Abstract
In anautogenous mosquitoes, vitellogenesis, which includes production of yolk protein precursors, requires blood feeding. Consequently, mosquitoes transmit many diseases. Understanding the molecular mechanisms of vitellogenesis regulation will contribute significantly to vector control strategies. Newly emerged Aedes aegypti females require 3 days before becoming competent to activate vitellogenesis in response to a blood-meal-initiated, elevated titer of 20-hydroxyecdysone (20E). An orphan nuclear receptor gene βFTZ-F1 is transcribed in the fat body of newly emerged mosquito females; however, the βFTZ-F1 protein is only found 3 days later. Dramatically increased titer of the juvenile hormone III (JH III) is essential for the acquisition of 20E competence. In vitro fat body culture experiments have shown that βFTZ-F1 protein appears after exposure to JH III. Injection of double-stranded RNA complementary to βFTZ-F1 into newly emerged females attenuated expression of the early genes EcR-B, E74B, and E75A and the target YPP gene Vg, in response to a blood meal. Thus, βFTZ-F1 is indeed the factor defining the acquisition of competence to 20E in the mosquito fat body. Moreover, this is achieved through JH III-mediated posttranscriptional control of βFTZ-F1.
In oviparous animals, vitellogenesis is a key event in egg maturation, which involves the production of yolk protein precursors (YPPs) predominantly by extraovarial tissues and their uptake by developing oocytes. Numerous signals are involved in a precise coordination of vitellogenic tissues. In anautogenous mosquitoes, initiation of vitellogenesis requires a blood meal. As a consequence, mosquitoes are vectors of many devastating infectious diseases, including malaria, dengue fever, and lymphatic filariasis (1-4). Unraveling the molecular mechanisms of vitellogenesis regulation will aid the development of new strategies for more efficient vector control.
In the yellow fever mosquito Aedes aegypti, blood feeding triggers a signaling cascade culminating in the elevation of titers of ecdysteroids, which is closely correlated with the production of YPPs in the fat body, a tissue functionally analogous to vertebrate liver (5-7). Also, the genes encoding two major YPPs, vitellogenin (Vg) and vitellogenic carboxypeptidase (VCP), are activated in fat bodies cultured in vitro on addition of the physiologically active ecdysteroid 20-hydroxyecdysone (20E), which suggests that the Vg and VCP genes are regulated by this hormone (8).
The steroid hormone 20E controls larval molting and metamorphosis in many insects, and it functions during embryonic development and adult reproduction. The molecular mechanism of 20E action has been dissected in detail during Drosophila metamorphosis (9). As 20E titers are rising and dropping again, unique sets of genes are turned on and off at distinct stages. Additional factors are therefore essential for the achievement of such a precise control of gene expression. For instance, cuticle proteins, a major component of the insect exoskeleton, are controlled by the interaction of two insect hormones, 20E and juvenile hormone (JH). 20E can stimulate an insect to shed its old exoskeleton and molt to form a larger larva, or it can cause an insect to metamorphose from a larva to a pupa. The specific effect of 20E is regulated by JH released from the corpora allata (10, 11). When JH is present at high concentrations, the new cuticle will be larval; when JH is absent, as in the final larval instar, 20E will initiate metamorphosis by causing a switch in the cuticular program. The subsequent production of the pupal cuticle by 20E, however, occurs in the presence of JH that prevents the imaginal discs, such as the wings and genitalia, from imaginal differentiation. Subsequently, adult-cuticle formation is initiated in the absence of JH. During Drosophila metamorphosis, the stage specificity of the genetic response to 20E is set up by βFTZ-F1, an orphan nuclear receptor. Ectopic βFTZ-F1 expression leads to enhanced levels of transcription of the 20E-induced BRC, E74, and E75 early genes and premature induction of the stage-specific 93F early-puff and E93 transcription. βFTZ-F1 mutants pupate normally in response to the late-larval 20E pulse but display defects in stage-specific responses to the subsequent 20E pulse in prepupae (12-14).
In A. aegypti, a previtellogenic preparatory period is required for the adult female to attain competence for blood feeding and for the mosquito fat body to become competent for massive yolk protein synthesis and secretion. This process is manifested by several cellular events in the fat body, such as development of the endoplasmic reticulum and Golgi complexes, ribosome proliferation, and an increase in ploidy (15-17). JH III titers rise 10-fold during the first 2 days after emergence and then slowly decline during the next 5 days. Blood ingestion causes an immediate decline of JH, which falls to its lowest level at 24 h post-blood meal (PBM; ref. 18). Activation of fat body nucleoli for ribosomal RNA production and ribosomal production is blocked by removal of the corpora allata in newly enclosed adult females, but it can be restored by either implantation of corpora allata or topical application of JH III to allatectomized females. This indicates that these events are controlled by JH from the corpora allata (16, 19). Furthermore, the exposure of a newly emerged female mosquito to JH III has been shown to be essential for the fat body to become responsive to 20E (20-22).
