Abstract
Rationale
Disruption of CB1 receptor signaling through the use of CB1 (-/-) mice or the CB1 receptor antagonist rimonabant (SR141716) has been demonstrated to impair extinction of learned responses in conditioned fear and Morris water maze tasks. In contrast, CB1 (-/-) mice exhibited normal extinction rates in an appetitively motivated operant conditioning task.
Objectives
The purpose of this study was to test whether rimonabant would differentially disrupt extinction learning between fear-motivated and food-motivated tasks.
Materials and methods
Separate groups of C57BL/6J mice were trained in two aversively motivated tasks, conditioned freezing and passive avoidance, and an appetitively motivated operant conditioning task at a fixed ratio (FR-5) schedule of food reinforcement. After acquisition, the respective reinforcers in each task were withheld, and an intraperitoneal injection of vehicle or rimonabant was given 30 min before each extinction session.
Results
Rimonabant (3 mg/kg) treatment significantly disrupted extinction in both the conditioned freezing and passive avoidance tasks but failed to affect extinction rates in the operant conditioning task, whether using daily or weekly extinction sessions. Interestingly, rimonabant (3 mg/kg) prevented the significant increases in lever pressing (i.e., extinction burst) that occurred during the first extinction session of the operant conditioning task.
Conclusions
These results support the hypothesis that the CB1 receptor plays a vital role in the extinction of aversive memories but is not essential for extinction of learned responses in appetitively motivated tasks.
Keywords: Freezing, Working memory, Passive avoidance, Fear conditioning, Extinction, Cannabinoid, Operant conditioning task, Marijuana, SR141716 (rimonabant), Endocannabinoid
Introduction
The central endocannabinoid system, consisting of CB1 receptors (Herkenham et al. 1991; Matsuda et al. 1993) as well as endogenous ligands that stimulate those receptors, anandamide (Devane et al. 1992), and 2-AG (Mechoulam et al. 1995; Sugiura et al. 1995), has been implicated in several physiological systems including pain modulation (Calignano et al. 1998), feeding (Di Marzo et al. 2001), lipogenesis (Osei-Hyiaman et al. 2005), and cognition (Terranova et al. 1996). In particular, the high density of CB1 receptors in brain regions associated with learning and memory (Herkenham et al. 1991) is consistent with the well-documented effects of cannabinoids on cognition in humans (Chait and Pierri 1992; Miller and Branconnier 1983) and in laboratory animals (Lichtman et al. 2002). Importantly, the CB1 receptor antagonist rimonabant (SR141716) blocked the disruptive effects of cannabinoid agonists on both long-term potentiation (Terranova et al. 1995) and memory (Lichtman and Martin 1996; Mallet and Beninger 1998; Varvel et al. 2001), indicating a CB1 receptor mechanism of action.
Rimonabant has been used to demonstrate that the memory disruptive effects of cannabinoid agonists in a variety of operant (Brodkin and Moerschbaecher 1997; Hampson and Deadwyler 2000; Mallet and Beninger 1998; Winsauer et al. 1999) and spatial tasks (da Silva and Takahashi 2002; Lichtman and Martin 1996; Varvel et al. 2001) are mediated via a CB1 receptor mechanism of action. The administration of this drug has also been used to infer the tonic involvement of the endocannabinoid system in mnemonic processes. In particular, rimonabant impaired extinction learning in fear conditioning (Marsicano et al. 2002;Suzuki etal. 2004) and spatial memory procedures (Varvel et al. 2005) without affecting normal learning. Additionally, CB1 (-/-) mice exhibit similar extinction deficits (Marsicano et al. 2002; Varvel et al. 2005).
The extent to which the CB1 receptor modulates extinction in different memory paradigms is unclear. In contrast to their extinction deficits in aversively motivated tasks, CB1 (-/-) mice were found to extinguish at the same rate as their wild type littermates in an operant nose-poke food-motivated task (Holter et al. 2005). Thus, these investigators concluded that the CB1 receptor was not critical for extinction in this appetitively-motivated task. However, there are two phenotypes displayed by CB1 (-/-) mice that complicate the interpretation of those findings. First, CB1 (-/-) mice acquired the operant response at a slower rate than the CB1 (+/+) mice and required an increased food deprivation schedule to ensure that they achieved criteria (Holter et al. 2005). Second, the mice in that study were between 11 and 14 weeks old, an age range in which a subsequent report found that CB1 (-/-) mice displayed significant learning deficits in operant and other tasks (Bilkei-Gorzo et al. 2005). To avoid motivational and age-related behavioral deficits associated with CB1 (-/-) mice, we evaluated whether rimonabant would affect extinction rates in wild type mice trained in an appetitively motivated operant conditioning task (i.e., lever pressing for sweetened condensed milk). Additionally, we investigated the effects of rimonabant on extinction learning in two aversively motivated tasks, conditioned freezing and passive avoidance tests.
