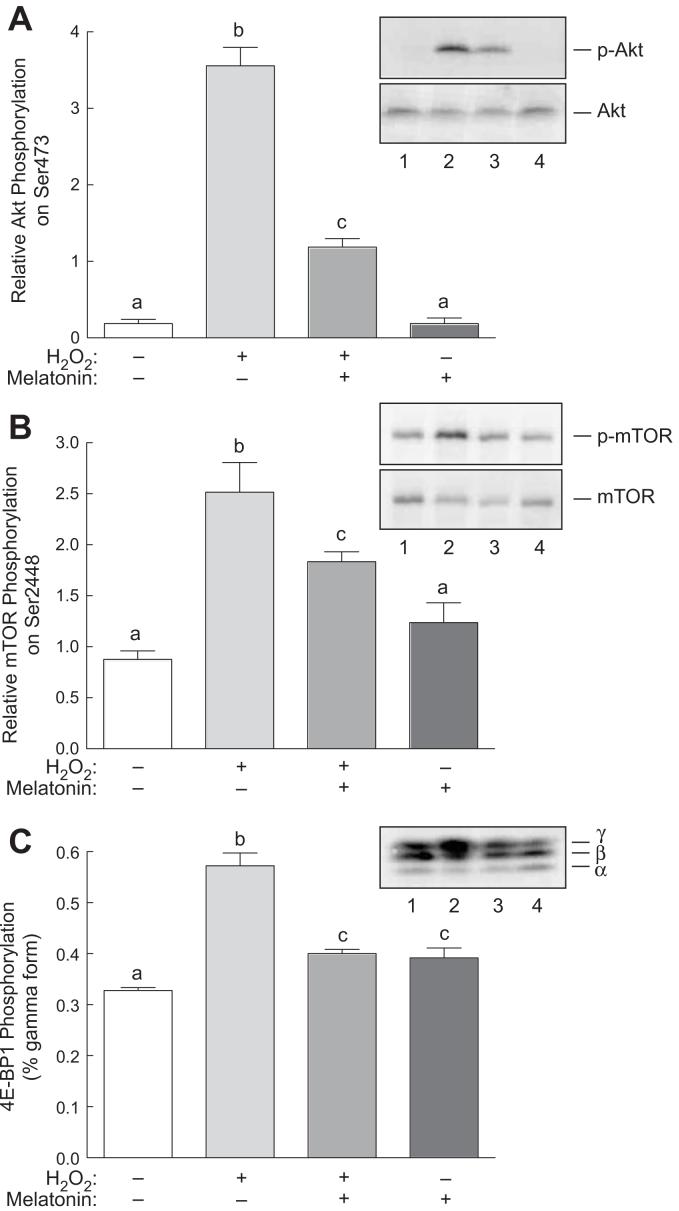
Previous studies have shown that Ras activates not only the ERK1/2 signaling pathway, but also the pathway downstream of phosphoinositide 3-kinase (PI-3K) [36]. Therefore, to assess possible effects of melatonin on H2O2-induced activation of the PI-3K pathway, changes in phosphorylation of Akt and a downstream target of the pathway, mTOR, were examined. As shown in Fig. 6A, phosphorylation of Akt was significantly increased in H2O2-treated compared to control cells. Melatonin alone had little or no significant effect on phosphorylation of Akt, but attenuated the H2O2-induced increase in phosphorylation. To assess whether or not the observed changes in Akt phosphorylation were sufficient to alter Akt activity, phosphorylation of mTOR on Ser2448, a residue directly phosphorylated by Akt [37, 38], was examined. As shown in Fig. 6B, phosphorylation of mTOR on Ser2448 was induced by H2O2 and melatonin attenuated the induction. Moreover, phosphorylation of the mTOR substrate 4E-BP1 was similarly induced by H2O2, an event that was reversed by melatonin. Thus, in addition to attenuating H2O2-induced activation of the p38 and ERK1/2 signaling pathways, melatonin largely abates H2O2-induced signaling through the Akt signaling pathway.
Fig. 6.
