Abstract
The subventricular zone (SVZ) of the adult mouse brain is a narrow stem cell niche that lies along the length of the lateral wall of the lateral ventricles. The SVZ supports neurogenesis throughout adulthood; however, with increasing age, the ventral SVZ deteriorates and only the dorsolateral SVZ remains neurogenic. Associated with the elderly dorsolateral SVZ, we reported previously an increased number of astrocytes interposed within the adjacent ependymal lining. Here, we show that astrocytes integrated within the ependyma are dividing, BrdU-labeled astrocytes that share cellular adherens with neighboring ependymal cells. By tracking BrdU-labeled astrocytes over time, we observed that, as they incorporated within the ependyma, they took on antigenic and morphologic characteristics of ependymal cells, suggesting a novel form of SVZ-supported “regenerative” repair in the aging brain. A similar form of SVZ-mediated ependyma repair was also observed in young mice after mild ependymal cell denudation with low dosages of neuraminidase. Together, this work identifies a novel non-neuronal mechanism of regenerative repair by the adult SVZ.
Keywords: subventricular zone, neural stem cells, neural progenitor cells, ependymal cells, repair, aging
Introduction
The adult subventricular zone (SVZ) directs stem/progenitor cell fate decisions and is thought to regulate primarily the production of new neurons in the adult brain. Within the narrow adult SVZ, intercellular signaling, dictated by a highly organized cytoarchitecture, orchestrates cell fate decisions and ultimately regulates the regenerative potential of the niche. However, the exact cell signaling mechanisms and cell–cell interactions governing these processes remain unclear. High-resolution electron microscopy has revealed that the organization of the SVZ niche is based on four major cell types: ependymal cells, SVZ astrocytes, transitory amplifying progenitor cells (Type C cells), and neuroblasts (Doetsch et al., 1997; Garcia-Verdugo et al., 1998). An ependyma lines the ventricles and provides a barrier and filtration system for the CSF (Bruni, 1998). SVZ astrocytes, the putative neural stem cells (NSCs), lie subadjacent to the ependyma and surround newly generated Type C cells and ensheath highly migratory neuroblasts (Chiasson et al., 1999; Doetsch et al., 1999b; Laywell et al., 2000). Additional evidence has linked adult SVZ astrocytes/NSCs to the embryonic precursor, radial glia, and suggested that SVZ-mediated adult neurogenesis is a modified version of radial glia-mediated embryonic neurogenesis (Rakic and Sidman, 1968; Gaiano et al., 2000; Malatesta et al., 2000; Alvarez-Buylla et al., 2001; Hartfuss et al., 2001; Miyata et al., 2001; Noctor et al., 2001; Tamamaki et al., 2001; Doetsch, 2003b; Tramontin et al., 2003; Anthony et al., 2004; Merkle et al., 2004). Besides radial glia-mediated neurogenesis, a recent study demonstrated that radial glia also give rise to ependymal cells during embryonic development (Spassky et al., 2005). Together, these findings suggest that SVZ astrocytes (NSCs) may have a similar potential for both neurogenesis and ependymogenesis in the adult SVZ.
Unlike other epithelial membranes, the ependyma is not regenerative and ependymal cells do not divide in the adult brain (Bruni et al., 1985; Bruni, 1998; Doetsch et al., 1999a,b; Spassky et al., 2005). In studies examining the ependyma under pathological conditions, limited repair was detected but only in regions associated with an underlying SVZ (Garfia et al., 1980). The mechanism of repair was not evaluated in these models. Previously, we reported increased numbers of SVZ astrocytes interposed within the ependyma in the aged mammalian brain (Luo et al., 2006). Here, we examined the mechanism and extent of SVZ-associated ependymal replacement/repair using the process of normal aging as our initial model repair system. We predicted a modest need for ependymal repair in healthy aged mice, required after attrition or to prevent gaps attributable to distention of the ependymal walls (e.g., age-related hydrocephalus). We now provide evidence that dividing SVZ astrocytes incorporate within the ependyma, establish cellular adherens with neighboring ependymal cells, and over time take on characteristics of ependymal cells. This novel form of “regenerative” replacement/repair was found associated only with ependyma with an adjacent SVZ. In addition, studies in young mice of limited ependymal cell loss after intraventricular injection of neuraminidase resulted in a repair response that mimicked repair in our aging model.
Materials and Methods
Animals
Male CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA) and aged in our vivarium. In all the following experiments, 2- to 4-month-old mice were designated young adult, whereas 22- to 26-month-old mice were designated elderly. Animal procedures were performed under protocols approved by the Institutional Animal Care and Use Committee of the University of Connecticut and conform to National Institutes of Health guidelines.
Immunohistochemistry
Mice were perfused transcardially with 0.9% saline followed by 3% paraformaldehyde (Electron Microscopy Science, Hatfield, PA) in PBS. Brains were removed, fixed overnight in the same fixative at 4°C, and washed in PBS three times for 40 min the next day before cutting into 50 μm sections with a vibratome (VT-1000S; Leica, Wetzlar, Germany). Free-floating sections were then permeabilized with 0.1% Triton X-100 (Sigma, St. Louis, MO) in PBS for 10 min, blocked in 10% goat serum (Invitrogen, Carlsbad, CA) in PBS/0.1% Triton X-100 for 1 h, and incubated with the following primary antibodies: mouse anti-acetylated tubulin (1:500; Sigma); rabbit anti-β-catenin (1:100; Cell Signaling Technology, Beverly, MA); rat anti-bromodeoxyuridine (BrdU) (1:100; Accurate Chemical & Scientific, Westbury, NY); mouse anti-BrdU (1:100; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); rat anti-CD24 (1:250; BD PharMingen, San Diego, CA); mouse anti-γ-tubulin (1:500; Sigma); mouse anti-glial fibrillary acidic protein (GFAP) (1:400; Millipore Bioscience Research Reagents, Temecula, CA); rat anti-GFAP (1:250; Zymed Laboratories, South San Francisco, CA); mouse anti-RC2 (1:5; Developmental Studies Hybridoma Bank); and rabbit anti-s100β (1:500; Dako, Carpinteria, CA). After washing three times in PBS, sections were incubated with appropriate Alexa Fluor dye-conjugated secondary antibodies (Invitrogen) for 1 h. Sections were then washed in PBS and incubated for 5 min in 2 μg/ml Hoechst 33342 (Sigma) for counterstaining of nuclei. Secondary antibodies alone were used as a control. Stained sections were washed for 5 min in PBS, coverslipped using aquapolymount (Polysciences, Warrington, PA), and imaged on either a Zeiss Axioskop 2 plus microscope (Carl Zeiss, Thornwood, NY), using a Retiga 1300 EX digital camera (QImaging, Surrey, British Columbia, Canada), or on a Leica TCS SP2 confocal laser-scan microscope.
