Abstract
How mechanical information is encoded in the vestibular periphery has not been clarified. To begin to address the issue we examined the intrinsic firing properties of postnatal mouse vestibular ganglion neurons using the whole cell, tight-seal technique in current-clamp mode. We categorized two populations of neurons based on the threshold required to evoke an action potential. Low-threshold neurons fired with an average minimum current injection of −43 pA, whereas high-threshold neurons required −176 pA. Using sine-wave stimuli, we found that the neurons were inherently tuned with best frequencies that ranged up to 40 Hz. To investigate the membrane properties that contributed to the variability in firing properties we examined the same neurons in voltage-clamp mode. High-threshold neurons had larger cell bodies and whole cell capacitances but a resting conductance density of 0.18 nS/pF, nearly identical to that of low-threshold neurons, suggesting that cell size was an important parameter determining threshold. We also found that vestibular ganglion neurons expressed a heterogeneous population of potassium conductances. TEA-sensitive conductances contributed to the position of the tuning curve in the frequency domain. A 4-AP–sensitive conductance was active at rest and hyperpolarized resting potential, limited spontaneous activity, raised threshold, and prevented repetitive firing. In response to sine-wave stimulation 4-AP–sensitive conductances prevented action potential generation at low frequencies and thus contributed to the high-pass corner of the tuning curve. The mean low-pass corner (about 29 Hz) was determined by the membrane time constant. Together these factors contributed to the sharply tuned, band-pass characteristics intrinsic to postnatal vestibular ganglion neurons.
INTRODUCTION
To illuminate fundamental mechanisms of sensory signaling in the vestibular periphery, we examined the primary sensory neurons that encode and convey vestibular information from the inner ear organs of balance to the brain stem. The study was designed to characterize the intrinsic firing patterns of postnatal mouse vestibular ganglion neurons isolated from their peripheral and central targets and to begin to identify the membrane properties that shape the neuronal response to vestibular input.
In mammals, the utricle and saccule detect linear acceleration and gravity and three semicircular canals detect rotational head movements in orthogonal planes, all critical for maintaining balance and, through the vestibuloocular reflex, a stable visual world. Low-frequency stimuli (up to a few hertz) are generated by intentional head movements, whereas higher frequencies (tens of hertz) are generated by vibrational stimuli that occur during vigorous activity, such as running (Armand and Minor 2001; Grossman et al. 1988).
The vestibular ganglia contain bipolar neurons with efferents that project to the vestibular nuclei of the brain stem and afferents that contact mechanosensory hair cells distributed among the five sensory end organs. Within the sensory epithelium of each end organ there are three classes of afferent contacts: 1) bouton afferents are concentrated in the periphery and contact cylindrical type II hair cells; 2) calyx terminals engulf flask-shaped type I cells concentrated in central regions; and 3) dimorphic afferents are distributed throughout the epithelium and have both bouton and calyceal terminals (Fernández et al. 1988; Goldberg et al. 1990; Lysakowski and Goldberg 1997).
The firing properties of the three classes of vestibular afferents have been characterized using sharp electrodes to record from semi-intact preparations (Goldberg 2000; Highstein et al. 2005). A continuum of afferent responses have been described that range from regular to irregular based on spontaneous firing rates. Irregular neurons have significant variability in interspike interval, larger diameters with calyx terminals, greater sensitivity, and phasic responses. Regular neurons have less variable interspike intervals, smaller diameters with bouton terminals, lower sensitivity, and tonic responses. Dimorphic afferents have intermediate properties. Although the firing properties have been well described, whether the diversity in responses arises from differences within the sensory hair cells, the afferent synapses, or within the neurons themselves has not been clarified.
Several studies have examined sensory signaling in neonatal mouse vestibular hair cells (Eatock et al. 2002; Géléoc et al. 2004; Holt et al. 1998, 1999) but the afferent synapse and the firing properties of rodent vestibular ganglion neurons have received little attention until recently (Autret et al. 2005; Limón et al. 2005; Rennie and Streeter 2006). Here we report the first characterization of the whole cell firing properties of postnatal mouse vestibular ganglion neurons. We found that the neurons had diverse responses to injected currents and that they were inherently tuned to frequencies ≤40 Hz, which spans the sensitive range of mammalian vestibular organs (Grossman et al. 1988, 1989; Hullar and Minor 1999). We hypothesize that some of the diversity in the firing properties of vestibular ganglion neurons arises from the intrinsic membrane properties of the neurons that, in turn, result from a heterogeneous population of potassium conductances. Limón et al. (2005) identified a calcium-dependent potassium current in rat vestibular neurons and suggested that differential expression of the current may contribute to firing diversity. Chabbert et al. (2001) characterized three distinct potassium currents in postnatal mouse vestibular neurons, two of which were inhibited by the potassium channel antagonists tetraethylammonium chloride (TEA) and 4-aminopyridine (4-AP). Consistent with previous reports, we found that the potassium channel antagonists TEA and 4-AP revealed three populations of potassium conductances: a TEA-sensitive conductance, a 4-AP–sensitive conductance, and a TEA- and 4-AP–insensitive conductance. Here we describe how the TEA- and 4-AP–sensitive conductances contribute to the diversity in the firing patterns of postnatal mouse vestibular ganglion neurons.
METHODS
Tissue culture
Vestibular ganglia were excised from Swiss Webster mice (Hilltop Lab Animals, Scottdale, PA or Taconic Farms, Germantown, NY), ranging in age from 0 to 12 days postnatal (P0–P12), in accordance with a protocol (#3123) approved by the University of Virginia Animal Care and Use Committee. Mice were decapitated and superior vestibular ganglia were removed by severing the peripheral afferent fibers and the fiber tracts of the vestibular branch of the eighth cranial nerve. No enzymatic dissociation was used. The ganglia were gently teased apart to expose the central region and placed as explant cultures onto glass coverslips coated with Cell-Tak (BD Biosciences 354240) and maintained in vitro overnight at 37°C to allow for recovery from the dissection process. Dissections and culture were performed in MEM solution (Invitrogen, 41090-036) supplemented with 25 mg ampicillin and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4.
Immunofluorescence
To examine the morphology of the vestibular neurons, cultures were fixed and stained with neurofilament 200 monoclonal antibody (NF200, Sigma), as described by Mo and Davis (1997). Briefly, ganglia were fixed in 100% methanol at −20°C for 6 min followed 0.1% Triton-X for 15 min. The tissue was incubated in a blocking solution that contained 5% normal goat serum in phosphate-buffered saline (PBS) at room temperature for 2 h. The primary antibody was applied at 1:400 and incubated overnight at 4°C. The secondary antibody, goat anti-mouse IgG1 FITC (Sigma), was applied at 1:40 for 1 h at room temperature. Between each step, the tissue was washed with 0.1 M PBS three times for 5 min. For control experiments, the primary antibody was omitted. The tissue was mounted onto glass slides and imaged with a Zeiss LSM510 confocal microscope (Oberkochen, Germany).
To facilitate identification of neurons, the diameter of neuronal cell bodies was estimated in Adobe Photoshop 7.0 using confocal images of fixed samples and compared with differential interference contrast (DIC) images of living samples. Cell diameters at their largest dimension were measured directly from the digital images.
Electrophysiology
Vestibular ganglion neurons were placed into a recording chamber and viewed with a Zeiss Axioskop FS equipped with a 63× water-immersion lens and DIC optics. Recording pipettes were pulled from borosilicate capillary glass (R-6, Garner Glass) with resistances that ranged from 3 to 6 MΩ. The pipette tips were coated with ski wax to reduce pipette capacitance.
The whole cell, tight-seal recording technique was used in both voltage- and current-clamp modes. Neurons were held at −84 mV and data were acquired at room temperature (22–24°C) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA), filtered at 1 kHz with a low-pass Bessel filter, digitized at 5 kHz with a 12-bit acquisition board, and acquired using pClamp 8.0 software (Axon Instruments). The mean series resistance was 13 ± 7.8 MΩ (n = 60) and was compensated post hoc.
Data were included in the analysis if they met the following criteria in voltage-clamp mode: expression of neuronal-type Na+ currents and a holding current of positive to −100 pA at −84 mV. In current-clamp mode, the criteria for data analysis included stable resting potentials at hyperpolarized levels (≤−50 mV) as well as the ability to fire action potentials. Action potentials were characterized by voltage spikes with a clear upward inflection and an amplitude of ≥30 mV. If at any point during a recording the cell failed to meet these criteria, subsequent data were excluded from analysis. We observed no morphological or physiological evidence of the formation of reciprocal connections between neurons in culture.
Solutions
For electrophysiological recordings, cells were bathed in a standard extracellular solution that contained (in mM): 140 NaCl, 5 KCl, 1.3 CaCl2, 1 MgCl2, 10 HEPES, vitamins (1:100), and amino acids (1:50; Invitrogen, Carlsbad, CA) as in MEM. The solution was adjusted to pH 7.4 with NaOH and an osmolarity of 303 mOsmol/kg. Recording pipettes were filled with an intracellular solution containing (in mM): 135 KCl, 2.5 Mg-ATP, 0.1 CaCl2, 3.5 MgCl2, 5 ethylene glycol-bis (b-aminoethyl ether)–N,N,N′,N′-tetraacetic acid, and 10 HEPES, adjusted to pH 7.4 with KOH and an osmolarity of 277 mOsmol/kg.
Antagonists were dissolved in the standard extracellular solution and applied and washed out using bath exchange. To block Na+ currents, 1 µM tetrodotoxin (TTX; Sigma, T-5657) was used. Potassium currents were blocked using 10 mM 4-aminopyridine (4-AP; Sigma, A-0152) and/or 10 mM tetraethylammonium chloride (TEA; Sigma, T-2265).
Analysis
Analysis was performed using Clampfit 8.1 software (Axon Instruments) and Origin 7.1 (MicroCal Software, Northampton, MA). All membrane potentials were adjusted for a 4-mV junction potential. Data are presented as means ± SD, unless otherwise noted, and independent, two-sample t-tests were used to determine statistical significance.
To determine the activation and inactivation range for Na+ and K+ conductances, we used a Boltzmann equation of the type
| (1) |
where Gmax and Gmin represent the maximum and minimum conductances, respectively; V1/2 is the half-maximal voltage, and s represents the slope factor.
