Abstract
Fine differences in the phosphorylation and acylation of lipooligosaccharide (LOS) from Neisseria species are thought to profoundly influence the virulence of the organisms and the innate immune responses of the host, such as signaling through toll-like receptor 4 (TLR4) and triggering receptor expressed on myeloid cells (TREM). MALDI time-of-flight (TOF) mass spectrometry was used to characterize heterogeneity in the native LOS from Neisseria gonorrheae and N. meningitidis. A sample preparation methodology previously reported for Escherichia coli lipopolysaccharide (LPS) employing deposition of untreated LOS on a thin layer of a film composed of 2,4,6-trihydroxyacetophenone and nitrocellulose was used. Prominent peaks were observed corresponding to molecular ions and to fragment ions primarily formed by cleavage between the 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) and the lipid A (LA). Analyses of these data and comparison with spectra of the corresponding O-deacylated or hydrogen fluoride-treated LOS enabled the detection of novel species that apparently differed by the expression of up to three phosphates with one or more phosphoethanolamine (PEA) groups on the LA. We found that the heterogeneity profile of acylation and phosphorylation correlates with the induction of proinflammatory cytokines in THP-1 monocytic cells. This methodology enabled us to rapidly profile components of structural variants of native LOS that are of importance biologically.
Keywords: lipooligosaccharide, endotoxin, Neisseria gonorrhoeae, Neisseria meningitidis, MALDI, phosphorylation, acylation, structural, mass spectrometry
The lipooligosaccharide (LOS) of Neisseria gonorrheae and N. meningitidis is structurally related to the lipopolysaccharide (LPS) of enteric Gram-negative bacteria but does not have repeating O-antigens (1). LOS and LPS have conserved inner cores composed of L-glycero-D-manno-heptose (Hep) and 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo), which are anchored in the outer membrane by lipid A (LA). The structure of the LA of gonococci and meningococci, although based on a limited number of studies, generally differs from that of Escherichia coli in the acylation and the chain length of the fatty acid residues. With regard to phosphorylation, a limited number of studies have shown that gonococci and meningococci elaborate LOS containing a LA, which is phosphorylated with variable numbers of phosphate (P) and phosphoethanolamine (PEA) residues. The recent report of a PEA transferase specific for the LA on meningococcal LOS creates the possibility of phase variation in phosphorylation (2). Thus, the LA expressed by these organisms exhibit heterogeneity in acylation and phosphorylation, both of which have been shown to influence the endotoxicity in E. coli LPS (3).
The innate immune response to bacterial pathogens relies on the detection of pathogen-associated molecular patterns by pattern recognition molecules. Toll-like receptor 4 (TLR4) is one of the best characterized of the family of mammalian TLRs that represents the most extensively studied class of pattern recognition molecules (4). Recently, a second family of innate immune receptors responsive to bacterial pathogens has been termed triggering receptor expressed on myeloid cells (TREM) (5). One of the TLRs, TLR4, as well as TREM1 and TREM2 have been shown to engage LPS and LOS and are required for an efficient immune response to Gram-negative bacterial infections.
We have observed major differences in the proinflammatory TLR4-mediated signal elicited by various LOS purified from meningococcal and gonococcal strains (6). In particular, we have observed significant variation in the TNF-α and IL-8 responses in THP-1 monocytic cells stimulated by LOS from different Neisserial strains. Recently, our laboratory reported strain-specific binding of gonococcal LOS to TREM-2, which was significantly reduced by O-deacylation (7). Although it is recognized that LPS from different genera of bacteria differ in their endotoxic activity (8, 9), it was an unexpected finding that LOS from isolates within a species and from two such closely related species elicited such a broad spectrum of activity. Similarly, a wide range of ability of Neisserial LOS to clot Limulus amebocyte lysate has been reported (10). These differences in clotting activity were shown to be associated with the lipoidal portion of the LOS rather than the oligosaccharide (OS) terminus, as the purified lipid showed a similar potency to the parent LOS.
There have been several reported mutational or enzymatic alterations of the acylation pattern of the Neisserial LA leading to modulation of its endotoxic activity as measured by various assays (11–13). Inactivation of genes lpxL1 or lpxL2 involved in acylation of the LA resulted in a lipid, which was pentaacylated or tetraacylated, respectively, rather than hexaacylated, and in both cases the LOS induced less TNF-α from a human macrophage cell line (13). Neisserial LOS deacylated by the enzyme acyloxyacyl hydrolase from neutrophils was less potent in the Limulus amebocyte lysate assay, in the ability to stimulate neutrophil adherence to endothelial cells, and in mitogenic ability for splenocytes (12). In addition, Ellis et al. (14) found that mutational disruption of the expression a single lauric fatty acid residue in the LA resulted in a mutant with a markedly reduced ability to induce proinflammatory cytokines from human macrophages and neutrophils. Another modification of the LA that may play a role in altering endotoxic activity is the fatty acid chain length. Steeghs et al. (15) reported that Neisserial LOS showed reduced endotoxic activity when the Neisserial lpxA gene was substituted with that from other Gram-negative bacteria expressing either shorter or longer O-linked 3-OH fatty acids. Taken together, these data indicate that structural differences in the Neisserial LA result in substantial differences in biological potency.
Our current focus is on the relationship between the structural heterogeneity in Neisserial LOS and its biological activity. Thus, in this study we undertook the characterization of native LOS by MALDI mass spectrometry using a thin-layer method for deposition of the matrix, which is composed of a mixture of 2,4,6-trihydroxyacetophonone (THAP) with nitrocellulose to determine whether this methodology would provide a more thorough analysis of LOS structural variation than previous approaches (16). We report that this method enables rapid profiling of the structural heterogeneity in native LOS and that the heterogeneity profile correlates with the induction of proinflammatory cytokines in THP-1 monocytic cells.
MATERIALS AND METHODS
Bacterial strains and LOS extraction
N. gonorrheae strains 1291, DOV, and GC56, and N. meningitidis serogroup A strains 7880 and 7889 are clinical isolates and have been described previously (6). The LOS was extracted and purified by a modification of the hot phenol-water method (17, 18).
