Abstract
Processed meat intake is associated with increased risk of colorectal cancer. This association may be explained by the endogenous formation of N-nitroso compounds (NOC). The hypothesis that meat intake can increase fecal NOC levels and colon carcinogenesis was tested in 175 F344 rats. Initiation was assessed by the number of aberrant crypt foci (ACF) in the colon of rats, 45 days after the start of a high-fat bacon-based diet. Promotion was assessed by the multiplicity of ACF (crypt/ACF), in rats given experimental diets for 100 d, starting 7 d after an azoxymethane injection. Three promotion studies were done, each in 5 groups of 10 rats, whose diets contained 7, 14 or 28% fat. Tested meats were bacon, pork, chicken and beef. Fecal and dietary NOC were assayed by thermal energy analysis. Results show that feces from rats fed bacon-based diets contained 10 to 20 times more NOC than feces from control rats fed a casein-based diet (all p<0.0001 in four studies). In bacon-fed rats, amount of NOC input (diet) and output (feces) were similar. Rats fed a diet based on beef, pork or chicken meat had less fecal NOC than controls (most p<0.01). No ACF were detected in the colon of bacon-fed uninitiated rats. After azoxymethane injection, unprocessed but cooked meat-based diets did not change the number of ACF, nor the ACF multiplicity, compared with control rats. In contrast, the bacon-based diet consistently reduced the number of large ACF per rat, and the ACF multiplicity in the three promotion studies by 12, 17 and 20 % (all p<0.01). Results suggest that NOC brought by dietary bacon would not enhance colon carcinogenesis in rats.
Keywords: Animals; Azoxymethane; toxicity; Carcinogens; Cattle; Chickens; Colonic Neoplasms; chemically induced; etiology; prevention & control; Dietary Fats; administration & dosage; Disease Models, Animal; Feces; chemistry; Female; Food Handling; Meat; adverse effects; analysis; Nitroso Compounds; adverse effects; analysis; Random Allocation; Rats; Rats, Inbred F344; Risk Factors; Swine
Keywords: cancer, colorectal, prevention, promotion, initiation, toxicology, toxicity, processed meat, cured meat, ham, bacon, rat, NOC, nitrosamine, ACF, preneoplastic lesions, biomarkers, N-nitroso compounds, aberrant crypt foci, TEA, thermal energy analyser, diet, dietary, food, TEA-responsive compounds, ATNC
Introduction
Epidemiological studies suggest that dietary factors are important determinants of colon cancer risk (1). Many studies have implicated red meat and processed meat intake as risk factors, while white meat or fish intake would reduce the risk of developing colon cancer (review in ref. 2). One of the causative agent from processed meat may be N-nitroso compounds (NOC) either brought by the meat, or generated endogenously. But epidemiologic evidence of the carcinogenic potential of dietary NOC, and of precursor nitrates and nitrites, remains inconclusive with regard to the risk of stomach and esophageal cancers (3, 4). Volatile nitrosamines are detected in cured meat processed with nitrite, like bacon. In some fried bacon, concentrations of NOC could vary from 430 to 6800 μg(N-NO)/kg (5). NOC are alkylating agents, and they are a class of potent animal carcinogen that occur in the environment. But NOC are also found in the colon. They are formed endogenously, because the amines and the amides produced primarily by bacterial decarboxylation of amino acids can be N-nitrosed in the presence of a nitrosating agent such as nitrite in food (6). A relationship between NOC formation catalysed by bacteria, chronic urinary tract infections, and an increased risk for squamous cell carcinoma of the bladder is supported by studies using analytical methods and storage conditions that exclude artifactual NOC formation (7). Furthermore, the mutations that are common in human colorectal cancers are consistent with the alkylating effect of NOC (8). The effect of red meat and white meat consumption on fecal NOC levels have already been study in humans (9, 10). The authors showed a 3-fold increase in fecal NOC levels when volunteers are given 600 g of red meat per day. In contrast white meat and fish intakes do not increase fecal NOC levels in volunteers (9).