Genetic analysis of the 5′ upstream regulatory region of the Vg gene has revealed binding sites for the ecdysone receptor and the transcriptional regulators E74 and E75 (23). This analysis has suggested that the 20E regulatory hierarchy that is used in A. aegypti vitellogenesis is similar to that of Drosophila metamorphosis. At the top of the hierarchy is the 20E receptor, a heterodimer consisting of two members of the nuclear receptor superfamily, ecdysone receptor (EcR) and Ultraspiracle (USP), a homologue of vertebrate retinoid X receptor (24-29). On the binding of the ligand, the 20E receptor complex up-regulates a small number of primary-response early genes, including E74, E75, and the Broad Complex (BRC) (refs. 30 and 31; L.C. and A.S.R., unpublished data). More recently, chromatin immunoprecipitation experiments have displayed the occupancy of the EcR/USP receptor on the Vg promoter shortly after blood meal, which suggests that the Vg gene is the target of direct and indirect regulation by 20E (32).
To dissect the molecular mechanism governing the acquisition of competence for the vitellogenic ecdysteroid response in the mosquito fat body, we have cloned and characterized the mosquito homologue of the Drosophila ecdysteroid-response competence factor βFTZ-F1 (22). Mosquito βFTZ-F1 is transcribed highly in the late pupa and in the adult female fat body during pre- and postvitellogenic periods, when ecdysteroid titers are low, yet the transcripts nearly disappear in midvitellogenesis, when ecdysteroid titers are high. Each rise in the level of βFTZ-F1 transcripts is preceded by a high expression of another nuclear receptor (HR3) that coincides with the 20E peaks (22, 33). This observation is consistent with the role of HR3 in Drosophila, which facilitates induction of βFTZ-F1 in midprepupae (34, 35).
Here we present evidence showing that exposure of the newly emerged female adult to JH III has distinct effects on 20E early responsive genes and YPP genes. We also demonstrate that the presence of the βFTZ-F1 protein is closely correlated with acquisition of 20E responsiveness by YPP genes. In addition, βFTZ-F1 is regulated by JH III at the posttranscriptional level. Functional analysis of βFTZ-F1 by the RNA interference (RNAi) technique suggests that βFTZ-F1 is in fact a competence factor that defines the stage-specific 20E response during vitellogenesis in the mosquito.
Materials and Methods
Hormonal Treatment. 20E and JH III (Sigma) were dissolved in ethanol and acetone, respectively. A medium containing JH III was prepared as described by Riddiford et al. (36), and containers and culture plates were coated with Sigmacote (Sigma). The abdominal walls with adhering fat bodies (hereafter referred to as the fat body) were incubated in an organ-culture system as described (8) in the presence of 20E, JH III, or solvent alone (ethanol or acetone).
RNA Extraction, Reverse Transcription, and Real-Time PCR. Dissected fat bodies were homogenized with a motor-driven pellet pestle mixer (Kontes) and lysed by Trizol reagent (Invitrogen). RNA was isolated according to the manufacturer's protocol. Contaminating genomic DNA was removed by treatment with RNase-free DNase I (Invitrogen). Reverse transcription was carried out by using an Omniscript reverse transcriptase kit (Qiagen, Valencia, CA) in a 20-μl reaction mixture, containing random primers and 1 μg of total RNA at 37°C for 1 h. To quantitatively measure the levels of mRNA, real-time PCR was performed by using the iCycler iQ system (Bio-Rad). Reactions were performed in 96-well plates with a QuantiTect SYBR PCR kit (Qiagen). The sequences of the primer pairs for each of the specific RNA transcripts assayed are listed in Table 1. Quantitative measurements were performed in triplicate and normalized to the internal control of β-actin mRNA for each sample.
Table 1. Primers used in real-time PCR.
| Gene/primer | Sequence, 5′—3′ |
|---|---|
| Actin | |
| Forward | GACTACCTGATGAAGATCCTGAC |
| Reverse | GCACAGCTTCTCCTTAATGTCAC |
| EcR-B | |
| Forward | CGAAGAACGGAAAGGATAGCAGC |
| Reverse | TCACACCACTACTCCCACACAC |
| E74B | |
| Forward | GACCTCGTTCGCAAACACCTC |
| Reverse | AAGCCACCTGTTGATCGTCTTC |
| E75A | |
| Forward | CAGACATCTCGTACCAACAGTCG |
| Reverse | TTCGACCAACCGTGGTACTGTTC |
| βFTZ-F1 | |
| Forward | CGGCAGTAGCAAGGATGATGAAC |
| Reverse | ACAATCGCTTCGCTTCGCTCG |
| Vg | |
| Forward | GCAGGAATGTGTCAAGCGTGAAG |
| Reverse | ACGAGGACGAAGAATCGGAAGAG |
Protein Analysis. Nuclear proteins were isolated from fat body of 100 female adults for each time point as described (47). Total proteins were prepared by boiling fat-body samples for 5 min in lysis buffer [5% SDS/0.03% bromophenol blue/20% glycerol/5% 2-mercaptoethanol/0.5 M Tris·HCl (pH 6.8)].
cDNA fragments encoding the A/B domain of AaβFTZ-F1 were subcloned in pGEX-4T-1 (Amersham Pharmacia) to create a GST fusion. Polyclonal antibodies were then raised against the bacterially expressed fusion protein in New Zealand White rabbits. Monoclonal anti-β-actin antibody was purchased from Sigma and was used as suggested.