To examine the time constant for Na+ channel inactivation, we used a first-order exponential function
| (2) |
where Imax is the current amplitude, t is the time constant of decay, and IO is the offset step.
RESULTS
Morphology of vestibular ganglion neurons
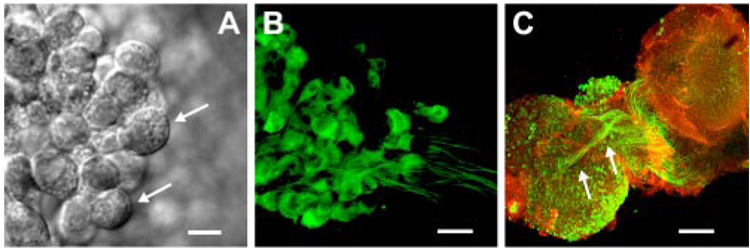
To facilitate identification of vestibular ganglion neurons, we examined their morphology with DIC and confocal microscopy. Acutely excised, living vestibular ganglia were placed in a chamber and viewed with DIC optics, which revealed neuronal cell bodies with diameters that ranged between 13.7 and 25.6 µm with an average diameter of 14.9 ± 2.9 µm (n = 145 neurons from 38 ganglia; Fig. 1A). In a separate series of experiments the tissue was excised, fixed, and stained with NF200 to illuminate neuronal cell bodies and neurites (Fig. 1B). The neurons were bipolar and the fixed cell bodies had an average diameter of 12.4 ± 2.8 µm (n = 101 neurons from six ganglia), slightly smaller than the live cells, as might be expected as the result of shrinkage of the fixed tissue. Neurons were easily distinguished from glial cells based on their morphology. In DIC, glial cells had cell bodies with an average diameter of 9.7 ± 1.2 µm (n = 11) and lacked the bipolar branching pattern that typified vestibular neurons. We were unable to specify which vestibular end organ each neuron innervated (utricle, saccule, or semicircular canal) based on visualization of the ganglia alone. However, for our electrophysiological studies we targeted neurons within the central region of the superior ganglia to increase the probability of selecting neurons that innervated the utricle. Previously, Maklad and Fritzsch (1999, 2003) used retrograde markers and showed that the central region of superior vestibular ganglia contained, primarily, neurons that projected to the utricle. In a few experiments we excised the vestibular ganglion together with the neuronal connections to the utricle sensory epithelium still intact. For those experiments we stained with NF200 to illuminate the neurons and phalloidin to highlight the actin-rich hair bundles in the sensory epithelium (Fig. 1C). Using this approach, we found that many of the neuronal cell bodies within the central region of the superior vestibular ganglia projected to the utricle. Although utricle projection could not be confirmed in the neurons we examined electrophysiologically, their central location within the superior vestibular ganglion suggested that they most likely projected to the utricle.
FIG. 1. Morphology of vestibular ganglion neurons.
A: differential interference contrast (DIC) image of postnatal day 2 (P2) vestibular ganglion neurons. Scale bar = 10 µm. Top arrow points to neuron with diameter of 16.4 µm; bottom arrow indicates a neuron with a diameter of 13.7 µm. B: P1 neurons viewed with confocal microscopy. Neurons were bipolar and were stained with neurofilament 200 (NF200). Scale bar = 20 µm. C: coculture of the superior vestibular ganglion (bottom left) and the utricle (top right) stained with NF200 (green) and phalloidin (red). Scale bar = 200 µm. Arrows indicate fiber tracts from neuronal cell bodies within the central region of the superior vestibular ganglion that project to the utricle.
Both neurons and glial cells were present within our vestibular ganglia explant preparations and each displayed a unique K+ current profile (data not shown). In addition to the morphological differences, we distinguished neurons and glia based on amplitude and kinetics of the K+ current they expressed, as well as the presence or absence of Na+ currents. Glial cells displayed fast activating/fast inactivating K+ currents that were small in amplitude and they lacked Na+ currents entirely. In subsequent sections we present data obtained from cells within the central region of the superior vestibular ganglia that we identified as neurons based on their expression of neuronal type K+ and Na+ currents and their ability to fire action potentials.
Firing properties of vestibular ganglion neurons
Vestibular ganglia were excised from postnatal mice ranging in age from postnatal day 0 to 12 (P0–P12) and maintained in culture overnight. We used a whole cell, tight-seal technique to record voltage-dependent currents in voltage-clamp mode and membrane potential in current-clamp mode. At developmental stages older than P5, we found that the neuronal cell bodies were myelinated and inaccessible to our recording pipettes. In a few cases we were able to break through the myelin sheath of older neurons, although the resulting data did not meet our stringent criteria (see METHODS) and thus were excluded from this study. Of 159 mouse vestibular ganglion neurons aged P0–P5, 79 met criteria and were analyzed in detail. On average, the neurons had a resting potential of −64 ± 6 mV (n = 61). We observed no spontaneous firing of action potentials in current-clamp mode. Spontaneous firing has been widely reported elsewhere based on extracellular recordings from both adult and postnatal vestibular afferent fibers (Baird et al. 1988; Curthoys 1982; Goldberg 2000; Hirvonen et al. 2005; Honrubia et al. 1989; Hullar and Minor 1999; McCue and Guinan 1995; Romand and Dauzat 1982). There are two differences between our preparation and those from which spontaneous firing has been reported, either of which may account for the apparent discrepancy: 1) our experiments were conducted at room temperature (22–24°C), as opposed to mammalian temperatures; and 2) our preparation took advantage of the isolated ganglion in which the synaptic contacts with hair cells have been severed. We favor the latter explanation, which is consistent with the previous suggestion (Goldberg 2000) that spontaneous activity arises from tonic activity at the hair cell afferent synapse rather than within the vestibular neurons themselves. Regardless, the absence of spontaneous activity prevented the categorization of our neurons as regular or irregular, and thus we categorized our neurons based on the following distinction.
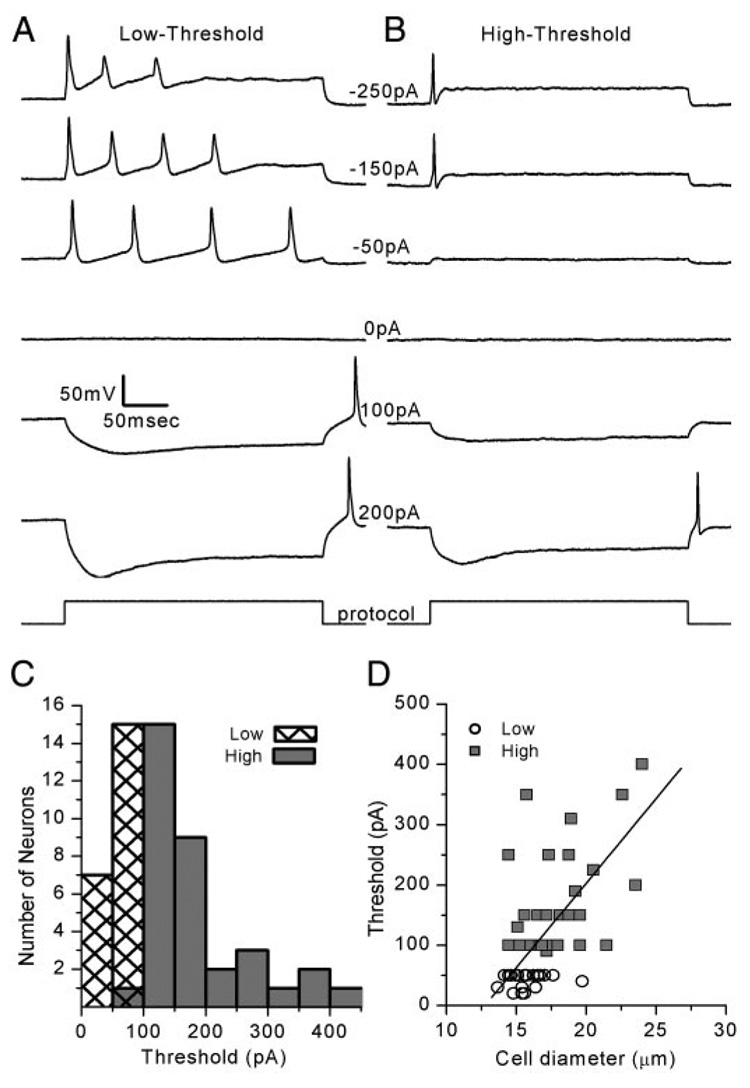
Action potentials were evoked in response to current injections. We injected 300-ms current steps ranging from −250 to 400 pA and recorded the membrane potential response. We grouped the neuronal responses into two broad categories: those that fired action potentials at a low threshold (Fig. 2A) and those that fired at a high threshold (Fig. 2B). Low-threshold neurons required a minimum of −43 ± 12 pA (n = 22) on average to evoke an action potential and 16 of 22 low-threshold neurons fired two or more action potentials with larger current injections. High-threshold neurons required a minimum of −176 ± 106 pA (n = 35), on average, to evoke an action potential and never fired more than one spike in response to step stimuli (Fig. 2B). The thresholds observed for the entire population of low- and high-threshold neurons are depicted in the histogram in Fig. 2C. The distribution was not bimodal but a skewed normal distribution (toward the high-threshold end). Thus our low- and high-threshold distinction was arbitrarily set at 75 pA. However, as we show in subsequent sections, a number of neuronal firing properties paralleled this distinction, which raised the possibility that there may be a biological basis.
FIG. 2. Vestibular ganglion neurons were categorized into 2 groups: low- and high-threshold.
A: membrane potentials of a representative low-threshold neuron in response to current steps at the amplitudes indicated (Cell 20030717E, P2). Multiple action potentials were evoked in 16 of 22 cells. Scale bars apply to all traces. B: membrane potential of a representative high-threshold neuron, which required larger current steps to evoke action potentials (Cell 20030717A, P2). C: histogram of the minimum threshold required to evoke at least one action potential. Mean threshold was −43 ± 12 pA (n = 22) for low-threshold cells and −176 ± 106 pA (n = 35) for high-threshold cells. D: threshold current required to evoke an action potential is plotted as a function of cell diameter. Low-threshold neurons (circles) and high-threshold neurons (squares) were fit with a linear regression with a slope of 28 pA/µm and a correlation coefficient of 0.68.