BrdU injection paradigms and immunohistochemistry
Mice were injected with BrdU at 50 mg/kg on specified days as indicated (10 d in elderly animals and 3 d in young injured animals) to label a large population of dividing cells. Mice were then killed at 0, 3, 6, and 13 weeks after the last BrdU injection for immunostaining, as described previously (Lie et al., 2002). Confocal imaging of BrdU, GFAP, and s100β cells along the lateral wall of the lateral ventricle was performed using a Leica TCS SP2 confocal laser-scan microscope. All BrdU-positive (BrdU+) cells along the dorsolateral wall of the lateral ventricle were counted in 31 brain sections, from coordinates −0.40–1.13 relative to bregma using Adobe Photoshop 7 (Adobe Systems, San Jose, CA).
Whole mounts
Whole mounts of the entire lateral wall of the lateral ventricles were prepared as described previously (Doetsch and Alvarez-Buylla, 1996) and immunostained for acetylated tubulin (Sigma), β-catenin (Cell Signaling Technology), and GFAP (Zymed Laboratories). Whole mounts were placed onto depressed glass slides and coverslipped with CoverWell imaging chambers (Grace Bio-Labs, Bend, OR). Samples were imaged on a Carl Zeiss Axiovert 200M Inverted microscope with an ApoTome attachment and Axiovision 4.6 software (Carl Zeiss).
Neuraminidase injection
Neuraminidase from Clostridium perfringens (Roche Diagnostics, Indianapolis, IN) was dissolved in sterile saline at a concentration of 0.5 mg/ml. Young CD-1 mice were anesthetized with isofluorane and placed in a stereotaxic apparatus. One microliter of diluted neuraminidase (1, 10, 50, 100, and 500 ng) was delivered unilaterally to the anterior lateral ventricle via a stereotaxically positioned capillary needle using a microinjector (IM-9B; Narishige, Greenvale, NY) (coordinates: 0 mm anterior/posterior, 0.65 mm lateral, and 2.65 mm ventral relative to bregma). Saline injection was performed in parallel as a control.
Brain histology
CD-1 mice were perfused, and brains were postfixed, as described above. Brains were washed in ddH20 followed by a series of ethanol dehydration (50, 70, 80, 95, 100, and 100% ethanol; 45 min each). Brains were then infiltrated with xylene (Spectrum Medical, Great Falls, MT) and embedded in paraffin (Kendall, Miami, FL). Thereafter, brains were cut into 10 μm sections with a microtome (Microm HM325), and coronal sections were mounted onto Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA) and dried overnight. Before hematoxylin and eosin staining, samples were deparaffined in Citrisolv (Thermo Fisher Scientific) and hydrated gradually in a series of ethanol (100, 100, 95, 80, and 70% ethanol; 3 min each). Slides were then placed in hematoxylin (Sigma) for 1 min to stain cell nuclei, washed with running tap water, and dehydrated gradually (70, 80, and 95% ethanol). Sections were then stained with eosin (Sigma) for 10 s, and the dehydration steps continued with 95 and 100% ethanol. Finally, slides were rinsed in Citrisol, mounted in DPX (Electron Microscopy Science), and coverslipped. Coronal sections were imaged on a Carl Zeiss Imager Z1 microscope with an AxioCam MRm camera and Axiovision 4.5 software (Carl Zeiss).
Electron microscopy
Transmission electron microscopy.
Mice were perfused and processed for electron microscopy as described previously (Luo et al., 2006). Electron micrographs were taken on a Fei Technai G2 Spirit Biotwin electron microscope and digitized using a CCD AMT XR40 (4 megapixel) camera. Montages of the SVZ were constructed using Adobe Photoshop 7. Cell types were identified based on previously described criteria (Doetsch et al., 1997). Briefly, ependymal cells contain spherical nuclei, lipid droplet, basal bodies of cilia, microvilli, some reticular fibers, apical mitochondria, and usually form tight junctions with each other along their apical surface. Astrocytes typically have irregular nuclei, thick bundles of intermediate filaments, and light cytoplasm. Neuroblasts are small, elongated cells with dark scant cytoplasm. They form clusters with intercellular spaces between cells. Transitory amplifying progenitor cells (Type C) have very large, irregular nuclei with deep invaginations and many mitochondria in the cytoplasm.
Scanning electron microscopy.
CD-1 mice were perfused transcardially with 1× Ringer's solution followed by 3% paraformaldehyde/1.25% glutaraldehyde (Electron Microscopy Science) in 0.12 m sodium phosphate buffer. Heads were further fixed by immersion in the same fixative overnight. The next day, brains were removed, and whole mounts of the entire lateral wall of the lateral ventricle were prepared, as described previously (Doetsch and Alvarez-Buylla, 1996). Samples were then postfixed with 1% OsO4 in 0.12 m sodium phosphate buffer overnight followed by a graded ethanol dehydration. Specimens were then dried in a Polaron (Hertfordshire, UK) E3000 Critical Point Dryer for 2.5 h and mounted onto aluminum specimen mounts (Ted Pella) using silver paint (Ernest F. Fullam, Clifton Park, NY). Each mount was sputter coated with gold palladium (60% gold, 40% palladium) using a Polaron E5100 Sputter Coater. Samples were examined and photographed using a Leo DSM982 SE (sealed emission) scanning electron microscope.
