Abstract
Lifelong substance abuse is often initiated during adolescence; yet, most pre-clinical research in this area has been conducted in adult animals. Substantial evidence exists that the brain development that continues throughout adolescence may result in pharmacological responses that differ in a crucial manner from those of adults. The goal of this study was to evaluate age differences in motor activity following acute and repeated administration of drugs that are commonly abused by adolescents, including cocaine, Δ9-tetrahydrocannabinol (Δ9-THC), and the club drugs, ketamine and 3,4-methylenedioxymethamphetamine (MDMA). Adolescent and adult male rats were injected once daily with saline or with a dose of one of the test drugs for two 5-day dosing periods, separated by a 2-day drug holiday during which they remained in their home cages. Following each injection, rats were placed in a locomotor chamber for a 20-minute session. The potencies of cocaine, ketamine and MDMA for producing motor stimulation were less in male adolescents than in male adults. Furthermore, sensitization to the club drug, ketamine, developed after repeated dosing in adults, but not adolescents. In contrast, adolescents were initially more sensitive to the stimulatory effects of low doses of Δ9-THC than were adults, although rapid tolerance occurred. These results suggest that adolescents are less sensitive to the acute and repeated stimulant effects of some, but not all, of the drugs that are preferentially abused by this age group. This differential sensitivity may contribute to the different patterns of use that have been noted in adolescent versus adult drug abusers.
Keywords: Adolescence, cannabinoid, club drugs, ketamine, MDMA, sensitization
Adolescent mammals of many species, including humans, exhibit a characteristic pattern of behaviors that reflect a reproductively necessary change in orientation from interaction with parents to interaction with peers. Typical behaviors include increased play and exploration, greater risk taking and heightened novelty seeking (Spear & Brake 1983; Spear 2000). Accompanying these alterations in behavior are physiological changes associated with development of sexual maturity as well as neuronal re-organization and receptor pruning (Spear 2000; Chambers, Taylor & Potenza 2003). Some of the most notable brain changes occur within the dopamine system. Autoradiography has shown that levels of both D1 and D2 receptors in the nucleus accumbens and caudate putamen rose steadily after birth to a peak at postnatal day (PN28) (Tarazi & Baldessarini 2000). As adolescence progressed, however, significant pruning of D1 and D2 dopamine receptors was observed in these brain regions. In contrast, D1 and D2 receptor levels continued to rise steadily in rat cortical and hippocampal areas up until PN60; i.e. no dopamine receptor pruning was observed (Tarazi & Baldessarini 2000). Due to these differential developmental patterns of dopamine receptors across brain region, predominance of dopamine D1 and D2 receptor functioning undergoes a major shift from subcortical to cortical (and particularly frontal) areas during early adolescence (for a review, see Spear 2000). Given these substantial developmental changes in brain and behavior, it is hardly surprising that the probability of experimentation with illicit drugs increases dramatically during adolescence and that long-term substance abuse often has its origin here.
Although many adolescents try licit and illicit substances of abuse, relatively few progress to regular use during adulthood. Those that do, however, tend to have more recalcitrant substance abuse problems. In humans, many sociocultural, genetic and psychological, as well as neurobiological factors, may affect progression from initial use to abuse. The degree to which this process may vary during adolescence (versus adulthood) has not been investigated extensively. To this end, two issues related to the neurobiology of drug addiction are of particular importance. The first issue is the extent to which adolescents and adults are differentially sensitive to the acute effects of abused substances. Subsequent behavior is governed to a large extent by the consequences of previous behavior; hence, differential sensitivity to these initial ‘consequences’ (e.g. positive or negative effects of drugs) might reasonably be expected to affect whether or not the drug is used again. Second, given that progression to substance abuse implies chronic administration of the abused substance, examination of age differences in changes in sensitivity over time with repeated exposure to the drug is also crucial. Changes in sensitivity may take the form of tolerance or sensitization – decreased or increased sensitivity, respectively, to the drug’s initial effects. Sensitization of the locomotor activating effects of selected abused drugs is the focus of this study. In this context, sensitization is a phenomenon whereby initial drug-induced stimulation of locomotor activity in a rodent model is enhanced following repeated administration of the abused drug. While sensitization to effects other than locomotor activity certainly may occur, close correspondence between dopamine pathways that govern ambulatory motor activity and those involved in reward has led to the hypothesis that sensitization to this measure represents a form of neural adaptation that concomitantly results in an increase in the sensitivity of reward pathways to stimulation by dopamine (Robinson & Berridge 1993, 2003). This hypersensitivity is progressive and may lead to an increase in subjectively felt ‘wanting’ or ‘craving’ for the drug (Robinson & Berridge 1993, 2003), resulting in continued or increased use.