After the first spike, neurons that fired multiple action potentials (n = 16) displayed a decrement in spike amplitude with subsequent spikes. The spike amplitude decrement occurred predominantly with larger depolarizations. This was evident in the response to the −250- and −150-pA injections in the example of Fig. 2A. For low-threshold neurons that fired three or more action potentials, we calculated the interspike interval, defined as the time between the peaks of adjacent action potentials. In the eight neurons that fired three or more action potentials, we observed a decrease in the mean interspike interval with larger current injections. We speculate that the larger current steps evoked larger depolarizations in the DC component of the membrane potential to the point where there was significant activation of a high-voltage–activated K+ conductance. Activation of a high-voltage–activated K+ conductance might explain both the decrease in spike amplitude and the shorter interspike interval (Fig. 2A), the latter attributed to a decrease in membrane resistance and thus a faster membrane time constant.
At the termination of hyperpolarizing current steps we often noted a rebound spike (Fig. 2, A and B). Interestingly, low-threshold neurons fired rebound spikes after smaller hyperpolarizing current injections (75 ± 44 pA; n = 17). High-threshold neurons that fired rebound spikes required significantly (P < 0.0001) more current to generate the spike: 145 ± 48 pA (n = 18). The larger hyperpolarization and the presence of a rebound spike in the low-threshold neurons for 100- and 200-pA steps suggested that the low-threshold neurons had a higher input resistance (Fig. 2, A and B). Indeed, the input resistance was significantly different (P = 0.02) between the two populations. We used the current evoked by a 5-mV step from −84 mV to estimate input resistance. Low-threshold neurons had a mean input resistance of 549 ± 221 MΩ (n = 22), whereas high-threshold neurons had a lower mean input resistance (416 ± 192 MΩ; n = 39), suggesting that high-threshold neurons had a greater conductance active at rest.
Previously, the magnitude of a Ca2+-activated K+ conductance was correlated with cell size (Limón et al. 2005). Thus we wondered whether cell size might contribute to the differences we noted between low- and high-threshold neurons. We plotted threshold as a function of cell diameter and fit the data with a linear relationship (r = 0.68; Fig. 2D). The cell bodies of neurons that were classified as low-threshold cells had an average diameter of 15.7 ± 1 µm (n = 21), whereas high-threshold cells were larger with an average diameter of 18 ± 3 µm (n = 37). If we assumed a specific capacitance of 0.01 pF/µm2 we predicted that low-threshold neurons would have an average whole cell capacitance of 7.7 pF and high-threshold neurons would have an average capacitance of 10.2 pF, a difference of about 2.5 pF. Actual capacitance measures paralleled our predictions but were roughly 30% larger, perhaps the result of a contribution from partial neurites that were retained in our preparations. Low-threshold neurons had a mean capacitance of 10 ± 4 pF (n = 22) and high-threshold neurons had a mean capacitance of 13 ± 6 pF (n = 37).
To calculate the conductance density at rest we used the actual capacitance measures and input resistances for low- and high-threshold neurons. To our surprise we discovered that the resting conductance density was identical in the two populations of cells: 0.18 nS/pF. Because both cell diameter and cell capacitance were correlated with threshold and because the resting conductance density was identical for the two populations, we suggest that cell size may be an important parameter for determining neuronal threshold. The contribution of voltage- dependent Na+ and K+ conductances to the conductance active at rest and to the firing properties of low- and high-threshold neurons will be considered in subsequent sections.
To explore the frequency responses of vestibular ganglion neurons we injected sinusoidal currents, which may approximate the input to these neurons at the hair cell afferent synapse, and measured membrane potential in current-clamp mode. Much of the work on vestibular afferents has examined their response to low-frequency, sinusoidal stimulation (<2 Hz; Goldberg 2000). Recently, Hullar et al. (2005) extended the range and examined the responses of rodent vestibular afferent neurons at frequencies up to 16 Hz. Interestingly, they reported a rise in sensitivity as a function of frequency but found no evidence for a decline in sensitivity at the high-frequency end. Thus we opted to examine a broad range of frequencies (0.7–175 Hz) with peak-to-peak amplitudes from 20 to 800 pA in intervals as small as 20 pA.
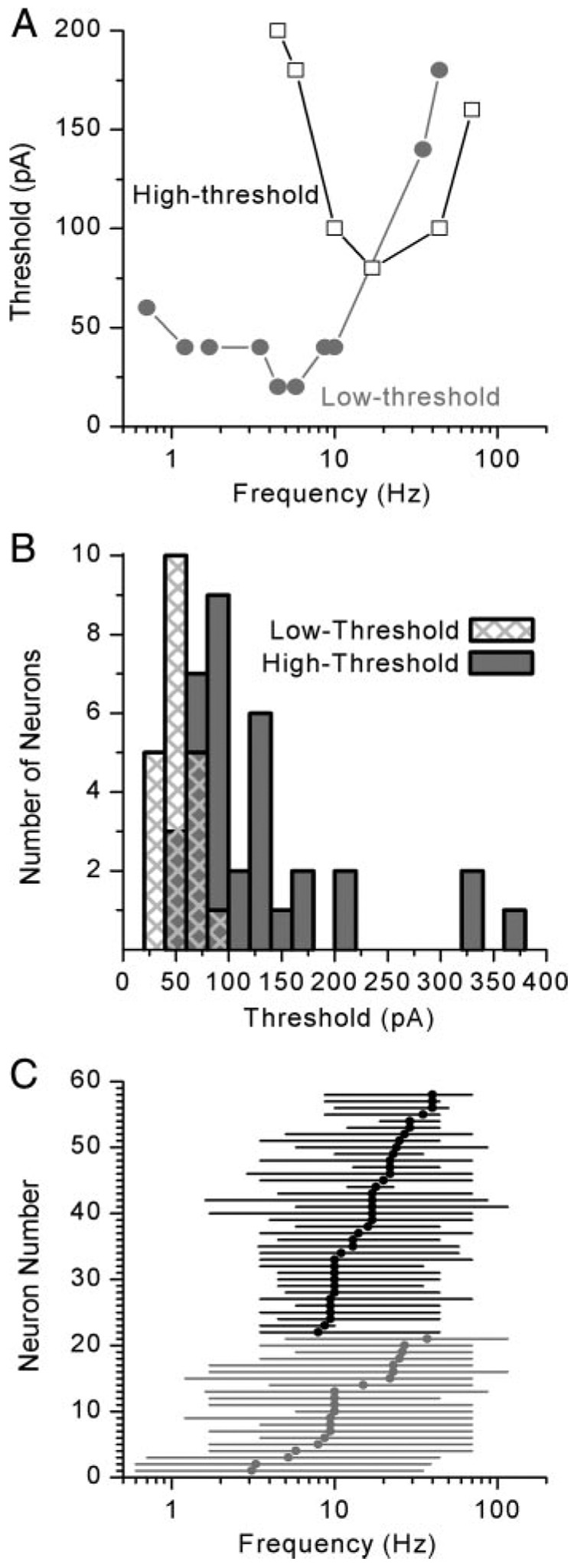
The responses of representative low- and high-threshold cells are shown in Fig. 3. In general, low-threshold neurons required less current to evoke action potentials at low to midrange frequencies (Fig. 3A) than high-threshold neurons (Fig. 3B). The gray areas of Fig. 3 highlight the responses that were phase locked to the stimulus; i.e., the cell responded with at least one action potential during each cycle of a five-cycle burst. Thus the gray area defines the parameter space for which the neurons were responsive. For each neuron we generated a tuning curve by plotting the minimum current required to evoke an action potential during each cycle of the stimulus as a function of stimulus frequency. The tuning curves of two different, yet representative, cells are shown in Fig. 4A. We found that vestibular ganglion neurons were responsive over a broad range of frequencies but were often tuned and had a single best frequency or a narrow range of best frequencies. The high-threshold cell had a sensitive range between 4.5 and 70 Hz and a single best frequency of 17 Hz, whereas the low-threshold neuron was responsive to frequencies between 0.7 and 44 Hz. For cells that responded over a range of frequencies at the minimum threshold, we assigned the middle of the range as the best frequency, 5.2 Hz in the case of the low-threshold neuron in Fig. 4A. Our categorization of low- and high-threshold neurons based on their response to step stimuli held true for sine-wave stimuli as well: the current threshold at the best frequency was lower for low-threshold neurons (Fig. 4A). Low-threshold neurons required an average of −43 ± 17 pA (n = 21) for generation of an action potential during each phase of the five-cycle burst, whereas high-threshold neurons had a mean threshold of −129 ± 107 pA (n = 36) at their best frequency (Fig. 4B).
FIG. 3. Low- and high-threshold neurons were tuned differently to sinusoidal stimuli.
A: voltage traces from a representative low-threshold neuron (Cell 20030717E, P2). Stimulus frequency is indicated along the top and peak-to-peak stimulus amplitude is indicated at the left. B: voltage traces from a representative high-threshold neuron (Cell 20030717A, P2). Shaded boxes highlight the responses that included at least one action potential for each depolarizing phase of the stimulus waveform.
FIG. 4. Tuning characteristics of vestibular ganglion neurons.
A: tuning curves for representative low- (gray) and high-threshold (black) neurons. Low-threshold neurons required less current to evoke an action potential and often fired at lower frequencies than high-threshold neurons (Low-threshold: Cell 20030717E, P2; High-threshold: Cell 20030514A, P2). B: histogram illustrating the threshold for action potential generation for low- (gray bars) and high- (black filled bars) threshold neurons. Low-threshold neurons had a mean threshold of −43 ± 17 pA (n = 21); −129 ± 107 pA (n = 36) for high-threshold neurons. C: sensitive range (lines) and best frequency (symbols) are plotted for each neuron. Low-threshold cells are shown in gray and high-threshold cells in black. Low-threshold neurons were more broadly tuned, with a mean sensitive range that spanned 67 ± 16 Hz (n = 21), whereas high-threshold neurons had a mean sensitive range of 49 ± 21 Hz (n = 37).