O-deacylation of native LOS
Native or intact LOS was O-deacylated prior to MS analysis as reported previously (19). Briefly, LOS (∼0.3 mg) was placed in a 1.5 ml polypropylene tube, and 200 μl of anhydrous hydrazine was added. The sample was kept in a water bath at 37°C for 20 min and intermittently vortexed. The solution was cooled, and 1 ml of chilled acetone (−20°C) was added dropwise. The precipitated O-deacylated LOS was centrifuged at 12,000 g for 20 min, and the supernatant was removed. The pellet was washed with cold acetone, centrifuged again, and the supernatant was removed. Water (200–500 μl) was added to the pelleted O-deacylated LOS, and the sample was lyophilized.
HF treatment of native LOS
Phosphoesters were partially removed by mild hydrogen fluoride (HF) treatment to aid in distinguishing between N-acetylhexosamine (HexNAc; 203 Da) and phosphate (P or HPO3, 80 Da) plus PEA (123 Da) substitutions. Native LOS was reacted with 48% aqueous HF to preferentially remove phosphoester moieties. From 0.1 to 1 mg of LOS was placed in a 1.5 ml polypropylene tube, and cold 48% aqueous HF was added to make a 5–10 mg/ml solution, which then was allowed to react at 4°C for 16–20 h. Excess HF was removed using a SpeedVac (Thermo Savant) with an in-line trap.
Sample preparation for MS analysis
Samples were prepared for MS using a method previously described for analysis of native rough (R)-type bacterial LPS similar in size to the LOS from Neisseria species (16). Purified, HF-treated, or O-deacylated LOS was suspended (1–2 μg/μl) in a mixture of methanol:water (1:1) containing 5 mM EDTA. An aliquot was desalted on Parafilm with a few cation-exchange beads (Dowex 50WX8-200) that had been converted to the ammonium form. The desalted sample was mixed with an equal volume of dibasic ammonium citrate (20 mM), and 0.5 μl was deposited on the mass spectrometry sample plate on top of a thin layer of matrix. The matrix layer was formed by deposition of drops (0.3–0.9 μl) of a solution composed of THAP (200 mg/ml; Sigma-Aldrich, St. Louis, MO) in methanol, with nitrocellulose transblot membrane (15 mg/ml; Bio-Rad, Hercules, CA) in acetone:isopropanol (1:1 v/v) mixed in a 4:1 v/v ratio.
MALDI-TOF mass spectrometry
MALDI mass spectrometry was performed in the linear mode on a Voyager-DE STR model time-of-flight (TOF) instrument equipped with a 337 nm nitrogen laser and delayed extraction. Spectra were obtained in the negative-ion mode with an average of 500 pulses per spectrum. The acceleration voltage was −20 kV. Spectra were processed with digital smoothing and baseline correction using Data Explorer software. External calibration was performed using the average masses of the molecular ions of the peptides porcine renin substrate, bovine insulin, and oxidized insulin chain B (Sigma-Aldrich). A two-point internal calibration of the mass spectrum of native LOS from N. meningitidis strain 7889 was performed using the average masses of 2 molcular ions with previously reported structural components (20), a diphosphoryl LA with a single PEA substituent (calculated m/z 1,836.27) and an LOS (calculated m/z 3,434.54) with a 2-hexose (Hex) α-chain in addition to two PEA groups on the LOS inner core (2Kdo, 2Hep, 1HexNAc) and a hexaacylated LA. A two-point internal calibration of the mass spectra of LOS from N. gonorrheae strains GC56 and DOV was performed using the average masses of the fragment ions for a diphosphoryl LA with (m/z 1,836.27) and without (calculated m/z 1,713.22) a PEA group. A two-point internal calibration of the spectrum of the LOS from N. meningitidis 7880 was carried out using the calculated masses for LA at m/z 1,836.27, and molecular ions (m/z 3,596.69) with a composition consistent with previous data indicative of the presence of an alternative 3-Hex α-chain with a nonreducing galactose (Gal)α1→4 substituent on lactosyl [Galβ1→4 glucose (Glc)] with the LOS inner core and PEA substituents (20, 21).
Proinflammatory cytokine response of THP-1 cells
Human monocyte-like cell line THP-1 was obtained from the American Type Culture Collection (Manassas, VA) and has been characterized previously (6). Cells were cultured in RPMI-1640 containing 10% fetal bovine serum. Prior to LOS treatment, cells were first differentiated with phorbol myristate acetate (Sigma-Aldrich) at a concentration of 10 ng/ml for 24 h. Differentiated THP-1 cells were seeded at 1 × 105 cells per well in 96-well plates and treated with 100 ng/ml of LOS for 18 h. Control wells were treated with culture media only. Supernatants from the LOS-treated and untreated cells were assayed for TNF-α, IL-1β, and IL-6 using a bead-based multiplex cytokine kit (Invitrogen, Carlsbad, CA). Samples were processed as recommended by the manufacturer and analyzed using a Bio-Plex 200 system (Bio-Rad).
Statistical analysis
Statistical analyses were performed using SigmaStat for Windows version 3.11 (Systat Software, San Jose, CA). Groups of data were analyzed by the Tukey test for multiple pairwise comparisons. Values of P < 0.05 were considered significant for all comparisons.