Most prior pre-clinical studies that have examined sensitization have used adult animals. Only recently has there been increased interest in possible differential effects of substances of abuse in adolescent (versus adult) animals. The primary focus of many of these studies has been on nicotine and ethanol (Slotkin 2002; Barron et al. 2005; Spear & Varlinskaya 2005). While nicotine and ethanol are certainly among the drugs frequently abused by adolescents (and adults), other drugs are known to be prevalent in use especially among adolescents and young adults (Banken 2004), including marijuana, ‘club drugs’ and inhalants. ‘Club drugs’ incorporate drugs from multiple pharmacological classes and, among others, include methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA), ketamine, gamma-hydroxybutyric acid (GHB), rohypnol and lysergic acid diethylamide (LSD). These drugs are popular and readily accessible in dance club settings (hence, the category name). Of these, MDMA and ketamine are arguably the most ‘dangerous’, as each of these drugs has been associated with toxic effects on the nervous system (Scallet et al. 2004; Warren et al. 2006). Δ9-Tetrahydrocannabinol (Δ9-THC) was also investigated because it is the primary psychoactive substituent of marijuana, one of the most frequently used illicit substance during adolescence. Results for all drugs were compared with those obtained with cocaine, a prototypic psychostimulant. The hypothesis of this study is that the initial and repeated effects of marijuana and club drugs on locomotor activity in adolescent rats will differ from those observed in adult rats. Furthermore, as these age differences may reflect underlying differences in development of brain dopamine systems, as noted above, they are likely to mediate differences in the consequences of substance abuse in adolescents versus adults.
MATERIALS AND METHODS
Subjects
Adult female Long-Evans rats (Harlan, Dublin, VA, USA) were impregnated by adult male Long-Evans rats (Harlan) in our animal room facility. After breeding, dams were individually housed in clear plastic cages in a temperature-controlled (20–22°C) environment with a 12-hour light-dark cycle (lights on at 7 am). Plenty of sawdust bedding was available in each cage for nesting. The dams were left undisturbed except for providing food, water, and fresh bedding until they gave birth (postnatal day 0, PN0). Pups were sexed and culled to no more than 10 pups per litter. Pups that were not used in this study were used in other independent studies. They remained with their dams until weaning at PN21. On PN21, pups were separated from the dam and were pair-housed with a same-sex rat that received the same treatment. Male pups were randomly selected (one per litter) for each of the drug treatment groups described below. The rat pups were tested for 10 days between ages PN27 and PN38 (adolescence). Although the exact time span for adolescence in rats varies somewhat dependent upon sex and strain, the period from PN28 to PN42 has been most frequently cited (Spear 2000) and has been used here. Drug naïve male rats (Harlan) in the ‘adult’ condition (>PN65) were also pair-housed with a same sex rat that would receive the same treatment. They were allowed to acclimate to the animal facility for at least 1 week before testing. (Hence, testing of adult rats did not begin until they were at least 70 days of age.) Throughout the experiment, all rats had free access to food and water in their home cages. Ten rats were used for each group dosed with saline, cocaine and ketamine. Six rats were tested with each dose of MDMA and Δ9-THC. The studies reported in this manuscript were carried out in accordance with guidelines published in guide for the care and use of laboratory animals (National Research Council 1996) and were approved by our Institutional Animal Care and Use Committee.
Apparatus
Clear plastic rat cages (22.5 cm width × 44 cm length × 20 cm height) were housed in sound-attenuating cabinets and were used as locomotor chambers. Each cabinet contained up to 12 chambers, with a maximum of two per shelf. Chambers did not contain bedding and were wiped with alcohol solution between sessions. Sessions occurred in darkness (i.e. with the cabinet doors closed). A cage rack system with 4 × 8 equally spaced photocell beams on the X- and Y-axes (Lafayette Instrument, Lafayette, IN, USA) was placed around each chamber (4.5 cm from bottom of cage). Two measures of locomotor activity were obtained: ambulatory activity (operationally defined as total number of beam breaks of sequential photocells) and fine movements (operationally defined as consecutive breaks of the same photocell beam with a refractory period of 1 second after each count).
Procedure
Male pups and adult rats were randomly assigned to receive saline or one dose of one of the test drugs (see drug section). Litter was used as the unit of analysis for the pups. Rat pups in the different dose groups for each drug were chosen from different litters. Dosing and testing began on PN27 (adolescent rats) or PN70 or later (adults). On test days, each rat was transported to the laboratory, was injected with its assigned dose of drug or saline and placed in one of the locomotor chambers for a 20-minute session. After the session, the rat was returned to its home cage. For the next 4 days, this sequence of drug injection followed by locomotor activity assessment was repeated. On days 6 and 7 of the experiment (PN32—PN33), rats were left undisturbed in their home cages in the vivarium. Subsequently, the rats received five more daily injection and testing sessions (PN34—PN38). Throughout the dosing regimen, an individual rat was always tested in the same locomotor chamber. The purpose of the interruption in daily drug injections was to determine the effects of intermittent (versus continuous) administration, as the former has been shown to enhance development of sensitization to locomotor stimulants (Himnan 1984; Wiaderna & Tomas 2000, 2002). In order to complete all testing during the short duration of adolescence in rats (approximately 2 weeks), a couple of modifications were made to the experimental design, as compared with those used in previous studies. First, the delay between drug administration periods was shortened to 2 days. Second, habituation to the locomotor chambers prior to drug administration was not included in the study design. Nevertheless, ambulatory activity remained relatively constant across all days of the study in rats of both age groups that received saline.
Drugs
Cocaine [National Institute on Drug Abuse (NIDA), Bethesda, MD] and MDMA (NIDA) were dissolved in saline. Ketamine (Phoenix Scientific, Inc., St. Joseph, MO, USA) was diluted with saline from a commercial stock of 100 mg/ml. Δ9-THC (NIDA) was mixed in a vehicle of absolute ethanol, Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ, USA), and saline in a ratio of 1:1:18. All injections were administered intraperitoneally at a volume of 1 ml/kg. Except for Δ9-THC, placement in the locomotor chambers occurred immediately after injection. Rats were placed in the locomotor chambers 30 minutes after injection with Δ9-THC. Drugs and doses tested were saline, cocaine (7 and 15 mg/kg), ketamine (3 and 10 mg/kg), MDMA (3 and 10 mg/kg at both ages and 30 mg/kg in adolescents only), and Δ9-THC (0.03, 0.1, 0.3 and 1 mg/kg).