Phosphorylation of Akt and 4E-BP1 in response to H2O2 and/or melatonin. H4IIE cells were incubated with or without 3 mM melatonin for 60 min, and one-half of the dishes then received 0.5 mM H2O2. Sixty min later, phosphorylation of (A) Akt, (B) mTOR, and (C) 4E-BP1 was assessed by Western blot analysis as described under “Materials and Methods”. Representative blots are shown as insets to the panels. Lane 1, control cells; lane 2, cells incubated for 60 min with 0.5 mM H2O2; lane 3, cells incubated for 60 min with 3 mM melatonin followed by a 60 min incubation with both melatonin and H2O2; lane 4, cells incubated for 2 h with 3 mM melatonin. Bars not sharing the same letter are significantly different, p<0.05.
DISCUSSION
ROS have been shown to exhibit mitogenic effects at low concentrations and act as second messengers that stimulate various intracellular signaling pathways [39]. Conversely, when produced in excess, ROS have harmful effects that impede normal cellular functions [40]. As shown previously in other cell types [33], in the present study, the MAP kinases ERK1/2 and p38 were activated in response to oxidative stress. Thus, H2O2, a precursor for oxygen-derived free radicals, promoted the phosphorylation of both ERK1/2 and p38. The mechanism through which oxidative stress activates the MAPK signaling pathways is unclear, but may involve activation of Ras. Thus, in the present study, the proportion of Ras in the active GTP-bound form was greater in cells treated with H2O2 compared to control cells. Although not examined in the present study, previous studies suggest that Ras may be directly affected by oxidative stress. For example, Adachi et al. [34] showed that induction of ROS production in rat vascular smooth muscle cells leads to increased disulfide bond formation between glutathione and multiple Cys residues in Ras including Cys118 in the GTP binding domain. Exogenous expression of a Ras variant in which Cys118 is changed to Ser prevents ROS-mediated activation of p38 and Akt, but does not prevent ERK1/2 activation. A caveat to those findings is that under the conditions used in that study, ERK1/2 may have been activated not only through Ras/MEK/ERK signaling, but also through other pathways [34]. Interestingly, in the present study, melatonin not only prevented the H2O2-induced activation of Ras, it also significantly decreased the amount of Ras associated with GTP in control cells. This finding may suggest that basal Ras activation is in part modulated through a redox-dependent mechanism.
In the present study, phosphorylation of S6K1 on Thr421/Ser424 was increased five-fold in cells treated with H2O2, an effect that was completely reversed by melatonin. Previous studies have shown that phosphorylation of these residues in response to various inducers [e.g. interleukin-6 [41] or ultraviolet radiation [42]] is blocked completely by inhibition of the ERK1/2 signaling pathway. However, in the present study, two distinct inhibitors of MEK, PD98059 and UO126, attenuated, but did not prevent the H2O2-induced S6K1 phosphorylation, suggesting that the ERK1/2 pathway contributed, but was not the sole determinant of increased S6K1 phosphorylation. Phosphorylation of S6K1 is also regulated through the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt pathway. Activated Akt phosphorylates, and thereby inhibits, the GTPase activating protein, TSC2 (a.k.a. Tuberin), that acts to increase the GTPase activity of the Ras homolog enriched in brain (Rheb) [43-46]. Binding of the Rheb·GTP, but not the Rheb·GDP, binary complex to the protein kinase referred to as the mammalian target of rapamycin (mTOR), results in mTOR activation and subsequent phosphorylation of downstream targets such as S6K1 and eukaryotic initiation factor (eIF)4E binding protein (4E-BP)1 [47, 48]. Previous studies have shown that increased Rheb·mTOR signaling increases the sensitivity of Drosophila to oxidative stress [49]. Thus, low-level exogenous expression of Rheb or mTOR dramatically increases the sensitivity of flies to oxidative stress. Importantly, S6K1 is required for the Rheb/mTOR-induced increase in sensitivity. However, previous studies have shown that the H2O2-induced phosphorylation of S6K1 on Thr421/Ser424 is attenuated, but not prevented by either the PI 3-kinase inhibitor, wortmannin, or the mTOR inhibitor, rapamycin [50], suggesting that, similar to the ERK1/2 pathway, the Akt/mTOR signaling pathway contributes to, but is not the sole determinant of S6K1 phosphorylation. Thus, in the present study it is likely that H2O2-induced signaling through both the ERK1/2 and Akt/mTOR pathways is involved in the observed changes in S6K1 phosphorylation.