RESULTS
MALDI TOF of native 1291 LOS
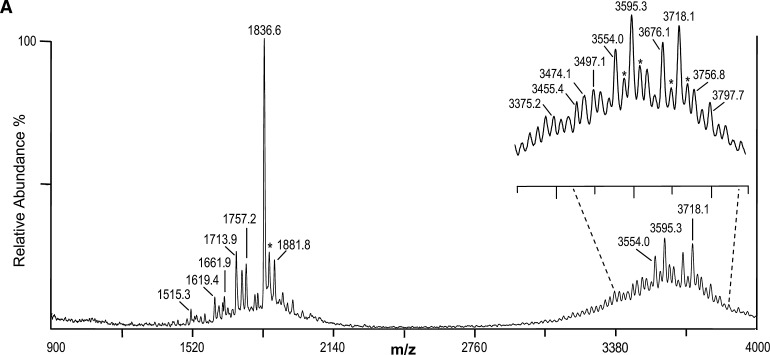
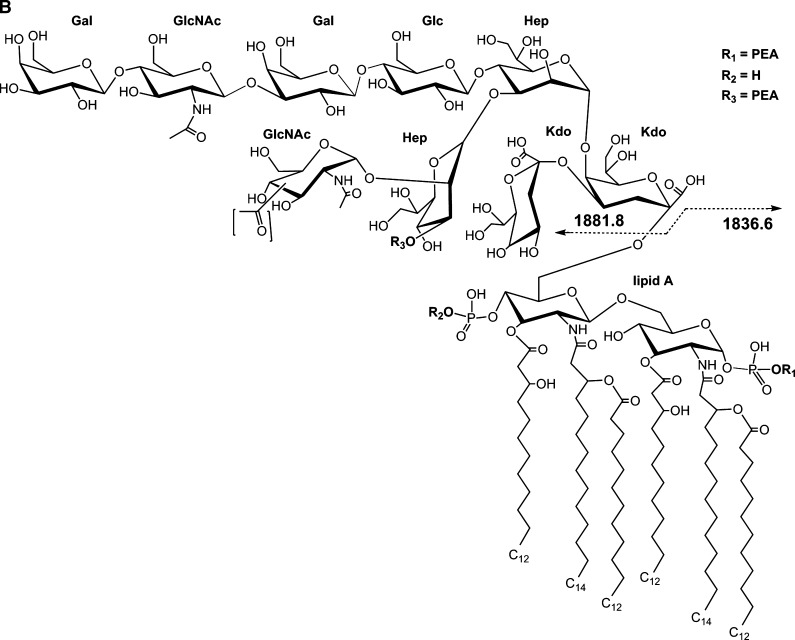
Our initial analysis of native LOS using the thin-layer of THAP/nitrocellulose matrix for negative-ion MALDI TOF was performed on a well-characterized LOS from N. gonorrheae strain 1291 (22, 23). This yielded several apparent molecular ions (M-H)-, which differed by masses corresponding to differences in PEA, P, or O-acetyl (Ac, 42 Da) groups. The resulting spectrum is presented in Fig. 1A, and potential compositions for the ions observed are presented in Table 1. A structure for the molecular ions at m/z 3,718.1, which is consistent with the spectrum, and a conserved inner core for gonococcal LOS is presented in Fig. 1B.
Fig. 1.
Negative-ion MALDI time-of-flight (TOF) mass spectrum of lipooligosaccharide (LOS) from N. gonorrheae strain 1291 in the linear mode (A) and structure of the LOS derived from the current, previously published data, and the prototypical Neisserial lipid A (LA) (B). The (M-H)- peak at m/z 3,718.1 has two phosphates (P), two phosphoethanolamines (PEA), and an acetyl (Ac), and differs by 123 Da corresponding to addition of a PEA group from the peak at m/z 3,595.3. The peak at m/z 3,554.0 is consistent with loss of a PEA plus an Ac from the peak at m/z 3,718.1. Additional peaks can be seen in the inset of A. In the lower mass range (< m/z 2,140) are a series of peaks for prompt fragment ions corresponding to the LA or OS portion of the molecule, with the most prominent at m/z 1,836.6, consistent with a diphosphoryl LA with a single PEA group. The brackets around the Ac group (B) are indicative of the lack of information regarding the specific site of linkage to the terminal N-acetylglucosamine (GlcNAc) residue. The asterisks in the spectrum indicate peaks for sodiated (M+Na-2H)- ions.
TABLE 1.
Masses of (M-H)- ions of 1291 lipooligosaccharide (LOS)
| Experimental (M-H)- | Calculated ± moiety | Calculated (M-H)- |
|---|---|---|
| 1,515.3 | −OS, −OHC12 | 1,514.9 |
| 1,619.4 | −LA, −Ac, Kdo | 1,619.4 |
| 1,661.9 | −LA, −Kdo | 1,661.4 |
| 1,713.9 | −OS, −PEA | 1,713.2 |
| 1,757.2 | −OS, −HPO3 | 1,756.3 |
| 1,836.6 | −OS | 1,836.3 |
| 1,881.8 | −LA | 1,881.6 |
| 3,375.2 | −Kdo, −PEA | 3,375.6 |
| 3,455.4 | −Ac, −Kdo | 3,456.7 |
| 3,474.1 | −Ac, −HPO3, −PEA | 3,473.8 |
| 3,554.0 | −Ac, −PEA | 3,553.8 |
| 3,595.3 | −PEA | 3,595.8 |
| 3,676.1 | −Ac | 3,676.8 |
| 3,718.1a | 3,718.9a | |
| 3,756.8 | −Ac, +HPO3 | 3,756.8 |
| 3,797.7 | +HPO3 | 3,798.9 |
PEA, phosphoethanolamine.
Structure shown in Fig. 1B.
The two most prominent molecular (M-H)- ions at m/z 3,718.1 and 3,595.3 differ by 123 Da, which is consistent with a single PEA group. At m/z 1,836.6 and 1,713.9 are peaks that also differ by a PEA and that appear to correspond to LA Y-type fragments of the molecular ions at m/z 3,718.1 and 3,595.3. The glycosidic oxygen is retained by the LA in Y-type fragmentation (24). The masses are in accordance with the presence of a diphosphoryl (2P) hexaacylated LA with or without a single PEA, but otherwise similar to previous reports on the LA from N. gonorrheae (23, 25). Another LA fragment ion peak in the spectrum at m/z 1,757.2 is apparently due to monophosphorylated molecules with a single PEA.
In addition, in the spectrum shown in Fig. 1A, there is a peak for nonreducing terminal B-type fragment ions at m/z 1,881.8, which could arise from the OS portion of the ions at either m/z 3,718.1 or 3,595.3. The m/z 1,881.8 ions are consistent with the previously reported structure for the 1291 OS which is O-3 linked to HepI of the inner core and which has an Ac group on the GlcNAc that is attached to O-2 of HepII and an α-chain lacto-N-neotetraose group (22, 23).
The spectrum of the 1291 native LOS also provides evidence of heterogeneity in the LA and the OS. The small molecular ion peak (M-H)- at m/z 3,797.7 is in accordance with a third P group, presumably on the LA, added to molecules detected at m/z 3,718.1. The peak at m/z 3,756.8 is in agreement with the presence of a third P if the ions are missing the Ac group present on the OS of the ions at m/z 3,718.1 and 3,797.7. Lower mass ions can observed in the spectrum that are due to B-type fragments, which contain the OS, but with additional losses of the labile Kdo, or both Kdo and Ac at m/z 1,661.9 and 1,619.4, respectively.
A peak at m/z 1,515.3 could arise from pentaacyl LA ions that are missing one of the 3-hydroxylauric acid groups (198 Da), as previously reported (25). Alternatively, these could be due to prompt fragmentation causing loss of 3-hydroxylauric acid (−198 Da) from the diphosphoryl LA ions at m/z 1,713.9, or the loss of both PEA and 3-hydroxylauric acid from the diphosphoryl PEA LA ions at m/z 1,836.6.