The present study was designed to investigate the effects of a diet containing processed meat, red meat or white meat on fecal NOC levels in rats. Since the fat content of diet may modify the effect of diet on colon carcinogenesis (11), the effect of meat was tested in both a low-fat and a high-fat context. We have examined three hypotheses: i) that red meat consumption increases NOC production in the rat as it does in man; ii) that processed meat intake increases fecal NOC excretion; and, iii) that these high intestinal NOC levels may initiate or promote the growth of aberrant crypt foci (ACF). The rat/azoxymethane model of experimental colon carcinogenesis was used to assess the validity of these hypotheses.
Materials and methods
Animals
A total of 175 female Fischer 344 rats (F344) obtained from Iffa-Credo (Lyon, France) at 4 weeks of age, were used. The animals were housed two rats per stainless steel wire drop-bottom cage, at 22°C on a 12-hour light:12-hour dark cycle. Before randomization, the rats were allowed free access to standard rodent chow (UAR, Villemoisson, France) and to water. After randomization, the rats were fed dry powdered diets based on a modified AIN-76 formula, provided by UAR.
Experimental procedures
We did three sequential rodents studies. #1: A 45d initiation study in three groups of rats; #2: A 100d promotion study in 10 groups of rats, fed high-fat diets. #3: A 100d promotion study in 5 groups of rats fed low-fat diets. Both ACF and NOC data of studies #1 and #3 are reported here. The ACF data of study #2 have already been reported in a previous article (12), and the results of NOC analysis are presented here.
Study #1: Initiation
Twenty-five rats were allocated to three groups after five days of acclimatisation (Fig. 1). Five positive control rats in group 1 were given a single i.p. injection of azoxymethane (Sigma, St Quentin, France) at a dose of 5 mg/kg in NaCl 9g/l. These rats and ten control rats were fed a high fat AIN76-based diet containing 28% fat and 40% protein (Table 1.a). Ten experimental rats were fed, ad libitum, a diet containing 60% bacon during 30 days (Table 1.a). The bacon-based diet and the control diet contained identical fat and protein levels. Then, all rats received the high-fat control diet for 15 more days (Fig. 1).
Figure 1.
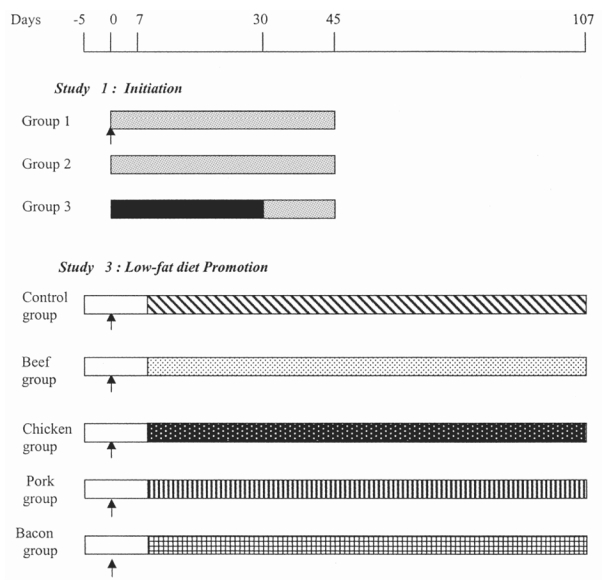
Experimental protocol. Arrow, azoxymethane injection (20 mg/kg body wt ip). Open bars, basal diet; gray bars, high-fat control diet; black bars, high-fat bacon diet; hatched bars, low-fat control diet; open stippled bars, beef diet; filled stippled bars, chicken diet; striped bars, low-fat bacon diet; checkered bars, pork diet. Experimental design of Study 2 has been published elsewhere (12).
Table 1.