Data analysis
Ambulatory activity was operationally defined as total number of interruptions of sequential photocell beams during the 20-minute session. Fine motor movement was operationally defined as total number of consecutive interruptions of the same photocell beam (with 1 second refractory period after each count) during the 20-minute session. Mean (±SEM) values for the dependent measures were calculated across dose and time for each age separately. A single group of rats of each age was tested with saline. Data for the age-appropriate saline group were used in each ANOVA and are presented on each figure for ease of comparison. Drug data for each age were analyzed separately through the use of separate mixed factorial ANOVAs (dose × repeated time) for each drug. Comparisons of patterns of differences (versus respective saline control) were used to assess age-dependence of the effects. Due to experimenter error, the computer interface for the photocell beams was not operational on day 4 of dosing for five of the six adult rats that received 0.3 mg/kg Δ9-THC. In order to proceed with data analysis, data for this day only were omitted from analysis for the adult rats that received any dose of Δ9-THC, resulting in nine timepoints for the analysis. Means for this day are also not included on the graph (Fig. 4). When any ANOVA was significant, Tukey–Kramer post hoc tests (α = 0.05) were used to compare individual means.
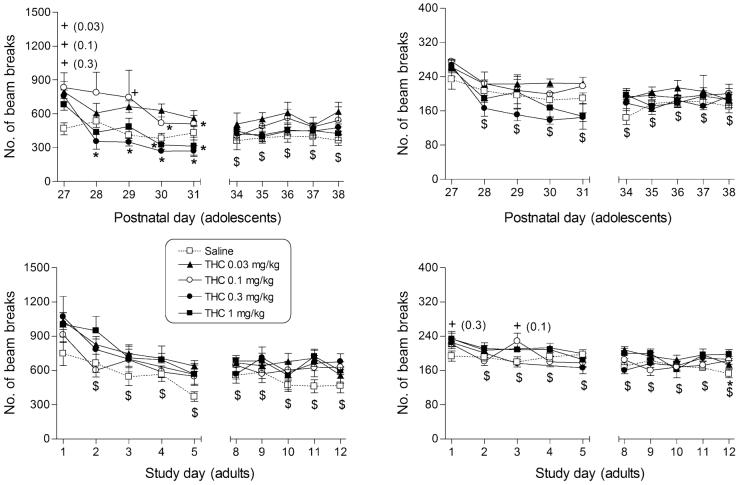
Figure 4.
Effects of Δ9-THC on ambulation (left panels) and fine motor counts (right panels) in male rats during adolescence (top panels) and in adulthood (bottom panels). Points represent mean (±SEM) locomotor counts during the 20-minute session, separated into counts of sequential breaks of adjacent photocell beams for ambulation and counts of consecutive breaks of the same photocell beam for fine movements. For each drug dose group, n = 6 and for each saline group, n = 10. *indicates significant difference (P < 0.05) from day 1. +indicates significant dose—time interaction with post hoc difference from saline. #indicates significant main effect of dose (P < 0.05), as compared with saline. $indicates significant main effect of time (P < 0.05), as compared with PN27 for adolescents or day 1 for adults.
RESULTS
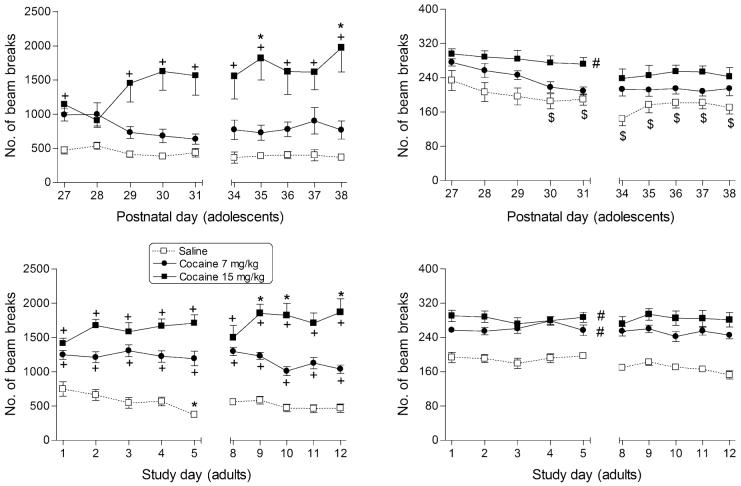
Adolescents were less sensitive to the locomotor stimulation produced by cocaine than were adults. Whereas both 7 and 15 mg/kg significantly increased ambulatory activity (compared with saline) in adult rats (Fig. 1, bottom left panel; main effect for dose: F2,240 = 96.2, P < 0.05), activity in adolescent rats was only increased at the 15 mg/kg dose (Fig. 1, top left panel; main effect for dose: F2,242 = 21.0, P < 0.05). Furthermore, significant dose—time interactions were obtained for both age groups (F18,240 = 2.9, P < 0.05 and F18,242 = 2.6, P < 0.05 for adults and adolescents, respectively). Post hoc analysis revealed that adults showed increased ambulatory activity (compared with saline) for both doses initially and across all test days. In contrast, initial increases in ambulation (compared with saline) were observed only for the 15 mg/kg dose of cocaine in adolescents. While 15 mg/kg cocaine significantly increased ambulatory activity on everyday except the second test session, 7 mg/kg cocaine did not significantly affect activity in adolescents during any session over the entire course of the experiment. Activity increases in the ambulation measure (compared with day 1) that were indicative of sensitization occurred at both ages, although they were more statistically reliable in adults than in adolescents. Nevertheless, overall magnitudes of the difference between initial ambulatory activity and that observed during the final test session were approximately similar across ages following repeated injection with 15 mg/kg cocaine. Hence, both adults and adolescents exhibited sensitization to the stimulating effects of 15 mg/kg cocaine on ambulatory activity.