Electrophoretic mobility-shift assays were carried out as described by Li et al. (22). The nucleotide sequence of the F1RE used was 5′-GCAGCACCGTCTCAAGGTCGCCGAGTAGGAGAA-3′ (37).
Synthesis of Double-Stranded (ds) RNA and Microinjection. Synthesis of dsRNA was accomplished by simultaneous transcription of both strands of template DNA with a HiScribe RNAi Transcription kit (NEB, Beverly, MA). The DNA fragment encoding the A/B domain of mosquito βFTZ-F1 was inserted into LITMUS 28i cloning vector (NEB) at the EcoRI site, whereas the plasmid LITMUS 28iMal, containing a nonfunctional portion of the Escherichia coli malE gene that encodes maltose-binding protein, was used to generate control dsRNA. After RNA synthesis, the samples were digested with DNase I for 15 min at 37°C, followed by phenol/chloroform extraction and ethanol precipitation. The dsRNA was then suspended in diethyl pyrocarbonate-treated Aedes physiological saline with a final concentration of 10 μg/μl. Formation of dsRNA was confirmed by running 0.2 μl of these reactions in a 1.0% agarose gel in TBE [90 mM Tris-borate/2 mM EDTA (pH 8.0)]. A Picospritzer II (General Valve, Fairfield, NJ) was used to introduce 50 nl of dsRNA into the thorax of CO2-anesthetized mosquito females, at 12 h after eclosion. After 48 h, these mosquitoes were subjected to a second injection. They were allowed to recover for 24 h and then were given a blood meal. The fat bodies were collected at different times for RNA analysis.
Results
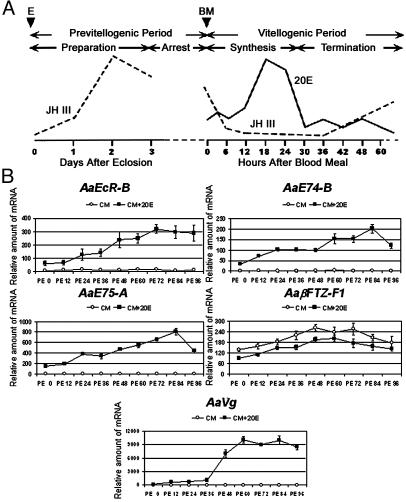
20E Responsiveness in the Fat Body of Previtellogenic Female Mosquitoes. To evaluate the influence of JH III on 20E responsiveness, we first analyzed the 20E induction of genes encoding a component of the ecdysone receptor (EcR), two 20E-responsive early genes (E74 and E75), and the target late gene (Vg), representing each level of the 20E regulatory hierarchy. We examined responses of these genes in the fat body both before and after the elevation of JH III titer. The fat bodies, collected from adult females at different time points posteclosion (PE), were cultured in vitro for 6 h in medium in the absence or presence of 1 × 10-6 M 20E. mRNA levels of individual genes were monitored by real-time PCR after RNA isolation and reverse transcription.
For EcR, E74, and E75, we measured isoforms that were induced shortly after blood feeding: EcR-B, E74-B, and E75-A. Transcription of these genes was stimulated in the fat body of adult females immediately after eclosion (Fig. 1B). However, this induction was low, but as the mosquitoes matured, they became more responsive to 20E in terms of the amount of transcript induced. A 5- to 7-fold increase in responsiveness was observed for the fat body of mosquitoes at 72-84 h PE as compared with those at 0 h PE. In parallel, the induced expression of Vg was negligible at the early previtellogenic stage. However, it was strikingly boosted after 36 h PE, when the JH III titers in the hemolymph had risen dramatically (18). Similar results were obtained when the fat bodies were incubated in vitro for 9 or 12 h (data not shown). These results showed that the 20E responsiveness of the genes of the 20E hierarchy correlates with the previtellogenic JH III pulse and that all genes reached their maximal responsiveness after exposure to the maximal JH III titer.
Fig. 1.
20E responsiveness in the fat body of previtellogenic female mosquitoes. (A) Hormonal titers during the first vitellogenic cycle of the anautogenous mosquito, A. aegypti. BM, blood meal; E, eclosion; JH III titers [modified from Shapiro et al. (18)]; 20E titers [modified from Hagedorn et al. (6)]. (B) Fat bodies from mosquitoes were isolated at 12-h intervals after eclosion and cultured in vitro for 6 h in medium with or without the presence of 1 × 10-6 M 20E. The expression of the genes involved in the 20E signal pathway was determined by real-time PCR and was normalized to β-actin expression. Representative data (mean ± SEM) from at least three independent experiments are shown. CM, culture medium.
