Abstract
UBE1L is the E1-like ubiquitin-activating enzyme for the interferon-stimulated gene, 15 KDa protein (ISG15). The UBE1L-ISG15 pathway was previously proposed to target lung carcinogenesis by inhibiting cyclin D1 expression. This study extends prior work by reporting UBE1L promotes a complex between ISG15 and cyclin D1 and inhibited cyclin D1, but not other G1 cyclins. Transfection of the UBE1L-ISG15 deconjugase, ubiquitin specific protein 18 (UBP43), antagonized UBE1L-dependent inhibition of cyclin D1 and ISG15-cyclin D1 conjugation. A lysine-less cyclin D1 species was resistant to these effects. UBE1L transfection reduced cyclin D1 protein, but not mRNA expression. Cycloheximide (CHX) treatment augmented this cyclin D1 protein instability. UBE1L knock-down increased cyclin D1 protein. UBE1L was independently retrovirally transduced into human bronchial epithelial (HBE) and lung cancer cells. This reduced cyclin D1 expression and clonal cell growth. Treatment with the retinoid X receptor (RXR) agonist bexarotene induced UBE1L and reduced cyclin D1 immunoblot expression. A proof of principle bexarotene clinical trial was independently examined for UBE1L, ISG15, cyclin D1 and Ki-67 immunohistochemical expression profiles in pre- versus post-treatment tumor biopsies. Increased UBE1L with reduced cyclin D1 and Ki-67 expression occurred in human lung cancer when a therapeutic bexarotene intratumoral level was achieved. Thus, a mechanism for UBE1L-mediated growth suppression was found by UBE1LISG15 preferentially inhibited cyclin D1. Molecular therapeutic implications are discussed.
Keywords: UBE1L, ISG15, cyclin D1, lung cancer, growth suppression
Introduction
Lung cancer is the leading cause of cancer-related mortality for men and women in the United States (1). Despite advances in chemotherapy, radiation therapy and surgery, only a minority of lung cancer patients are cured (1). Novel targets for lung cancer therapy and chemoprevention are needed. Prior work with classic and non-classic retinoid receptor agonists found G1 cyclins were pharmacologic targets for lung carcinogenesis (2–8). Aberrant expression of cyclin D1 and cyclin E in human preneoplastic and malignant lung lesions implicated these species as therapeutic or chemopreventive targets (9).
That aberrant cyclin expression caused lung carcinogenesis was found in transgenic mice with the human surfactant C promoter targeting cyclin E expression in the lung. This caused chromosome instability, hedgehog pathway activation, appearance of pulmonary dysplasia and multiple lung adenocarcinomas along with other changes that recapitulated features of clinical lung carcinogenesis (10). Together, these findings implicated cyclin deregulation as an early step in lung carcinogenesis and as an anti-neoplastic target. That view was supported by results of clinical proof of principle trials with the epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) erlotinib or the retinoid X receptor (RXR, rexinoid) agonist bexarotene where intratumoral repression of cyclin D1 was uncovered as a pharmacodynamic marker of anti-neoplastic response (11,12). Reduced cyclin D1 expression was also detected in post- versus pre-treatment buccal swabs following combined erlotinib and bexarotene treatments (13).
One cancer chemoprevention mechanism already identified involved induced proteasomal degradation of cyclin D1 and cyclin E by retinoids and rexinoids (3–8). This confers G1 arrest and permits repair of genomic DNA damage by carcinogens (2,3). Another mechanism engaged a previously unrecognized retinoid target gene, which inhibited cyclin D1 (14,15). Microarray analyses of all-trans-retinoic acid (RA) treated human bronchial epithelial (HBE) and acute promyelocytic leukemia (APL) cells revealed UBE1L (ubiquitin-activating enzyme E1-like) induction (14–16). UBE1L conjugates the interferon (IFN) stimulated gene, 15 kDa protein (ISG15), a member of the ubiquitin-like protein family, which is also retinoid induced (15). UBE1L is located near a chromosome 3 region deleted in lung cancers (17). UBE1L mRNA expression is often reduced in lung cancer cells, but its genomic structure is intact (18,19). Prior work established UBE1L as a retinoid target gene conferring PML/RARα repression in APL cells (14) and reduced cyclin D1 expression in HBE cells (16). These and other findings implicated UBE1L as a growth or tumor suppressive species.
This study was undertaken to uncover UBE1L-dependent mechanisms for lung cancer growth suppression. Findings reported here identify a complex between ISG15 and cyclin D1, which provides a basis for UBE1L-mediated inhibition of cyclin D1 protein, but not mRNA expression. UBE1L transduction suppressed cyclin D1 expression and growth of HBE and lung cancer cells. In contrast, UBE1L knockdown increased cyclin D1 expression. UBE1L confers growth suppression by preferentially targeting cyclin D1. This was confirmed by UBE1L transfection and treatment with cycloheximide (CHX), which increased cyclin D1 protein instability. To ascertain clinical relevancy, immunohistochemical expression profiles of UBE1L, ISG15, cyclin D1, and Ki-67 were each examined in a bexarotene proof of principle lung cancer trial. These and other findings highlight UBE1L-ISG15 as a distinct growth suppressive pathway exerting anti-neoplastic effects by targeting cyclin D1 for repression. Implications for cancer therapy and chemoprevention are discussed.