Results
SVZ astrocytes integrate within the aging ependyma
Ultrathin, coronal sections of the anterior forebrain were prepared for and imaged by transmission electron microscopy (TEM). Contiguous EM micrographs were assembled into montages of the entire lateral wall of the lateral ventricle for young (2 month) and elderly (22 month) mice. Cell morphological criteria was then used to define individual SVZ cell types, as described previously (Doetsch et al., 1997; Garcia-Verdugo et al., 1998; Conover et al., 2000). A representative portion of the montages from each age group is shown in Figure 1a–d. TEM-based reconstructions of the elderly SVZ revealed a diminished SVZ and a significant increase in the number of astrocytes positioned within the ependymal monolayer and contacting the ventricular lumen (Fig. 1, compare a with b, c) (Luo et al., 2006). Astrocytes were identified based on morphological characteristics, such as light cytoplasm and the presence of bundles of intermediate filaments (Fig. 1e, asterisks), whereas ependymal cells contained only a few reticular fibers (data not shown). Astrocyte contact with the ventricle has been suggested as a means to activate the neural stem cell phenotype (Doetsch et al., 1999b; Conover et al., 2000), and occasionally a thin astrocytic process can be detected within the ependyma of young mice (Doetsch et al., 1999b; Conover et al., 2000; Luo et al., 2006). In contrast to astrocytic processes with ventricle contact seen in young animals, high-resolution EM micrographs showed astrocytes firmly established within the ependyma of elderly mice (Luo et al., 2006). In coronal sections, we observed an average of 24 ± 10 SD fully integrated astrocytes/μm in 22-month-old mice, whereas only an occasional thin astrocytic process was found in matched, coronal sections from younger 2-month-old mice. It is important to note that the small size of the astrocytic process contacting the ventricle may result in an underestimation of their number. In sharp contrast, astrocytes integrated within the aged ependyma were cuboidal in shape; their nuclei aligned with ependymal cell nuclei, and they exhibited intercellular adherens (zonulae adherens) with either neighboring ependymal cells (Fig. 1b, arrowheads) or other interposed astrocytes (Fig. 1b, arrow). Astrocytes fully incorporated within the ependyma were never found in young adult mice. A surprising observation was the detection of several basal bodies of cilia in the apical cytoplasm of many of the astrocytes integrated within the aged ependyma (Fig. 1f,g, white arrowheads). This grouping of basal bodies was reminiscent of the organization of tufts of cilia along the apical surface of mature ependymal cells (Bruni et al., 1985; Bruni, 1998; Luo et al., 2006). In addition, cilia in the apical cytoplasm of integrated astrocytes showed the 9 + 2 microtubule arrangement specific to motile cilia of ependymal cells (Fig. 1g, arrows), not the 9 + 0 organization of the primary cilium (Alvarez-Buylla et al., 2001; Spassky et al., 2005).
Figure 1.
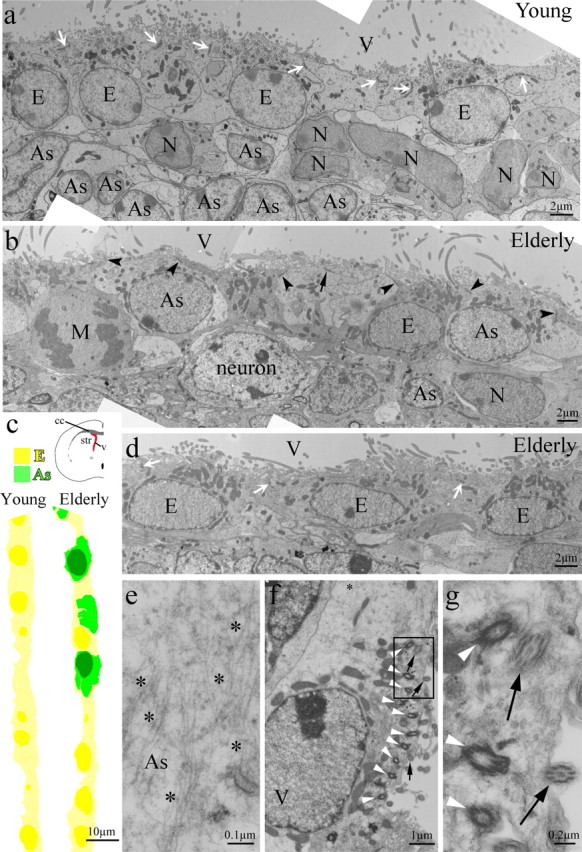
Astrocytes found within the ependymal wall of elderly mice. a, An EM photomontage of the lateral wall of the lateral ventricle of a young mouse with a normal ependymal layer. White arrows point to adherens junctions between ependymal cells. b, In the elderly ependyma, four astrocytes (2 showing only cytoplasm) possess adherens with neighboring ependymal cells (arrowheads) and other interpolated astrocytes (arrow). Mature striatal neurons are observed close to the greatly reduced SVZ. c, The ependymal layer of young (a) and elderly (b, d) mice shown in colorized overview. d, A continuation of the ependymal wall in b reveals some areas with an intact ependymal cell monolayer. White arrows indicate intercellular adherens between ependymal cells. e, Integrated astrocytes are identified by light cytoplasm and thick bundles of intermediate filaments (asterisks in e and f). f, Basal bodies (white arrowheads) and cilia (black arrows) found in an astrocyte within the ependymal wall. g, Cross sections of the cilia (arrows, from the boxed area in f) of the interposed astrocyte reveal a 9 + 2 structure, different from 9 + 0 primary cilium organization occasionally observed in SVZ astrocytes of young mice (data not shown). As, Astrocytes; E, ependymal cells; N, neuroblasts; M, cell in mitosis; V, lateral ventricle.
Evaluation of the apical surface of lateral wall of the lateral ventricle through aging
Scanning electron microscopy (SEM) provides a means to assess the integrity of the apical surface of the ependymal wall in young (2 month) and elderly (22 month) animals. We prepared whole mounts of the entire lateral wall of the lateral ventricle for SEM analysis. In young mice, we observed a carpet of short microvilli and tufts of cilia covering the apical surface of the entire ventricle lining (Fig. 2a), representing a normal, healthy ependymal lining. In contrast, the ependymal lining in elderly mice revealed some bare patches devoid of cilia and microvilli among relatively normal appearing areas replete with both microvilli and tufts of cilia (Fig. 2b).
Figure 2.