To graphically represent the 58 neurons for which we obtained frequency responses, we plotted the sensitive range and best frequency for each cell: low-threshold neurons in gray and high-threshold neurons in black (Fig. 4C). The population of low-threshold neurons from which we recorded were sensitive to frequencies that ranged from 0.6 to 116 Hz. Best frequencies for individual cells ranged between 3.1 and 37 Hz with a median best frequency of 10 Hz (n = 21). The population of high-threshold neurons was sensitive to a similar range of frequencies: from 1.6 to 116 Hz. Yet, the individual cells had narrower sensitive ranges that spanned 49 ± 21 Hz (n = 37), on average, as opposed to the low-threshold cells, which had sensitive ranges that spanned 67 ± 16 Hz (n = 21), on average (P = 0.002). Thus low-threshold cells were more broadly tuned. High-threshold neurons were tuned with best frequencies between 7.9 and 40 Hz with a median best frequency of 17 Hz (n = 37). The broader tuning of low-threshold neurons was also apparent in estimates of the quality of tuning or Q-values. To generate Q-values we divided the best frequency by the bandwidth 40 pA above threshold. The mean Q-value for low-threshold neurons was 0.44 ± 0.04 and 0.73 ± 0.15 for high-threshold cells, indicating that the low-threshold cells were more broadly tuned, but not significantly.
Voltage-dependent sodium currents
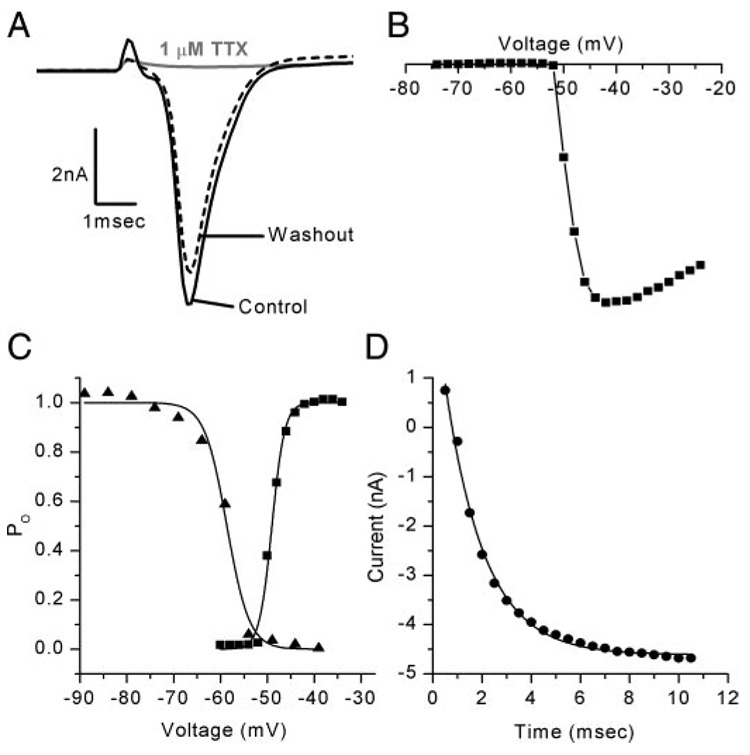
To identify the factors that contribute to the differences in firing properties, we characterized the voltage-dependent conductances in voltage-clamp mode. To examine the Na+ conductance, a 100-ms prepulse to −104 mV was applied to relieve Na+ channel inactivation, followed by voltage steps that ranged from −74 to −24 mV every 2 mV. Depolarization evoked fast activating/fast inactivating inward currents that were reversibly blocked by 1 µM TTX (Fig. 5A) consistent with vestibular ganglion neuron Na+ currents described previously (Chabbert et al. 1997). Maximal inward Na+ currents were evoked by voltage steps that ranged between −50 and −40 mV and reached their mean peak value of −6.6 ± 2.5 nA (n = 61) within 1 ms of the onset of the step. The Na+ currents activated positive to −55 mV (Fig. 5, B and C) and had a mean V1/2 of activation of −52.9 ± 5.5 mV (n = 61). Inactivation was studied by applying voltage steps that ranged from −129 to −29 mV in 5-mV intervals for 50 ms followed by a step to −44 mV. The peak current evoked by the step to −44 mV was plotted as a function of prepulse potential (Fig. 5C, triangles) and was fitted with a Boltzmann equation (Eq. 1) with a V1/2 of inactivation of −65.1 ± 8 mV (n = 59). To investigate the time course of recovery from inactivation we first applied a voltage step to −24 mV for 2 ms to inactivate the Na+ current. To relieve inactivation a family of steps to −104 mV, ranging in duration from 0.5 to 10 ms in 0.5-ms intervals, was applied. A step back to −24 mV followed and revealed the fraction of Na+ current that recovered from inactivation during the step to −104 mV. The peak inward currents at −24 mV were plotted as a function of prepulse duration in Fig. 5D. Recovery from inactivation was well described by an exponential function (Eq. 2) that had a mean time constant of 1.3 ± 0.5 ms (n = 58).
FIG. 5. Sodium currents in vestibular ganglion neurons had homogeneous properties.
A: Na+ currents, evoked by a step to −57 mV, were reversibly blocked by 1 µM tetrodotoxin (TTX; Cell 20030311D, P3). B: current–voltage (I–V) relationship from a representative neuron. Na+ current in this cell activated at −52 mV (Cell 20030731A, P2). Current scale bar of A also applies to B. C: activation (squares) and inactivation (triangles) relationships for a representative family of Na+ currents. Data were fitted with a Boltzmann curve (Eq. 1) with a V1/2 of activation of −49 mV with a slope (s) of 1.4 and a V1/2 of inactivation of −59 mV with s = 2.4 (Cell 20030731A, P2). D: recovery from inactivation fitted with a single exponential (Eq. 2) with a time constant of 1.6 ms (Cell 20030731A, P2).
The properties of the vestibular ganglion neuron Na+ currents were remarkably homogeneous in terms of their amplitude, sensitivity to TTX, voltage dependency of activation, and recovery from inactivation and were similar to those described previously in dissociated vestibular ganglion neurons (Chabbert et al. 1997). As such, we conclude that the heterogeneous firing properties of vestibular ganglion neurons and, in particular, the differences between low-threshold and high-threshold neurons cannot be attributed to differences in the properties of the Na+ currents they express.
Voltage-dependent potassium currents
Next, we analyzed the properties of voltage-dependent K+ currents. Figure 6 illustrates data from three representative cells showing the raw currents (left), peak and steady-state current–voltage (I–V) relations (middle), and activation curves (right). Voltage steps that ranged from −124 to 76 mV every 10 mV evoked outward currents that had peak amplitudes between 2.9 and 14 nA with a mean of 7.5 ± 2.8 nA at 76 mV (n = 61) (Fig. 6). The reversal potential of the outward current was determined using tail currents that were evoked by a family of voltage steps that ranged from −104 to −14 mV every 5 mV and followed a depolarizing step to 16 mV to fully activate the outward current. The mean reversal potential was −74 ± 6 mV (n = 54), which was consistent with the currents being carried primarily by K+. Indeed, the Nernst potential for K+ was −81 mV.
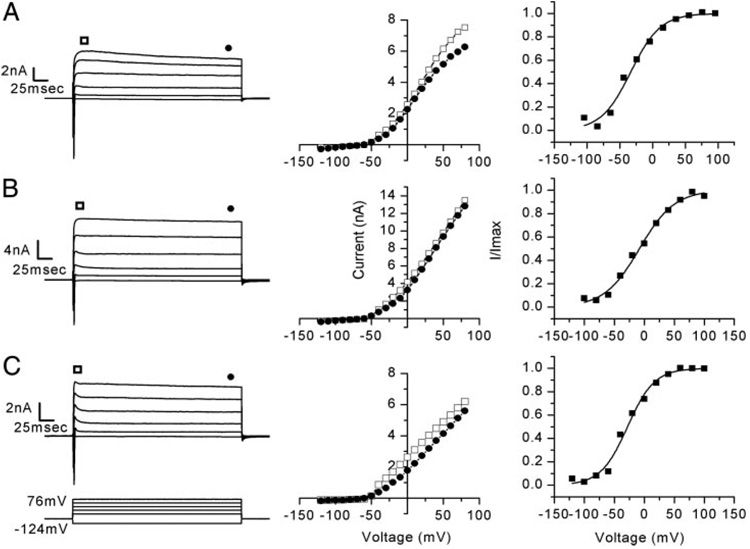
FIG. 6. Vestibular ganglion neurons expressed a heterogeneous population of K+ currents.
A: family of outward K+ currents dominated by slow activation and slow inactivation, seen in many of the neurons examined (56 of 79 cells) (Cell 20040109B, P2). Corresponding peak (squares) and steady-state (circles) I–V relations and an activation curve (V1/2 = −33 mV, s = 23) are shown at the right. B: fast activating K+ currents with no inactivation were observed in 11 of 79 neurons. I–V relation and activation curve are shown (V1/2 = −12 mV, s = 29; Cell 20031209C, P2). C: remaining 12 of 79 neurons revealed currents that had fast activation and fast inactivation that relaxed to a steady-state level. I–V relation and activation curve are shown (V1/2 = −32 mV, s = 22; Cell 20030704D, P4).
To generate activation curves (Fig. 6, A–C, right), we used a family of voltage steps that ranged from −124 to 96 mV in 20-mV intervals, followed by a step to −54 mV. The tail current measured at the instant of the step to −54 mV was divided by the driving force (20 mV) to yield conductance. The mean maximal whole cell conductance was 22.8 ± 16.9 nS (n = 61). Conductance was then plotted as a function of prepulse potential and fitted with a first-order Boltzmann equation (Eq. 1; Fig. 6, right). K+ conductances had a mean V1/2 of activation of −31.2 ± 16 mV and a slope (s) of 19.5 ± 5.4 mV (n = 61). The broad activation range is not consistent with expression of a single variety of K+ conductance but instead suggested that the whole cell conductance may have consisted of several K+ conductances with overlapping activation ranges. When comparing low- and high-threshold neurons, differences in the V1/2 of activation and slope were apparent. The mean V1/2 of activation in low-threshold neurons was −25.6 ± 15.2 mV with s = 18.1 ± 3.7 mV (n = 22). High-threshold neurons had a significantly more negative V1/2 of activation of −34.3 ± 15.8 mV (P = 0.04) and a slightly broader slope of 20.3 ± 6.1 mV (n = 39). The difference in both V1/2 and slope between low- and high-threshold neurons suggested that the specific complement of K+ conductances expressed by these neurons may be different and thus may underlie some of the differences we observed in firing properties.