Figure 1.
Effects of cocaine on ambulation (left panels) and fine motor counts (right panels) in male rats during adolescence (top panels) and in adulthood (bottom panels). Points represent mean (±SEM) locomotor counts during the 20-minute session, separated into counts of sequential breaks of adjacent photocell beams for ambulation and counts of consecutive breaks of the same photocell beam for fine movements. Each early adolescent and adult dose group contained 10 male rats. *indicates significant difference (P < 0.05) from day 1. +indicates significant dose—time interaction with post hoc difference from saline. #indicates significant main effect of dose (P < 0.05), as compared with saline. $indicates significant main effect of time (P < 0.05), as compared with PN27 (for adolescents).
In contrast, neither age group displayed sensitization (or tolerance) to the stimulatory effects of cocaine on fine motor movements, although a significant time-related decrease in fine movements was noted for the adolescent saline group (Fig. 1, upper right panel; main effect of time: F9,242 = 9.2, P < 0.05). In addition, overall sensitivity to these effects was less in adolescents. Whereas both 7 and 15 mg/kg doses of cocaine increased fine movements in adult rats (Fig. 1, lower right panel; main effect of dose: F2,240 = 82.1, P < 0.05), only the higher dose was effective in adolescents (Fig. 1, upper right panel; main effect of dose: F2,242 = 11.5, P < 0.05).
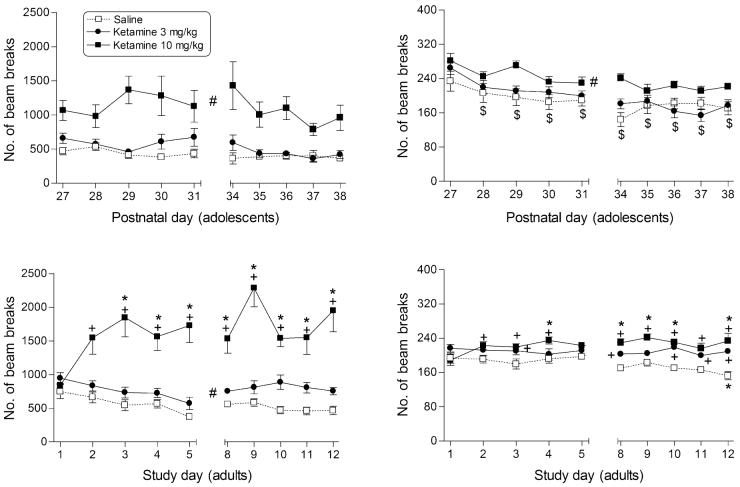
As presented in Fig. 2, ketamine also produced an age-dependent pattern of ambulatory activity increases (left panels). Just as adolescents were less sensitive to the loco-motor stimulatory effects of cocaine, they were also less sensitive to those of ketamine (Fig. 2, top left panel). Adults showed significantly increased locomotor activity at doses of 3 and 10 mg/kg ketamine (compared with saline; main effect for dose: F2,243 = 108.0, P < 0.05), albeit the magnitude of the increase was considerably smaller for the 3 mg/kg than for the 10 mg/kg dose (Fig. 2, bottom left panel). In contrast, ambulatory activity was significantly increased only by 10 mg/kg ketamine in the adolescents (main effect for dose: F2,241 = 19.5, P < 0.05). Furthermore, sensitization to ketamine occurred in adults (dose–time interaction: F18,243 = 3.0, P < 0.05), but not in adolescents (i.e. dose–time interactions were not significant, P > 0.05). In adults, the initial injection with 10 mg/kg ketamine resulted in saline levels of ambulatory activity. By the second session, however, activity was significantly higher than that seen with saline. On day 3 (and continuing to the final day of the dosing regimen), 10 mg/kg ketamine increased ambulatory activity compared with its initial absence of effect on day 1.
Figure 2.
Effects of ketamine on ambulation (left panels) and fine motor counts (right panels) in male rats during adolescence (top panels) and in adulthood (bottom panels). Points represent mean (±SEM) locomotor counts during the 20-minute session, separated into counts of sequential breaks of adjacent photocell beams for ambulation and counts of consecutive breaks of the same photocell beam for fine movements. Each early adolescent and adult dose group contained 10 male rats. *indicates significant difference (P < 0.05) from day 1. +indicates significant dose—time interaction with post hoc difference from saline. #indicates significant main effect of dose (P < 0.05), as compared with saline. $indicates significant main effect of time (P < 0.05), as compared with day 27 (for adolescents).