Materials and methods
Subjects
Male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were employed as subjects in these experiments. The subjects were between 8 and 12 weeks of age and were housed in a temperature- and humidity-controlled room (20–22°C) with a 12:12 light/dark cycle. Mice trained for the operant conditioning task were housed individually, and those used in all other experiments were housed in groups of four mice per cage. The mice had ad libitum access to food and water in their home cages. The Institutional Animal Care and Use Committee at Virginia Commonwealth University approved all experimental procedures.
Drugs
Rimonabant (SR 141716) was obtained from the National Institute on Drug Abuse (Rockville, MD). The drug was dissolved in a 1:1:18 solution of ethanol/emulphor/saline. All injections were given through the intraperitoneal (i.p.) route of administration in a volume of 0.1 ml/10 g.
Behavioral procedures
Conditioned freezing procedure
The conditioned freezing apparatus consisted of four sets of conditioning apparatuses with stainless steel bars (3.2-mm diameter and spaced 7.6 mm apart) spanning the width of the floor, through which an electric shock could be delivered (Hamilton-Kinder, Poway, CA). Four-sided black plastic conditioning chambers were placed on top of the steel bars (21.1×21.1×20.4 cm). Each conditioning apparatus was kept in an individual sound-attenuating chamber with an array of adjustable white light-emitting diodes as a light source located on the side. A digital camera (Fire-i digital camera, Unibrain) and a piezo buzzer (80 dB) were both located on the ceiling of the sound-attenuating chamber. In the retrieval/extinction portion of the experiment, clear Plexiglas boxes (42.7×21.0×20.4 cm) were placed on top of a smooth plastic floor. Anymaze (Stoelting, Wood Dale, IL) software was used to track the animals and analyze immobility. We have found that the software’s immobility detection is highly correlated with freezing counts observed and recorded manually (unpublished data).
Subjects were acclimated to the experimental room for 2 h before all the experiments. On the first day of the experiment, mice were placed in the conditioning chamber. After a 3-min acclimation period, a 20-s tone (80 dB) was presented that co-terminated with a scrambled 2-s (0.7 mA, alternating current [AC]) electric foot shock. Mice were returned to their home cage 1 min later. On the second day of the experiment, subjects were given an injection of vehicle or rimonabant (3 mg/kg) 30 min before being placed in the extinction context. After a 3-min acclimation period, the tone was presented for 3 min, and extinction was evaluated within the single session. Freezing to the tone was recorded and analyzed digitally. Two minutes after tone presentation, the mice were removed from the apparatus and returned to their respective home cages.
Passive avoidance procedure
The passive avoidance apparatus consisted of two adjoining compartments (each 30.5×20.5×19 cm), one dark with black walls and one illuminated with white walls, separated by a guillotine door. The floor of each compartment consisted of steel rods capable of delivering an electric shock to the animal’s feet (Lafayette Instrument, Lafayette, IN). The procedure included an acclimation session, a conditioning session, and ten extinction sessions. During the first 60 s of each session, a guillotine door blocked the entryway between the illuminated and dark chambers. Subjects were always placed in the light chamber for 60 s before the door was lifted for a 4-min period. The day after the acclimation session, the guillotine door was shut immediately after the mouse entered the dark chamber, and 30 s later the subject received a total of three scrambled 1.7-mA (AC) grid shocks that were 1 s in duration, with 20 s interval between each shock. The subject was then removed after the third shock.
In the first experiment, we evaluated the dose—response relationship of rimonabant on extinction. Thirty minutes before each extinction session, the subjects were given an i.p. injection of vehicle or rimonabant (0.3, 1, or 3 mg/kg), placed in the light chamber for 60 s as described above, and the guillotine door was then lifted. If the subject entered the dark chamber, the guillotine door was lowered, and 2 min later, the animal was removed from the chamber. If a mouse failed to enter the dark chamber by the end of the 4-min choice period, it was gently guided into the dark chamber by the experimenter. The guillotine door was lowered, and 2 min later, the animal was removed from the chamber. The latency to enter the dark chamber was recorded on the conditioning day and each extinction session.