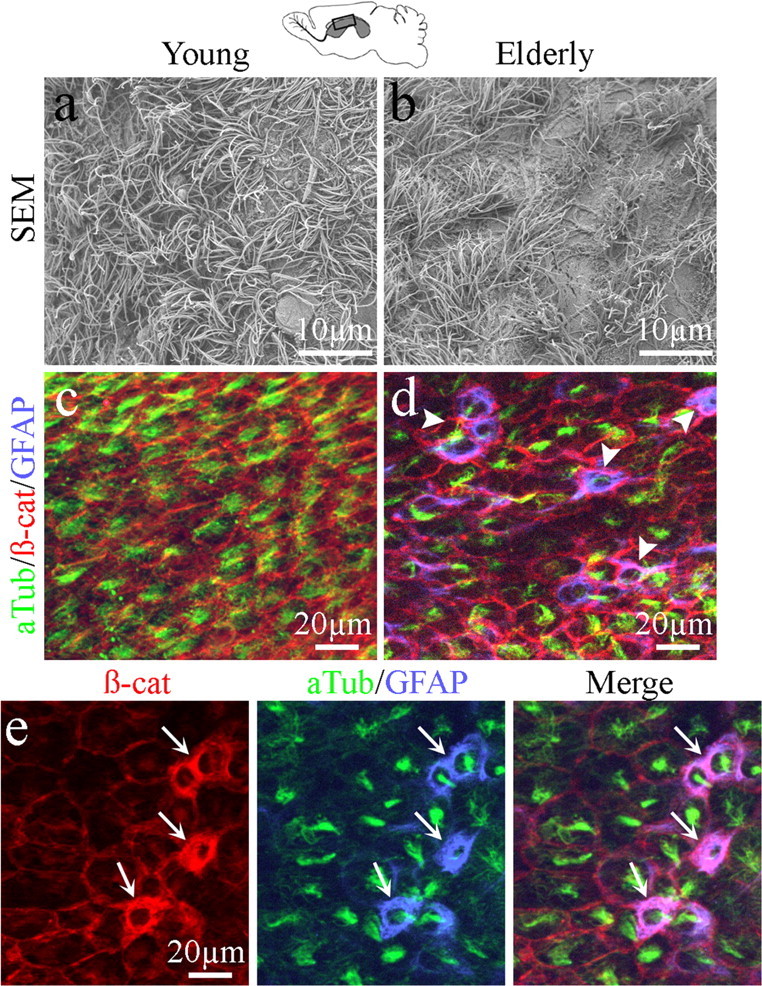
Comparison of the lateral wall ependymal surfaces for young and elderly mice. a, b, Scanning EM images of whole mounts of the lateral walls of the lateral ventricles from young and elderly mice. Patches devoid of cilia and microvilli were found along the apical surface of the elderly ependymal wall (b). c, d, Whole mounts were immunostained with acetylated tubulin (aTub), β-catenin (β-cat), and GFAP. Ependymal cells were defined by adherens junctions (β-cat) between cells and the presence of cilia (aTub) in young mice; GFAP immunostaining was not detected along the lateral wall surface (c). In elderly mice, aTub+/β-cat+/GFAP+ cells (arrowheads) were observed within the ependymal wall (d). e, Higher magnification of aTub+/β-cat+/GFAP+ triple-labeled cells (arrows) along the apical surface of the elderly ependymal wall clearly indicates that GFAP+ cells integrate within the ependyma and possess cilia.
Whole-mount preparations of the apical surface of the lateral wall of the lateral ventricle allowed immunohistochemical comparisons between young and elderly ventricle linings. We stained for markers of adherens junctions (β-catenin) (Perez-Moreno and Fuchs, 2006), cilia (acetylated tubulin) (McClintock et al., 2008), and intermediate filaments of astrocytes (glial fibrillary acidic protein, GFAP). In young mice, β-catenin staining revealed the cellular outline of the ependymal cells, whereas acetylated tubulin decorated tufts of cilia associated with each ependymal cell (Fig. 2c). GFAP staining of the lateral wall apical surface in young mice was rare and restricted to only an occasional thin process along the surface, similar to what we observed using TEM (Luo et al., 2006). In contrast, immunostaining of the lateral ventricle lining of elderly mice revealed GFAP and β-catenin coexpression in several cuboidal cells on the ventricle surface. The majority of GFAP+/β-catenin+ cells also possessed cilia, as defined by acetylated tubulin detection (Fig. 2d,e, arrows). Together, these studies indicated that cells with characteristics of both ependymal cells and astrocytes were found within the ependymal layer of the elderly lateral wall of the lateral ventricle.
Dividing SVZ astrocytes integrate within the aged ependyma
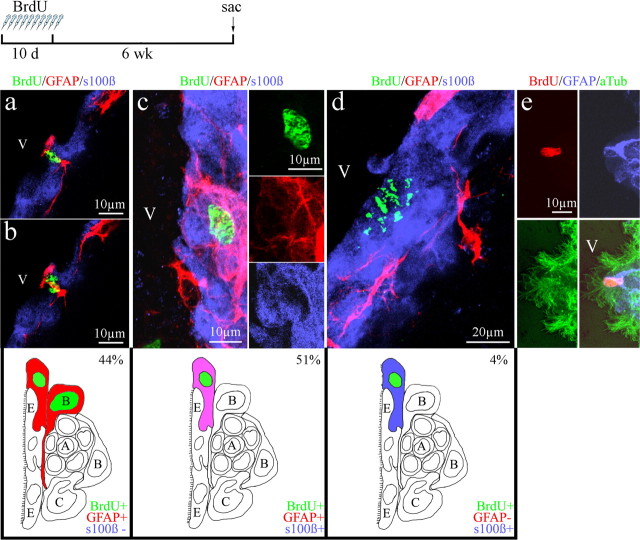
To label a large population of dividing SVZ progenitor cells, we injected 2- and 24-month-old mice daily with BrdU (50 mg/kg) for 10 d. Not knowing the frequency or rate at which astrocytes incorporated within the ependyma of elderly mice, we initially examined the ependymal lining for BrdU+ cells 6 weeks after the last BrdU injection. Previously, others and our laboratory had determined that 6 weeks was sufficient time to allow newly generated BrdU+ neuroblasts to migrate from the SVZ into the olfactory bulb, leaving primarily BrdU+ astrocytes in the SVZ of young mice. During examination of the ependymal layer, we found that the majority (95%) of BrdU+ cells incorporated within the ependyma after 6 weeks colabeled for the astrocyte marker GFAP. Of these, 44% labeled for BrdU and GFAP alone (Fig. 3a,b), and we occasionally observed dividing astrocytes with their cleavage plane parallel to the ependyma, as seen in Figure 3, a and b. This orientation suggested the possibility that one daughter cell became incorporated within the ependyma, whereas the other remained in the SVZ (see schematic below Fig. 3a,b). Of the remaining dividing cell population in the ependyma, 51% were triple labeled for BrdU/GFAP/s100β (Fig. 3c, Table 1). Using an antibody to CD24 as an alternative marker of ependymal cells, we observed similar numbers of BrdU+/GFAP+/CD24+ cells within the ependymal layer (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Antibodies to both s100β and CD24 labeled ependymal cells and did not label adult SVZ astrocytes (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) (Spassky et al., 2005; Kuo et al., 2006; Raponi et al., 2007). Therefore, the presence of BrdU/GFAP/s100β and BrdU/GFAP/CD24 triple-labeled cells within the ependyma suggested that previously dividing cells possessed both an astrocyte and ependymal cell phenotype 6 weeks after BrdU injection. It is noteworthy that, at this time point, only a few BrdU+ cells colabeled for s100β alone (4%) (Fig. 3d, Table 1). As expected, some BrdU+ astrocytes remained within the SVZ. These label-retaining astrocytes, putative precursors/NSCs, were clearly distinguishable by their characteristic long astrocytic processes (supplemental Fig. 3, available at www.jneurosci.org as supplemental material), making them distinct from the cuboidal astrocytes that were found integrated within the ependymal wall and that often possessed multiple cilia (Fig. 3e).