Composition of experimental diets used in initiation (#) 1 and in promotion (#3) studies
| Studies | Initiation #1 | Promotion #3 | |||||
|---|---|---|---|---|---|---|---|
| Diets | high fat | low fat | |||||
| Group Ingredienta | 1 and 2 | 3 | Control | Beef | Chicken | Pork | Bacon |
| beef b | - | - | - | 30 | - | - | - |
| chicken c | - | - | - | - | 28 | - | - |
| pork d | - | - | - | - | - | 30 | - |
| bacon | - | 60e | - | - | - | - | 30f |
| casein | 40 | 13.9 | 25 | - | - | 2.73 | 1.58 |
| lard | - | - | 5 | - | - | 3.42 | 0.29 |
| chicken fat | - | - | - | - | 3.3 | - | - |
| corn oil | 28 | - | - | - | - | - | - |
| sucrose | 6 | 6 | 43 | 43 | 43 | 43 | 43 |
| corn starch | 10.2 | 10.2 | 15 | 15 | 15 | 15 | 15 |
| cellulose | 6.7 | 6.7 | 5 | 5 | 5 | 5 | 5 |
| choline bitartrate | 0.26 | 0.26 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| methionine | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| corn oil | 2.5 | 2.5 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| mineral mix | 4.68 | 4.68 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| vitamin mix | 1.33 | 1.33 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
Notes: values are g/100g diet
Freeze-dried cooked beef contained 11.1% fat and 83.8% protein
Freeze-dried cooked chicken contained 6.1% fat and 89.0% protein
Freeze-dried cooked pork contained 15.7% fat and 78.1% protein
Freeze-dried cooked bacon contained 47.1% fat and 44.4% protein
Freeze-dried cooked bacon contained 5.3% fat and 74.3% protein
Study #2: High-fat diet Promotion
As previously reported (12), one hundred F344 rats were given given a single i.p. injection of azoxymethane (20 mg/kg, Sigma, St Quentin, France), then randomized to 10 different AIN76-based high-fat diets, given ad libitum for 100 days. Five diets were adjusted to 14% fat and 23% protein, and 5 other diets to 28% fat and 40% protein. Fat and protein were supplied by 1) lard and casein, 2) olive oil and casein, 3) beef, 4) chicken with skin, and 5) bacon. The three types of meat were cooked in an oven for 15 minutes at 180-185°C. Each ovenproof glass dish contained 500 g of meat, 1 cm thick. During cooking, the bottom of meat portions was dipping in melted fat from meat. After cooling, the portions were minced, frozen for 24 hours at −20°C, then freeze-dried. Dry powdered cooked meats were included to make 30% or 60% of the diet.
Study 3: Low-fat diet Promotion
The effect of the high-fat meat-based diets on the promotion of ACF was so unexpected (12) that we decided to duplicate the meat study in a low-fat diet context. Fifty rats were given a single i.p. injection of azoxymethane (20 mg/kg, Sigma, St Quentin, France) after five days of acclimatization to the animals colony. One week later, the rats were randomly allocated to 5 groups (n = 10 in each group) and given the experimental low-fat diets for 100 days (Fig. 1). Control rats were fed an AIN76 based low-fat control diet containing 2% corn oil, 5% lard, and 25 % casein (Table 1.b). Four experimental groups were given diets with the same fat and protein contents, containing 30% meat. Low-fat beef (hamburger, Carrefour), chicken (fillet, Père Dodu), pork (Carrefour), and lean bacon (Carrefour) were obtained from a local supermarket (Carrefour Purpan, Toulouse, France). Meat was cooked as previously described. After analysis of fat and protein contents in each type of meat, meat diets were supplemented with casein to reach the 25 or 40% protein target. The percentage of fat was adjusted with lard for bacon and pork diets, and with chicken fat for chicken diet (Table 1.b).
In the three studies, the animals were observed daily and weighed weekly. The consumption of experimental diets and water were recorded on two occasions, for three days. The 24h fecal excretion (weight and number of pellets) was monitored for 3 days, on three occasions. Fecal moisture was also recorded on feces collected for 24h. All rats were killed by carbon dioxide asphyxiation. When the animals were sacrificed, feces in colon were removed for NOC assay, and immediately put in ice until preparation of fecal sample.
Assay of ACF
At the termination of the studies, the colon were excised, flushed with Kreb’s Ringer solution (Sigma), then opened longitudinally and fixed flat between coded filter papers in 10% buffered formalin (Sigma). ACF were scored using the procedure of Bird (13). The colons were stained with methylene blue (0.1%) for 6 min, then the mucosal side was observed at 32 × magnification. ACF were distinguished by their slit-like opening, increased staining, size and pericryptal zone. The number of ACF per colon and the number of aberrant crypts in each focus were recorded. All colons were scored blindly by a single observer. Initiation was assessed by the number of ACF. Promotion was assessed by the number of large ACF, and by the multiplicity of aberrant crypt foci (number of crypts per ACF).