To distinguish between extinction and simple forgetting of the avoidance behavior, a second experiment was conducted that included three groups. The first two groups were treated with vehicle and rimonabant (3 mg/kg), respectively, on each of the ten extinction sessions as described above. The third group was treated with vehicle on each extinction day but remained in the lit compartment for 4 min with the guillotine door closed (i.e., had no access to the dark chamber). On the tenth day, this control group was given access to the dark chamber, as described above, and the latency to enter the dark chamber was recorded.
Operant conditioning procedure
The operant conditioning apparatus (Med Associates, St. Albans, VT) consisted of individual chambers (18×18×18 cm each). Each operant conditioning chamber was equipped with a house light, two levers (left and right), and a hole where a motor-driven dipper arm delivered sweetened milk. The sweetened milk consisted of dry fat-free milk, sugar, and water in a ratio of 1:1:3.3, respectively. The mice were trained to press the right lever to receive the reward. A dipper arm delivered the sweetened milk in a 0.05-ml cup, which was available for 5 s. Each daily session was 15 min in duration, and the response rates on each lever were recorded. All mice in this experiment were kept at 90% of the original body weight. All mice had free access to water. Immediately after the daily session, the mice were given their daily food rations.
The operant conditioning procedure included an acclimation session, approximately 21–27 training sessions, and five extinction sessions. The subjects were trained on a fixed ratio (FR-5) schedule of food reinforcement and were required to achieve stable rates of responding during training, in which daily response rate was within 25% of the mean for five consecutive days. All mice met the criteria. On each extinction day, the subjects received an i.p. injection of vehicle or rimonabant (1 or 3 mg/kg) 30 min before the session; however the dipper containing the sweetened milk was withheld. In the first experiment, daily 15-min extinction sessions were given, and in the second experiment, weekly 15-min extinction sessions were given. These schedules (daily and weekly) were chosen because rimonabant delayed extinction with weekly probe extinction sessions in the Morris water maze but not with daily probes (Varvel et al. 2005). The total number of lever presses on the reinforced lever as well as on the non-reinforced lever across each 15-min session was recorded. Subjects rarely pressed the non-reinforced lever once achieving stable baseline criteria (i.e., 5±0.7 and 6±0.7 total responses for the entire 15-min session for experiments 1 and 2, respectively), and no significant differences in lever pressing on the non-reinforced lever occurred during either the daily (p=0.33) or weekly (p=0.53) extinction sessions. Therefore, the lever-pressing data on only the reinforced lever are presented. Finally, we assessed the effects of rimonabant (1 mg/kg) on operant conditioning behavior in well-trained mice when the reinforcer (e.g., sweetened milk) was made available.
Assessment of locomotor activity
The mice were brought up to the lab 1 h before testing. The mice were given i.p. injections of vehicle or rimonabant (1 or 3 mg/kg) and 30 min later placed in clean 28×16 cm plastic cages inside of the sound-attenuating chambers where the traveled distance, the speed, and the time spent immobile were recorded for 15 min and analyzed by an Anymaze (Stoelting, Wood Dale, IL) video tracking system.
Statistical analysis
The percentage time spent freezing in the conditioned freezing task, the latency to enter the dark chamber in the passive avoidance task, and the total number of lever presses in the operant conditioning task were used as dependent measures. The traveled distance, the speed, and the percentage time spent immobile were used as dependent measures in the locomotor activity task. The data in each experiment were analyzed using one- or two-way analysis of variances (ANOVA) in which drug treatment was a between-subject factor and session (where appropriate) was a within-subject measure. Dunnett’s test was used for post hoc analysis when appropriate. A p value of less than 0.05 was considered as being significant.
Results
Rimonabant impairs within-session extinction in the conditioned freezing test
As can be seen in Fig. 1, both vehicle-treated and rimonabant-treated mice exhibited an equal magnitude of freezing behavior during the first minute of the extinction test, indicating an equivalent degree of memory. Whereas the vehicle-treated mice exhibited significant decreases in freezing behavior across the 3-min session, the drug-treated mice continued to freeze across the entire session, suggesting a deficit in extinction as previously reported (Marsicano et al. 2002). Accordingly, a significant two-way interaction between injection and time was found, F[2,28]=4.2, p<0.05. Planned comparisons confirmed that the vehicle-treated mice froze significantly less than the rimonabant-treated mice during both the second (p<0.05) and third (p<0.001) minutes of the extinction session. Additionally, the vehicle-treated mice spent significantly less time freezing during the third minute than the first minute of the extinction session (p<0.05).
Fig. 1.