Figure 3.
Confocal images of BrdU+ cells within the ependymal wall of elderly mice 6 weeks after BrdU injection. a, b, BrdU+ cell that coexpressed GFAP undergoing division with its cleavage plane parallel to the ependymal wall. c, BrdU+/GFAP+/s100β+ triple-labeled cell within the ependyma. d, BrdU+/s100β+ cell in the ependyma. Schematics under a–d depict each BrdU+ cell type found in the ependyma at 6 week period with its relative percentage based on total BrdU+ cells found in the ependyma. e, Multiple cilia (aTub) are found to colocalize with a BrdU+/GFAP+ cell in the ependyma. V, Lateral ventricle.
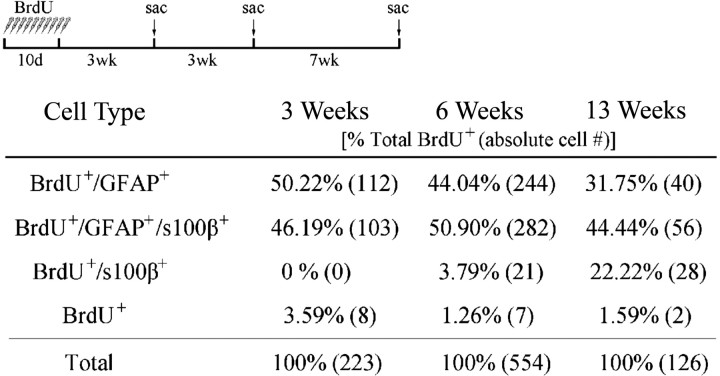
Table 1.
BrdU cell populations in the aged ependyma
To eliminate the possibility that ependymal cells were dividing, we examined the ependymal lining immediately after BrdU injection. At this time point, BrdU+ cells within the ependyma were primarily GFAP+ astrocytes; an occasional triple-labeled BrdU+/GFAP+/s100β+ cell was found, but no BrdU+ cells labeled for s100β alone. To establish a time course for the incorporation and conversion of BrdU-labeled cells in the ependymal wall of elderly mice, we examined the ependymal lining 3 and 13 weeks after BrdU injection (in addition to the 6 weeks described above). Three weeks after BrdU administration, 96% of BrdU+ cells colabeled for GFAP. Of these, 46% labeled for both GFAP/s100β (triple-labeled) (Table 1). These numbers were similar to those found at the 6 week period. However, we found no cells immunoreactive for BrdU/s100β alone at the 3 week time period, and only 4% of the BrdU+ cells immunolabeled for s100β alone at the 6 week period (Table 1). The absence of BrdU+/s100β+ cells immediately after and 3 weeks after BrdU injection indicated that ependymal cells do not divide in the elderly brain, similar to findings in the young mammalian brain (Bruni et al., 1985; Bruni, 1998; Doetsch et al., 1999a,b; Spassky et al., 2005). At 13 weeks after BrdU injection, the majority of BrdU+ cells (44%) were triple labeled (costaining for GFAP/s100β), and 32% were BrdU+/GFAP+ astrocytes. Most significant was our finding that the number of BrdU+ cells in the ependyma that colabeled for s100β alone increased from 0% (3-weeks) to 4% by 6 weeks and eventually rose to 22% at the 13 week time point (Table 1), suggesting conversion of SVZ astrocytes to ependymal-like cells.
Integration of proliferating astrocytes into the ependyma is dependent on an active SVZ
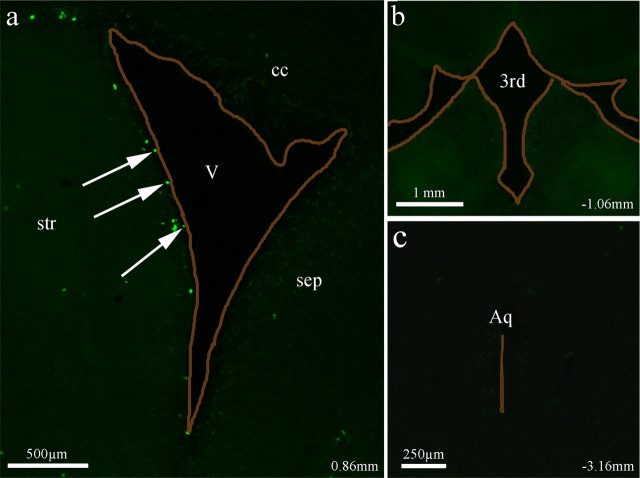
To determine whether dividing astrocytes contribute to ependymal repair of other ventricles in elderly mice, we examined the third ventricle and the cerebral aqueduct for proliferating cells that could be used in ependymal repair. After the same BrdU injection scheme outlined above (a daily injection of BrdU for 10 d followed by a 6 week period), we observed BrdU+ cells along the ventricle lining of the lateral wall of the lateral ventricle (Fig. 4a, arrows); however, no BrdU+ cells were detected along the third ventricle or the cerebral aqueduct in elderly mice (Fig. 4b,c). BrdU+ cells were also not observed within the ependyma of the medial wall of the lateral ventricles (Fig. 4a). These results suggest an SVZ-driven supply of dividing astrocytes for the ependyma of the lateral wall of the lateral ventricle in elderly mice.
Figure 4.
Examination of BrdU-labeled cells along different ventricular surfaces in elderly mice. a, BrdU+ cells were found only along the lateral walls of the lateral ventricles. Arrows indicate BrdU+ cells directly in contact with the ventricle. b, c, BrdU+ cells were not found along the apical surface of either the third ventricle (b) or cerebral aqueduct (c) in elderly mice. All ventricles are outlined in brown. cc, Corpus callosum; str, striatum; sep, septum; V, lateral ventricle; 3d, the third ventricle; Aq, aqueduct.