Analysis of Fecal Nitrate and Nitrite
Fresh fecal samples were weighed and mixed with 4 volumes of distilled water. After shaking the mixture for 20 min and centrifugation at 20000 rpm, the supernatant was removed. One part was treated with sulfamic acid to destroy nitrite, and stored at −20°C until analysis of fecal NOC. The other part was used to measure nitrate and nitrite. Fecal nitrate were converted to nitrite a Nitralyser™ (World Precision Instruments, Inc). Nitrite concentration was determined colorimetrically: 200μl of reduced supernatant were added to 200μl of Griess reactive (0.1% β naphthylethylenediamine in 60% acid acetic/1% sulfanilic acid in 30% acid acetic (V/V)). The absorbance was read at λ =550nm.
Fecal NOC Assay
Fecal total NOC were determined using thermal energy analysis (TEA) (14). In brief, this method involves direct chemical denitrosation of the sample followed by chemiluminescence detection of the released nitric oxide (NO). In procedure A, the fecal sample was injected directly into refluxing ethyl acetate (50 ml) containing acetic acid 0.1% V/V hydrochloric acid and the thermo- and acetic acid labile TEA-responsive compounds (TAC) were determined. In procedure B, the fecal sample were injected directly into refluxing ethyl acetate (50 ml) containing hydrogen bromide (15% in glacial acetic acid) and both TAC and NOC were determined simultaneous. The difference between the two procedures (B-A) represents the concentration of NOC in fecal sample. The concentrations of NOC were calculated from the ratio of the peak area of the sample to that of a standard sodium nitrite solution. Results are expressed as μmol NOC/kg feces.
Statistical Methods
Values are means ± SD, and P values are two sided. Data were analysed by one-way factorial analysis of variance (ANOVA: factor: type of meat). When ANOVA showed a significant difference between groups (f test < 0.05), multiple comparisons were done by the Dunnett’s test comparing each group with the control group.
Results
General Observations
In the initiation study #1, the feeding of bacon did not modify the mean body weight (Table. 2), and the food intake (data not shown). Mean daily food intake was 8.2 g/d for each high-fat diets. Study #2 results have already been reported in details (ref. 12, table 3). In the promotion study #3, the feeding of meat increased significantly the rats mean body weight (p=0.001, Table. 2). In contrast, the mean daily food intake was the same in all groups (10 g/d).
Table 2.
Effect of feeding meat diets on body weight, water intake and fecal values in F344 rats
| Group | Treatment and diet | n | Body Weight (g) | Water intake (ml) | Fecal Weight (g/d) | Water in Feces(%) |
|---|---|---|---|---|---|---|
| study #1: initiation | ||||||
| 1 | AOM + control high-fat | 5 | 166 ± 6a | 15.7 ± 1.2 | 1.0 ± 0.5 | 16.2 ± 8.4 |
| 2 | control high-fat diet | 10 | 167 ± 8 | 15.0 ± 1.5 | 1.1 ± 0.2 | 10.7 ± 1.3 |
| 3 | bacon high-fat | 10 | 169 ± 5 | 21.2 ± 1.2b | 1.8 ± 0.6b | 40.0 ± 3.7b |
| study #3: promotionc | ||||||
| control | AOM + control low-fat | 10 | 187 ± 13 | 11.7 ± 0.5 | 0.67 ± 0.14 | 11.2 ± 0.9 |
| beef | AOM + beef low-fat | 10 | 204 ± 8b | 12.7 ± 0.7 | 0.82 ± 0.05 | 14.4 ± 1.4 |
| chicken | AOM + chicken low-fat | 10 | 204 ± 9b | 12.9 ± 0.9 | 0.73 ± 0.05 | 12.8 ± 2.0 |
| pork | AOM + pork low-fat | 10 | 204 ± 12b | 12.4 ± 1.3 | 0.76 ± 0.05 | 14.1 ± 5.7 |
| bacon | AOM + bacon low-fat | 10 | 200 ± 12b | 21.2 ± 1.6b | 0.90 ± 0.10b | 24.3 ± 5.2b |
Mean ± SD
Significantly different from the control by Dunnet test (p<0.01)
ACF data from study#2 have been published (12)
Table 3.