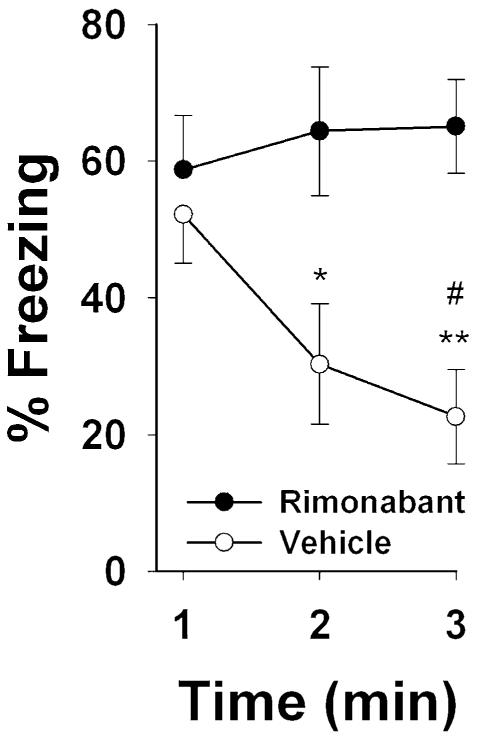
Rimonabant disrupts extinction of a conditioned freezing response. Mice that were administered rimonabant (3 mg/kg) failed to extinguish freezing to a 3-min tone, which had been paired with foot shock on the preceding day. *p<0.05 and **p<0.01 for the rimonabant-treated group compared to the vehicle-treated group at the same time point; #p<0.05 for the vehicle group at the third minute compared to the first minute. Results shown as mean time spent immobile±SEM; n=8 mice per group
Rimonabant disrupts extinction in a passive avoidance task
During the conditioning session, all subjects entered the dark chamber within the 4-min choice period (mean±SEM latency to enter=29±5 s). The data depicted in Fig. 2a show the effects of vehicle and rimonabant on the latency to enter the dark chamber across each of the ten 4-min extinction sessions. For the sake of clarity, only the groups treated with vehicle and rimonabant (3 mg/kg) are shown in Fig. 2a. Irrespective of drug treatment, subjects demonstrated that they learned the avoidance task as indicated by their failure to enter the dark chamber on the first post-acquisition session (i.e., extinction day 1). Rimonabant disrupted extinction, as indicated by a significant interaction between dose and day, F[3,27]=2.1, p<0.01. Subsequent one-way repeated measures ANOVAs revealed a differential rate of extinction for each injection condition. Whereas subjects treated with repeated injections of vehicle or rimonabant (0.3 or 1 mg/kg) exhibited significant decreased latencies across extinction sessions to enter the dark chamber (p<0.001), the mice given daily injections of 3 mg/kg rimonabant failed to exhibit any significant effects across the ten extinction sessions (p=0.15).
Fig. 2.
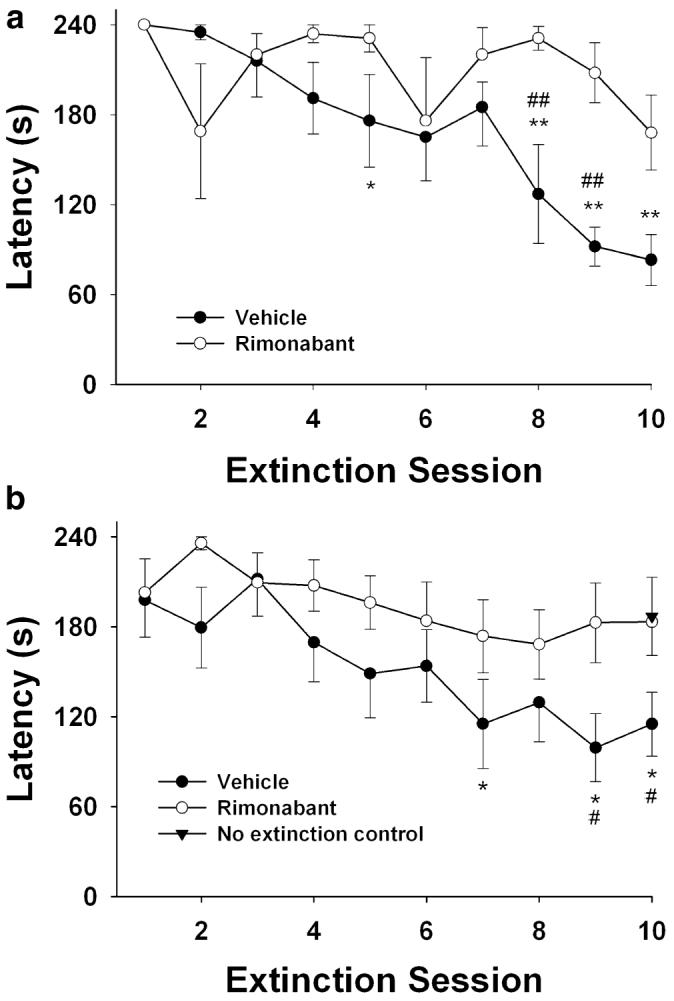
Rimonabant retards extinction learning in a passive avoidance task. a Latencies to enter the chamber associated with shock in vehicle-treated mice gradually decreased across extinction sessions, whereas rimonabant dose-dependently disrupted extinction. To optimize the clarity of the data presentation, the vehicle and the 3-mg/kg rimonabant conditions are shown here (statistics of the dose—response study are reported in the results section). b Repeated exposure to the chamber associated with shock is necessary for extinction of the avoidance behavior. *p<0.05 and **p<0.01 for each respective group versus its first extinction session; #p<0.05 and ##p<0.01 for rimonabant (3 mg/kg) versus vehicle on extinction days. Results shown as mean latency to enter the chamber associated with shock (s)±SEM; n=6 mice per group in a; n=10–11 mice per group in b