Materials and Methods
Cell Culture
BEAS-2B HBE cells were cultured in LHC-9 media (Biofluids, Rockville, MD) (2,3). The H358 lung cancer cell line was cultured in RPMI 1640 media (Invitrogen Corporation, Carlsbad, CA) containing L-glutamine, 10% fetal bovine serum and 1% antibiotic-antimycotic solution (Cellgro, Herndon, VA) (11). Cells were incubated at 37°C in a humidified incubator with 5% CO2. Bexarotene treatments of HBE and lung cancer cells were accomplished as previously described (12,13). Culture conditions for the murine ED-1 lung cancer cell line are described elsewhere (20).
Expression Plasmids and Transient Transfection
The pSG5-UBE1L expression vector was previously described (14). The pcDNA3-UbcH8 plasmid was obtained from Dr. Robert M. Krug (University of Texas, Austin, TX). His6-tagged pcDNA3-ISG15 and pcDNA3-His-UBP43 expression vectors were previously reported (15,21,22). The HA-tagged pRcCMV-cyclin D1 plasmid was provided by Dr. Steven Dowdy (Washington University School of Medicine). The lysine-less HA-tagged cyclin D1 species was previously described (23). Transient transfection of BEAS-2B cells was accomplished using Effectene transfection reagent (Qiagen, Valencia, CA) and optimized methods (23). Transfections of pSG5-UBE1L, insertless pSG5 vector or with small interfering RNAs (siRNAs) were accomplished using established techniques (16,20,22).
SiRNAs targeting UBE1L or a RISC-free control siRNA were synthesized (Dharmacon). Different siRNAs were designed to target UBE1L: UBE1L siRNA1 (5’- CGACAACTTTCTCCCGTTA-3’) and siRNA2 (5’-CCTCGGAGTTAGGGCGAAT-3’). Transfection efficiency was monitored by transfecting siGLO Green Transfection Indicator (Dharmacon, Lafayette, CO). Percent of cells transfected was assayed by flow cytometry.
Engineering Retroviral UBE1L Expression
MSCV-IRES-UBE1L-GFP retrovirus expressing human UBE1L has been reported (14). Five µg of MSCVIRES-UBE1L-GFP and an empty vector were independently transfected into the Phoenix Amphotropic packaging cell line (ATCC, Manassas, VA) using Fugene 6 (Roche, Indianapolis, IN), and the vendor’s recommended procedures. Forty-eight hours later, viral supernatants were harvested from respective transduced BEAS-2B HBE or H358 lung cancer cells with 4µg/ml polybrene (Sigma). Forty-eight hours later, cells positive for GFP expression were harvested using a FACStar Plus (Becton Dickinson, San Jose, CA) high speed sorting cytometer. This was repeated one week later to enrich for studied transductants, as in prior work (14).
Immunoblot Analyses
Cells were lysed with ice-cold radioimmunoprecipitation (RIPA) lysis buffer, using established techniques (15,16,22,23). Lysates were size-fractionated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) assays before transfer to nitrocellulose membranes (Schleicher and Schuell Bioscience, Inc., Keene, NH). A polyclonal antibody recognizing the UBE1L amino terminus of UBE1L (14’16) was used for immunoblot and immunohistochemical assays. Other primary antibodies for immunoblot assays included a rabbit polyclonal antibody recognizing cyclin D1 (M-20) (Santa Cruz Biotechnology, Santa Cruz, CA), a murine monoclonal antibody against hemagglutinin (HA)-tagged proteins (Babco, Richmond, CA) and a goat polyclonal antibody recognizing actin (Santa Cruz Biotechnology). Anti-mouse and anti-rabbit antisera were purchased (Amersham Biosciences, Piscataway, NJ) as was anti-goat antisera (Santa Cruz Biotechnology) and these were used as respective secondary antibodies. Membranes used for immunoblot analyses were treated with the MemCode™ reversible stain (Pierce, Rockford, IL). Treatment with the proteasome inhibitor ALLN was used (24). Quantification of signal intensities was scored as before (22–24). To assess cyclin D1 protein stability following UBE1L transfection, cells were treated with or without CHX (40µg/ml), as in prior work (23).
Immunoprecipitation and Pull-Down Assays
After BEAS-2B cells were transiently transfected with indicated expression vectors, transfectants were lysed with RIPA buffer for immnunoprecipitation or for Ni-NTA-agarose (Invitrogen) pull-down using optimized procedures (22). Anti-HA antibody (Santa Cruz Biotechnology) and protein A/G beads (Santa Cruz Biotechnology) were used. Ni-NTA-agarose pull-down assays were performed as described (22).