MALDI TOF of native 7889 LOS
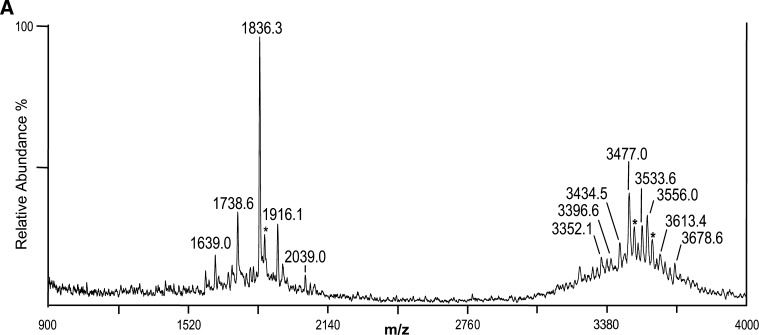
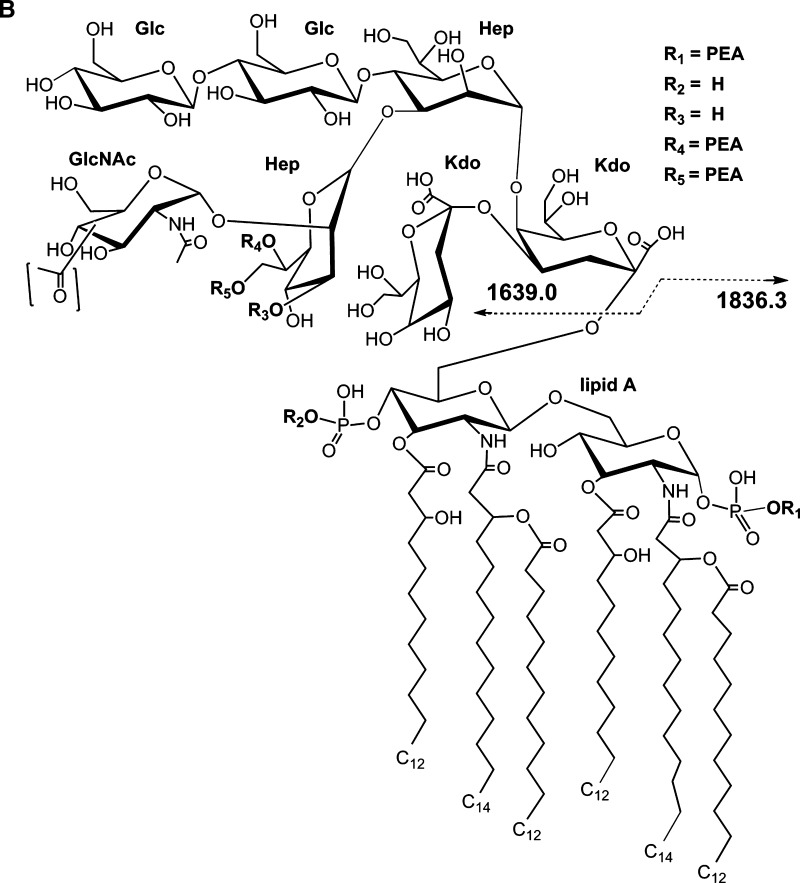
We next applied the thin-layer MALDI method to native meningococcal LOS with a partially characterized structure (20, 26). LOS from N. meningitidis strain 7889 was prepared on thin layers of THAP/nitrocellulose matrix and subjected to negative-ion MALDI TOF analysis. The spectrum is presented in Fig. 2A, and potential compositions for the ions observed are presented in Table 2. A structure for the molecular ions at m/z 3,477.0, which is consistent with the spectrum and a conserved inner core for N. meningitidis LOS, is presented in Fig. 2B2.
Fig. 2.
Negative-ion MALDI TOF mass spectrum of native LOS from N. meningitidis strain 7889 in the linear mode (A) and structure of the LOS derived from the current, previously published data, and the prototypical Neisserial LA (B). The mass of the peak at m/z 3,477.0 is consistent with a structure with two P, three PEA, and one Ac group. Peaks for prompt fragments ions for the LA or OS portion of the molecule can be observed in the lower mass range. The single PEA on the LA shown in the structure on the reducing terminal GlcN can be on either terminus. A Gly or PEA substituent has been reported previously to occur on O7 (R5) of L-glycero-D-manno-heptose (HepII) (see text). Lower mass prompt fragment ions can be observed (< m/z 2,140), with the most abundant ions at m/z 1,836.3, consistent with a diphosphoryl LA with a single PEA substituent. The asterisks in the spectrum indicate peaks for sodiated (M+Na-2H)- ions.
TABLE 2.
Masses of (M-H)- ions of 7889 LOS
| Experimental (M-H)- | Calculated ± moiety | Calculated (M-H)- |
|---|---|---|
| 1,639.0 | −LA | 1,639.3 |
| 1,738.6 | −OS, −H3PO4 | 1,738.3 |
| 1,836.3 | −OS | 1,836.3 |
| 1,916.1 | −OS, +HPO3 | 1,916.3 |
| 2,039.0 | −OS, +HPO3, +PEA | 2,039.3 |
| 3,352.1 | −PEA | 3,353.5 |
| 3,396.6 | −HPO3 | 3,396.6 |
| 3,434.5 | −Ac | 3,434.5 |
| 3,477.0a | 3,476.6a | |
| 3,533.6 | +Gly | 3,533.6 |
| 3,556.0 | +HPO3 | 3,556.6 |
| 3,613.4 | +Gly, +HPO3 | 3,613.6 |
| 3,678.6 | +HPO3, +PEA | 3,679.6 |
Ac, Acetyl.
Structure shown in Fig. 2B.
The mass of the most prominent molecular ion peak in the spectrum at m/z 3,477.0 is in accordance with a composition containing an OS with a 2-Hex α-chain on the N. meningitidis conserved inner core, 2 PEA groups, an Ac, and a diphosphoryl LA with a single PEA group. This proposed composition is supported by the presence of the prominent peak for Y-type fragments for the diphosphoryl LA with a single PEA at m/z 1,836.3, and the related but much less prominent peak for B-type fragment ions containing the OS portion of the molecule at m/z 1,639.0 as shown in Fig. 2B. These data are consistent with previous analyses of the LA from N. meningitidis, which revealed P groups at positions 1 and 4` of the GlcN disaccharide with nonstoichiometric PEA substitutions on both (13, 27) and the electrospray mass spectrometric analysis of the 7889 O-deacylated LOS (20).