Ketamine’s pattern of effects on fine motor movements mimicked its effects on ambulation. In adolescents, ketamine produced increases in fine movements at the 10 mg/kg dose, but not at 3 mg/kg (Fig. 2, upper right panel; main effect for dose: F2,241 = 7.1, P < 0.05). In adults, both doses of ketamine increased fine movements and sensitization to this effect was observed at the 10 mg/kg dose (Fig. 2, lower right panel; time–dose interaction: F18,243 = 2.4, P < 0.05). The magnitude of these increases, although significant, was small as compared with those observed with cocaine.
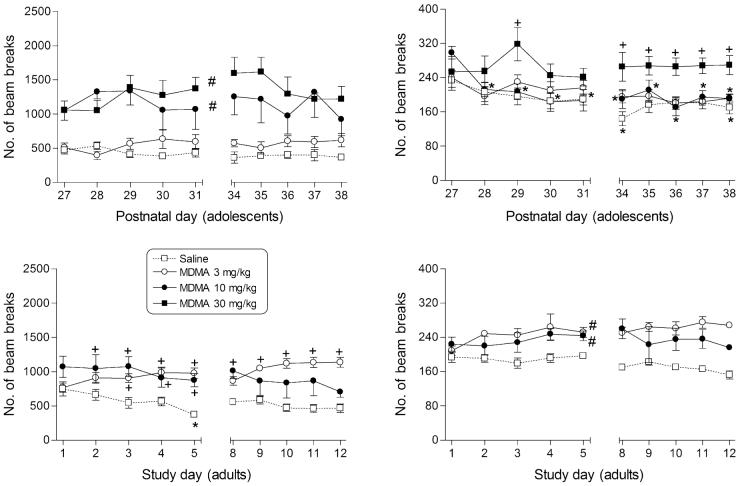
MDMA significantly increased ambulatory activity in adolescents at doses of 10 and 30 mg/kg (main effect for dose: F3,215 = 18.8, P < 0.05; Fig. 3, top left panel). In adults, neither dose tested (3 or 10 mg/kg) significantly affected ambulatory activity (compared with saline) after acute injection; however, increases in ambulation (compared with saline, but not to day 1) were observed at both 3 and 10 mg/kg on a number of subsequent tests throughout the 2-week dosing regimen (main effect for dose: F18,171 = 2.6, P < 0.05; Fig. 3, bottom left panel). Dosing with 30 mg/kg MDMA could not be completed in adults due to toxicity (including lethality) within the first week of administration. In contrast, lethality following repeated administration of this high dose of MDMA did not occur in adolescents and all were able to complete the entire dosing regimen. Sensitization to the effects of MDMA on ambulation (compared with respective day 1) did not occur at either age.
Figure 3.
Effects of MDMA on ambulation (left panels) and fine motor counts (right panels) in male rats during adolescence (top panels) and in adulthood (bottom panels). Points represent mean (±SEM) locomotor counts during the 20-minute session, separated into counts of sequential breaks of adjacent photocell beams for ambulation and counts of consecutive breaks of the same photocell beam for fine movements. For each drug dose group, n = 6 and for each saline group, n = 10. *indicates significant difference (P < 0.05) from day 1. +indicates significant dose—time interaction with post hoc difference from saline. #indicates significant main effect of dose (P < 0.05), as compared with saline.
MDMA affected fine motor movements in both age groups. In adolescents, MDMA stimulated fine motor movements primarily during the second week of administration and only at the 30 mg/kg dose (Fig. 3, upper right panel; dose—time interaction: F27,242 = 1.6, P < 0.05). Lower doses (3 and 10 mg/kg) decreased fine movements over time (compared with PN27, but not to saline), suggesting development of tolerance. Post hoc analysis of the main effect for dose for adults indicated that both 3 and 10 mg/kg MDMA increased fine motor movements (F2,171 = 30.2, P < 0.05; Fig. 3, lower right panel).
A significant dose—time interaction was obtained for the Δ9-THC ambulation data in adolescents (F36,260 = 1.9, P < 0.05; Fig. 4, top left panel), but not in adults (F32,231 = 1.2, P > 0.05; Fig. 4, bottom left panel). In adolescents, the lower doses of Δ9-THC initially increased ambulatory activity, with significant increases (compared with saline) noted at 0.03, 0.1 and 0.3 mg/kg. By the second injection, however, ambulatory activity had returned to saline levels at all doses and continued at such with one exception (0.1 mg/kg on PN29). In contrast, Δ9-THC did not significantly affect ambulatory activity of adult male rats over the dose range tested. A significant main effect of time was noted in adults, with post hoc determination of decreased ambulation (compared with day 1) on all subsequent test days (F32,231 = 26.5, P < 0.05; Fig. 4, bottom left panel).
Δ9-THC produced minimal effects on fine motor movements in either age group. Adolescents in the saline group showed a time-related decrease in fine movements, which was not altered by administration of Δ9-THC (main effect of time: F9,260 = 12.1, P < 0.05; Fig. 4, upper right panel). In adults, significant increases in fine movements were observed following Δ9-THC injection at isolated times through the first week of dosing; however, the general pattern was one of time-related decrease in their occurrence without dose dependence (dose—time interaction: F32,231 = 2.1, P < 0.05; Fig. 4, lower right panel).