The kinetics of the outward K+ currents also varied considerably from cell to cell. Consistent with a previous report (Chabbert et al. 2001), we were able to distinguish three components of the outward K+ currents based on their kinetics of activation and inactivation (Fig. 6). In 56 of 79 neurons (71%) the most prominent K+ currents had fast activation and slow inactivation (Fig. 6A) as determined by eye. In the second group, 11 of 79 neurons (14%), the most prominent K+ current displayed similar activation but did not inactivate (Fig. 6B). The lack of inactivation in this group of neurons was also evident by the superimposed peak and steady-state I–V relations (Fig. 6B, middle). The remaining 15% of vestibular neurons (12 of 79) revealed currents dominated by fast activation and fast inactivation that relaxed to a steady-state level (Fig. 6C). Most of the neurons we examined (both low- and high-threshold) expressed all three varieties of K+ conductance, although to varying degrees.
To examine the properties of these three K+ conductances more quantitatively and to begin to tease apart their contribution to the firing properties of vestibular ganglion neurons we applied the K+ channel blockers 4-AP and TEA and recorded the responses in both voltage-clamp and current-clamp mode. Both 4-AP and TEA were previously shown to block K+ currents in dissociated primary vestibular neurons (Chabbert et al. 2001; Limón et al. 2005). In our experiments, we found that 4-AP and TEA had similar effects in nondissociated, acutely excised vestibular ganglion neurons to those reported previously. Although the specificity of 4-AP and TEA is broad, we opted to use concentrations that we found to be effective for isolating physiologically distinct potassium conductances in our vestibular ganglion neurons (Fig. 7).
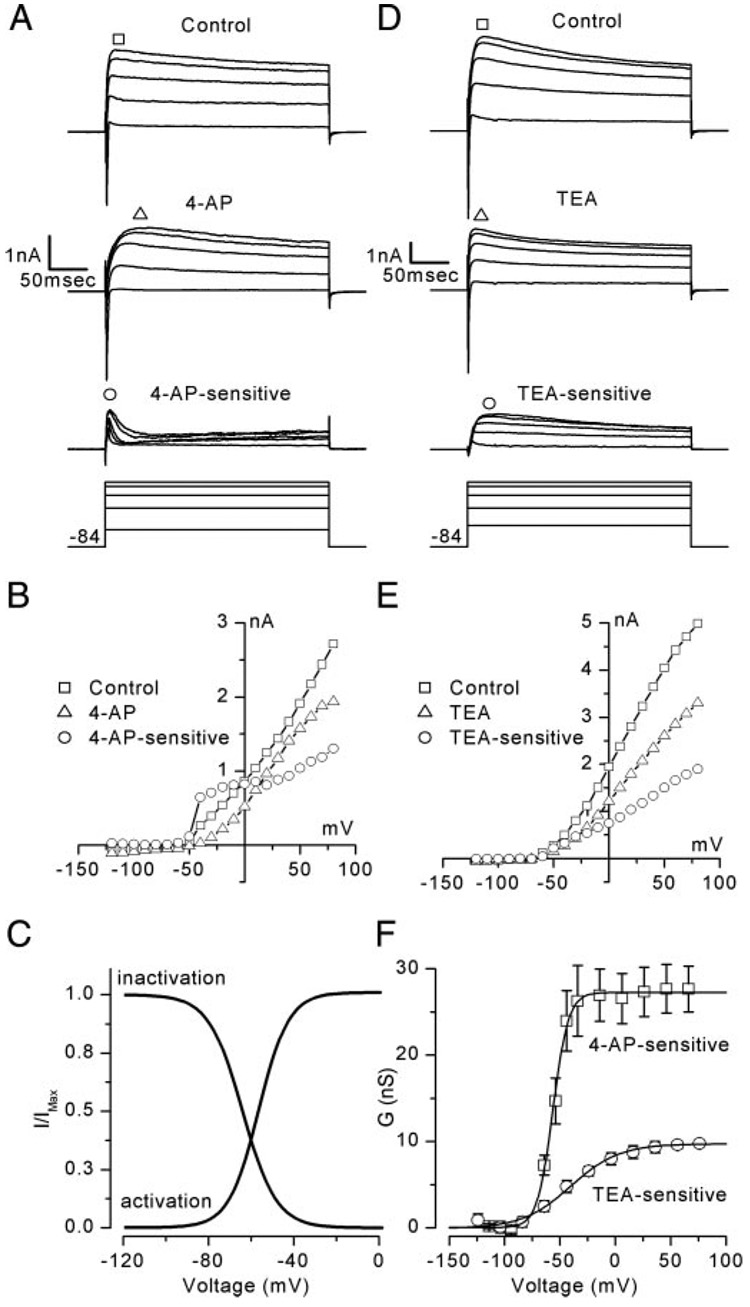
FIG. 7. K+ channel antagonists 4-aminopyridine (4-AP) and tetraethylammonium chloride (TEA) revealed different components of the outward K+ current present within vestibular ganglion neurons.
A: 4-AP revealed fast activating/fast inactivating K+ currents (4-AP–sensitive = Control– 4-AP; Cell 20030702D, P1). Bottom traces: voltage protocol with steps (in mV) to −34, 6, 36, 56, and 66. B: peak I–V relation for the data shown in A. C: activation and inactivation curves for the 4-AP–sensitive conductance. Activation curve was generated from a Boltzmann fit (Eq. 1) to the data shown in F (squares) and had a V1/2 = −56 mV, s = 6.8 mV, and Gmax = 27 nS. Inactivation curve was generated as described in the text and had a V1/2 = −64 mV, s = 7.9 mV, and Gmax = 27 nS. D: TEA revealed slowly activating K+ currents with slow inactivation (TEA-sensitive = Control–TEA; Cell 20030624A, P2). Bottom traces: same voltage protocol as in A. E: peak I–V relations from the data shown in D. F: activation curves for the 4-AP–sensitive (squares) and TEA-sensitive (circles) conductances. Means ± SE were fit with Boltzmann equations (Eq. 1) with a V1/2 = −56 mV, s = 6.8 mV, and Gmax = 27 nS for the 4-AP–sensitive conductance and a V1/2 = −41 mV, s = 16 mV, and Gmax = 9.6 nS for the TEA-sensitive conductance.
Both 4-AP and TEA applied separately and together revealed that the outward K+ currents were composed of at least three distinct conductances, which we refer to as the 4-AP– sensitive conductance, the TEA-sensitive conductance, and the 4-AP– and TEA-insensitive conductance. For this study we characterized the properties of the 4-AP–sensitive and TEA-sensitive conductances in voltage-clamp mode and their contribution to vestibular ganglion firing properties in current-clamp mode. In 24 of 28 cells, 10 mM 4-AP blocked the fast activating/fast inactivating component of the K+ current (Fig. 7A). There was no effect in the other four cells. By subtracting the currents that remained in the presence of 4-AP from the control currents we isolated the 4-AP–sensitive component (Fig. 7A). In the example of Fig. 7A, addition of 4-AP blocked 56% of the control outward K+ current. On average, 4-AP blocked 44 ± 16% (n = 28) of the peak whole cell current measured at 76 mV. The peak I–V relationships for the control, 4-AP–sensitive, and 4-AP–insensitive components are shown in Fig. 7B. Because the 4-AP–sensitive currents displayed significant inactivation we were unable to generate activation curves using our tail current protocol. Thus we opted to use an alternate method to estimate the activation range for the 4-AP– sensitive conductance. The peak currents from the I–V relation for the 4-AP–sensitive currents were divided by driving force to yield conductance and plotted as a function of voltage. Although this method was subject to possible contamination from open channel rectification, we were able to estimate that the 4-AP–sensitive current had a V1/2 of activation of −56 ± 1.5 mV (means ± SE, n = 16) with a slope of 6.8 ± 1.4 mV (Fig. 7F). The mean 4-AP–sensitive conductance was 27 ± 4 nS, or 64% of the control conductance in the same cells (42 ± 17 nS).
To examine the inactivation range of the 4-AP–sensitive conductance we designed a protocol with prepulses that stepped from −124 to −4 mV in 10-mV increments followed by a step to −24 mV. The peak current at −24 mV was plotted as a function of prepulse potential and revealed the fraction of the conductance that was available to be activated at −24 mV. The data were fit with a Boltzmann equation (Eq. 1) that had a slope of 7.9 ± 1.0 and a V1/2 of −64 ± 4.5 mV (means ± SE; n = 5; Fig. 7C). Overlap in the activation and inactivation curves revealed a window between −80 and −40 mV within which the 4-AP–sensitive conductance was activated but not completely inactivated. Within this voltage window 4-AP–sensitive currents were tonically activated and therefore available to influence resting potential, input resistance, and membrane time constant.
4-AP blocked a similar conductance in both low- and high-threshold neurons. However, the density of the 4-AP–sensitive conductance differed depending on neuronal threshold. Low-threshold neurons had a lower 4-AP conductance density (1.11 ± 0.54 nS/pF; n = 8) than that of high-threshold neurons (1.86 ± 0.95 nS/pF; n = 13). The difference approached statistical significance (P = 0.057) and may have contributed to the difference we observed in firing properties between the two populations of neurons (see the following section).
In 16 of 19 cells, 10 mM TEA blocked a slowly activating current that displayed slow inactivation (Fig. 7D). The TEA-sensitive component was estimated by subtracting the currents in the presence of TEA from the control currents. The peak I–V relations for the control, TEA-insensitive, and TEA-sensitive currents are shown in Fig. 7E. With addition of TEA, 44% of the outward K+ currents were blocked relative to the control currents (Fig. 7D). On average TEA blocked 46 ± 9% (n = 19) of the peak whole cell current measured at 76 mV. We used our standard tail current protocol to generate activation curves for the TEA-sensitive conductance. The V1/2 of the TEA-sensitive component was −41 ± 6.2 mV (means ± SE, n = 14), 15 mV more positive than the V1/2 of the 4-AP–sensitive component. The slope was 16.1 ± 5.2 and the mean maximal conductance was 9.6 ± 2.6 nS (Fig. 7F). TEA blocked a similar component in both low- and high-threshold neurons. The mean TEA-sensitive conductance density differed slightly (P = 0.2) between low- (0.85 ± 0.4 nS/pF; n = 2) and high-threshold (1.71 ± 0.68 nS/pF; n = 12) neurons and also may have contributed to the difference in firing properties that we observed as discussed in the next section.