Translational Research Studies
Paraffin-embedded and formalin-fixed tissues were obtained from an Institutional Review Board-approved proof of principle bexarotene lung cancer trial (12). Tissues were examined for cyclin D1 (11–13),UBE1L (16), ISG15 (25), and Ki-67 (11–13) immunohistochemical expression profiles.
Clonal Growth Assays
Clonal growth assays (2) were performed using 5 × 102 BEAS-2B and 1 × 103 H358 cells. These cells were independently engineered to over-express UBE1L or a control vector. Colonies were treated with bexarotene or vehicle (dimethyl sulfoxide, DMSO) to determine dose-responsive effects. Two weeks later, visible colonies were fixed and stained with Diff Quik solution (Baxtor, McGaw Park, IL) and quantified with the Col Count instrument (Oxford Optronix, Oxford, UK).
Results
Our prior work implicated UBE1L as a molecular pharmacologic target inhibiting cyclin D1 (16). This provided a mechanism for the hypothesized tumor suppressive role of UBE1L (17–19,21,22). To determine whether UBE1L affected cyclin expression, BEAS-2B cells were co-transfected with UBE1L and independently with cyclin D1, cyclin D2, cyclin D3 or cyclin E. Only cyclin D1 was inhibited by UBE1L and actin expression was unaffected, as quantified in Figure 1A
Figure 1. Effects of UBE1L on individual co-transfected G1 cyclins in human bronchial epithelial (HBE) cells.
(A) Effects of UBE1L on individual transiently transfected cyclin D1, cyclin D2, cyclin D3, and cyclin E species. UBE1L transfection (+) or insertless control vector transfection (−) was accomplished as was immunoblot analyses, as in the Materials and Methods. Actin expression served as a loading control. Quantification of each signal is displayed. The percent (%) change in expression of each of these cyclins relative to actin expression is displayed. (B) Dose-dependent effects of UBP43 on exogenously expressed cyclin D1 are shown by “+” depicting 0.3µg of transfected UBP43 and “++” depicting 0.5µg of transfected UBP43 expression plasmid. Transfected UBP43 increased cyclin D1 relative to actin protein expression. Quantification of signals is provided. (C) Effects of UBP43 co-transfection on UBE1L-mediated inhibition of cyclin D1. UBP43 antagonized UBE1Lmediated inhibition of cyclin D1 while having no appreciable effect on actin expression. Quantification of signals is provided. (D) Effects of UBE1L transfection on transfected HA-tagged cyclin D1 immunoblot expression in the presence (+) or absence (−) of cycloheximide (CHX, 40µg/ml) treatment. UBE1L destabilized (versus actin control) exogenous cyclin D1 protein detected by an anti-HA antibody. This was enhanced by cycloheximide (CHX) treatment. Quantification of signals is provided.
Immunoblot experiments were conducted following transfection of UBP43, the enzyme leading to ISG15 deconjugation (26). Figure 1B examined dose-dependent effects in BEAS-2B cells of transient UBP43 transfection on cyclin D1 protein. Cyclin D1 expression increased as UBP43 transfection dosage increased. Quantifications appear in Figure 1B. UBE1L transfection inhibited cyclin D1 expression in BEAS-2B cells, but UBP43 co-transfection antagonized this effect in Figure 1C. Quantifications are displayed. To establish UBE1L affected cyclin D1 protein stability, UBE1L was co-transfected with HA-tagged cyclin D1 into BEAS-2B cells in the presence and absence of CHX. Figure 1D revealed UBE1L reduces exogenous cyclin D1 protein stability following CHX treatment. UBE1L transfection also reduced endogenous cyclin D1 protein, but not cyclin D1 mRNA expression in BEAS-2B cells (Supplemental Figures 1A and 1B).
It was hypothesized the ubiquitin-like protein ISG15 would complex with cyclin D1. BEAS-2B cells were transiently transfected with or without cyclin D1 and with or without UBE1L and ISG15 expression vectors, as in Figure 2. Lysates were subjected to immunoprecipitation before immunoblot analyses. Figure 2 revealed two major conjugatates of cyclin D1 (arrows) following co-transfection of UBE1L, ISG15 and cyclin D1. Transfection of UbcH8, the E2 enzyme for ISG15ylation (27) did not appreciably change this conjugation (data not shown), indicating endogenous UbcH8 expression was not limiting. Treatment with the proteasome inhibitor ALLN stabilized ISG15ylated species, as in Figures 2A and 2B. These ISG15-conjugated cyclin D1 species were reduced by UBP43 (28) co-transfection in Figures 2C and 2D.
Figure 2. Complex between ISG15 and cyclin D1 and the role of UBP43 in inhibiting this in BEAS-2B human bronchial epithelial (HBE) cells.