DISCUSSION
Cocaine was used as the prototypic psychomotor stimulant to verify the effectiveness of the procedure used in the present study to induce sensitization. In the present study, cocaine produced increases in ambulatory activity when administered acutely to male adult rats and produced sensitization to this locomotor stimulation with repeated administration, as has been shown previously in a wide variety of procedures (Vanderschuren & Kalivas 2000). Previous studies have also found that acute cocaine increases ambulation in adolescent rats, albeit initial sensitivity to the stimulant effects of cocaine and other catecholamine agonists was less in adolescents than in adults (present study; Spear & Brake 1983; Laviola et al. 1995). Investigations of locomotor sensitization following repeated cocaine in adolescent rats are contradictory (Laviola et al. 1995; Collins & Izenwasser 2002; Frantz, O’Dell & Parsons 2007), although this may have resulted, in part, due to differences in the ages at which sensitization induction and testing occurred: with both induction and assessment within the adolescent period when sensitization was reported (present study; Frantz et al. 2007; Laviola et al. 1995) or separately during adolescence and after puberty when it was not (Collins & Izenwasser 2002). Cocaine doses used to produce sensitization were also substantially higher in the latter study and only a single uninterrupted subchronic dosing period was used, which has been shown to produce less sensitization than multiple dosing periods separated by a ‘drug holiday’ (as in the present study; Davidson et al. 2002). Together, these results suggest that interactions of conditioning factors, age, dosing regimen and age-dependent differences in initial sensitivity may play a large role in whether or not sensitization develops.
The club drug ketamine (Special K, Vitamin K, K) is a dissociative anesthetic that, at subanesthetic doses, produces a dissociated, ‘out of body’ state, tactile sensory distortion, mild hallucinatory effects, and sedative properties (Krystal et al. 1994). Although ketamine interacts with multiple receptors in the central nervous system, its action as a non-competitive N-methyl-D-aspartate (NMDA) receptor channel blocker is believed to mediate its behavioral effects (Bennett, Bernard & Amrick 1988; Narita et al. 2001). In the present study, ketamine’s stimulant effects in adolescents were less pronounced than its effects in adults. Of particular interest is the finding that ketamine did not produce sensitization in male adolescent rats under conditions where it produced pronounced sensitization in adult male rats. Furthermore, this effect was not solely a function of differences in the acute effects of ketamine, as the absolute magnitude of ketamine-induced activity observed in adult male rats at later time points exceeded that seen in adolescent rats at any time during the dosing regimen. It was also not a function of the baseline rate of behavior, as adolescents also showed less sensitivity and no sensitization to ketamine’s stimulatory effects on fine movement, a behavior that occurred less frequently than did ambulation. Interestingly, some of ketamine’s effects (e.g. emergence delirium) are also age dependent in humans, with sensitivity beginning during later adolescence (White, Way & Trevor 1982; Reich & Silvay 1989). In female rats (males were not tested), sensitivity to the neurotoxic effects of the NMDA channel blocker MK-801 did not begin to appear until PN45 (Farber et al. 1995), suggesting that decreased sensitivity to this class of compounds during adolescence may encompass more than their effects on locomotion. Although adolescent rodents are less sensitive to some effects of NMDA antagonists, long-term consequences of adolescent exposure to these drugs have been demonstrated in adult rats. For example, male rats that received subchronic phencyclidine twice daily for 7 days beginning at PN42 exhibited decreased locomotion, disturbed social behavior, and increased number of errors in a spatial learning task when tested as adults (>PN70) (Schwabe, Klein & Koch 2006). In addition, glutamate has been shown to be involved in timing of puberty (Parent, Matagne & Bourguignon 2005), raising the possibility that subchronic blockade at NMDA receptors may affect this hallmark of adolescent development.
The mechanism of action of MDMA (XTC, ecstasy, X, Adam) differs from that of ketamine. Acute MDMA increases the carrier-mediated release and inhibition of reuptake of 5-HT and DA. With chronic use, MDMA administration is associated with decreased levels of synthesis and decreased reuptake of 5-HT, effects that have been shown to be neurotoxic to these neurons in multiple species (Easton & Marsden 2006). (Broening, Bacon & Slikker 1994) found that adult rats were more sensitive to the neurotoxic effects of MDMA on 5-HT neurons than were adolescent rats, a finding that is consistent with increased lethality of MDMA in adult rats in the present study. Similarly, male adult rats showed greater sensitivity to the locomotor stimulant effects of MDMA, with increased ambulation and increases in fine movements occurring at 3 and 10 mg/kg doses in adult rats, but only at doses of 10 mg/kg and higher in adolescents. While sensitization did not occur at either age, adult rats that received 10 mg/kg MDMA were less responsive to its stimulant effects on ambulation during the second week of administration than during the first week. Similarly, adolescents were less sensitive to the stimulatory effects of this dose of MDMA on fine movements at later time points. This decreased response to MDMA with repeated dosing could be suggestive of tolerance development. Alternatively, it is possible that repeated dosing with the higher 10 mg/kg dose of MDMA was increasingly toxic to the adult animals, resulting in a non-specific decrease in ambulation. MDMA-induced increases in fine movements in adults, however, were not similarly affected. Consistent with the present results, a previous study also reported that adolescents were less sensitive to the loco-motor stimulant effects of MDMA than were adults (Aberg et al. 2007). Interestingly, exposure to MDMA during adolescence (but not during adulthood) enhanced the later value of cocaine as a conditioned reinforcer (Fone et al. 2002; Achat-Mendes, Anderson & Itzhak 2003; Aberg et al. 2007). In contrast, adolescent exposure to 30 mg/kg MDMA in the present study did not confer protection against the toxicity of this dose when rats were re-tested as adults (data not shown).