Effects of potassium channel blockers on firing properties
To gain insight into the contribution of the various K+ conductances to the firing properties of vestibular ganglion neurons, we examined the responses of the neurons in current-clamp mode in the presence of 4-AP or TEA. The resting potential of each neuron was obtained in current-clamp mode for both control and drug-treated cells. Before 4-AP addition, control neurons had a mean resting potential of −64 ± 5 mV (n = 19), which shifted significantly (P = 0.001) to −56 ± 9 mV on application of 4-AP. The shift suggested that 4-AP blocked a K+ conductance that was active at rest. Indeed, the input resistance of the control cells was 452 ± 198 MΩ before and 551 ± 318 MΩ after addition of 4-AP, consistent with the block of conductance that had a reversal potential negative to the resting potential. TEA, on the other hand, did not have a significant effect on resting potential or input resistance, which was not surprising given the more positive activation range of the TEA-sensitive conductance. Before addition of TEA, control neurons had a mean resting potential of −63 ± 6 mV (n = 11) with an input resistance of 369 ± 136 MΩ. After TEA application the resting potential was −62 ± 9 mV and the input resistance was 418 ± 140 MΩ.
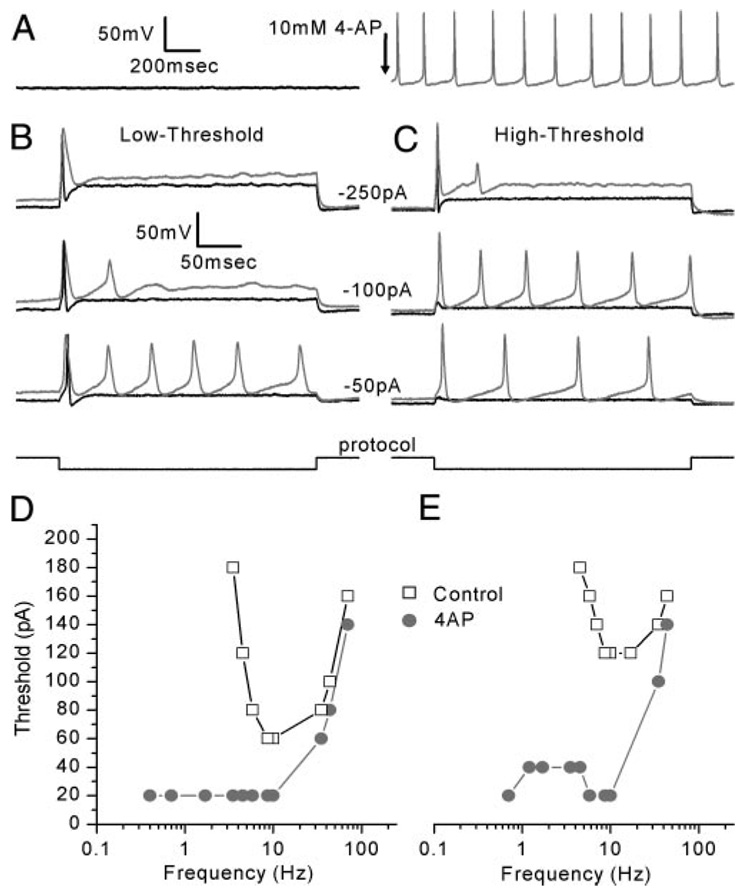
Interestingly, in four of 19 cells, application of 4-AP induced spontaneous firing (Fig. 8A). In the remaining cells, 4-AP lowered the threshold in both low- and high-threshold neurons and increased the number of action potentials evoked per stimulus in 16 of 19 (84%) neurons. In response to current steps, control low-threshold neurons had a threshold of −43 ± 13 pA (n = 7; Fig. 8B, black traces). In the presence of 4-AP, the threshold was lowered by 29 pA to −14 ± 24 pA (Fig. 8B, gray traces). High-threshold neurons fired action potentials with a mean minimum current injection of −177 ± 85 pA (n = 11) before drug application. The threshold was lowered by 132 pA to −45 ± 35 pA in the presence of 4-AP (Fig. 8C). We attribute the 4-AP effect on threshold to block of the 4-AP–sensitive conductance we observed in voltage-clamp mode. Because the 4-AP–sensitive conductance was active at rest, with a V1/2 of activation of −50 mV, block of the conductance eliminated its hyperpolarizing influence on resting potential and increased the input resistance. Thus depolarization of the resting potential brought the cells closer to threshold and the greater input resistance resulted in larger depolarizations for a given current injection. Together these factors significantly lowered threshold and, in the case of the four cells that fired spontaneously, threshold was lowered to zero.
FIG. 8. 4-AP lowered the threshold of both low- and high-threshold neurons and shifted their sensitive ranges to lower frequencies.
A: representative example of the 4 of 19 cells in which 4-AP induced spontaneous firing (Cell 20040114C, P3). Arrow indicates addition of 4-AP. B: 4-AP lowered the threshold and induced multiple action potentials in low-threshold neurons (control: black, 4-AP: gray, Cell 20030702D, P2). C: 4-AP had similar effects on high-threshold neurons (control: black, 4-AP: gray, Cell 20030731A, P2). D and E: tuning curves for low- (D) and high- (E) threshold neurons. In both cases, 4-AP lowered the threshold. Interestingly, both low- and high-threshold neurons behaved as low-pass filters in the presence of 4-AP (Low-threshold: Cell 20031113B, P3; high-threshold: Cell 20030709C, P3).
Application of 4-AP also altered the tuning characteristics of low- and high-threshold neurons such that 14 of 18 neurons behaved as low-pass filters (Fig. 8, D and E). In standard solutions, low-threshold neurons had best frequencies that ranged from 5.8 to 22 Hz with a median best frequency of 9.4 Hz (n = 7). In the presence of 4-AP most neurons lacked a single best frequency but fired at a lower threshold and over a broad range of frequencies that extended from the low-pass corner frequency of 28 ± 7 Hz (n = 5) to <0.4 Hz (Fig. 8D). High-threshold neurons had best frequencies that ranged between 7.9 and 40 Hz with a median best frequency of 10 Hz (n = 11) in standard solutions. Similar to low-threshold neurons, 4-AP converted high-threshold neurons from tuned neurons into low-pass neurons with corner frequencies of 15 ± 5 Hz (n = 9). The effect of 4-AP suggests that the 4-AP–sensitive conductance functions to limit the neuronal response at the low-frequency end and helps shape the band-pass characteristics of the vestibular ganglion neurons. The activation kinetics of the 4-AP–sensitive conductance was sufficiently rapid to overcome the depolarizing phases of low-frequency sine-wave stimuli and thereby prevented generation of action potentials at those frequencies (Fig. 8, D and E).
Application of 10 mM TEA had little effect on the threshold of low-threshold neurons, but evoked multiple action potentials for step current-injections between −50 and −250 pA (Fig. 9A). High-threshold neurons, on the other hand, required a minimum of −150 ± 61 pA (n = 9) on average to evoke an action potential, but in the presence of TEA the threshold was lowered to −72 ± 26 pA (Fig. 9B). TEA evoked multiple action potentials in only one of nine high-threshold neurons but increased action potential width in both low- and high-threshold neurons. Measured at threshold, the mean action potential width (3.6 ± 2 ms) nearly doubled in the presence of TEA (6.2 ± 3.7 ms; n = 11). Finally, in high-threshold neurons we also noted that TEA reduced the action potential peak by 20 ± 9.7 mV (n = 4) at intermediate steps (about −100 pA).
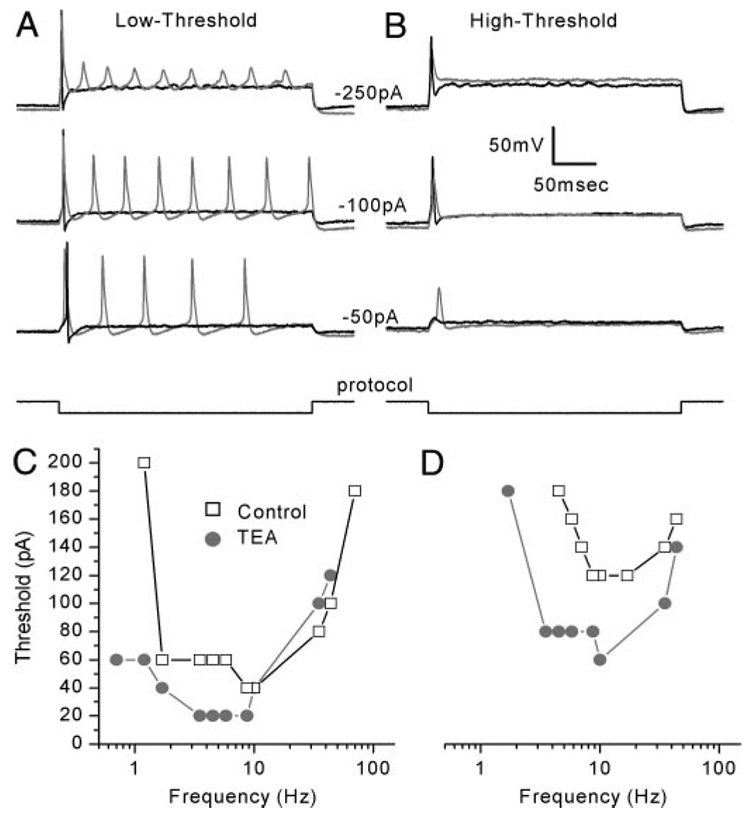
FIG. 9. TEA lowered the threshold of low- and high-threshold neurons and shifted their sensitive ranges to lower frequencies.
A: TEA application lowered the threshold for action potential firing, induced multiple action potentials, and increased AP width in a representative low-threshold neuron (control: black, TEA: gray, Cell 20030624A, P2). B: TEA lowered the threshold for action potential generation and broadened the width of the spike in a representative high-threshold neuron (control: black, TEA: gray, Cell 20030715A, P1). C and D: tuning curves for low- (C) and high- (D) threshold neurons. In the presence of TEA, both neurons had a lower threshold and fired action potentials at lower frequencies relative to controls (Low-threshold: Cell 20030702D, P2; high-threshold: Cell 20030709C, P3).