(A) Immunoprecipitation with an anti-HA antibody followed by immunoblot with a second anti-HA antibody revealed ISG15-tagged cyclin D1 complexes. The arrows depict positions of larger molecular weight cyclin D1 complexes with ISG15 and the slightly smaller molecular weight species in lanes 1, 5, and 6 represent a non-specific band. Co-transfection of UBE1L promoted ISG15 cyclin D1 complex formation. Treatment with the proteasome inhibitor, ALLN, stabilized these species. (B) Independent Ni-NTA pull-down of these tagged ISG15 species. The same sized species as in panel A were independently identified with this pull-down assay. Co-transfection with UBE1L and ISG15 expression vectors led to ISG15-cyclin D1 complexes. Arrows display positions of ISG15-cyclin D1 complexes. Cyclin D1-HA conjugates were identified with an anti-HA antibody. (C) UBE1L-dependent ISG15 complex formation with cyclin D1 was inhibited by transfection of the deconjugase, UBP43. Immunoprecipitation with an anti-HA antibody followed by immunoblot with a second anti-HA antibody revealed ISG15-tagged cyclin D1 complexes formed in HBE cells and were inhibited by UBP43 co-transfection. Arrows depict positions of ISG15-cyclin D1 complexes. (D) Results in panel C were independently confirmed in HBE cells by Ni-NTA pull-down of these tagged ISG15 species. Cyclin D1-HA conjugates were identified with an anti-HA antibody. Arrows depict positions of ISG15-cyclin D1 complexes. To confirm equal amounts of input protein lysates loaded, MemCode™ stained membrane filters were shown. Positions of molecular weight size markers (Kilodaltons, KDa) are displayed for each experiment.
Specific lysines in cyclin D1 affect cyclin D1 protein stability (23). Whether a lysine-less cyclin D1 species was resistant to UBE1L-mediated inhibition of cyclin D1 was studied and found in Figure 3A. This was an expected outcome since absence of lysine residues prevented ISG15ylation. To confirm this inhibition involved a complex between ISG15 and cyclin D1, transfected lysine-less cyclin D1 was immunoprecipitated with anti-HA or pulled-down with Ni-NTA, respectively, before immunoblotting with an anti-HA antibody. Figures 3B and 3C revealed UBE1L-inhibition of cyclin D1 depended on conjugation to lysine(s) within cyclin D1.
Figure 3. Effects of cyclin D1 lysine mutations on ISG15-cyclin D1 complex formation and consequences of UBE1L transfection on wild-type and lysine-less cyclin D1 species.
(A) Wild-type and lysine-less cyclin D1 species were independently transfected into BEAS-2B human bronchial epithelial (HBE) cells in the presence or absence of co-transfected UBE1L. As compared to effects on wild-type cyclin D1, UBE1L did not appreciably inhibit lysine-less cyclin D1 expression. Quantification of signals is provided. (B) ISG15-cyclin D1 complexes were detected in wild-type cyclin D1 species (arrows). Immunoprecipitation with an anti-HA antibody followed by immunoblot with a second anti-HA antibody revealed prominent ISG15-tagged complexes detected in HBE cells with transfected wild-type, but not lysine-less cyclin D1 species. (C) Results in panel B were confirmed in HBE cells by Ni-NTA pull-down of these tagged ISG15 species. Arrows depict positions of cyclin D1 species complexed with ISG15. ISG15 complex formation occurred with transfected wild-type, but not with lysine-less cyclin D1. In panels B and C, positions of molecular weight size markers are displayed (Kilodaltons, KDa). To confirm equal amounts of input protein loaded, MemCode™ stained membrane filters were provided.