To distinguish between extinction learning and forgetting, a separate experiment was performed in which we assessed whether mice treated daily with vehicle, placed in the lit compartment for 4 min, and not given access to the dark chamber would re-enter the dark chamber when given access to it after 10 days. Once again, as shown in Fig. 2b, rimonabant disrupted extinction of the avoidance behavior across days, as indicated by a significant main effect of the drug, F[1,20]=5.3, p<0.05. Subsequent one-way ANOVAs revealed that, whereas subjects treated with repeated injections of vehicle exhibited significant decreased latencies across extinction sessions to enter the dark chamber (p<0.01), the group treated with daily injections of 3 mg/kg rimonabant failed to exhibit any significant decreases in latency across the ten extinction sessions (p=0.45). Importantly, the control group, which was treated with vehicle and not allowed access to the shock-associated chamber for 9 days, virtually failed to enter the dark chamber on day 10. The mean latency for this group to enter the dark chamber on day 10 was significantly higher than that of the group treated with vehicle and given daily extinction sessions (p<0.05) and was indistinguishable from the rimonabant-treated group, consistent with the notion that rimonabant disrupts extinction in the passive avoidance task.
Rimonabant does not affect extinction rates in an appetitively motivated operant conditioning task
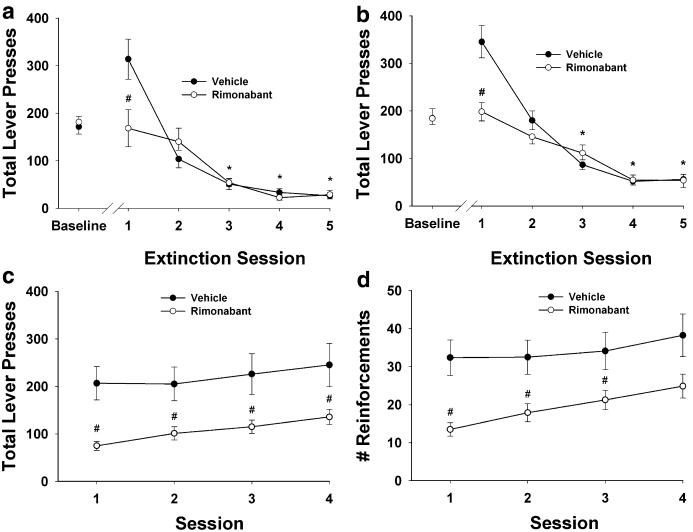
Mice were initially shaped to lever press on a continuous reinforcement schedule, with the ratio requirement systematically increased to an FR-5 schedule of food reinforcement over several days. Once stable response rates were achieved (178±7 and 187±11 responses±SEM for experiments 1 and 2, respectively), mice were given an injection of vehicle or rimonabant (1 or 3 mg/kg) 30 min before each extinction session. The data depicted in Fig. 3a and b show the effects of vehicle and rimonabant on the daily (experiment 1) and weekly (experiment 2) extinction sessions, respectively. For the sake of clarity, only the groups treated with vehicle and rimonabant 3 mg/kg are shown here. Neither rimonabant nor the extinction schedule (daily or weekly) affected the extinction rate. One-way repeated measures ANOVA revealed that all groups, irrespective of drug treatment and extinction condition, exhibited a significant decrease in responding rate by the third extinction session (p<0.001). Interestingly, there was a significant interaction of drug across extinction sessions in both the daily and weekly schedule, F[10,105]=3.5, p<0.001 and F[10,180]=3.7, p<0.001, respectively. In particular, on the first day of extinction, the vehicle-treated groups displayed an extinction burst in which lever pressing was increased by more than 80% compared to their stable baseline level, whereas the group treated with 3 mg/kg of rimonabant exhibited significantly fewer responses than the vehicle-treated mice. There were no other significant differences between the groups on subsequent extinction sessions.