Upon TEA addition, the frequency response to sine-wave stimuli was shifted to lower frequencies in both low- and high-threshold neurons. Two low-threshold neurons had best frequencies of 8.7 and 9.4 Hz and sensitive ranges between 1.2 and 70 Hz. TEA shifted the best frequencies of those neurons to 5.4 and 6.8 Hz and the sensitive ranges to between 0.7 and 44 Hz (Fig. 9C). High-threshold neurons had best frequencies that ranged between 9.4 and 25 Hz (n = 9) with a median of 12 Hz. In the presence of TEA, the range of best frequencies was between 4.7 and 11 Hz and the median best frequency was 8.1 Hz (Fig. 9D). The sensitive ranges shifted from between 3.5 and 70 Hz to between 0.7 and 44 Hz. Unlike 4-AP, TEA application rarely caused the neurons to behave as a low-pass filter. Only one of 11 cells was broadly tuned with a low-pass corner frequency of 19 Hz.
We attributed the effect of TEA on firing properties to block of the slowly activating/slowly inactivating, TEA-sensitive conductance. Because the TEA-sensitive conductance was not appreciably activated (V1/2 = −34 mV) at the resting potential (−62 mV), we were not surprised that TEA had little effect on resting potential or on threshold, particularly for low-threshold cells. More interesting was the effect of TEA on tuning properties. Block of the TEA-sensitive conductance did not convert tuned neurons into low-pass filters, but instead, shifted the best frequency and sensitive range to lower values.
DISCUSSION
Determining how information carried by the graded hair cell receptor potential is transmitted and encoded into trains of action potentials by vestibular ganglion neurons is critical for a comprehensive understanding of peripheral vestibular physiology. Here we present the first whole cell, tight-seal characterization of the firing properties of postnatal mouse vestibular ganglion neurons. The study was designed to examine postnatal neurons isolated from their peripheral and central targets to begin to identify the membrane properties inherent to the neurons and how they contribute to vestibular signaling.
We found that postnatal mouse vestibular neurons in the isolated ganglion lacked spontaneous firing, which suggests that the spontaneous activity recorded from vestibular afferents in other species and organs (Goldberg 2000) may arise elsewhere in the system, perhaps within the hair cells or at the afferent synapse. Alternatively, this difference may be a result of the differences in age of the preparations. Although Curthoys (1982) reported mature responses in vestibular neurons that innervate the rat semicircular canal by P6, we cannot rule out the possibility that developmental changes could account for the lack of spontaneous activity. For example, we found that inhibition of the 4-AP–sensitive conductance induced spontaneous firing in some neurons. If the 4-AP conductance was lost as a function of development, intrinsic spontaneous activity may arise. Although the lack of spontaneous firing in our postnatal neurons prevented a direct comparison between our data and those obtained from extracellular recordings from the intact vestibular afferents (Goldberg 2000), it is tempting to speculate that our high-threshold neurons correspond to irregular neurons based on cell size. Irregular neurons have calyceal afferent terminals with larger axons, which may correspond to the larger cell bodies of our high-threshold neurons. Furthermore, if the spontaneous activity reported for vestibular afferents results from activity at the afferent synapse, the lower input resistance and higher thresholds of the high-threshold neurons may contribute to the irregularity of the resting discharge rate in those neurons.
Interestingly, even in the absence of peripheral input, we found that the neurons had diverse firing properties and were tuned with best frequencies that ranged up to 40 Hz. We went on to investigate the biophysical basis of these differences and found that a heterogeneous population of K+ conductances contributed to the intrinsic firing patterns of postnatal vestibular neurons. Whether the properties of the neurons become more refined as a function of postnatal development remains to be determined. Yet, because hair cell transduction appears mature at its onset around embryonic day 17 (Géléoc and Holt 2003) and because functional synaptogenesis begins around the same time (Mbiene et al. 1988), vestibular ganglion neurons may convey sensory information even at early postnatal stages. Similarities between the data presented here and preliminary data from juvenile and young adult mice (unpublished observations) suggest that the general principles of tuning and a diversity of firing properties may be relevant to mature vestibular ganglion neurons as well. Methods for whole cell recording from the myelinated cell bodies of adult neurons will need to be developed to further test these hypotheses.
Vestibular neuron potassium conductances
Consistent with a previous report (Chabbert et al. 2001), we found at least three kinetically and pharmacologically distinct K+ conductances. Chabbert and colleagues characterized K+ currents they termed ITEA, IBDS, and IDTX, based on their sensitivity to TEA, blood depressing substance (BDS), and α-dendrotoxin (DTX), respectively. The currents we studied had similar biophysical properties in many respects and a few differences. Our TEA-sensitive currents resembled their ITEA in terms of amplitude, activation, and inactivation kinetics but differed in the voltage range of activation. In our neurons, the TEA-sensitive current had a V1/2 of −41 mV, as opposed to −16 mV in the Chabbert et al. study. Several differences between the two studies may account for the apparent discrepancy. First, the previous work examined K+ currents in enzymatically dissociated cells in which the channel proteins may have suffered enzymatic degradation. We used a semi-intact ganglion preparation without enzymatic dissociation. Second, we used a different method for generation of the activation curve that avoided contamination from open-channel rectification. Using this protocol we measured tail currents evoked by a step to −54 mV that followed a family of voltage steps from −124 to 96 mV in 20-mV intervals. Finally, we used 10 mM TEA, whereas Chabbert et al. used 40 mM. Because TEA is less selective at higher concentrations, we cannot be confident that TEA blocked an identical population of K+ channels in the two studies.
The properties of our 4-AP–sensitive currents were similar to those described by Chabbert et al. (2001), both activated rapidly and with a similar V1/2 of activation. A notable difference was the magnitude of inactivation. We observed significant inactivation, whereas they reported that 4-AP blocked an inactivating current and a noninactivating current at different concentrations. Whether these were distinct conductances or incomplete block of a single conductance was not clear.
A recent report from Limón et al. (2005) suggested that enzymatically dissociated postnatal rat vestibular ganglion neurons express calcium-dependent potassium conductances that consist of BK and SK channels. We did not test the calcium sensitivity of the K+ conductances in our preparation and thus cannot rule out the possibility that BK and SK channels contribute to the diversity of responses we describe in mouse vestibular neurons.
The ion channel subunits that make up the physiologically and pharmacologically defined potassium conductances in vestibular ganglion neurons have not yet been identified. However, based on the biophysical and pharmacological similarities with K+ conductances in other neurons we can begin to narrow in on potential candidates. Our fast activating/fast inactivating, 4-AP–sensitive currents were similar to IA observed in other neuronal systems. Elsewhere IA is blocked by 4-AP, activates quickly at subthreshold voltages, and displays fast inactivation (Baldwin et al. 1991; Schroter et al. 1991; Serôdio et al. 1994, 1996; Stuhmer et al. 1989). Kv1.4, Kv3.4, and Kv4 subunits are sensitive to 4-AP (Coetzee et al. 1999) and thus may contribute to 4-AP–sensitive currents in vestibular ganglion neurons. On the other hand, 10 mM TEA blocks members of both the Kv1 and Kv3 subfamilies (Erisir et al. 1999; Glazebrook et al. 2002; Wang et al. 1998). Although it is premature to suggest the molecular identity of vestibular neuron K+ conductances, we propose that these subunits can be placed on the list of potential candidates.
Role of potassium conductances in vestibular neurons
We found relatively homogeneous expression of a TTX-sensitive Na+ conductance with little variability in magnitude, voltage dependency, kinetics of activation and inactivation, and recovery from inactivation. As such, we conclude that the diverse firing properties we observed in vestibular ganglion neurons cannot be attributed to cell-to-cell variability in the TTX-sensitive Na+ conductance.
Factors we identified that may influence the firing patterns include cell size, input resistance, and the magnitude and properties of each of three biophysically distinct K+ conductances. We found a strong correlation between neuronal cell body diameter and our classification of neurons as low or high threshold. To our surprise, when we examined resting conductance and normalized for cell capacitance we discovered that both low- and high-threshold neurons had the same conductance density: 0.18 nS/pF. Because threshold depends largely on resting conductance, we suggest that the size of the vestibular ganglion neuron cell body can predict the relative threshold: i.e., smaller neurons have lower thresholds.
Our observation that vestibular ganglion neurons are inherently tuned with best frequencies that span the sensitive range of the rodent vestibular system (Hullar and Minor 1999; Hullar et al. 2005) is novel. We identified several factors that determined best frequency and the position of the tuning curve in the frequency domain. We found that a TEA-sensitive K+ conductance shifted the tuning curve to higher frequencies. Because the TEA-sensitive component was not appreciably activated at rest, it had little influence on resting potential or threshold. However, voltage-dependent activation of the conductance during an action potential limited neuronal firing, particularly in low-threshold neurons, and contributed to the afterhyperpolarization. In both low- and high-threshold cells, TEA shifted the sensitive range and best frequency to lower values. Although the slow kinetics of the TEA-sensitive conductance limited its activation during each phase of the sine-wave stimulus (AC component) above a few hertz, cumulative activation of the TEA-sensitive conductance may result from the DC component of the membrane potential even at higher frequencies. Cumulative activation may in turn decrease membrane resistance and thereby extend the sensitive range to higher frequencies. Indeed, block of the TEA-sensitive conductance shifted the sensitive range to lower frequencies.
The contribution of the 4-AP–sensitive conductance to the response to step stimuli was threefold: 1) 4-AP depolarized the resting potential by an average of 8 mV; 2) block of the conductance significantly lowered the threshold and in four cells induced spontaneous firing; and 3) 4-AP induced repetitive firing. These observations suggested that a conductance active at rest was blocked by application of 4-AP. Indeed, the V1/2 of activation for the 4-AP–sensitive conductance was −56 mV, consistent with significant activation at the mean resting potential of −64 mV. Although the 4-AP–sensitive conductance displayed considerable inactivation, it was not complete. 4-AP–sensitive currents in other neuronal systems display an overlap in their voltage range of activation and inactivation, which can produce a voltage window (Dilks et al. 1999; McCormick 1991). Likewise, we identified a voltage window between −80 and −40 mV (Fig. 7C) within which the conductance was tonically activated and thus available to influence membrane properties such as resting potential, threshold, spontaneous and repetitive firing. Therefore we propose that the tonic activity of the 4-AP–sensitive conductance maintains negative resting potentials, limits spontaneous activity, raises the threshold for generation of action potentials, and prevents repetitive firing.