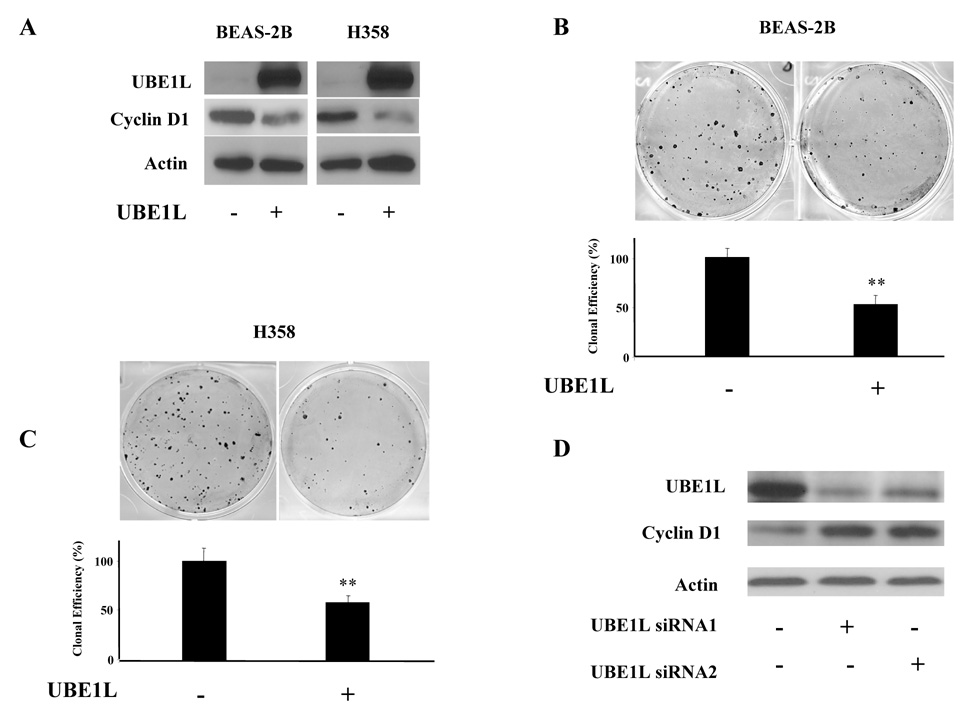
Effects of UBE1L expression on BEAS-2B HBE and H358 lung cancer cell growth were studied. Retroviral UBE1L expression was independently accomplished in BEAS-2B and H358 cells in Figure 4A. Exogenous UBE1L reduced endogenous cyclin D1 expression in both transduced cell lines (relative to controls). A limiting dilution clonal growth assay confirmed UBE1L over-expression conferred a marked repression of clonal growth. Representative experiments are displayed in Figures 4B and 4C. Standard deviation bars are provided for each transductant. Similar results were obtained in a replicate experiment (data not shown). BEAS-2B and H358 UBE1L transductants exhibited a significant repression (** depicts P < 0.001) of clonal growth versus insertless controls. Whether knock-down of UBE1L affected cyclin D1 expression, two independent siRNAs targeting UBE1L and a control siRNA were independently transfected into ED-1 murine lung cancer cells (20), which exhibit high basal UBE1L protein expression in Figure 4D Over 90% of these cells are transiently transfected (data not shown). Knock-down of UBE1L by these siRNAs increased cyclin D1 immunoblot expression in ED-1 cells in Figure 4D.
Figure 4. UBE1L transduction and UBE1L knock-down in BEAS-2B human bronchial epithelial (HBE), human H358 or murine ED-1 lung cancer cell lines.
(A) Independent immunoblot analyses in BEAS-2B and H358 UBE1L transductants (+) versus an insertless (−) retrovirus. Engineered UBE1L expression inhibited endogenous cyclin D1 expression. (B) UBE1L (+) reduced clonal growth (upper panel) in BEAS-2B HBE cells. Cloning efficiency relative to an insertless control vector (−) was quantified in the lower panel. A significant difference was depicted by the symbol “**” (P < 0.001). Standard deviation bars are shown. (C) UBE1L (+) reduced clonal growth in H358 cells. Cloning efficiency relative to an insertless control vector (−) was quantified in the lower panel using established techniques. A significant difference was depicted by the symbol “**” (P < 0.001). Standard deviation bars are shown. (D) Transient transfection of two independent UBE1L targeting (+) siRNAs relative to control (−) siRNA reduced UBE1L and increased cyclin D1 proteins in ED-1 cells.
Prior work revealed prolonged RA-treatment augmented UBE1L expression in BEAS-2B cells (16). Studies were undertaken to assess UBE1L expression after bexarotene treatments of BEAS-2B and H358 cells since bexarotene was also used in a proof of principle lung cancer trial (12). Bexarotene repressed cyclin D1 protein in BEAS-2B and H358 cells (12). Bexarotene (1µM) prominently increased UBE1L immunoblot expression following 10 days treatment of BEAS-2B cells versus vehicle treatment in Figure 5A. Similar findings were obtained in H358 cells (data not shown). β-actin expression served as loading control. Bexarotene treatment caused a dose-dependent decline of BEAS-2B clonal growth in Figure 5B.
Figure 5. Effects of bexarotene treatment on UBE1L expression and clonal growth of BEAS-2B human bronchial epithelial cells.
(A) In vitro treatment (+) of BEAS-2B cells with bexarotene (1µM) for 10 days. This increased UBE1L immunoblot expression versus vehicle treated (−) cells. Actin expression served as a loading control (B) Dose-dependent inhibition of BEAS-2B cell growth by bexarotene.
Prior work revealed cyclin D1 immunohistochemical expression declined when high bexarotene levels were measured in lung tumors (12). Whether this repression occurred with an increased UBE1L expression in bexarotene post- versus pretreatment lung cancer biopsies was studied. ISG15 immunohistochemical expression was similar in post- and pre-bexarotene treatment biopsies of the lung cancer cases in Figure 6. In contrast, cyclin D1 expression declined in post- versus pre-treatment biopsies when high bexarotene plasma (1.49µM) and intratumoral (0.31µM) levels were measured (12) as in Figure 6A. In this case, UBE1L immunohistochemical expression increased with bexarotene treatment and proliferation, as assessed by Ki-67 immunostaining, decreased in post- versus pretreatment biopsies.