Previous analyses of OS from meningococcal LOS also have revealed O-acetyl substitution on the nonreducing terminal GlcNAc, as well as variable PEA substituents on the O-3, O-6, or O-7 position of HepII (28–30). The specific sites of acetylation and phosphorylation of the OS of strain 7889 have not been determined yet. Thus, the structure of the 7889 LOS that is presented in Fig. 2B shows potential locations for the Ac and PEA groups based on previous reports on N. meningitidis and the hypothesis that it has a conserved inner core structure. We have indicated the PEA groups detected on the OS as linked to the O-6 (R4) and O-7 (R5) of HepII, and the Ac on the O-3 of the terminal GlcNAc moiety according to reports on other meningococcal serotypes (30, 31).
The spectrum of the native 7889 LOS also contained a series of molecular ions at m/z 3,533.6 and 3,613.4, which differed from m/z 3,477.0 by masses consistent with the addition of glycine (Gly, 57 Da), or Gly plus a P substituent, respectively. Gly has been previously reported in an ester linkage at O-7 of HepII (R5 in Fig. 2B) in a number of LOS immunotypes of N. meningitidis (29, 32), but not in the L11 type LOS expressed by strain 7889. Having an O-linkage, the Ac and Gly groups would be expected to be labile to O-deacylation, and accordingly neither were reported in the previous analysis of the O-deacylated LOS (20).
Lower mass peaks at m/z 3,434.5, 3,396.6, and 3,352.1 may be due to prompt fragmentation of the molecular ions at m/z 3,477.0, causing a loss of Ac, P, and PEA, respectively, or the presence of heterogeneous species. However, the occurrence of higher mass (M-H)- ions at m/z 3,556.0, 3,613.4, and 3,678.6 are indicative of molecules containing the addition of a third P (m/z 3,556.0), or the addition of a third P plus Gly (57 Da; m/z 3,613.4) or PEA (m/z 3,678.6), respectively.
Supporting the presence of a third P moiety on a subset of the LA are the peaks that can be seen at m/z 1,916.1 and 2,039.0. These are in accordance with the diphosphoryl LA with a single PEA plus an additional P group, or the additional P plus a second PEA group, respectively. Thus, the data all support the existence of a heterogenous population of molecules some of which contain a previously unreported third P moiety on the LA of the 7889 LOS.
MALDI TOF of HF-treated 7889 LOS
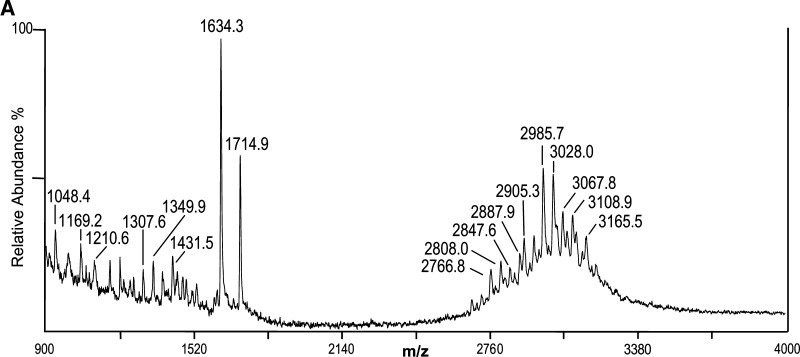
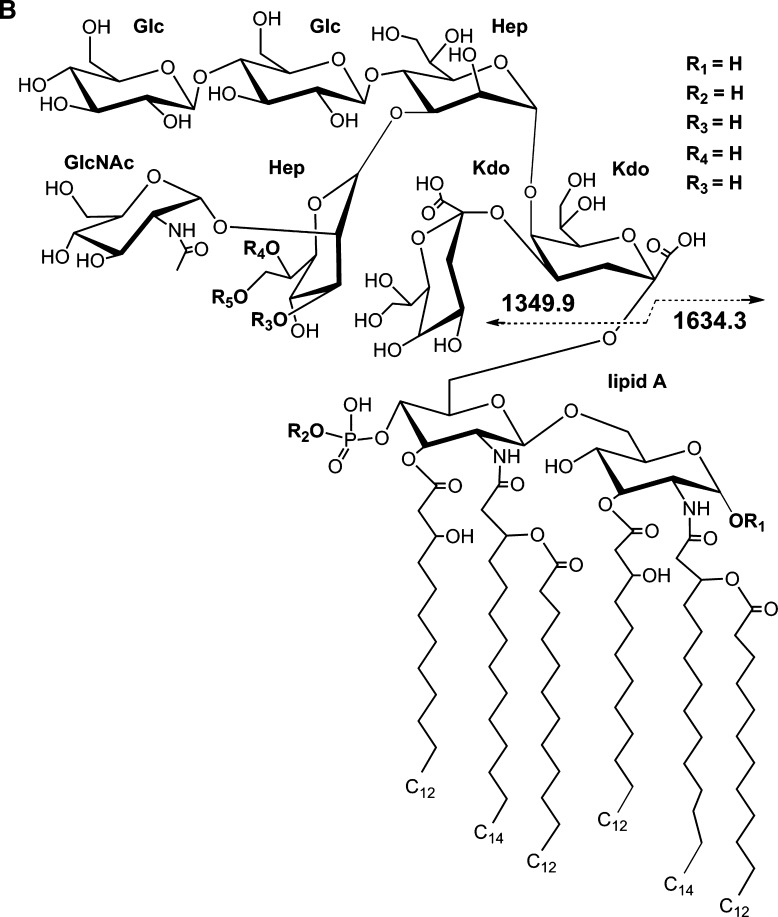
To help distinguish apparent P(80 Da) plus PEA (123 Da) from possible HexNAc (203 Da) substitutions that could not be resolved sufficiently with the mass accuracy of the MALDI analysis, the native 7889 LOS was treated with HF to preferentially cleave phosphoester linkages and then analyzed using negative-ion MALDI TOF. The resulting spectrum is presented in Fig. 3A, and potential compositions for the ions observed are presented in Table 3. A structure for the molecular ions at m/z 2,985.7, which is in agreement with the spectrum, and a conserved inner core for N. meningitidis LOS is presented in Fig. 3B.