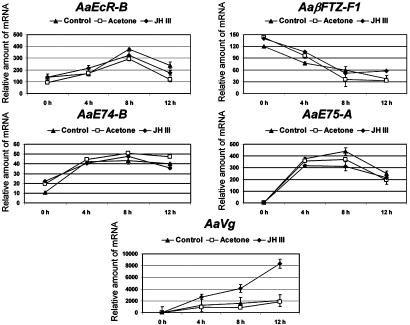
Exposure of Mosquito Fat Bodies to JH III in Vitro Enhanced Induction of Vg by 20E. Next, we tested the hypothesis that the exposure of the fat body to JH III during the previtellogenic period is essential for attaining 20E responsiveness. To determine whether early treatment with JH III would enhance Vg induction by 20E, fat bodies from newly emerged female mosquitoes (6 h PE) were either cultured directly in medium with 1 × 10-6 M 20E or incubated first for 12 h in medium with 1 × 10-5 M JH III or acetone (solvent of JH III) and then cultured in medium with 20E. At 4-h intervals, the fat bodies were collected and the transcription of genes of interest was examined by real-time PCR. Pretreatment with either acetone or JH III treatment had no noteworthy effects on the mRNA levels of EcR-B, E74-B, E75-A, and βFTZ-F1 (Fig. 2). In contrast, 20E-induced expression of Vg was considerably amplified by the preceding incubation with JH III, although not by incubation with acetone alone. These results demonstrate that early and late genes are differentially affected by JH III. JH III titers in the previtellogenic fat body are essential for Vg to become competent for 20E responsiveness.
Fig. 2.
JH III augments activation of the Vg gene by 20E. Fat bodies from newly emerged female mosquitoes were exposed to 1 × 10-5 M JH III (♦) or acetone (□) in vitro for 12 h, followed by culture in the presence of 1 × 10-6 M 20E. Fat bodies were also incubated directly with 20E as a control (▴). The expression of the indicated genes was determined by real-time PCR and was normalized to β-actin expression. Arbitrary units are plotted against time of incubation in medium with 20E. Representative data (mean ± SEM) from at least three independent experiments are shown.
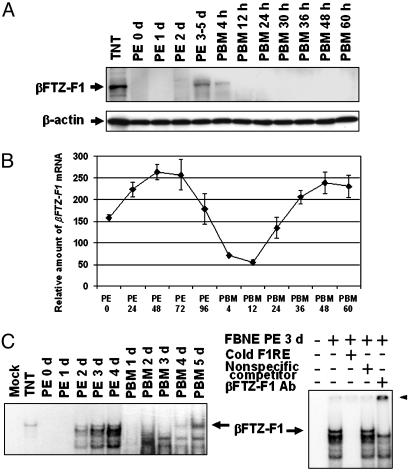
The Presence of βFTZ-F1 Protein Closely Correlates with the Competence for 20E Response. By using electrophoretic gel mobility shift assay, our previous study showed that active βFTZ-F1 protein was detected only in the fat bodies of previtellogenic female adult at 3-5 d PE (22). To further study its regulation, we raised antibodies that specifically recognized A. aegypti βFTZ-F1. Western blot analysis has shown that βFTZ-F1 protein is not detectable in the fat body nuclear extracts of newly emerged and 1-day-old mosquitoes. βFTZ-F1 was barely detectable at 2 d PE (Fig. 3A), reached the maximum at 3-5 d PE, and faded away shortly after blood feeding. In contrast, the mRNA level of βFTZ-F1 was abundant throughout the entire previtellogenic stage, with a climax at 2-3 d PE (Fig. 3B). These findings suggest that regulation of βFTZ-F1 in the previtellogenic stage occurred principally through a posttranscriptional mechanism.
Fig. 3.
Mosquito βFTZ-F1 protein in the fat body during vitellogenesis. (A) Fat-body nuclear extracts were separated on SDS/10% polyacrylamide gel, transferred to polyvinylidene fluoride membrane, and analyzed with rabbit polyclonal anti-AaβFTZ-F1 antiserum. The antibodies bound were then stripped, and the membrane was reprobed with monoclonal antibody against β-actin. TNT, in vitro synthesized AaβFTZ-F1. (B) βFTZ-F1 mRNA in the fat body of adult females was measured by real-time PCR. Representative data (mean ± SEM) from at least three independent experiments are shown. (C) βFTZ-F1 in the fat-body nuclear extracts was examined with electrophoretic gel mobility shift assay as described by Li et al. (22). The arrowhead indicates the supershifted band. F1RE, consensus binding site of βFTZ-F1; FBNE, fat-body nuclear extracts.