Figure 6.
Pre- and post-bexarotene treatment biopsies of representative lung cancer cases accrued in a proof of principle trial where plasma and intratumoral bexarotene (panel A) levels were high or these bexarotene levels were low (panel B) (12). High bexarotene levels reduced cyclin D1 expression (12). Immunohistochemical assays for cyclin D1, UBE1L, and ISG15 were performed. (A) Bexarotene treatment (+) versus pre-treatment (−) had little effect on ISG15 expression, but bexarotene reduced cyclin D1 and Ki-67 while increasing UBE1L expression in this lung cancer case. (B) Bexarotene treatment did not augment UBE1L, decrease cyclin D1 and Ki-67, or appreciably change ISG15 immunohistochemical expression profiles in this lung cancer case.
Another representative case was examined. The case shown in Figure 6B had low plasma (0.13µM) and intratumoral (0.09µM) bexarotene levels (12). UBE1L and cyclin D1 immunohistochemical expression profiles were not appreciably altered by bexarotene treatment. Repression of Ki-67 immunostaining was not observed. A total of 5 cases were examined with only 1 having high intratumoral bexarotene levels and also regulation of UBE1L, cyclin D1 and Ki-67 expression. The response rate for UBE1L is 20% (95% CI 1, 72). The odds ratio (OR) assessing association between intratumoral bexarotene concentration and UBE1L increase is 6 (95% asymptotic CI) (0.1, 354.9). This OR indicates a high probability of UBE1L induction in tumors with high bexarotene compared to tumors with low bexarotene levels.
Discussion
UBE1L is the ubiquitin-activating E1-like enzyme for ISG15. Prior work implicated the UBE1L-ISG15 pathway as a molecular pharmacologic target (16). The current study advances prior work by reporting UBE1L directly inhibits cyclin D1 by destabilizing cyclin D1 protein (Figure 1 and Figure 2).This confers anti-proliferative effects in Figure 4 and Figure 5, and clinical anti-neoplastic activity in lung cancer in Figure 6. These observations build on previous work implicating UBE1L as exerting tumor suppressive effects (16–19). These findings provide a mechanism for UBE1L triggering cyclin D1 repression since a complex forms between cyclin D1 and ISG15 in Figure 2. The dependency on UBE1L for cyclin D1 complex formation with ISG15 was shown by transfection of the UBE1L-ISG15 deconjugase, UBP43, which inhibited this complex that depended on cyclin D1 lysines as in Figure 3. An inverse relationship between UBE1L and cyclin D1 expression was found after UBE1L retroviral transduction and UBE1L knock-down experiments in cells, as shown in Figure 4. Bexarotene-treatment of HBE and lung cancer cells as well as clinical lung cancer augmented UBE1L expression in Figure 5 and Figure 6.
Cyclin D1 is a proposed anti-neoplastic target (29). Both tumor cell differentiation and growth suppression are linked to induced cyclin D1 degradation (2–8). It is important to elucidate involved mechanisms. Prior work using retinoids as pharmacologic tools uncovered specific cyclin D1 residues regulating proteasomal degradation (23). A retinoic acid receptor (RAR) agonist and a rexinoid (RXR) agonist each conferred cyclin D1 proteolysis (3–8). UBE1L is a retinoid target gene (14–16). This study directly linked UBE1L expression to destabilization of cyclin D1 by showing reduced cyclin D1 protein expression followed UBE1L transfection and CHX treatment (Figure 1D). These findings, coupled to presence of ISG15-cyclin D1 complexes, implicate this complex as regulating cyclin D1 protein stability. These findings add to prior work (16,29,30,31) by highlighting UBE1L-dependent mechanisms as affecting cyclin D1 protein.
This UBE1L-ISG15-cyclin D1 pathway is affected by expression of the deconjugase, UBP43, as in Figure 1 and Figure 2. UBP43 is a proposed pharmacologic target (29,30). Notably, UBP43 transfection inhibited ISG15 complex formation and reduction of cyclin D1 protein, as in Figure 3 and Figure 4. It is hypothesized an inhibitor of UBP43 would promote conjugation of ISG15 to cyclin D1 and enhance cyclin D1 inhibition. Clinical relevance of the UBE1L-ISG15 pathway was shown by results of a proof of principle clinical trial (12) where increased UBE1L within bexarotene treated lung cancer was linked to cyclin D1 inhibition, as in Figure 6.
Combination therapy is a tenet guiding cancer therapy and chemoprevention (32). An optimal combination regimen is one where a critical oncogenic target, such as cyclin D1, is affected by different pharmacologic mechanisms converging on it. Future work should determine whether cross-talk between ubiquitination and ISG15ylation pathways affect cyclin D1. Preliminary studies indicate UBE1L transfection affects ubiquitination in HBE cells (data not shown). Pharmacologic targeting of the UBE1L-ISG15 pathway through inhibition of the deconjugase, UBP43, is an appealing approach to repress cyclin D1 protein. Perhaps use of agents that induce cyclin D1 proteasomal degradation or those that inhibit activity of cyclin D1 binding partners, cyclin dependent kinase 4 and 6, would cause cooperative clinical anti-neoplastic effects (29). Future work should explore this possibility by testing in proof of principle trials agents engaging these cyclin-dependent pathways.