Fig. 3.
Negative-ion MALDI TOF mass spectrum of LOS from HF-treated N. meningitidis strain 7889 LOS in the linear mode (A) and structure of the HF-treated LOS derived from the current, previously published data, and the prototypical Neisserial LA (B). The (M-H)- peak at m/z 2,985.7 is consistent with a single P substituent, and higher mass (M-H)- ions are indicative of additional Ac, P, PEA, and Gly groups. Lower mass prompt fragment ions can be observed (< m/z 2,000), with the most abundant ions at m/z 1,634.3, consistent with a monophosphoryl LA with no PEA groups, and those at m/z 1,714.9, consistent with a diphosphoryl LA also without PEA.
TABLE 3.
Masses of (M-H)- ions of HF-treated 7889 LOS
| Experimental (M-H)- | Calculated ± moiety | Calculated (M-H)- |
|---|---|---|
| 1,048.4 | −LA, −Hex, −Kdo, +PEA, −CO2 | 1,047.9 |
| 1,169.2 | −LA, −Hex, −H2O | 1,171.0 |
| 1,210.6 | −LA, −Kdo, +PEA, −CO2 | 1,210.0 |
| 1,307.6 | −LA, −CO2 | 1,307.2 |
| 1,349.9 | −LA | 1,351.2 |
| 1,431.5 | −LA, +PEA, −CO2 | 1,430.2 |
| 1,634.3 | −OS | 1,633.2 |
| 1,714.9 | −OS, +HPO3 | 1,713.2 |
| 2,766.8 | −Kdo | 2,765.2 |
| 2,808.0 | +Ac, −Kdo | 2,807.3 |
| 2,847.6 | +HPO3, −Kdo | 2,845.2 |
| 2,887.9 | −Kdo, +PEA | 2,888.3 |
| 2,905.3 | −HPO3 | 2,905.4 |
| 2,985.7a | 2,985.4a | |
| 3,028.0 | +Ac | 3,027.5 |
| 3,067.8 | +HPO3 | 3,065.4 |
| 3,108.9 | +PEA | 3,108.5 |
| 3,165.5 | +Gly, +PEA | 3,165.5 |
Hex, hexose.
Structure shown in Fig. 3B.
The composition of the most prominent of the molecular ions in the spectrum at m/z 2,985.7 is consistent with the loss of all three PEA groups, the presence of a monophosphoryl LA, and no Ac. Nearly as prominent is the peak at m/z 3,028.0, which is in accordance with the presence of an Ac, and which is less than the most prominent molecular ion peak in the native 7889 spectrum at m/z 3,477.0 by 449 Da. This mass is consistent with the loss of a P and three PEA groups due to the HF treatment.
In addition, there are minor molecular ion peaks at m/z 3,067.8, 3,108.9, and 3,165.5, which are consistent with the additions of P, PEA, and PEA with Gly, respectively, to the molecular ions at m/z 2,985.7. However, there are no significant peaks for molecular ions that are 203 Da more than the peaks at m/z 2,985.7 or 3,028.0, which would be indicative of an additional HexNAc moiety on the LOS that should be less susceptible to HF cleavage than a PPEA group. Although some glycosidic bond cleavage can occur from HF treatment, it is more specific to phosphoester linkages.
The peak at m/z 2,905.3 represents fragment ions that have lost a P from the molecular ion at m/z 2,985.7. Lower mass peaks in the molecular ion range at m/z 2,887.9, 2,847.6, 2,808.0, and 2,766.8 all represent fragment ions that have lost Kdo (220 Da), but for the former three peaks this is in the context of molecules that are larger than those at m/z 2905.3 by the addition of either a PEA, P, or an Ac group.
The calculated masses of two peaks observed in the spectrum of the HF-treated 7889 LOS vary from that observed by more than 2 Da as shown in Table 3, whereas in the spectra of the native 1291 and 7889 there are none that differ by more than 1.5. This greater discrepancy could be due to the presence of a number of smaller, unresolved peaks on the shoulders of some of the molecular ion peaks such as the one at m/z 3,067.8.
In this spectrum, as in those of native 1291 and 7889 LOS, the most prominent peaks represent Y-type fragments for the LA as exemplified by the monophosphoryl LA at m/z 1,634.3 and the diphosphoryl LA at 1,714.9. In addition, as in the spectra of the native LOS, there exists a less prominent peak for corresponding B-type fragment ions containing the OS that can be seen at m/z 1,349.9 (Fig. 3B).
The lower mass fragment ion peaks at m/z 1,307.6 and 1,210.6 represent portions of the OS moiety, with loss of CO2 (44 Da) and loss of CO2 and Kdo (220 Da) on molecules with an additional PEA group, respectively. An additional fragmentation has occurred with the loss of a nonreducing terminal Hex (162 Da) plus H2O (18 Da) from the ions at m/z 1,349.9 leading to the ions at m/z 1,169.2, with the loss of a Kdo and CO2 from molecules with an additional PEA producing the ions at m/z 1,048.4. We have found similar ions showing loss of Kdo and CO2 due to HF treatment previously (33). There are a number of even less abundant fragment ion peaks that can be seen in the region of m/z 900 to 1,520, which are apparently due to various one- or two-bond cleavages of the OS coupled with losses of CO2.