Fig. 3.
The effects of rimonabant in an operant conditioning task. Rimonabant (3 mg/kg) reduces the extinction burst (p<0.05) but fails to alter extinction rate in mice given daily (a) or weekly (b) extinction sessions. To optimize the clarity of the data presentation, the vehicle and the 3 mg/kg rimonabant conditions are shown here (statistics for the 1 mg/kg rimonabant group are reported in the results section). Irrespective of drug treatment, all groups exhibited significant decreases in response rates by extinction day 3 for both schedules (p <0.001). The baseline values represent the average of five stable training days with daily sessions that were 15 min in duration. No injections were given on the training days. c Rimonabant (1 mg/kg) significantly reduced the mean lever pressing for sweetened milk in well-trained mice (p<0.05). d Rimonabant-treated mice obtained significantly fewer reinforcers than the vehicle-treated mice during the 15-min session. Asterisk denotes significant difference from the baseline (p<0.05), and number sign denotes significant difference from the corresponding vehicle group (p<0.05). Results shown as mean values±SEM for each 15-min session; n=8 mice per group for a,c, and d; n=15–16 mice per group for b
Additionally, the effects of rimonabant (1 mg/kg) were assessed in well-trained mice when the reinforcer (e.g., sweetened milk) was made available. Rimonabant (1 mg/kg) significantly reduced the lever-pressing response (Fig. 3c) as indicated by a significant main effect of rimonabant, F[1,14]=8, p<0.05. Consequently, rimonabant-treated mice received significantly fewer reinforcers (Fig. 3d) than the vehicle-treated group, F[1,14]=8, p<0.05. On each of the four test days, the rimonabant-treated mice made significantly fewer lever presses and consequently received fewer reinforcers than the control group (p<0.05).
Rimonabant does not affect gross locomotor activity
As shown in Table 1, there were no significant differences between vehicle- and rimonabant-treated mice in the mean distance traveled (p=0.72), speed (p=0.75), and percentage of time spent immobile (p=0.47) throughout the 15-min test session.
Table 1.
Rimonabant failed to have significant effects on gross locomotor behavior (n=7–9 mice per group)
| Treatment | Total distance traveled (m±SEM) |
Speed (cm/s±SEM) |
% Immobility (mean±SEM) |
|---|---|---|---|
| Vehicle | 21.63±3.24 | 2.4±0.4 | 4.1±1.4 |
| Rimonabant (1 mg/kg) |
17.87±3.09 | 2.0±0.3 | 6.4±1.5 |
| Rimonabant (3 mg/kg) |
19.10±3.22 | 2.1±0.4 | 7.3±2.1 |
Discussion
The results of the present study bolster the claim that the endocannabinoid system plays a selective role in facilitating the extinction of fear-conditioned behaviors but not of appetitively motivated behaviors. Whereas vehicle-treated mice underwent extinction in the conditioned freezing and passive avoidance tasks, rimonabant-treated mice failed to exhibit any evidence of extinction in either of these fear-motivated tasks. The failure of rimonabant-treated mice to extinguish freezing behavior to a tone that had been paired with foot shock replicates work by Marsicano et al. (2002), who previously demonstrated that wild type mice treated with this antagonist as well as CB1 (-/-) mice failed to display extinction in a similar procedure. Additionally, the results presented here are the first demonstration that rimonabant impairs extinction in a passive avoidance task, which requires that the mice remain in a brightly lit chamber and withhold their inclination to enter a dark chamber in which they previously received three foot shocks. Even ten extinction sessions were insufficient to elicit extinction learning in the drug-treated mice. Also shown in the present study is that repeated exposures to the dark chamber are necessary for the avoidance behavior to extinguish. The observation that rimonabant-treated mice and vehicle-treated mice extinguished the lever-press at the same rate was consistent with the results of Holter et al. (2005), who found no differences in extinction rate between CB1 (-/-) mice and CB1 (+/+) mice in another operant conditioning task. Thus, the results of the present study support the notion that the endocannabinoid system modulates the extinction of behaviors motivated by aversive stimuli but not appetitively motivated behaviors.