The vitellogenic process is cyclic, because female mosquitoes can take several blood meals during their life span, each followed by oviposition of a new batch of eggs. To examine the behavior of the active FTZ-F1 protein during vitellogenic cycles, we repeated electrophoretic gel mobility shift assays with fat-body nuclear proteins, extending this analysis from the eclosion of the adult female to the second blood meal. This analysis demonstrated that the βFTZ-F1 protein capable of DNA binding indeed returns at 4-5 d PE, when the female adult is ready for another blood meal (Fig. 3C), indicating that the presence of βFTZ-F1 parallels cyclicity of the competence to respond to 20E by the fat body. The specificity of this protein-DNA interaction was confirmed by (i) competition with double-strand oligonucleotides containing the consensus binding site of βFTZ-F1 (F1RE) and (ii) supershifting by using the mosquito anti-βFTZ-F1 antibodies (Fig. 3C).
JH III-Stimulated βFTZ-F1 Synthesis in the Fat-Body Culture. The βFTZ-F1 protein appeared after the elevation of JH III titers, which prompted us to explore whether or not JH III was implicated in posttranscriptional regulation of βFTZ-F1. Fat bodies from female mosquitoes at 6 h PE were isolated and incubated for 18 h in medium with JH III or acetone. Nuclear proteins were then extracted from these fat bodies and subjected to Western blot analysis. The βFTZ-F1 protein, undetectable in the fat bodies of newly emerged mosquitoes, became evident after the fat bodies of the same age were incubated in vitro with JH III (Fig. 4). The yield of βFTZ-F1 in this experiment was dose-dependent. In contrast, the protein was not observed in the fat bodies cultured in medium alone or in medium with acetone. Therefore, JH III in the fat body was likely the crucial factor modulating the production of the βFTZ-F1 protein.
Fig. 4.
JH III stimulates synthesis of βFTZ-F1 in fat bodies cultured in vitro. Fat bodies of newly emerged female adults (6 h PE) were cultured in medium for 18 h in the presence of JH III or acetone. Western blot analyses were performed on fat-body nuclear extracts by using antibodies against AaβFTZ-F1 and β-actin. Fat bodies taken directly from female mosquitoes at 6 h PE and 72 h PE were used as controls. Representative data from three independent experiments are shown. CM, culture medium.
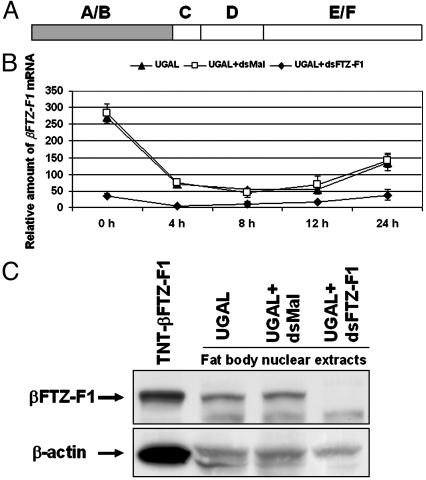
Functional Analysis of the βFTZ-F1 Role in 20E Competence by RNA Interference Assay. To study its function during vitellogenesis, βFTZ-F1 was silenced by RNA interference. dsRNA complementary to the A/B domain of βFTZ-F1 was synthesized in vitro and injected into the thorax of newly emerged female mosquitoes. In parallel, dsRNA complementary to the bacterial malE gene was used as negative control. After recovery, these mosquitoes were given a blood meal, and the fat bodies were subsequently isolated at various points in time. Compared with the naïve female adult, the mRNA and protein levels of βFTZ-F1 declined substantially in mosquitoes treated with dsRNA corresponding to βFTZ-F1 but not in those treated with control dsRNA, which indicates that βFTZ-F1 was selectively inhibited by RNA interference (Fig. 5 B and C).
Fig. 5.
The effect of RNAi on βFTZ-F1 expression in A. aegypti. (A) Schematic diagram of nuclear receptor βFTZ-F1 showing the A/B domain that was used to generate complementary dsRNA. (B) dsRNAs were injected into thoraces of female mosquitoes as described in Materials and Methods. βFTZ-F1 mRNA was measured at the indicated time after blood feeding by real-time PCR and was normalized to β-actin expression. Representative data (mean ± SEM) from at least three independent experiments are shown. ▴, uninjected A. aegypti Rockefeller/UGAL strain; □, injected with dsRNA complementary to malE; ♦, injected with βFTZ-F1 dsRNA. (C) βFTZ-F1 protein in unfed mosquitoes at 84 h PE detected by Western blot analyses. The signals corresponding to βFTZ-F1 were measured with VersaDoc (Bio-Rad) and normalized to the actin-loading control. βFTZ-F1 proteins in mosquitoes injected with control dsRNA and βFTZ-F1 dsRNA were 95% and 9%, respectively, of those in untreated female mosquitoes. TNT, in vitro synthesized βFTZ-F1 protein.