In the present study, a pharmacological dose of melatonin (3 mM) was used to inhibit the effects of H2O2-induced oxidative stress. Reiter et al. [51] reported that the antioxidant properties of melatonin manifest at pharmacological, rather than physiological, doses. Further, Reiter et al. [24] showed that for exogenously administered melatonin to have antioxidant effects, the indoleamine has to be provided at a dose that increases the blood concentration up to 100,000-fold above the physiological dose. These results suggest that many of the rapid antioxidant properties of melatonin are unrelated to its function as a hormone.
It should be noted that in the present study, the effect of melatonin on H2O2-induced oxidative stress was examined in H4IIE hepatoma cells. Results from a variety of studies [52] suggest that the response of transformed cells to melatonin may be different compared to non-transformed cells. For example, in contrast to the anti-apoptotic effect melatonin exhibits in non-transformed cells, the hormone often acts to enhance apoptosis in cancer cells [52]. Moreover, melatonin may act in concert with anti-tumor drugs to decrease the growth and proliferation of cancer cells. Thus, the effect of melatonin in normal cells may differ from those presented herein.
Overall, the results of the present study suggest that H2O2-induced oxidative stress upregulates phosphorylation of the ERK1/2 and p38 MAPK family of protein kinases through a ras-raf-MEK-dependent pathway. Activated ERK1/2 promotes phosphorylation of p90RSK and S6K1 and its downstream target, rpS6. In addition, activated ERK1/2 and p38 promote the phosphorylation of eIF4E. H2O2-induced oxidative stress also upregulates phosphorylation of Akt, its downstream substrate mTOR, and the mTOR substrate 4E-BP1, probably through a ras-PI3K-mediated mechanism. Melatonin, alone or in combination with H2O2-induced oxidative stress, suppresses the activation of ras, which results in the downregulation of all the changes induced by oxidative stress. Overall, the results demonstrate that melatonin is an efficient agent in neutralizing the effects of oxidative stress and modulating changes in the MAPK and mTOR signal pathways that are induced in response to H2O2, at least in H4IIE hepatoma cells.
ACKNOWLEDGEMENTS
The authors would like to thank Lydia Kutzler for technical assistance. This project was supported by National Institutes of Health grant DK-13499.
REFERENCES
- 1.LANDER HM. An essential role for free radicals and derived species in signal transduction. FASEB J. 1997;11:118–124. [PubMed] [Google Scholar]
- 2.LO YYC, CRUZ TF. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J Biol Chem. 1995;270:11727–11730. doi: 10.1074/jbc.270.20.11727. [DOI] [PubMed] [Google Scholar]
- 3.MEIER B, RADEKE HH, SELLE S, et al. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FINLAY GA, THANNICKAL VJ, FANBURG BL, et al. Platelet-derived growth factor-induced p42/44 mitogen-activated protein kinase activation and cellular growth is mediated by reactive oxygen species in the absence of TSC2/Tuberin. Cancer Res. 2005;65:10881–10890. doi: 10.1158/0008-5472.CAN-05-1394. [DOI] [PubMed] [Google Scholar]
- 5.SUNDARESAN M, YU Z-X, FERRANS VJ, et al. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 6.SRINIVASAN V, PANDI-PERUMAL SR, CARDINALI DP, et al. Melatonin in Alzheimer’s disease and other neurodegenerative disorders. Behav Brain Funct. 2006;2:15. doi: 10.1186/1744-9081-2-15. doi: doi:10.1186/1744-9081-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.POP-BUSUI R, SIMA A, STEVENS M. Diabetic neuropathy and oxidative stress. Diab Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- 8.SAKAGUCHI S, FURUSAWA S. Oxidative stress and septic shock: metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Immunol Med Microbiol. 2006;47:167–177. doi: 10.1111/j.1574-695X.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 9.KLAUNIG JE, KAMENDULIS LM. The role of oxidative stress in carcinogenesis. Ann Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 10.SUBRAMANIAN P, MIRUNALINI S, DAKSHAYANI KB, et al. Prevention by melatonin of hepatocarcinogenesis in rats injected with N-nitrosodiethylamine. J Pineal Res. 2007;43:305–312. doi: 10.1111/j.1600-079X.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- 11.REITER RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocrin Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 12.KENNAWAY DJ, WRIGHT H. Melatonin and Circadian rhythms. Curr Topic Med Chem. 2002;2:199–209. doi: 10.2174/1568026023394380. [DOI] [PubMed] [Google Scholar]