MALDI TOF of O-deacylated 7889 LOS
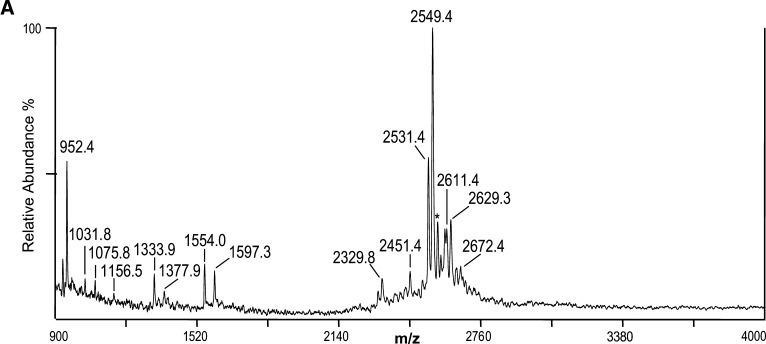
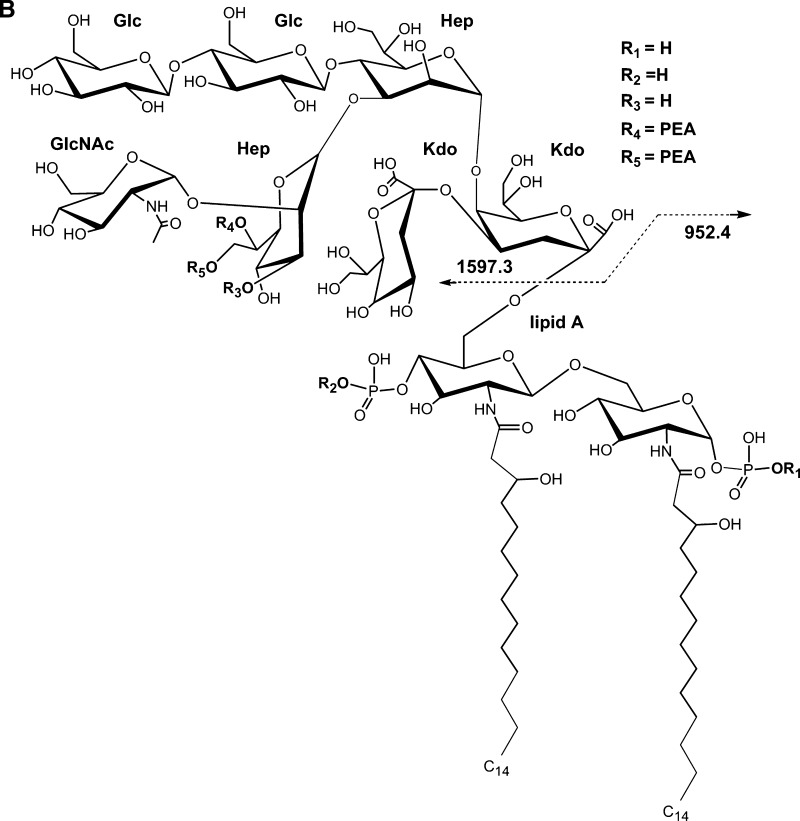
The 7889 LOS was O-deacylated and analyzed by negative-ion MALDI TOF to provide additional information on the structure of the 7889 LOS as a basis for comparison with the data derived using the current method with that previously reported using electrospray mass spectrometry of the O-deacylated 7889 LOS (20). The resultant spectrum is shown in Fig. 4A, and the corresponding structure based on these analyses and the concept of a conserved inner core is shown in Fig. 4B. Table 4 presents proposed compositions for the ions observed in the spectrum of the O-deacylated LOS.
Fig. 4.
Negative-ion MALDI TOF mass spectrum of LOS from O-deacylated N. meningitidis LOS strain 7889 in the linear mode (A) and structure of the O-deacylated LOS derived from the current, previously published data, and the prototypical Neisserial LA (B). The mass of the molecular ions at m/z 2,549.4 is in agreement with an O-deacylated LOS with two P and two PEA substituents. The peak for the prompt fragment ions for the O-deacylated diphosphoryl LA is at m/z 952.4. The asterisk in the spectrum indicates a peak for sodiated (M+Na-2H)- ions.
TABLE 4.
Masses of (M-H)- ions of O-deacylated 7889 LOS
| Experimental (M-H)- | Calculated ± moiety | Calculated (M-H)- |
|---|---|---|
| 952.4 | −OS | 952.0 |
| 1,031.8 | −OS, +HPO3 | 1,032.0 |
| 1,075.8 | −OS, +PEA | 1,075.0 |
| 1,156.5 | −OS, +HPO3, +PEA | 1,155.0 |
| 1,333.9 | −LA, −Kdo, −CO2 | 1,333.1 |
| 1,377.9 | −LA, −Kdo | 1,377.1 |
| 1,554.0 | −LA, −CO2 | 1,553.3 |
| 1,597.3 | −LA | 1,597.3 |
| 2,329.8 | −H3PO4, −PEA | 2,329.2 |
| 2,451.4 | −H3PO4 | 2,452.3 |
| 2,531.4 | −H2O | 2,532.3 |
| 2,549.4a | 2,550.3a | |
| 2,611.4 | +HPO3, −H2O | 2,612.2 |
| 2,629.3 | +HPO3 | 2,630.3 |
| 2,672.4 | +PEA | 2,673.3 |
Structure shown in Fig. 4B.
The base peak in the spectrum shown in Fig. 4A at m/z 2,549.4 represents the singly-charged form corresponding to the doubly- and triply-charged molecular ions observed in the previously reported electrospray analysis (20). The molecular ion at m/z 2,549.4 is consistent with a composition containing a diphosphoryl LA, with an OS consisting of two Kdo, two Hep, one HexNAc, two Hex, and two PEA groups. In the MALDI spectrum there is a significant peak at m/z 2,531.4, which is 18 Da less (M-H2O-H)- than the ions at m/z 2,549.4. Similarly, in the previously reported electrospray spectrum there were significant peaks for the doubly- and triply-charged ions with loss of H2O (20).
The molecular ions in the spectrum at m/z 2,611.4, 2,629.3, 2,672.4 are in accordance with an addition of P with loss of H2O, P, and PEA, respectively, to the molecular ions at m/z 2,549.4. Thus, in the MALDI spectrum of the O-deacylated LOS, and in that of the native LOS but not in spectrum of the HF-treated material, there is evidence in the molecular ion region for the existence of a third P group on the LOS. Ions observed at m/z 2,451.4 and 2,329.8 are consistent with the loss of H3PO4 (98 Da), and the loss of H3PO4 and PEA (123 Da) from the molecular ions at m/z 2,549.4, respectively.
As in the other three spectra presented, there are Y-type and B-type fragment ions observed in the spectrum of the O-deacylated LOS that together span the entire molecule. The ions at m/z 952.4 represent Y-type fragments containing the diphosphoryl O-deacylated LA, and at m/z 1,597.3 is a peak for B-type fragments containing the OS. As in the native LOS, the mass of the OS is consistent with the presence of two PEA groups. Similarly, at m/z 1,554.0, 1,377.9, and 1,333.9 are other nonreducing terminal B-type fragment ions for the OS, but these molecules have also lost CO2, Kdo, and CO2 plus Kdo, respectively.