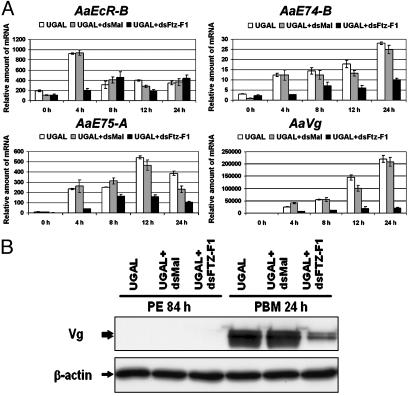
Next, we examined the expression of genes of the 20E hierarchy described above in the knock-down mosquitoes. The induction of EcR-B at 4 h PBM vanished in the βFTZ-F1 dsRNA-treated mosquitoes (Fig. 6A). The mRNA levels of E74-B and E75-A increased after blood feeding, but only ≈20-50% as much as in wild-type mosquitoes. A dramatic decline in the expression of the major YPP gene Vg was observed after blood feeding in the βFTZ-F1 dsRNA-treated mosquitoes (Fig. 6). On the other hand, mosquitoes harboring control dsRNA manifested mRNA profiles similar to those in untreated mosquitoes, indicating that these effects resulted from the inhibition of βFTZ-F1.
Fig. 6.
Expression of 20E-induced genes in mosquitoes subjected to βFTZ-F1 RNAi. (A) The transcripts of indicated genes in female mosquitoes were measured after blood feeding by real-time PCR and were normalized to β-actin expression. Arbitrary units are plotted against developmental time in hours PBM. Representative data (mean ± SEM) from three independent experiments are shown. Open bars, uninjected A. aegypti Rockefeller/UGAL strain; shaded bars, injected with dsRNA complementary to malE; solid bars, injected with βFTZ-F1 dsRNA. (B) Appearance of Vg protein in fat body was significantly inhibited by βFTZ-F1 RNAi. Proteins were extracted from mosquitoes at 84 h PE and 24 h PBM and were analyzed with monoclonal antibody against the Vg small subunit. The two bands represent different posttranslational modifications of the small subunit.
Discussion
In many insects, JH stimulates vitellogenin synthesis and uptake. In mosquitoes, 20E is responsible for the high synthetic rates of Vg, whereas JH primes the ecdysteroid response in the fat body and the ovary. Fat bodies removed from female A. aegypti between 0 and 52 h after emergence and incubated with 20E did not secrete Vg into the medium (21). However, between 56 and 64 h postemergence the fat bodies developed full competence to respond to the hormone. Using a similar in vitro culture system, we systematically examined the 20E response in the fat body of female adults at diverse times after eclosion. Transcription of the early genes EcR-B, E74B, and E75A was readily induced in the fat bodies from newly emerged female adults. It was found that these inductions gradually intensify as the mosquito matures, reaching the maximum at 72-84 h after emergence. In contrast, virtually no Vg activation was observed in the fat bodies of newly emerged mosquitoes, and it was astoundingly augmented in the fat bodies of older mosquitoes. Vg expression was stimulated in fat bodies from newly emerged mosquitoes if these fat bodies were incubated with JH III before 20E challenge. Together, these results imply that it is the exposure to JH that prompts the fat body to become fully competent to yield the tremendous amounts of YPPs and that individual genes from the 20E regulatory hierarchy react to JH III differently.
JH exerts pleiotropic functions during insect life cycles. Despite extensive studies, the mode of JH action is still not well understood. The receptor of JH is an area open to debate. Jones et al. (38, 39) have reported that USP can specifically bind JH and, in Sf9 cells, can activate transcription of the JH esterase core promoter, which is preceded by a DR12 enhancer. However, it is not clear whether USP acts in vivo as the bona fide receptor or as a component of the cognate receptor complex of this hormonal ligand. On the other hand, JHs have been documented as activating the transcription of many genes in diverse insects. The extent of most of the inductions has been relatively weak, and genetic analyses of the regulatory regions have failed to yield consensus on the JH response elements (40). Here, our results suggest that JH controls the synthesis of βFTZ-F1 protein in A. aegypti. This posttranscriptional control can be regulated at the level of gene expression by modulating the pre-mRNA splicing pattern, mRNA stability, mRNA transport, or the translation rate. The mechanism underlying this regulation remains obscure. Nevertheless, our findings add another step toward understanding of the mode of JH action. It appears that the posttranscriptional control of βFTZ-F1 occurs cyclically at the beginning of each vitellogenic cycle (Fig. 3). However, whether or not JH plays a similar role at the beginning of the second vitellogenic cycle requires further investigation.
RNAi refers to the introduction of homologous dsRNA to target specific mRNAs for degradation, resulting in null or hypomorphic phenotypes (41-43). By injecting dsRNA complementary to βFTZ-F1 cDNA, we successfully achieved post-transcriptional gene silencing in A. aegypti. The expression of β-actin mRNA and protein was not markedly altered by this treatment, which suggests that such inhibition is βFTZ-F1-specific (data not shown). The silencing of βFTZ-F1 provoked by injection of dsRNA was impressively effective. βFTZ-F1 mRNA dropped 80% versus the control group, whereas protein became undetectable under our experimental conditions. It is possible that the RNAi did not completely eliminate gene product, as it does in most instances in mammalian cells (44). Therefore, the inhibition must be considered as a ”knockdown” rather than a ”knockout” approach. Nonetheless, the silencing of βFTZ-F1 could result in phenotypes that mimic genetic knockouts.