While both cocaine and MDMA have direct effects on catecholamine neurotransmission, Δ9-THC’s effects are mediated via its activation of CB1 cannabinoid receptors in the brain (Devane et al. 1988; Compton et al. 1993). Acute Δ9-THC produces biphasic effects on locomotor activity in adult and adolescent rodents: at low doses it increases activity whereas it decreases activity at higher doses (Martin et al. 1991; Sañudo-Peña et al. 2000; Wiley et al. 2007). For the present study, lower doses of Δ9-THC were chosen for evaluation of sensitization development. Previously reported findings are conflicting regarding whether or not cannabinoid sensitization develops in adult rats (Arnold et al. 1998; Cadoni et al. 2001). In the present study, Δ9-THC produced initial increases in activity in adolescents, but not in adults. Interestingly, adolescents were also more sensitive to the initial suppression of activity induced by higher doses of Δ9-THC (Wiley et al. 2007). Sensitization to the stimulant effects of Δ9-THC did not develop at either age in the present study. In contrast, rapid tolerance developed to the acute increases in activity induced by low doses of Δ9-THC in adolescents (present study) and to the decreases in activity induced by higher doses of Δ9-THC in both adolescents and adults (Wiley et al. 2007). Together, these results suggest that locomotor activity undergoes modulatory regulation following repeated dosing with Δ9-THC; i.e. initial increases in activity produced by low doses disappeared by the second injection (present study) and initial decreases in activity produced by higher doses showed tolerance (Wiley et al. 2007).
Overall, the results of this study show that male adolescent rats tend to be less sensitive than adult male rats to the acute locomotor stimulant effects of abused substances with several different mechanisms of action (i.e. cocaine, ketamine and MDMA). In addition, adolescents exhibited less sensitization to ketamine. In contrast, adolescent rats were more sensitive to the initial stimulatory effects of Δ9-THC, although tolerance rapidly developed. Although possible, it is unlikely that pharmacokinetic factors are entirely responsible for the observed age differences in sensitivity, as decreased sensitivity in adolescents occurred with a subset of drugs having similar effects on behavior, but different metabolic pathways (Woolf & Adams 1987; Maurer et al. 2004, Maurer, Sauer & Theobald 2006). Differences in brain development and greater plasticity of the adolescent nervous system represent a more parsimonious explanation of the observed differential responsivity to substances of abuse between male adolescent and adults, a hypothesis that has received support from previous studies examining age-dependent differences in neurotransmission following administration of these abused substances to adolescent versus adult rodents (Collins & Izenwasser 2002; Morley-Fletcher et al. 2002; Badanich, Adler & Kirstein 2006; Frantz et al. 2007).
To the extent that these results can be applied to human adolescents, the present results have several translational implications. First, adolescents are likely to exhibit differential sensitivity to the initial effects of an abused drug and the nature of these differences may be dependent upon the specific drug or drug class. As shown in the present study, for example, male adolescents were less sensitive to the stimulatory effects of cocaine, MDMA and ketamine, but more sensitive to those of Δ9-THC. It would not be unreasonable to suppose that this differential sensitivity might have consequences for behavior upon initial use of the drug (e.g. decreased behavioral impairment while under the influence). Second, adolescents are also likely to show differential sensitivity to the effects of repeated administration of an abused drug. For example, in the present study male adolescents showed less sensitivity to sensitization induced by cocaine and ketamine and greater tolerance to the effects of Δ9-THC than did male adults. A previous study has shown that this decreased sensitization is not associated with decreased acquisition of cocaine self-administration or with amount of cocaine administered (Frantz et al. 2007), suggesting that the degree of sensitization may not reflect the degree of salience of the drug reinforcer as proposed by sensitization theory. Nevertheless, it is likely that decreased sensitization and increased tolerance is predictive of other differences in behavioral response to these abused drugs, including a differential course of adaptation to initial behavioral impairment. Furthermore, exposure to drugs of abuse during adolescence may have long-term consequences on subsequent substance abuse that are not necessarily apparent during adolescence. These results, combined with those of other pre-clinical developmental studies, emphasize the need for consideration of age differences in behavioral response to acute and repeated administration of abused substances in development of prevention and early intervention treatment approaches.
Acknowledgements
Research supported by National Institute on Drug Abuse grant DA-016644. Contributions of R.L.E and D.B.G. to this project occurred while each was on internship at VCU from the University of the West of England, Bristol, UK.