Voltage-dependent activation of the 4-AP–sensitive conductance limited the generation of action potentials at low frequencies and thus helped to shape the tuning curve. The activation kinetics of the 4-AP–sensitive conductance was sufficiently rapid to attenuate the depolarizing phase of sine-wave stimuli at low frequencies.Chabbert et al. (2001) reported activation time constants of 3.9 ms at −30 mV, which we estimate is fast enough to overcome stimuli below about 30 Hz. Therefore we conclude that the rapid activation of the 4-AP–sensitive conductance clips the peaks of the depolarizing phase of sine-wave stimuli at frequencies <30 Hz and thereby contributes to the high-pass corner by converting otherwise low-pass neurons into sharply tuned, band-pass neurons. Finally, we wondered what shaped the low-pass corner. We calculated the mean membrane time constant from the mean whole cell capacitance and input resistance, 5.5 ms, which allowed us to predict a low-pass corner frequency of about 29 Hz, remarkably similar to the actual value measured from the tuning curves: 28 Hz.
Not surprisingly, we found that activation of the 4-AP–sensitive conductance and the TEA-sensitive conductance contributed to the afterhyperpolarization (AHP). Indeed, block of those conductances altered the amplitude and kinetics of the AHP. Because the AHP requires voltage-dependent activation during an action potential, it probably has little effect on neuronal properties at rest such as threshold. However, consistent with a previous suggestion (Smith and Goldberg 1986), we suspect that cell-to-cell variation in the magnitude of both the 4-AP–sensitive and TEA-sensitive conductances may alter the magnitude and kinetics of the AHP and may thereby contribute to the diversity in firing regularity of vestibular afferent neurons.
We hypothesize that together with the broad range of responses that arise within the hair cells and at the afferent terminals, a heterogeneous population of neuronal K+ conductances contributes to the diversity in firing properties of vestibular afferent neurons. We propose that the precise composition of K+ conductances within each vestibular ganglion neuron determines that neuron’s threshold and best frequency. Collectively this diversity endows the vestibular ganglion with the ability to encode vestibular information at frequencies that span the physiological range of head movements.
ACKNOWLEDGMENTS
We thank members of the Holt laboratory for comments on a previous version of the manuscript.
GRANTS
This work was supported by National Institute of Deafness and Other Communication Disorders Grant DC-05439 to J. R. Holt and National Research Service Award Grant DC-008051 to J. R. Risner.
REFERENCES
- Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J Comput Neurosci. 2001;11:217–239. doi: 10.1023/a:1013771014232. [DOI] [PubMed] [Google Scholar]
- Autret L, Mechaly I, Scamps F, Valmier J, Lory P, Desmadryl G. The involvement of Cav3.2/α1H T-type calcium channels in excitability of mouse embryonic primary vestibular neurons. J Physiol. 2005;567:67–78. doi: 10.1113/jphysiol.2005.089342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernández C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol. 1988;60:182–203. doi: 10.1152/jn.1988.60.1.182. [DOI] [PubMed] [Google Scholar]
- Baldwin TJ, Tsaur ML, Lopez GA, Jan YN, Jan LY. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991;7:471–483. doi: 10.1016/0896-6273(91)90299-f. [DOI] [PubMed] [Google Scholar]
- Chabbert C, Chambard JM, Sans A, Desmadryl G. Three types of depolarization-activated potassium currents in acutely isolated mouse vestibular neurons. J Neurophysiol. 2001;85:1017–1026. doi: 10.1152/jn.2001.85.3.1017. [DOI] [PubMed] [Google Scholar]
- Chabbert C, Chambard JM, Valmier J, Sans A, Desmadryl G. Voltage-activated sodium currents in acutely isolated mouse vestibular ganglion neurons. Neuroreport. 1997;8:1253–1256. doi: 10.1097/00001756-199703240-00039. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Postnatal developmental changes in the response of rat primary horizontal semicircular canal neurons to sinusoidal angular accelerations. Exp Brain Res. 1982;47:295–300. doi: 10.1007/BF00239389. [DOI] [PubMed] [Google Scholar]
- Dilks D, Ling H, Cockett M, Sokol P, Numann R. Cloning and expression of the human Kv4.3 potassium channel. J Neurophysiol. 1999;81:1974–1977. doi: 10.1152/jn.1999.81.4.1974. [DOI] [PubMed] [Google Scholar]
- Eatock RA, Hurley KM, Vollrath MA. Mechanoelectrical and voltage-gated ion channels in mammalian vestibular hair cells. Audiol Neurootol. 2002;7:31–35. doi: 10.1159/000046860. [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Fernández C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol. 1988;60:167–181. doi: 10.1152/jn.1988.60.1.167. [DOI] [PubMed] [Google Scholar]
- Géléoc GSG, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;10:1019–1020. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GSG, Risner JR, Holt JR. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J Neurosci. 2004;24:11148–11159. doi: 10.1523/JNEUROSCI.2662-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook PA, Ramirez AN, Schild JH, Shieh C, Doan T, Wible BA, Kunze DL. Potassium channels Kv1.1, Kv1.2 and Kv1.6 influence excitability of rat visceral sensory neurons. J Physiol. 2002;541:467–482. doi: 10.1113/jphysiol.2001.018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res. 2000;130:277–297. doi: 10.1007/s002210050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Lysakowski A, Fernández C. Morphophysiological and ultrastructural studies in the mammalian cristae ampullares. Hear Res. 1990;49:89–102. doi: 10.1016/0378-5955(90)90097-9. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Abel LA, Lanska DJ, Thurston SE. Frequency and velocity of rotational head perturbations during locomotion. Exp Brain Res. 1988;70:470–476. doi: 10.1007/BF00247595. [DOI] [PubMed] [Google Scholar]
- Grossman GE, Leigh RJ, Bruce EN, Huebner WP, Lanska DJ. Performance of the human vestibuloocular reflex during locomotion. J Neurophysiol. 1989;62:264–272. doi: 10.1152/jn.1989.62.1.264. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Rabbitt RD, Holstein GR, Boyle RD. Determinants of spatial and temporal coding by semicircular canal afferents. J Neurophysiol. 2005;93:235–2370. doi: 10.1152/jn.00533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- Holt JR, Rüsch A, Vollrath MA, Eatock RA. The frequency dependence of receptor potentials in hair cells of the mouse utricle. Prim Sensory Neuron. 1998;2:233–241. [Google Scholar]
- Holt JR, Vollrath MA, Eatock RA. Stimulus processing by type II hair cells in the mouse utricle. Ann NY Acad Sci. 1999;28:15–26. doi: 10.1111/j.1749-6632.1999.tb09172.x. [DOI] [PubMed] [Google Scholar]
- Honrubia V, Hoffman LF, Sitko S, Schwartz IR. Anatomic and physiological correlates in bullfrog vestibular nerve. J Neurophysiol. 1989;61:688–701. doi: 10.1152/jn.1989.61.4.688. [DOI] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol. 2005;93:2777–2786. doi: 10.1152/jn.01002.2004. [DOI] [PubMed] [Google Scholar]
- Hullar TE, Minor LB. High-frequency dynamics of regularly discharging canal afferents provide a linear signal for angular vestibuloocular reflexes. J Neurophsyiol. 1999;82:2000–2005. doi: 10.1152/jn.1999.82.4.2000. [DOI] [PubMed] [Google Scholar]
- Limón A, Pérez C, Vega R, Soto E. Ca2+-activated K+ current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol. 2005;94:3751–3761. doi: 10.1152/jn.00177.2005. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol. 1997;389:419–443. doi: 10.1002/(sici)1096-9861(19971222)389:3<419::aid-cne5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Incomplete segregation of endorgan-specific vestibular ganglion cells in mice and rats. J Vestib Res. 1999;9:387–399. [PubMed] [Google Scholar]
- Maklad A, Fritzsch B. Development of vestibular afferent projections into the hindbrain and their central targets. Brain Res Bull. 2003;60:497–510. doi: 10.1016/s0361-9230(03)00054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene JP, Favre D, Sans A. Early innervation and differentiation of hair cells in the vestibular epithelia of mouse embryos: SEM and TEM study. Anat Embryol (Berl) 1988;177:331–340. doi: 10.1007/BF00315841. [DOI] [PubMed] [Google Scholar]
- McCormick DA. Functional properties of a slowly inactivating potassium current in guinea pig dorsal lateral geniculate relay neurons. J Neurophysiol. 1991;66:1176–1189. doi: 10.1152/jn.1991.66.4.1176. [DOI] [PubMed] [Google Scholar]
- McCue MP, Guinan JJ., Jr. Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat. J Neurophysiol. 1995;74:1563–1572. doi: 10.1152/jn.1995.74.4.1563. [DOI] [PubMed] [Google Scholar]
- Mo Z, Davis RL. Endogenous firing patterns of murine spiral ganglion neurons. J Neurophysiol. 1997;77:1294–1305. doi: 10.1152/jn.1997.77.3.1294. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Streeter MA. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol. 2006;95:26–32. doi: 10.1152/jn.00641.2005. [DOI] [PubMed] [Google Scholar]
- Romand R, Dauzat M. Modification of spontaneous activity in primary vestibular neurons during development in the cat. Exp Brain Res. 1982;45:265–268. doi: 10.1007/BF00235786. [DOI] [PubMed] [Google Scholar]
- Schroter KH, Ruppersberg JP, Wunder F, Rettig J, Stocker M, Pongs O. Cloning and functional expression of a TEA-sensitive A-type potassium channel from rat brain. FEBS Lett. 1991;278:211–216. doi: 10.1016/0014-5793(91)80119-n. [DOI] [PubMed] [Google Scholar]
- Serôdio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serôdio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Smith CE, Goldberg JM. A stochastic afterhyperpolarization model of repetitive activity in vestibular afferents. Biol Cybern. 1986;54:41–51. doi: 10.1007/BF00337114. [DOI] [PubMed] [Google Scholar]
- Stuhmer W, Ruppersberg JP, Schroter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Gan L, Forsythe ID, Kaczmarek LK. Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurons. J Physiol. 1998;509:183–194. doi: 10.1111/j.1469-7793.1998.183bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]