In βFTZ-F1 knock-down mosquitoes, activation in response to a blood meal of the early genes EcR-B, E74B, and E75A, and the target YPP gene Vg, was attenuated in vivo. These data suggest that A. aegypti βFTZ-F1 is essential for stage-specific 20E response in fat body during vitellogenesis, which is reminiscent of the role βFTZ-F1 plays in Drosophila melanogaster during the prepupae to pupae transition. In Drosophila, immunostaining with βFTZ-F1 antibodies has indicated that it targets many ecdysone-responsive loci along with EcR/USP on the polytene chromosome (45). Potential binding sites for βFTZ-F1 were indeed found in the regulatory regions of Vg in the vicinity of ecdysone response elements (J.Z. and A.S.R., unpublished results). Moreover, the presence of βFTZ-F1 was cyclic, in parallel with the attainment of competence before every blood meal. We have reported recently that the cyclicity of vitellogenic ecdysteroid-mediated signaling was modulated, in part, through alternative heterodimerization of the retinoid X receptor homologue USP (32, 46). Clearly, βFTZ-F1 and EcR/USP should work in concert to achieve the precise and dynamic production of YPPs after a blood meal. Future studies should test the importance of the interplay between βFTZ-F1 and the EcR/USP complex.
Acknowledgments
We thank Geoff M. Attardo and Ray E. Hardesty for editing the manuscript and anonymous reviewers for constructive comments on the manuscript. This work was supported by National Institutes of Health Grant AI-36959 (to A.S.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: YPP, yolk protein precursor; JH, juvenile hormone; PBM, post-blood meal; EcR, ecdysone receptor; USP, Ultraspiracle; dsRNA, double-stranded RNA; PE, posteclosion; 20E, 20-hydroxyecdysone; Vg, vitellogenin; VCP, vitellogenic carboxypeptidase.
References
- 1.Beier, J. C. (1998) Annu. Rev. Entomol. 43, 519-543. [DOI] [PubMed] [Google Scholar]
- 2.Beaty, B. J. (2000) Proc. Natl. Acad. Sci. USA 97, 10295-10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breman, J. G. (2001) Am. J. Trop. Med. Hyg. 64, 1-11. [DOI] [PubMed] [Google Scholar]
- 4.Wattam, A. R. & Christensen, B. M. (1992) Proc. Natl. Acad. Sci. USA 89, 6502-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagedorn, H. H. (1983) in Endocrinology of Insects, eds. Downer, R. G. H. & Laufer, H. (Liss, New York), pp. 271-304.
- 6.Hagedorn, H. H. (1985) in Comprehensive Insect Physiology, Biochemistry and Pharmacology, eds. Kerkut, G. & Gilbert, L. (Pergamon, Oxford), pp. 205-261.
- 7.Raikhel, A. S. & Dhadialla, T. S. (1992) Annu. Rev. Entomol. 37, 217-251. [DOI] [PubMed] [Google Scholar]
- 8.Deitsch, K. W., Chen, J. S. & Raikhel, A. S. (1995) Insect Biochem. Mol. Biol. 25, 449-454. [DOI] [PubMed] [Google Scholar]
- 9.Thummel, C. S. (2002) Insect Biochem. Mol. Biol. 32, 113-120. [DOI] [PubMed] [Google Scholar]
- 10.Williams, C. M. (1961) Biol. Bull. 116, 323-338. [Google Scholar]
- 11.Riddiford, L. M. (1994) Adv. Insect Physiol. 24, 213-274. [Google Scholar]
- 12.Woodard, C. T., Baehrecke, E. H. & Thummel, C. S. (1994) Cell 79, 607-615. [DOI] [PubMed] [Google Scholar]
- 13.Broadus, J., McCabe, J. R., Endrizzi, B., Thummel, C. S. & Woodard, C. T. (1999) Mol. Cell 3, 143-149. [DOI] [PubMed] [Google Scholar]
- 14.Lam, G. & Thummel, C. S. (2000) Curr. Biol. 10, 957-963. [DOI] [PubMed] [Google Scholar]
- 15.Raikhel, A. S. & Lea, A. O. (1983) Tissue Cell 15, 281-300. [DOI] [PubMed] [Google Scholar]
- 16.Raikhel, A. S. & Lea, A. O. (1990) Gen. Comp. Endocrinol. 77, 423-434. [DOI] [PubMed] [Google Scholar]
- 17.Dittmann, F., Kogan, P. H. & Hagedorn, H. H. (1989) Arch. Insect Biochem. 12, 133-143. [Google Scholar]