Supplementary Material
Acknowledgements
We thank Dr. Sutisak Kitareewan (Dartmouth Medical School) for helpful consultation, Dr. Ernest C. Borden (Cleveland Clinic Foundation) for the anti-ISG15 antibody, Dr. Robert M. Krug (University of Texas, Austin, TX) for the pcDNA3-UbcH8 expression vector, Dr. Steven Dowdy (Howard Hughes Medical Institute, Washington University School of Medicine) for the HA-tagged pRcCMV-cyclin D1 expression plasmid, and Dr. Zhongze Li, Dartmouth’s Norris Cotton Cancer Center for assistance in biostatistical analyses. This work was supported by National Institutes of Health (NIH) and National Cancer Institute (NCI) grants RO1-CA087546, (E.D.), RO1-CA111422 (E.D.), RO3-CA130102 (E.D.), R03-CA132166 (E.D.), a Samuel Waxman Cancer Research Foundation Award (E.D.), an American Cancer Society Clinical Research Professorship (E.D.), the American Lung Association (X.L.), a post-doctoral fellowship (PDF0503563) grant from the Susan G. Komen Breast Cancer Foundation (L.S.), and a Hitchcock Foundation grant (L.S.).
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Langenfeld J, Lonardo F, Kiyokawa H, et al. Inhibited transformation of immortalized human bronchial epithelial cells by retinoic acid is linked to cyclin E down-regulation. Oncogene. 1996;13:1983–1990. [PubMed] [Google Scholar]
- 3.Langenfeld J, Kiyokawa H, Sekula D, Boyle J, Dmitrovsky E. Posttranslational regulation of cyclin D1 by retinoic acid: a chemoprevention mechanism. Proc Natl Acad Sci (USA) 1997;94:12070–12074. doi: 10.1073/pnas.94.22.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonardo F, Dragnev KH, Freemantle SJ, et al. Evidence for the epidermal growth factor receptor as a target for lung cancer prevention. Clin Cancer Res. 2002;8:54–60. [PubMed] [Google Scholar]
- 5.Boyle JO, Langenfeld J, Lonardo F, et al. Cyclin D1 proteolysis: a retinoid chemoprevention signal in normal, immortalized, and transformed human bronchial epithelial cells. J Natl Cancer Inst. 1999;91:373–379. doi: 10.1093/jnci/91.4.373. [DOI] [PubMed] [Google Scholar]
- 6.Dragnev KH, Pitha-Rowe I, Ma Y, et al. Specific chemoprevention agents trigger proteasomal degradation of G1 cyclins: implications for combination therapy. Clin Cancer Res. 2004;10:2570–2577. doi: 10.1158/1078-0432.ccr-03-0271. [DOI] [PubMed] [Google Scholar]
- 7.Petty WJ, Dragnev KH, Dmitrovsky E. Cyclin D1 as a target for chemoprevention. Lung Cancer. 2003;41 Suppl 1:S155–S161. doi: 10.1016/s0169-5002(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 8.Spinella MJ, Freemantle SJ, Sekula D, Chang JH, Christie AJ, Dmitrovsky E. Retinoic acid promotes ubiquitination and proteolysis of cyclin D1 during induced tumor cell differentiation. J Biol Chem. 1999;274:22013–22018. doi: 10.1074/jbc.274.31.22013. [DOI] [PubMed] [Google Scholar]
- 9.Lonardo F, Rusch V, Langenfeld J, Dmitrovsky E, Klimstra DS. Overexpression of cyclins D1 and E is frequent in bronchial preneoplasia and precedes squamous cell carcinoma development. Cancer Res. 1999;59:2470–2476. [PubMed] [Google Scholar]
- 10.Ma Y, Fiering S, Black C, et al. Transgenic cyclin E triggers dysplasia and multiple pulmonary adenocarcinomas. Proc Natl Acad Sci (USA) 2007;104:4089–4094. doi: 10.1073/pnas.0606537104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petty WJ, Dragnev KH, Memoli VA, et al. Epidermal growth factor receptor tyrosine kinase inhibition represses cyclin D1 in aerodigestive tract cancers. Clin Cancer Res. 2004;10:7547–7554. doi: 10.1158/1078-0432.CCR-04-1169. [DOI] [PubMed] [Google Scholar]
- 12.Dragnev KH, Petty WJ, Shah SJ, et al. A proof-of-principle clinical trial of bexarotene in patients with non-small cell lung cancer. Clin Cancer Res. 2007;13:1794–1800. doi: 10.1158/1078-0432.CCR-06-1836. [DOI] [PubMed] [Google Scholar]
- 13.Dragnev KH, Petty WJ, Shah S, et al. Bexarotene and erlotinib for aerodigestive tract cancer. J Clin Oncol. 2005;23:8757–8764. doi: 10.1200/JCO.2005.01.9521. [DOI] [PubMed] [Google Scholar]