Mild denudation of the ependyma in young mice results in repair similar to that found in aging
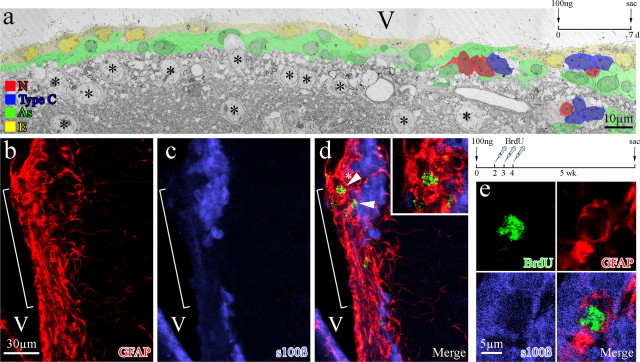
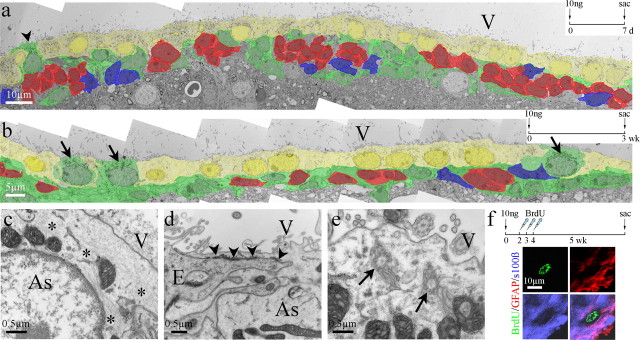
Sialoglycoproteins are involved in cell recognition and adhesion in the CNS (Rutishauser and Jessell, 1988; Kuchler et al., 1994; Figarella-Branger et al., 1995) and function in maintaining the integrity of the ependymal lining. Neuraminidase specifically cleaves sialic acid of sialoglycoproteins from the surface of ependymal cells, releasing cells from the ventricle lining and resulting in denudation of the ependymal layer (Grondona et al., 1996). To reproduce in young mice the limited ependymal cell loss found in elderly mice, we injected neuraminidase into the lateral ventricles of 2-month-old mice. Picnotic and detached ependymal cells were detected along the ventricle wall 30 min, 1 h, and 2 h after injection, with the level of denudation dependent on neuraminidase concentration (Grondona et al., 1996) (J. Luo, B. A. Shook, and J. C. Conover, unpublished observation). To identify a concentration of neuraminidase that resulted in minimal ependyma denudation, mimicking the level of replacement we found along the lateral wall of the lateral ventricle in elderly mice, we injected a single dose of neuraminidase (1 μl) into the lateral ventricle at concentrations ranging from 500 to 1 ng/μl (supplemental Fig. 4, available at www.jneurosci.org as supplemental material). We found that the higher concentrations of neuraminidase (500–100 ng/μl) resulted in wide stretches of ependymal cell loss and concluded that this level of extensive damage represented ependymal cell loss in excess of what would be expected in normal aged mice. Reducing the concentration of neuraminidase injected to 10 ng/μl resulted in scattered areas of individual ependymal cell loss, whereas injection of 1 ng/μl neuraminidase showed no obvious ependymal cell loss at the light microscope level. TEM analysis was used to observe the cytoarchitecture of the ependyma and adjacent SVZ after neuraminidase treatments. To generate extensive denudation, we injected 100 ng/μl neuraminidase, waited for a 1 week period, and observed a diminished SVZ, thinned ependyma, and increased numbers of astrocytes along the ependymal border and contacting the ventricle surface (Fig. 5a). Whereas 1 week after a dose of 10 ng of neuraminidase, we observed a relatively healthy ependyma and SVZ, with only a few astrocytes showing ventricle contact (Fig. 6a). By 3 weeks after neuraminidase treatment (10 ng), several astrocytes could be found fully integrated within the ependyma (Fig. 6b). These astrocytes were cuboidal in shape with their nuclei aligned with neighboring ependymal cell nuclei. Therefore, the level of repair after the 10 ng neuraminidase dosage was considered a close approximation of what was observed in elderly mice.
Figure 5.
High dosage neuraminidase treatment resulted in SVZ deterioration and glial scarring in young mice. a, Colorized EM montage of the lateral wall of the lateral ventricle of a young mouse 7 d after receiving an injection of 100 ng of neuraminidase. Note the close proximity of striatal neurons (asterisks) and associated neuropil to the SVZ. b–d, Immunostaining for GFAP and s100β along the lateral ventricle wall reveals regions of severe denudation 5 weeks after injection of 100 ng of neuraminidase and a 3 d BrdU injection paradigm. Denuded regions contained GFAP+ cells (brackets in b–d), many of which colabeled for BrdU (d, arrows and top inset). e, An occasional triple-labeled (BrdU+/GFAP+/s100β+) cell could be found, but only in regions in which the ependymal layer remained relatively intact. V, Lateral ventricle.
Figure 6.
Low dosage neuraminidase injection led to astrocyte incorporation similar to that found in aging. a, b, Colorized EM montages of the lateral walls 7 d (a) and 3 weeks (b) after a 10 ng neuraminidase treatment. An occasional astrocyte (arrowhead) was detected within the ependymal wall 7 d after treatment (a). In a representative section, three astrocytes (arrows in b) were found integrated within the ependymal lining by 3 weeks (b). c–e, These interposed astrocytes contained bundles of intermediate filaments (asterisks in c), formed adherens junctions with neighboring ependymal cells (arrowheads in d), and possessed basal bodies of cilia (arrows in e), demonstrating their similarity to astrocytes found in the elderly ependyma (see Fig. 1b,e–g). f, BrdU+/GFAP+/s100β+ cells were detected in the ependyma 5 weeks after 10 ng neuraminidase treatment and a 3 d BrdU injection paradigm. As, Astrocytes; E, ependymal cells; V, lateral ventricle.
We next evaluated incorporation of BrdU+ astrocytes into the ependymal wall after neuraminidase treatment at dosages of 1, 10, and 100 ng/μl. We reasoned that ependyma repair to replace lost ependymal cells would be necessary after treatment with all dosages of neuraminidase; however, repair-promoting conversion of SVZ astrocytes to cells with characteristics of ependymal cells may occur only after limited ependymal cell loss, similar to that found in aging. Four-month-old mice were injected with a single dose of neuraminidase, followed 2 d later with a daily injection of BrdU for 3 d. We evaluated the presence of BrdU immunoreactivity in the ependyma 5 weeks after the last BrdU injection. Five weeks after BrdU treatment, immunostaining for s100β and GFAP in mice injected with 100 ng of neuraminidase revealed broad stretches of ependymal denudation, as observed by the absence of s100β staining (Fig. 5b–d, bracket). These stretches showed increased numbers of GFAP+ astrocytes, a large percentage of which were BrdU+ (Fig. 5d, arrowheads). However, no triple-labeled (BrdU+/GFAP+/s100β+) cells were found in these or similar areas of extensive ependymal cell loss. Whereas, in other areas in which ependymal cell loss was less and a nearly intact ependyma was retained, we detected some triple-labeled cells within the ependyma lining (Fig. 5e). Using lower concentrations of neuraminidase (10 ng/μl), we detected minimal damage to the ependyma and SVZ (Fig. 6a). Three weeks after neuraminidase treatment, several astrocytes were found incorporated within the ependymal lining, as determined by TEM (Fig. 6b, arrows). Incorporated astrocytes, identified by their light cytoplasm and bundles of intermediate filaments (Fig. 6c, asterisks), shared cellular adherens with neighboring ependymal cells (Fig. 6d, arrowheads). In addition, basal bodies of cilia were found within the cytoplasm of the incorporated astrocytes (Fig. 6e, arrows). Immunohistochemistry revealed triple-labeled cells (BrdU+/GFAP+/s100β+) within the ependyma at our 5 week period after BrdU treatment (Fig. 6f). A reduced dosage of 1 ng/μl neuraminidase showed no observable ependymal cell loss at the light microscope level (supplemental Fig. 4a, available at www.jneurosci.org as supplemental material) but revealed some rare triple-labeled, BrdU+/GFAP+/s100β+ cells within the ependyma at the 5 week time period (data not shown).