Effect of meat-based diets on fecal NOC concentrations in rats§
| NOC concentration (nmol/g feces)a | ||||
|---|---|---|---|---|
| Studyb | 3 | 2 | 2 | 1 |
| Dietary Fat | 7% | 14% | 28% | 28% |
| Group | ||||
| lard and caseine | 1.38 ± 0.64 | 0.47 ± 0.18 | 1.10 ± 0.49 | |
| oil and caseine | 0.29 ± 0.10* | 0.72 ± 0.27* | 1.05 ± 0.11 | |
| beef | 0.28 ± 0.13 c | 0.20 ± 0.08** | 0.27 ± 0.20** | |
| chicken | 0.86 ± 0.78 | 0.43 ± 0.22 | 0.41 ± 0.18** | |
| pork | 0.50 ± 0.49c | |||
| bacon | 14.42 ± 5.43c | 9.24 ± 2.09c | 13.67 ± 3.08c | 22.04 ± 2.83c |
diet composition is given in Table 1
mean ± SD
No statistical comparison can be made between columns because studies were done at one year intervals.
Significantly different from control by Dunnet test (p<0,01)
Significantly different from control by Dunnet test (*, p<0,05;
p<0,01 without the bacon group.
NOC level in bacon-fed rats was high, thus the SD was large and masked the other differences.
The water intake was nearly the same in all groups except in bacon-fed rats (Table 2). As already reported (12), the water intake was 1.5 to 2-fold higher in rats fed bacon-based diets than in controls (p<0.0001). This is likely due to the salt in the bacon-based diets (3.5% and 2.6% NaCl in the diets containing bacon, study #1 and #3 respectively).
In the three studies, the feeding of bacon markedly increased the daily fecal weight and the fecal moisture (Table. 2 and ref. 12). Fecal excretion was 1.5 time higher, and fecal moisture more than doubled by the feeding of a bacon-based diet. The fecal weight and the fecal moisture were not different between the others groups (Table 2).
Effect of Different Types of Meat on Fecal NOC
Study #1 Initiation
The fecal NOC concentration was 21-fold higher in feces of bacon-fed rats than in feces of controls (p<0.001; Table. 3).
Study #2: High-fat diet Promotion
Rats fed 30% bacon had 20 times more NOC than control fed 14% lard, p<0.0001 (Table. 3). Rats given 60% bacon had 12 times more NOC than controls fed 28% lard, p<0.0001 (Table. 3). Rats fed with beef or chicken meat had less fecal NOC than controls (Table. 3), and this difference reached significance when we analysed the data set without the group of bacon-fed rats. Diets containing 30% and 60% bacon brought 4.3 and 12.2 nmol NOC daily to the rats, that excreted 8.6 and 12.3 nmol NOC/d respectively.
Study #3: Low-fat diet Promotion
Rats fed a bacon-based diet had 10.5 times more fecal NOC than control rats (p<0.0001) (Table. 3). Bacon-based diet brought 13.3 nmol NOC daily to the rats, that excreted 13.1 nmol NOC/d. Rats fed with beef or pork meat had less fecal NOC than control (Table. 3).
Fecal nitrite and nitrate levels were 3 times higher in rat fed the bacon based diet than in rat fed control diet: 321.3 ± 91.1 and 100.0 ± 32.3 nmol/g feces respectively (p<0.01). There were no significant difference between the others groups (beef: 146.1 ± 62.3; chicken: 116.3 ± 52.5; pork: 159.3 ± 72.9 nmol/g feces).
Effect of Different Type of Meat on ACF
Study #1 Initiation
No ACF were detected in saline-injected rats fed with a bacon-based diet (group 3) or a control diet (group 2). In contrast, many ACF were detected in each rats of group 1 treated with AOM (mean ACF number, 19; range: 7–154 ACF per colon).