References
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29:37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Anderson KL, Itzhak Y. Methylpheni-date and MDMA adolescent exposure in mice: long-lasting consequences on cocaine-induced reward and psychomotor stimulation in adulthood. Neuropharmacology. 2003;45:106–115. doi: 10.1016/s0028-3908(03)00135-7. [DOI] [PubMed] [Google Scholar]
- Arnold JC, Topple AN, Hunt GE, McGregor IS. Effects of pre-exposure and co-administration of the cannabinoid receptor agonist CP 55,940 on behavioral sensitization to cocaine. Eur J Pharmacol. 1998;354:9–16. doi: 10.1016/s0014-2999(98)00433-6. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Banken JA. Drug abuse trends among youth in the United States. Ann N Y Acad Sci. 2004;1025:465–471. doi: 10.1196/annals.1316.057. [DOI] [PubMed] [Google Scholar]
- Barron S, White A, Swartzwelder HS, Bell RL, Rodd ZA, Slawecki CJ, Ehlers CL, Levin ED, Rezvani AH, Spear LP. Adolescent vulnerabilities to chronic alcohol or nicotine exposure: findings from rodent models. Alcohol Clin Exp Res. 2005;29:1720–1725. doi: 10.1097/01.alc.0000179220.79356.e5. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Bernard PS, Amrick CL. A comparison of PCP-like compounds for NMDA antagonism in two in vivo models. Life Sci. 1988;42:447–454. doi: 10.1016/0024-3205(88)90083-5. [DOI] [PubMed] [Google Scholar]
- Broening HW, Bacon L, Slikker W., Jr Age modulates the long-term but not the acute effects of the serotonergic neurotoxicant 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1994;271:285–293. [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. Behavioural sensitization after repeated exposure to Delta 9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology (Berl) 2001;158:259–266. doi: 10.1007/s002130100875. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- Davidson C, Lazarus C, Lee TH, Ellinwood EH. Behavioral sensitization is greater after repeated versus single chronic cocaine dosing regimens. Eur J Pharmacol. 2002;441:75–78. doi: 10.1016/s0014-2999(02)01437-1. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol. 2006;20:194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, Peter H, Olney JW. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38:788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Fone KC, Beckett SR, Topham IA, Swettenham J, Ball M, Maddocks L. Long-term changes in social interaction and reward following repeated MDMA administration to adolescent rats without accompanying serotonergic neurotoxicity. Psychopharmacology (Berl) 2002;159:437–444. doi: 10.1007/s00213-001-0931-z. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, O’Dell LE, Parsons LH. Behavioral and neuro-chemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Himnan DJ. Tolerance and reverse tolerance to toluene inhalation: effects on open-field behavior. Pharmacol Biochem Behav. 1984;21:625–631. doi: 10.1016/s0091-3057(84)80048-9. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Maurer HH, Kraemer T, Springer D, Staack RF. Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis. Ther Drug Monit. 2004;26:127–131. doi: 10.1097/00007691-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Maurer HH, Sauer C, Theobald DS. Toxicokinetics of drugs of abuse: current knowledge of the isoenzymes involved in the human metabolism of tetrahydrocannabinol, cocaine, heroin, morphine, and codeine. Ther Drug Monit. 2006;28:447–453. doi: 10.1097/01.ftd.0000211812.27558.6e. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Bianchi M, Gerra G, Laviola G. Acute and carryover effects in mice of MDMA (‘ecstasy’) administration during periadolescence. Eur J Pharmacol. 2002;448:31–38. doi: 10.1016/s0014-2999(02)01904-0. [DOI] [PubMed] [Google Scholar]
- Narita M, Yoshizawa K, Nomura M, Aoki K, Suzuki T. Role of the NMDA receptor subunit in the expression of the discriminative stimulus effect induced by ketamine. Eur J Pharmacol. 2001;423:41–46. doi: 10.1016/s0014-2999(01)01089-5. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington DC: 1996. [Google Scholar]
- Parent AS, Matagne V, Bourguignon JP. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine. 2005;28:281–286. doi: 10.1385/ENDO:28:3:281. [DOI] [PubMed] [Google Scholar]
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36:186–197. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–274. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Scallet AC, Schmued LC, Slikker W, Jr, Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci. 2004;81:364–370. doi: 10.1093/toxsci/kfh224. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Klein S, Koch M. Behavioural effects of neonatal lesions of the medial prefrontal cortex and subchronic pubertal treatment with phencyclidine of adult rats. Behav Brain Res. 2006;168:150–160. doi: 10.1016/j.bbr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Nicotine and the adolescent brain: insights from an animal model. Neurotoxicol Teratol. 2002;24:369–384. doi: 10.1016/s0892-0362(02)00199-x. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D (1), D (2) and D (4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of pre-clinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Warren MW, Kobeissy FH, Liu MC, Hayes RL, Gold MS, Wang KK. Ecstasy toxicity: a comparison to methamphetamine and traumatic brain injury. J Addict Dis. 2006;25:115–123. doi: 10.1300/J069v25n04_11. [DOI] [PubMed] [Google Scholar]
- White PF, Way WL, Trevor AJ. Ketamine — its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- Wiaderna D, Tomas T. Effects of repeated exposure to toluene or amphetamine on locomotor activity in rats. Int J Occup Med Environ Health. 2000;13:317–324. [PubMed] [Google Scholar]
- Wiaderna D, Tomas T. Assessment of long-term effects of exposure to toluene based on the analysis of selected behavioral responses with particular reference to the ability to trigger behavioral hypersensitivity in rats. Int J Occup Med Environ Health. 2002;15:239–245. [PubMed] [Google Scholar]
- Wiley JL, O’Connell MM, Tokarz ME, Wright MJ., Jr Pharmacological effects of acute and repeated administration of Δ9-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther. 2007;320:1097–1105. doi: 10.1124/jpet.106.108126. [DOI] [PubMed] [Google Scholar]
- Woolf TF, Adams JD. Biotransformation of ketamine, (Z) -6-hydroxyketamine, and (E) -6-hydroxyketamine by rat, rabbit, and human liver microsomal preparations. Xenobiotica. 1987;17:839–847. doi: 10.3109/00498258709043993. [DOI] [PubMed] [Google Scholar]