A considerable amount of research has demonstrated that subjects undergoing extinction training initially exhibit marked increases in response frequencies (Lerman and Iwata 1996). Such an extinction burst was found in the vehicle-treated mice tested in the operant conditioning task (see Fig. 3a and b). A novel observation of the present study was that rimonabant significantly blocked this extinction burst. Similarly, Holter et al. (2005) reported a transient increase in the number of total responses during extinction that was more pronounced in CB1 (+/+) mice than in CB1 (-/-) mice, although the magnitude of this increase was not specified. It is thought that these initial increases in responding during extinction reflect a “frustration-like” response to the lack of expected reinforcement and are likely to be involved with the initiation of extinction learning. With this in mind, it is possible that rimonabant interfered with an early phase of extinction, although subsequent decreases in responding occurred at an identical rate to vehicle-treated mice. Alternatively, as described below, rimonabant may have blocked the extinction burst because it simply reduced the salience of the food reinforcer or motivation to work for food.
There are several explanations for the differential effects of rimonabant on appetitively and aversively motivated tasks. First, rimonabant may have produced its effects by reducing the animal’s propensity to perform an active response. Whereas extinction in both the conditioned freezing and passive avoidance tasks involves increased mobility, extinction in the operant conditioning task is characterized by a decrease in lever pressing. Moreover, the observation that rimonabant blocks extinction burst responding and decreases lever pressing for food further supports the notion that all the effects observed in these disparate learning paradigms are the result of a decrease in overall motor behavior. However, it is unlikely that all these effects are simply due to rimonabant-induced motor suppression because these doses of rimonabant failed to affect general locomotor activity (see Table 1). In addition, a similar rimonabant-induced extinction deficit has been described in the Morris water maze, which involves the perseverance of an active response (Varvel et al. 2005).
A second possibility for rimonabant’s differential effects on food-motivated behavior and fear-motivated behavior is that the drug decreased the salience of the food reward (Ward and Dykstra 2005) in the operant conditioning task but heightened aversive properties in the fear conditioning tasks. Rimonabant has been previously demonstrated to reduce operant responding for food (De Vry et al. 2004; Freedland et al. 2000) as well as consumption of a palatable sucrose solution (Higgs et al. 2003). Our observations that rimonabant reduced the extinction burst (see Fig. 3a and b) and operant responding for sweetened milk are consistent with this explanation (see Fig. 3c and d). Because the endocannabinoid system plays a modulatory role in feeding, it will be important to investigate the impact of disrupting CB1 receptor signaling in appetitively motivated tasks that do not involve food reinforcement. On the other hand, if rimonabant increases the intensity of aversive stimuli, conditioned fear responses would be expected to be resistant to extinction.
In addition to disrupting extinction in aversively motivated tasks, administration of rimonabant alone has been shown to elicit a variety of effects in different animal models of learning and memory, suggesting important yet complex roles for the endocannabinoid system. Upon systemic administration, this drug has been reported to enhance performance in several rodent memory paradigms including a social recognition task (Terranova et al. 1996), an eight-radial arm maze delay procedure (Lichtman 2000; Wolff and Leander 2003), and an elevated T-maze test (Takahashi et al. 2005). Interestingly, intrahippocampal infusion of rimonabant to black-capped chickadees improved performance in a food-storing task (Shiflett et al. 2004). Curiously, intrahippocampal infusion of the structurally related CB1 receptor antagonist AM251 immediately after acquisition disrupted memory in a passive avoidance task (de Oliveira Alvares et al. 2005). In contrast, systemic rimonabant administration failed to enhance memory in operant paradigms (Hampson and Deadwyler 2000; Mallet and Beninger 1998) as well as acquisition in the mouse Morris water maze model (Varvel et al. 2005). Disruption of CB1 receptors has been suggested to prolong or strengthen memory, which can come at the expense of learning new responses (Shiflett et al. 2004; Varvel and Lichtman 2002). More recently, the endocannabinoid system has been implicated in reconsolidation processes (Lin et al. 2006) and in the habituation of non-associative sensitized fear responses (Kamprath et al. 2006).
In conclusion, the results of the present study are consistent with the hypothesis of Holter et al. (2005) that the endocannabinoid system plays an important role in extinction of aversively motivated behaviors, but this system does not seem to be critically involved in extinguishing appetitively-motivated behaviors. It will be important to ascertain whether the effects of rimonabant on memory, fear-related behavior, extinction, and feeding behavior occur because it blocks endogenous cannabinoid tone or through its inverse agonist properties (Landsman et al. 1997; Sim-Selley et al. 2001). Additionally, it will be important to characterize thoroughly the effects of rimonabant on other appetitively-motivated tasks. Finally, it will also be important to understand the underlying processes by which rimonabant prevented the extinction burst (e.g., decreased salience of the food reinforcement or a decrease in frustration-like responses).