Study #2: High-fat diet Promotion
As already reported, the ACF multiplicity was reduced by 12% in rats fed the 30% bacon diet (3.21 ± 0.47 crypts/ACF in controls, and 2.84 ± 0.45 in bacon-fed rats), and by 20% in rats fed the 60% bacon diet (3.27 ± 0.38 and 2.62 ± 0.6; p<0.001) (12). In contrast, the ACF multiplicity was the same in the other groups fed casein and fat, or beef meat or chicken meat diets (p=0.7) (12). Bacon intake also decreased the number of large ACF (containing 7 or more crypts/focus). These large ACF (containing 7 crypts or more per ACF) are better predictors of cancers than total ACF. The number of large ACF was three times lower in rats fed a 60% bacon-diet than in control rats (2.1 ± 2.5 and 6.4 ± 4.9 large ACF/colon respectively, p=0.03), but the difference did not reach significance in rats given a 30% bacon-diet (5.0 ± 4.8 and 5.4 ± 4.1 large ACF/colon).
Study #3: Low-fat diet Promotion
The multiplicity of ACF which may be considered a marker for tumor promotion was diet dependent (p=0.0009; Table. 4). The multiplicity was nearly the same in all the groups fed various meats, except in the group of rats fed a low-fat bacon-based diet. Compared with the low-fat control diet, beef, chicken and pork diets did not change the growth of ACF. In contrast, compared with the low-fat control diet, the ACF multiplicity was reduced by 17% (p<0.01), and the number of large ACF was reduced by 44%, in rats fed the low-fat bacon-based diet (p=0.003; Table 4). The total number of ACF per rat was not changed by the diet (Table. 4).
Table 4.
Effect of diets containing 30% meat on number and multiplicity of ACF in the colon of F344 female rats, 100 days after a single azoxymethane injection (Study #3)
| Group | n | No. of ACF per colon | No. of aberrant crypt/focus | No. of ACF with 5 or more crypts/focus |
|---|---|---|---|---|
| control | 10 | 137 ± 26a | 2.9 ± 0.2 | 19.7 ± 6.8 |
| beef | 10 | 122 ± 60 | 2.9 ± 0.3 | 15.6 ± 9.8 |
| chicken | 10 | 151 ± 28 | 2.7 ± 0.2 | 18.6 ± 8.1 |
| pork | 10 | 151 ± 25 | 2.7 ± 0.3 | 18.1 ± 6.8 |
| bacon | 10 | 134 ± 21 | 2.4 ± 0.2b | 11.1 ± 4.4c |
Mean ± SD
Significantly different from the control by Dunnet test(p<0.01)
Significantly different from the control by Student t- test (p=0.003) and significantly different from pork group by Student t- test (p=0,014)
Discussion
The feeding of a diet containing 30 or 60% bacon to rats increased the fecal NOC level. Compared with control rats fed with casein and lard, the bacon-based diets led to a 10 to 20-fold increase in fecal NOC in three independent rat studies. Assuming that the median MW of fecal NOC is 150, the fecal concentration was 1.5 to 2.5 μg/g feces.
The present results can be contrasted with those of earlier studies: Previously, fecal NOC were found to be increased in humans increasing red meat consumption: a high red meat diet increases the fecal NOC level 3-fold in volunteers, to an estimated concentration of 2 μg/g feces (9). In contrast, here, a diet containing 60% beef meat did not increase the fecal NOC in rats. However, bacon-fed rats had a fecal NOC concentration similar to volunteers eating a high-meat diet. Two differences between humans and rats may explain the results: (i) The AIN76 rat-diet does not contain vegetables, in contrast with human diet. Most vegetables contain nitrate, which may combine with meat factors to make NOC. (ii) Humans and rats do not have the same gut flora: different bacteria may produce different metabolites. Indeed, epidemiological data suggest that intestinal bacterial activity may be related to colon cancer (17). The gut flora and its potential for metabolic transformations could play an important role in the genesis of carcinogens. Many bacteria are able to catalyze the formation of nitrosamines (18). Bacterial nitrate reductase reduces nitrate to nitrite, which generates nitrosating agents that, in turn, can react with nitrogenous compounds (amines, amides), in the presence of the microflora, to produce NOC. Nitrate reductase could be less active in rats than in humans, which may explain that beef-fed rats had lower fecal NOC levels than control rats. Moreover, in humans, the origin of NOC in feces is likely to be endogenous (9). In contrast, here, the rats excreted as much NOC as they consumed. Thus, in rats, fecal NOC seems to come, principally, from ingested NOC. However, preformed NOC in food seem to be efficiently metabolized in the liver by detoxification enzymes (15), and a small part of an ingested NOC dose should reach the colon. The nature of total NOC present or formed after nitrosation in colorectal content is unknown, so we cannot come to a conclusion about fecal NOC origin. In addition, Pignatelli et al (16), have shown that known NOC constitute only a minor fraction of total NOC that are formed or occur in diets. It is thus possible that a part of fecal NOC may be formed endogenously from meat amines or amides, and from bacon nitrate and nitrite.