- 13.REITER RJ, TAN DX, MAYO JC, et al. Melatonin as an antioxidant: biochemical mechanisms and pathophysiological implications in humans. Acta Biochim Pol. 2003;50:1129–1146. [PubMed] [Google Scholar]
- 14.GITTO EMD, REITER RPD, CORDARO SMD, et al. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: beneficial effects of melatonin. Am J Perinatol. 2004;21:209–216. doi: 10.1055/s-2004-828610. [DOI] [PubMed] [Google Scholar]
- 15.GITTO E, REITER RJ, AMODIO A, et al. Early indicators of chronic lung disease in preterm infants with respiratory distress syndrome and their inhibition by melatonin. J Pineal Res. 2004;36:250–255. doi: 10.1111/j.1600-079X.2004.00124.x. [DOI] [PubMed] [Google Scholar]
- 16.ALLEGRA M, REITER RJ, TAN DX, et al. The chemistry of melatonin’s interaction with reactive species. J Pineal Res. 2003;34:1–10. doi: 10.1034/j.1600-079x.2003.02112.x. [DOI] [PubMed] [Google Scholar]
- 17.MARTIN M, MACIAS M, ESCAMES G, et al. Melatonin-induced increased activity of the respiratory chain complexes I and IV can prevent mitochondrial damage induced by ruthenium red in vivo. J Pineal Res. 2000;28:242–248. doi: 10.1034/j.1600-079x.2000.280407.x. [DOI] [PubMed] [Google Scholar]
- 18.REITER RJ, TAN DX, MANCHESTER LC, et al. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 19.REITER RJ, TAN DX, GITTO E, et al. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Pol J Pharamacol. 2004;56:159–170. [PubMed] [Google Scholar]
- 20.JOU M-J, PENG T-I, YU P-Z, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 21.HARDELAND R, BACKHAUS C, FADAVI A, et al. N1-acetyl-5-methoxykynuramine contrasts with other tryptophan metabolites by a peculiar type of NO scavenging: cyclization to a cinnolinone prevents formation of unstable nitrosamines. J Pineal Res. 2007;43:104–105. doi: 10.1111/j.1600-079X.2007.00431.x. [DOI] [PubMed] [Google Scholar]
- 22.MANDA K, UENO M, ANZAI K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res. 2007;42:386–393. doi: 10.1111/j.1600-079X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- 23.TAN D-X, MANCHESTER LC, TERRON MP, et al. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 24.REITER RJ, TAN DX, MALDONADO MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res. 2005;39:215–216. doi: 10.1111/j.1600-079X.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 25.SAINZ RM, MAYO JC, REITER RJ, et al. Apoptosis in primary lymphoid organs with aging. Microsc Res Tech. 2003;62:524–539. doi: 10.1002/jemt.10414. [DOI] [PubMed] [Google Scholar]
- 26.ALVIRA D, TAJES M, VERDAGUER E, et al. Inhibition of the cdk5/p25 fragment formation may explain the antiapoptotic effects of melatonin in an experimental model of Parkinson’s disease. J Pineal Res. 2006;40:251–258. doi: 10.1111/j.1600-079X.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 27.MAZZON E, ESPOSITO E, CRISAFULLI C, et al. Melatonin modulates signal transduction pathways and apoptosis in experimental colitis. J Pineal Res. 2006;51:363–373. doi: 10.1111/j.1600-079X.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 28.SHIU SYW, XI SC, XU JN, et al. Inhibition of malignant trophoblastic cell proliferation in vitro and in vivo by melatonin. Life Sci. 2000;67:2059–2074. doi: 10.1016/s0024-3205(00)00792-x. [DOI] [PubMed] [Google Scholar]
- 29.SIU SWF, LAU KW, TAM PC, et al. Melatonin and prostate cancer cell proliferation: Interplay with castration, epidermal growth factor, and androgen sensitivity. Prostate. 2002;52:106–122. doi: 10.1002/pros.10098. [DOI] [PubMed] [Google Scholar]