Discussion
NSCs reside in two restricted zones in the adult brain and support continued neurogenesis through adulthood (Gage, 2000; Temple, 2001; Doetsch, 2003a; Alvarez-Buylla and Lim, 2004). This ability to generate new brain cells raises the exciting possibility that adult NSCs may be used for repair of brain injuries or neurological disease. However, brain pathologies are often associated with aging, and the process of aging diminishes the SVZ niche and compromises the potential for endogenous NSC-mediated regenerative repair. Our previous work (Luo et al., 2006) revealed that the SVZ deteriorates through aging along its ventral aspect, restricting neurogenesis to only the dorsolateral wall of the lateral ventricle. Associated with the remaining pockets of neurogenesis along the dorsolateral wall, we detected increased numbers of astrocytes incorporated within the ependyma in what appeared to be repair or remodeling of the ventricle lining. Ependymal cells provide a protective barrier and filtration system, separating brain parenchyma from CSF of the ventricles. We predict that young, healthy animals have a minimal requirement for ependyma repair; however, with increased age, the need for repair likely increases as new cells are needed to prevent gaps attributable to distention of the ependyma walls or after ependymal cell death. We show that, in elderly animals, astrocyte-mediated ependyma repair/remodeling is associated with an active SVZ that maintains a population of proliferative SVZ astrocytes or NSCs.
Shared radial glia origin of ependymal cells and SVZ astrocytes
The walls of the lateral ventricles consist of a single, continuous layer of cuboidal ependymal cells characterized by surface microvilli and cilia and tight adherens junctions between neighboring cells. Ependymal cells have a radial glia origin, with the majority of ependymal cells born between embryonic days 14 and 16 in mice (Rakic and Sidman, 1968; Spassky et al., 2005). However, their final maturation, including the development of cilia, occurs much later during the first postnatal week (Spassky et al., 2005). A radial glia lineage has also been ascribed to SVZ astrocytes, a subpopulation of which was identified as NSCs (Rakic and Sidman, 1968; Doetsch et al., 1999a; Alvarez-Buylla et al., 2001; Doetsch, 2003b; Tramontin et al., 2003; Merkle et al., 2004). The radial glia origin of SVZ astrocytes/NSCs suggests a continuum between the embryonic ventricular zone, its postnatal equivalent, and, finally, the more restricted germinal zone of the adult SVZ (Rakic and Sidman, 1968; Doetsch et al., 1999a; Alvarez-Buylla et al., 2001; Doetsch, 2003b; Tramontin et al., 2003; Merkle et al., 2004). It is important to note that some adult SVZ astrocytes maintain a thin process that intercalates between ependymal cells and contacts the ventricle, similar to the end foot of radial glia. Thus, it is attractive to hypothesize that this specific group of astrocytes contributes to ependymal repair. The lineage connection between SVZ astrocytes and radial glia presents the exciting possibility that some adult SVZ astrocytes still possess the ability to generate new ependymal cells in the adult brain, mimicking an earlier developmental program of radial glia.
Dividing SVZ astrocytes incorporate within the elderly ependymal wall
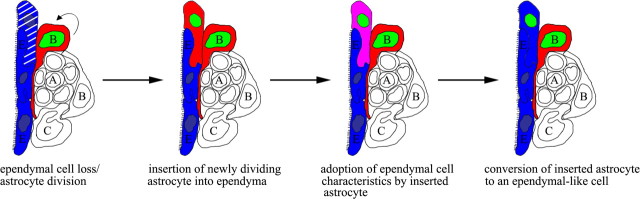
BrdU birthdating allowed us to track dividing BrdU+ cells within the ependyma over time. Our results suggest that the following sequence of events are involved in ependyma repair (Fig. 7): (1) ependymal cell loss or distension occurs along the elderly ventricle lining; (2) a dividing SVZ astrocyte (BrdU+/GFAP+), possibly one with ventricle contact via a thin process, inserts into ependyma to maintain the integrity of the ependyma barrier; (3) the inserted astrocyte (BrdU+/GFAP+) establishes cellular adherens with neighboring ependymal cells and takes on antigenic and morphologic characteristics of a mature ependymal cell (s100β+, triple-labeled); and (4) over time, the triple-labeled cell loses GFAP immunoreactivity. Consistent with the work of others (Bruni et al., 1985; Bruni, 1998; Doetsch et al., 1999a,b; Spassky et al., 2005), we found that ependymal cells do not divide in the elderly brain. In whole-mount preparations of the apical surface of the lateral wall of the lateral ventricle from elderly mice, we observed that the majority of intercalated GFAP+ cells possessed cilia; those that did not possibly represent an earlier transition stage. Together, our results suggest that BrdU+ astrocytes incorporated within the ependyma steadily lost GFAP immunoreactivity and over time took on characteristics of ependymal cells. However, it is not clear whether SVZ astrocytes with ependymal-like characteristics ultimately transform into fully functional ependymal cells. In addition, it is unknown whether distinct subpopulations of SVZ astrocytes are unipotent, capable of generating only a specific cell lineage (i.e., neuroblasts or ependymal-like cells), or are bipotent, supporting both neurogenic and ependymogenic pathways.
Figure 7.
Proposed sequence of events for SVZ-mediated ependymal repair.