Previously, Pence et al. showed that pyrolysed beef can significantly enhance colon carcinogenesis in rats when it is fed during, but not after, the dimethylhydrazine initiation (11). In this study, cooked meat diet was started a week after the carcinogen injections. The lack of tumor promotion in rats fed a cooked beef meat diet was thus consistent with Pence’s study. This lack of effect is also consistent with all other published rats studies: cooked red meat does not promote colon carcinogenesis in rodents (see refs. 2 and 12 for review).
Previously, specific NOC have been shown to produce colon tumors (19). In this study, a high level of fecal NOC was not associated with ACF initiation or ACF promotion in rats. However, Hasegawa could show the initiation of few colon tumors in rats given a total dose of 100 μmol of pure genotoxic NOC (19). He also showed a weak promotion of colon tumors in rats given a total dose of 1 mmol of non-genotoxic NOC (19). Here, each rat had received a total dose of 0.4 μmol of NOC (initiation study), or of 1.3 μmol of NOC (promotion studies). Hence, the NOC doses from bacon were too small to initiate or to promote ACF in the colon of rats. More, in rats fed bacon, NOC could have been diluted by the high water content of feces (Table 2). In addition, Peto et al. (20) have also demonstrated that preformed NOC was not consistently associated with large bowel cancer. Oral doses of nitrosamines in animal studies mainly result in liver and esophageal tumors. Moreover, we don’t know the nature of NOC in the bacon-based diet. According to the literature, the main N-nitroso compound found in fried bacon is nitrosothiazolidine, a non-carcinogenic nitrosamine (4). So, it is possible that the specific NOC in bacon and in feces of bacon-fed rats were not genotoxic or not tumor-promoting for the rat colonic mucosa.
Rats given the high-bacon diets consistently had smaller ACF than controls, as previously reported (12). This protective effect was found in two independent studies, using bacon from different suppliers. Bacon-based diets containing 7, 14 and 28% fat decreased significantly the ACF growth in rats colon, when compared with matched controls. A bacon–based diet thus appears to protect against carcinogenesis, perhaps because bacon contains 5% NaCl and increased the rats’ water intake. We have hypothesized that the high water intake could weakly increase the fecal moisture and allowed a dilution of toxic compounds, like bile acids, in the gut (12).
Beef, pork or chicken meat intake decreased fecal NOC excretion, and did not change ACF growth in rats. In contrast, a bacon-based diet increased 10-to 20-fold the fecal NOC level. But the intestinal concentration of NOC due to bacon-based diets did not initiate or promote colon carcinogenesis, assessed by the ACF assay in rats. Moreover, in three independent studies, a bacon-based diet decreased the colon ACF growth in rats. Taken together, the data do not support the hypothesis that NOC could explain the association of meat intake with colon cancer risk.
Acknowledgments
This work was supported in part by the Direction Générale de l’Enseignement et de la Recherche, the Ministère de l’Agriculture, France and by a grant from the Ligue Nationale Contre le Cancer, comity of Gers.