Acknowledgment
This work was supported by the National Institute on Drug Abuse grants DA015683, DA03672, and DA09789.
References
- Bilkei-Gorzo A, Racz I, Valverde O, Otto M, Michel K, Sastre M, Zimmer A. Early age-related cognitive impairment in mice lacking cannabinoid CB1 receptors. Proc Natl Acad Sci USA. 2005;102:15670–15675. doi: 10.1073/pnas.0504640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin J, Moerschbaecher JM. SR141716A antagonizes the disruptive effects of cannabinoid ligands on learning in rats. J Pharmacol Exp Ther. 1997;282:1522–1526. [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Chait LD, Pierri J. Effects of smoked marijuana on human performance: a critical review. In: Murphy L, Bartke A, editors. Marijuana/cannabinoids: neurobiology and neurophysiology. CRC Press; Boca Raton, FL: 1992. pp. 387–423. [Google Scholar]
- da Silva GE, Takahashi RN. SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuro-psychopharmacol Biol Psychiatry. 2002;26:321–325. doi: 10.1016/s0278-5846(01)00275-5. [DOI] [PubMed] [Google Scholar]
- de Oliveira L Alvares, de Oliveira LF, Camboim C, Diehl F, Genro BP, Lanziotti VB, Quillfeldt JA. Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Mem. 2005;83:119–124. doi: 10.1016/j.nlm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schreiber R, Eckel G, Jentzsch KR. Behavioral mechanisms underlying inhibition of food-maintained responding by the cannabinoid receptor antagonist/inverse agonist SR141716A. Eur J Pharmacol. 2004;483:55–63. doi: 10.1016/j.ejphar.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- Freedland CS, Poston JS, Porrino LJ. Effects of SR141716A, a central cannabinoid receptor antagonist, on food-maintained responding. Pharmacol Biochem Behav. 2000;67:265–270. doi: 10.1016/s0091-3057(00)00359-2. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs S, Williams CM, Kirkham TC. Cannabinoid influences on palatability: microstructural analysis of sucrose drinking after delta(9)-tetrahydrocannabinol, anandamide, 2-arachidonoyl glycerol and SR141716. Psychopharmacology (Berl) 2003;165:370–377. doi: 10.1007/s00213-002-1263-3. [DOI] [PubMed] [Google Scholar]
- Holter SM, Kallnik M, Wurst W, Marsicano G, Lutz B, Wotjak CT. Cannabinoid CB1 receptor is dispensable for memory extinction in an appetitively-motivated learning task. Eur J Pharmacol. 2005;510:69–74. doi: 10.1016/j.ejphar.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Lerman DC, Iwata BA. Developing a technology for the use of operant extinction in clinical settings: an examination of basic and applied research. J Appl Behav Anal. 1996;29:345–382. doi: 10.1901/jaba.1996.29-345. discussion 383–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. D9-Tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology. 1996;126:125–131. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Varvel SA, Martin BR. Endocannabinoids in cognition and dependence. Prostaglandins Leukot Essent Fat Acids. 2002;66:269–285. doi: 10.1054/plef.2001.0351. [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–321. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta-9-tetrahydrocannabinol or anandamide. Psychopharmacology. 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski N, Schatz A, Gopher A, Almog S, Martin B, Compton D, Pertwee R, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Miller LL, Branconnier RJ. Cannabis: effects on memory and the cholinergic limbic system. Psychol Bull. 1983;93:441–456. [PubMed] [Google Scholar]
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–12305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Rankin AZ, Tomaszycki ML, DeVoogd TJ. Cannabinoid inhibition improves memory in food-storing birds, but with a cost. Proc Biol Sci. 2004;271:2043–2048. doi: 10.1098/rspb.2004.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim-Selley LJ, Brunk LK, Selley DE. Inhibitory effects of SR141716A on G-protein activation in rat brain. Eur J Pharmacol. 2001;414:135–143. doi: 10.1016/s0014-2999(01)00784-1. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoyglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–275. doi: 10.1016/j.neulet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Terranova J-P, Michaud J-C, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716A, a selective antagonist of CB1 cannabinoid receptors. Nauyn-Schmiedeberg’s Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology. 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of delta9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157:142–150. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Dykstra LA. The role of CB1 receptors in sweet versus fat reinforcement: effect of CB1 receptor deletion, CB1 receptor antagonism (SR141716A) and CB1 receptor agonism (CP-55940) Behav Pharmacol. 2005;16:381–388. doi: 10.1097/00008877-200509000-00010. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]