- 30.XI SC, SIU SWF, FONG SW, et al. Inhibition of androgen-sensitive LNCaP prostate cancer growth in vivo by melatonin: Association of antiproliferative action of the pineal hormone with mt1 receptor protein expression. Prostate. 2001;46:52–61. doi: 10.1002/1097-0045(200101)46:1<52::aid-pros1008>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.SHIU SYW, LAW IC, LAU KW, et al. Melatonin slowed the early biochemical progression of hormone-refractory prostate cancer in a patient whose prostate tumor tissue expressed MT1 receptor subtype. J Pineal Res. 2003;35:177–182. doi: 10.1034/j.1600-079x.2003.00074.x. [DOI] [PubMed] [Google Scholar]
- 32.KIMBALL SR, JURASINSKI CV, LAWRENCE JCJ, et al. Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol. 1997;272:754–759. doi: 10.1152/ajpcell.1997.272.2.C754. [DOI] [PubMed] [Google Scholar]
- 33.MCCUBREY JA, LAHAIR MM, FRANKLIN RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 34.ADACHI T, PIMENTEL DR, HEIBECK T, et al. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem. 2004;279:29857–29862. doi: 10.1074/jbc.M313320200. [DOI] [PubMed] [Google Scholar]
- 35.HUANG C, LI J, KE Q, et al. Ultraviolet-induced phosphorylation of p70S6K at Thr389 and Thr421/Ser424 involves hydrogen peroxide and mammalian target of rapamycin but not Akt and atypical protein kinase C. Cancer Res. 2002;62:5689–5697. [PubMed] [Google Scholar]
- 36.RODRIGUEZ-VICIANA P, WARNE PH, DHAND R, et al. Phosphatidylinositol-3-OH kinase direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 37.NAVE BT, OUWENS DM, WITHERS DJ, et al. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 38.SEKULIC A, HUDSON CC, HOMME JL, et al. A direct linkage between the phosphoinositide 3-kinase-Akt signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3523. [PubMed] [Google Scholar]
- 39.AIKAWA R, KOMURO I, YAMAZAKI T, et al. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SINGH N, DHALLA AK, SENEVIRATNE C, et al. Oxidative stress and heart failure. Mol Cell Biochem. 1995;147:77–81. doi: 10.1007/BF00944786. [DOI] [PubMed] [Google Scholar]
- 41.SHI Y, HSU J-H, HU L, et al. Signal pathways involved in activation of p70S6K and phosphorylation of 4E-BP1 following exposure of multiple myeloma tumor cells to interleukin-6. J Biol Chem. 2002;277:15712–15720. doi: 10.1074/jbc.M200043200. [DOI] [PubMed] [Google Scholar]
- 42.ZHANG Y, DONG Z, NOMURA M, et al. Signal transduction pathways involved in phosphorylation and activation of p70S6K following exposure to UVA irradiation. J Biol Chem. 2001;276:20913–20923. doi: 10.1074/jbc.M009047200. [DOI] [PubMed] [Google Scholar]
- 43.INOKI K, LI Y, XU T, et al. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Develop. 2003;17:1829–1834. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.KWIATKOWSKI DJ, MANNING BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:R251–258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 45.LI Y, CORRADETTI MN, INOKI K, et al. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 46.LI Y, INOKI K, GUAN K-L. Biochemical and functional characterization of small GTPase Rheb and TSC2 GAP activity. Molec Cell Biol. 2004;24:7965–7975. doi: 10.1128/MCB.24.18.7965-7975.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LONG X, LIN Y, ORTIZ-VEGA S, et al. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 48.SMITH EM, FINN SG, TEE AR, et al. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 49.PATEL PH, TAMANOI F. Increased Rheb-TOR signaling enhances sensitivity of the whole organism to oxidative stress. J Cell Sci. 2006;119:4285–4292. doi: 10.1242/jcs.03199. [DOI] [PubMed] [Google Scholar]
- 50.TU VC, BAHL JJ, CHEN QM. Signals of oxidant-induced cardiomyocyte hypertrophy: Key activation of p70 S6 kinase-1 and phosphoinositide 3-kinase. J Pharmacol Exp Ther. 2002;300:1101–1110. doi: 10.1124/jpet.300.3.1101. [DOI] [PubMed] [Google Scholar]
- 51.KLATT P, LAMAS S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 52.SAINZ RM, MAYO JC, RODRIGUEZ C, et al. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Molec Life Sci. 2003;60:1407–1426. doi: 10.1007/s00018-003-2319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]