References
- 1.Doll R, Peto R. The causes of cancer. JNCI. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.Parnaud G, Corpet DE. Colorectal cancer: controversial role of meat consumption. Bull Cancer. 1997;84:899–911. [PubMed] [Google Scholar]
- 3.Eichholzer M, Gutzwiller F. Dietary nitrates, nitrites, and N-nitroso compounds and cancer risk: a review of the epidemiologic evidence. Nutr Rev. 1998;56:95–105. doi: 10.1111/j.1753-4887.1998.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 4.Lijinsky W. N-nitroso compounds in the diet. Mutation Res. 1999;443:129–138. doi: 10.1016/s1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 5.Massey RC, Key PE, Jones RA, Logan GL. Volatile, non-volatile and total N-nitroso compounds in bacon. Food Additives and Contaminants. 1991;8:585–598. doi: 10.1080/02652039109374012. [DOI] [PubMed] [Google Scholar]
- 6.Pignatelli B. Formation et distribution des composes N-nitroses CNO dans l’alimentation. Securite Alimentaire du Consommateur. 1995:178–207. [Google Scholar]
- 7.Ohshima H, Calmels S, Pignatelli B, Vincent P, Bartsch H. IARC Scientific Publication. 84. Lyon: 1987. N-nitrosamine formation in urinary-tract infections; pp. 384–390. [PubMed] [Google Scholar]
- 8.Bos JL, Fearon ER, Hamilton SR, Verlaan de Vries M, Van boom JH. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 9.Bingham SA, Pignatelli B, Pollock JRA, Ellul A, Malaveille C, O’Neill IK. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer? Carcinogenesis. 1996;17:515–523. doi: 10.1093/carcin/17.3.515. [DOI] [PubMed] [Google Scholar]
- 10.Silvester KR, Bingham SA, Pollock JRA, Cummings JH, Oneill IK. Effect of meat and resistant starch on fecal excretion of apparent N-nitroso compounds and ammonia from the human large bowel. Nutr Cancer. 1997;29:13–23. doi: 10.1080/01635589709514596. [DOI] [PubMed] [Google Scholar]
- 11.Pence BC, Landers M, Dunn DM, Shen CL, Miller MF. Feeding of a well-cooked beef diet containing a high heterocyclic amine content enhances colon and stomach carcinogenesis in 1,2-dimethylhydrazine-treated rats. Nutr Cancer. 1998;30:220–226. doi: 10.1080/01635589809514667. [DOI] [PubMed] [Google Scholar]
- 12.Parnaud G, Peiffer G, Tache S, Corpet DE. Effect of meat (beef, chicken, and bacon) on rat colon carcinogenesis. Nutr Cancer. 1998;32:165–173. doi: 10.1080/01635589809514736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bird RP. Observation and quantification of aberrant crypts in murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–151. doi: 10.1016/0304-3835(87)90157-1. [DOI] [PubMed] [Google Scholar]
- 14.Pignatelli B, Richard I, Bourhade M, Bartsch H. Improved group determination of total NOC in human gastric juice by chemical denitrosation and thermal energy analysis. Analyst. 1987;112:945–949. doi: 10.1039/an9871200945. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch H, Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5:1381–1393. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 16.Pignatelli B, Malaveille C, Thuillier P, Hautefeuille A, Bartsch H. Improved methods for analysis of N-nitroso compounds and applications in human biomonitoring. In: Loeppky RN, Michedja CJ, editors. Nitrosamines and related N-nitroso compounds – Chemistry and Biochemistry. Vol. 553. ACS Symposium Series; 1994. pp. 102–117. [Google Scholar]
- 17.Moore WEC, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Applied Environnem Microbiol. 1995;61:3202–3207. doi: 10.1128/aem.61.9.3202-3207.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowland I. The potential role of colonic bacteria in colorectal cancer. ECP News. 1997;31:10–12. [Google Scholar]
- 19.Hasegawa R, Futakuchi M, Mizoguchi Y, Yamaguchi T, et al. Studies of initiation and promotion of carcinogenesis by N-nitroso compounds. Cancer Lett. 1998;123:185–191. doi: 10.1016/s0304-3835(97)00417-5. [DOI] [PubMed] [Google Scholar]
- 20.Peto P, Brantom P, Grasso P. Nitrosamine carcinogenesis in 5120 rodents: chronic administration of sixteen different concentrations of NDEA, NDMA, NPYR and NPIP in the water of 4440 inbred rats, with parallel studies on NDEA alone of the effect of age of starting (3,6 or 20 weeks) and of species (rats, mices or hamsters)». IARC Scientific Publication. 1984;57:627–665. [PubMed] [Google Scholar]