- 18.Shapiro, A. B., Wheelock, G. D., Hagedorn, H. H., Baker, F. C., Tsai, L. W. & Schooley, D. A. (1986) J. Insect Physiol. 32, 867-877. [Google Scholar]
- 19.Raikhel, A. S. & Lea, A. O. (1991) Tissue Cell 23, 577-591. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan, T. R. & Hagedorn, H. H. (1977) Physiol. Entomol. 2, 173-178. [Google Scholar]
- 21.Ma, M., Zhang, J. Z., Gong, H. & Gwadz, R. (1988) J. Insect Physiol. 34, 593-596. [Google Scholar]
- 22.Li, C., Kapitskaya, M. Z., Zhu, J., Miura, K., Segraves, W. & Raikhel, A. S. (2000) Dev. Biol. 224, 96-110. [DOI] [PubMed] [Google Scholar]
- 23.Kokoza, V. A., Martin, D., Mienaltowski, M. J., Ahmed, A., Morton, C. M. & Raikhel, A. S. (2001) Gene 274, 47-65. [DOI] [PubMed] [Google Scholar]
- 24.Cho, W. L., Kapitskaya, M. Z. & Raikhel, A. S. (1995) Insect Biochem. Mol. Biol. 25, 19-27. [DOI] [PubMed] [Google Scholar]
- 25.Kapitskaya, M., Wang, S., Cress, D. E., Dhadialla, T. S. & Raikhel, A. S. (1996) Mol. Cell. Endocrinol. 121, 119-132. [DOI] [PubMed] [Google Scholar]
- 26.Wang, S. F., Miura, K., Miksicek, R. J., Segraves, W. A. & Raikhel, A. S. (1998) J. Biol. Chem. 273, 27531-27540. [DOI] [PubMed] [Google Scholar]
- 27.Wang, S. F., Li, C., Zhu, J. S., Miura, K., Miksicek, R. J. & Raikhel, A. S. (2000) Dev. Biol. 218, 99-113. [DOI] [PubMed] [Google Scholar]
- 28.Wang, S. F., Li, C., Sun, G. Q., Zhu, J. S. & Raikhel, A. S. (2002) Mol. Cell. Endocrinol. 196, 29-42. [DOI] [PubMed] [Google Scholar]
- 29.Martin, D., Wang, S. F. & Raikhel, A. S. (2001) Mol. Cell. Endocrinol. 173, 75-86. [DOI] [PubMed] [Google Scholar]
- 30.Pierceall, W. E., Li, C., Biran, A., Miura, K., Raikhel, A. S. & Segraves, W. A. (1999) Mol. Cell. Endocrinol. 150, 73-89. [DOI] [PubMed] [Google Scholar]
- 31.Sun, G. Q., Zhu, J. S., Li, C., Tu, Z. J. & Raikhel, A. S. (2002) Mol. Cell. Endocrinol. 190, 147-157. [DOI] [PubMed] [Google Scholar]
- 32.Zhu, J. S., Miura, K., Chen, L. & Raikhel, A. S. (2003) Proc. Natl. Acad. Sci. USA 100, 544-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kapitskaya, M. Z., Li, C., Miura, K., Segraves, W. & Raikhel, A. S. (2000) Mol. Cell. Endocrinol. 160, 25-37. [DOI] [PubMed] [Google Scholar]
- 34.Lam, G. T., Jiang, C. A. & Thummel, C. S. (1997) Development (Cambridge, U.K.) 124, 1757-1769. [DOI] [PubMed] [Google Scholar]
- 35.White, K. P., Hurban, P., Watanabe, T. & Hogness, D. S. (1997) Science 276, 114-117. [DOI] [PubMed] [Google Scholar]
- 36.Riddiford, L. M., Curtis, A. T. & Kiguchi, K. (1979) TCA Manual 5, 975-985. [Google Scholar]
- 37.Ohno, C. K., Ueda, H. & Petkovich, M. (1994) Mol. Cell. Biol. 14, 3166-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones, G. & Sharp, P. A. (1997) Proc. Natl. Acad. Sci. USA 94, 13499-13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, Y., Fang, F., Chu, Y., Jones, D. & Jones, G. (2002) Eur. J. Biochem. 269, 6026-6036. [DOI] [PubMed] [Google Scholar]
- 40.Bownes, M., Ronaldson, E. & Mauchline, D. (1996) Dev. Biol. 173, 475-489. [DOI] [PubMed] [Google Scholar]
- 41.Sharp, P. A. (2001) Genes Dev. 15, 485-490. [DOI] [PubMed] [Google Scholar]
- 42.Zamore, P. D. (2001) Nat. Struct. Biol. 8, 746-750. [DOI] [PubMed] [Google Scholar]
- 43.Hannon, G. J. (2002) Nature 418, 244-251. [DOI] [PubMed] [Google Scholar]
- 44.Shi, Y. (2003) Trends Genet. 19, 9-12. [DOI] [PubMed] [Google Scholar]
- 45.Lavorgna, G., Karim, F. D., Thummel, C. S. & Wu, C. (1993) Proc. Natl. Acad. Sci. USA 90, 3004-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu, J. S., Miura, K., Chen, L. & Raikhel, A. S. (2000) EMBO J. 19, 253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miura, K., Wong, S. F. & Raikhel, A. S. (1999) Mol. Cell. Endocrinol. 156, 111-120. [DOI] [PubMed] [Google Scholar]