- 14.Kitareewan S, Pitha-Rowe I, Sekula D, et al. UBE1L is a retinoid target that triggers PML/RARα degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci (USA) 2002;99:3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitha-Rowe I, Hassel BA, Dmitrovsky E. Involvement of UBE1L in ISG15 conjugation during retinoid-induced differentiation of acute promyelocytic leukemia. J Biol Chem. 2004;279:18178–18187. doi: 10.1074/jbc.M309259200. [DOI] [PubMed] [Google Scholar]
- 16.Pitha-Rowe I, Petty WJ, Feng Q, et al. Microarray analyses uncover UBE1L as a candidate target gene for lung cancer chemoprevention. Cancer Res. 2004;64:8109–8115. doi: 10.1158/0008-5472.CAN-03-3938. [DOI] [PubMed] [Google Scholar]
- 17.Kok K, Hofstra R, Pilz A, et al. A gene in the chromosomal region 3p21 with greatly reduced expression in lung cancer is similar to the gene for ubiquitin-activating enzyme. Proc Natl Acad Sci (USA) 1993;90:6071–6075. doi: 10.1073/pnas.90.13.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kok K, Van den Berg A, Veldhuis PM, Franke M, Terpstra P, Buys CH. The genomic structure of the human UBE1L gene. Gene Expr. 1995;4:163–175. [PMC free article] [PubMed] [Google Scholar]
- 19.McLaughlin PM, Helfrich W, Kok K, et al. The ubiquitin-activating enzyme E1-like protein in lung cancer cell lines. Int J Cancer. 2000;85:871–876. doi: 10.1002/(sici)1097-0215(20000315)85:6<871::aid-ijc22>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Sempere L, Galimberti F, et al. Uncovering growth suppressive microRNAs in lung cancer. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-08-1355. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, Li X-L, Hassel BA. Proteasomes modulate conjugation to the ubiquitin-like protein, ISG15. J Biol Chem. 2003;278:1594–1602. doi: 10.1074/jbc.M208123200. [DOI] [PubMed] [Google Scholar]
- 22.Shah SJ, Blumen S, Pitha-Rowe I, et al. UBE1L represses PML/RARα by targeting the PML domain for ISG15ylation. Mol Cancer Ther. 2008;7:905–914. doi: 10.1158/1535-7163.MCT-07-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Q, Sekula D, MüCller R, Freemantle SJ, Dmitrovsky E. Uncovering residues that regulate cyclin D1 proteasomal degradation. Oncogene. 2007;26:5098–5106. doi: 10.1038/sj.onc.1210309. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Feng Q, Sekula D, Diehl JA, Freemantle SJ, Dmitrovsky E. Retinoid targeting of different D-type cyclins through distinct chemopreventive mechanisms. Cancer Res. 2005;65:6476–6483. doi: 10.1158/0008-5472.CAN-05-0370. [DOI] [PubMed] [Google Scholar]
- 25.Andersen JB, Aaboe M, Borden EC, Goloubeva OG, Hassel BA, Orntoft TF. Stage-associated over expression of the ubiquitin-like protein, ISG15, in bladder cancer. Br J Cancer. 2006;94:1465–1471. doi: 10.1038/sj.bjc.6603099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KI, Zhang DE. UBP43, an ISG15-specific deconjugating enzyme: expression, purification, and enzymatic assays. Methods Enzymol. 2005;398:491–499. doi: 10.1016/S0076-6879(05)98040-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhao C, Beaudenon SL, Kelley ML, et al. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci (USA) 2004;101:7578–7582. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XL, Blackford JA, Judge CS, et al. RNase-L-dependent destabilization of interferon-induced mRNAs. A role for the 2–5A system in attenuation of the interferon response. J Biol Chem. 2000;275:8880–8888. doi: 10.1074/jbc.275.12.8880. [DOI] [PubMed] [Google Scholar]
- 29.Freemantle SJ, Liu X, Feng Q, et al. Cyclin degradation for cancer therapy and chemoprevention. J Cell Biochem. 2007;102:869–877. doi: 10.1002/jcb.21519. [DOI] [PubMed] [Google Scholar]
- 30.Yan M, Luo JK, Ritchie KJ, et al. Ubp43 regulates BCR-ABL leukemogenesis via the type I interferon receptor signaling. Blood. 2007;110:305–312. doi: 10.1182/blood-2006-07-033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic intervention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dmitrovsky E, Sporn MB. Pharmacology of cancer chemoprevention. In: Bertino J, editor. Encyclopedia of Cancer. 2nd Edition. St. Louis: Academic Press; 2002. pp. 449–455. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.