Ependymal repair occurs after mild damage in young mice
It has long been assumed that, after postnatal development, no regeneration by the adult brain ependyma occurs (Bruni et al., 1985; Bruni, 1998). The one exception appears to be along the lateral wall of the lateral ventricles. Betty (1977) reported areas of hypertrophy and hyperplasia of unknown etiology within the ependymal lining of the lateral ventricles of guinea pigs that received indirect thermal lesions of the cerebral hemisphere. In another study, partial ependymal denudation with neuraminidase resulted in large numbers of mitotic cells along the dorsolateral wall of the lateral ventricle, whereas no mitotic cells were found along the third ventricle or aqueduct (Grondona et al., 1996). Together, these studies suggested the possibility of limited repair of the lateral ventricle ependyma after injury, most likely through the activation of the subependyma or SVZ. However, the mechanism and extent of repair or whether gliotic scarring versus regenerative restoration occurred was not evaluated in these injury models.
In a recent study by Kuo et al. (2006), deletion of Numb/Numblike at P0 in an inducible Cre/loxP transgenic mouse system resulted in severe damage to the integrity of the ependyma wall overlying the SVZ and reduced SVZ neuroblast survival. Numb/Numblike are known to be key regulators of embryonic neurogenesis and neuroepithelial integrity. Therefore, this study revealed a novel role for Numb in the maintenance of the ependyma. Because the inducible deletion of Numb/Numblike did not target all SVZ progenitors, those that escaped eventually remodeled the ventricular wall and reconstituted the SVZ. Interestingly, the remodeled ventricular wall revealed cells that stained positive for both s100β and GFAP, a cell type not found within the adult neurogenic SVZ but which resembled the triple-labeled cells (BrdU+/s100β+/GFAP+) we observed in the elderly ependymal wall.
To mimic in young mice the moderate ependymal cell loss found in aging, we used dilute concentrations of neuraminidase (1–10 ng/μl) to initiate mild ependyma denudation. Three weeks after neuraminidase treatment, EM analysis revealed scattered astrocytes within the ependymal lining; these astrocytes developed cellular adherens with neighboring ependymal cells and many possessed basal bodies of cilia. BrdU labeling revealed that dividing astrocytes incorporated within the ependyma and many colabeled for s100β/CD24, similar to our findings in the elderly ependyma. Higher doses of neuraminidase (100 ng/μl) resulted in glial scars along the ventricular surface, and, although the majority of cells within the scar expressed BrdU and GFAP, they did not express s100β. These studies emphasized that the extent of ependymal damage dictated the type of repair. Mild denudation allowed SVZ astrocytes to take on characteristics of ependymal cells (e.g., adherens junctions, s100β immunoreactivity, and formation of cilia), whereas greater loss of contiguous ependymal cells prompted scar formation.
Concluding comments
Based on our findings, we propose that the transformation of dividing SVZ astrocytes into ependymal-like cells requires contact with existing ependymal cells, because fully denuded areas did not appear capable of regenerative repair. In addition, ependymal repair in regions other than the lateral wall of the lateral ventricle may not result in the astrocyte–ependymal cell conversion that we find adjacent to the SVZ. Interestingly, the dosages of neuraminidase used in this study to partially denude the lateral wall did not appear to affect either the medial or dorsal walls of the lateral ventricle. Injection of 100 ng/μl neuraminidase (the highest dose examined in detail) into the lateral ventricle resulted in ependymal cell loss only along the lateral wall of the lateral ventricle. This implies that the ependyma adjacent to the SVZ may be structurally and functionally different from other ependyma. It will be of interest to compare ependymal monolayers throughout the ventricular system and determine how SVZ-mediated ependymal repair affects SVZ neurogenesis during aging.
Together, these studies support for a novel form of SVZ astrocyte-mediated regenerative repair to the ependymal lining. Ependymal repair occurs at a moderate level in the elderly brain but can be activated in the ependyma of young animals after injury. It is important to note that the ependyma is inextricably involved in neurological and psychiatric illnesses that result in ventriculomegaly (i.e., schizophrenia), and malfunction or failure of the ependymal lining has been described as a cause of hydrocephalus. Therefore, it will be of considerable interest to determine how perturbation of ependymal repair may contribute to these disease conditions.
Footnotes
This work was supported by National Institutes of Health Grants NS45894 and NS050338 and the University of Connecticut Large Grant Awards (J.C.C.).
References
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Betty MJ. Ependymal hyperplasia in the lateral ventricle of the guinea-pig. J Comp Pathol. 1977;87:185–194. doi: 10.1016/0021-9975(77)90005-6. [DOI] [PubMed] [Google Scholar]
- Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. A review of the literature. Brain Res. 1985;356:1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat Neurosci. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003a;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003b;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci USA. 1999a;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999b;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Figarella-Branger D, Lepidi H, Poncet C, Gambarelli D, Bianco N, Rougon G, Pellissier JF. Differential expression of cell adhesion molecules (CAM), neural CAM and epithelial cadherin in ependymomas and choroid plexus tumors. Acta Neuropathol (Berl) 1995;89:248–257. doi: 10.1007/BF00309340. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Garfia A, Mestres P, Rascher K. Trinitrophenol lesions of the ventricular wall: a SEM-TEM study. Scan Electron Microsc. 1980:449–456. [PubMed] [Google Scholar]
- Grondona JM, Perez-Martin M, Cifuentes M, Perez J, Jimenez AJ, Perez-Figares JM, Fernandez-Llebrez P. Ependymal denudation, aqueductal obliteration and hydrocephalus after a single injection of neuraminidase into the lateral ventricle of adult rats. J Neuropathol Exp Neurol. 1996;55:999–1008. doi: 10.1097/00005072-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- Kuchler S, Graff MN, Gobaille S, Vincendon G, Roche AC, Delaunoy JP, Monsigny M, Zanetta JP. Mannose dependent tightening of the rat ependymal cell barrier. In vivo and in vitro study using neoglycoproteins. Neurochem Int. 1994;24:43–55. doi: 10.1016/0197-0186(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, Jan YN. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Dziewczapolski G, Willhoite AR, Kaspar BK, Shults CW, Gage FH. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000;127:5253–5263. doi: 10.1242/dev.127.24.5253. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Glasser CE, Bose SC, Bergman DA. Tissue expression patterns identify mouse cilia genes. Physiol Genomics. 2008;32:198–206. doi: 10.1152/physiolgenomics.00128.2007. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Subcommissural organ and adjacent ependyma: autoradiographic study of their origin in the mouse brain. Am J Anat. 1968;122:317–335. doi: 10.1002/aja.1001220210. [DOI] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia. 2007;55:165–177. doi: 10.1002/glia.20445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Jessell TM. Cell adhesion molecules in vertebrate neural development. Physiol Rev. 1988;68:819–857. doi: 10.1152/physrev.1988.68.3.819. [DOI] [PubMed] [Google Scholar]
- Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]