In addition to the Y-type fragment ions at m/z 952.4 for the diphosphoryl LA, peaks can be observed at m/z 1,031.8 and 1,075.8, which are consistent with the presence of an additional P and PEA, respectively, and represent Y-type fragments of the molecular ions observed at m/z 2,629.3 and 2,672.4. The small peak at m/z 1,156.5 is in accordance with a LA with an additional PPEA group. Thus, both the peaks at m/z 1,031.8 and 1,156.5 are consistent with the presence of a third P group that is located on the LA of the 7889 LOS.
MALDI TOF of LOS from N. gonorrheae strains GC56 and DOV and N. meningitidis 7880
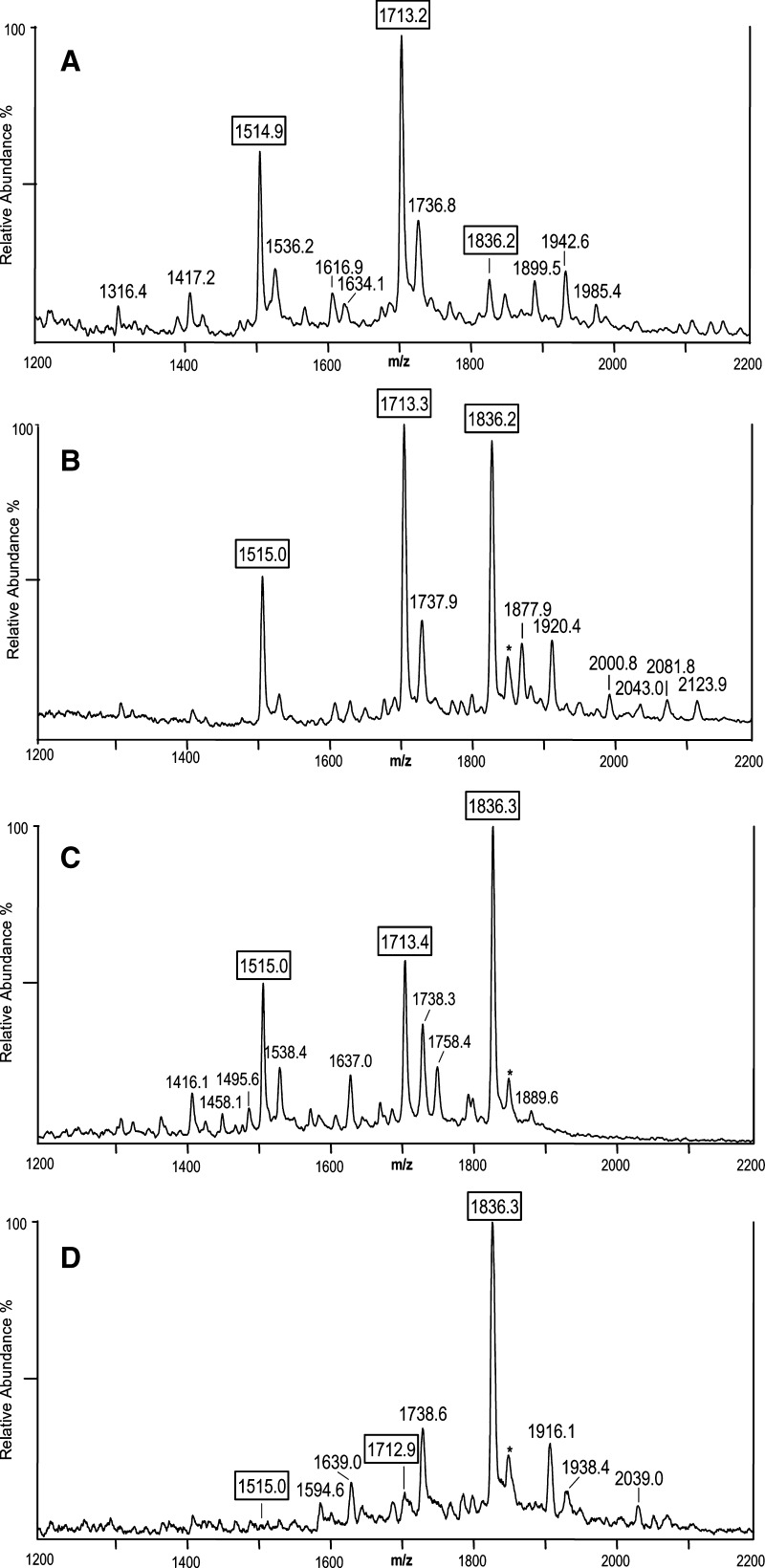
We next investigated the robustness of this methodology by utilizing it to characterize the LOS from three additional Neisserial strains, two gonococcal strains whose LOS structures had not been reported previously (6), and one meningococcal strain with a partially characterized LOS structure (20). The native LOS from N. gonorrheae strains GC56 and DOV as well as N. meningitidis 7880 were prepared on thin layers of THAP/nitrocellulose matrix and analyzed by negative-ion MALDI TOF. As we observed in the analysis of intact LOS from 1291 and 7889, these spectra contained a suite of molecular ion peaks from m/z 3,000–4,000 (results not shown), as well as the prominent lower mass peaks for Y-type fragments containing LA, and much less prominent B-type fragment ion peaks for the OS from m/z 1,200–2,200 (Fig. 5A–D). For example, in the spectrum of strain DOV (Fig. 5B) at m/z 1,877.9, 1,920.4, and 2,000.8 are three fragment ion peaks that are in agreement with the OS inner core (2 Kdo, 2 Hep, HexNAc) plus four Hex and one HexNAc with an (M-H)- average mass calculated to be 1,878.6 Da, plus Ac (calculated average mass of m/z 1,920.7) or PEA (calculated average mass of m/z 2,001.7). In the spectrum of 7880 (Fig. 5C), OS related peaks were observed at m/z 1,637.0 and 1,758.4, which are consistent with fragment ions for three Hex plus the inner core and either one (calculated m/z 1,636.4) or two PEA groups (calculated m/z 1,759.4), respectively.
Fig. 5.
Negative ion MALDI-TOF spectra from m/z 1,200–2,200 of intact LOS from N. gonorrheae strains GC56 (A) and DOV (B), and N. meningitidis strains 7880 (C) and 7889 (D). The 7889 spectrum derives from Figure 2A and is presented for comparison. Although there are three apparent LA peaks at m/z 1,515, 1,713, and 1,836 for the pentaacyl diphosphoryl LA, hexaacyl diphosphoryl LA, and hexaacyl diphosphoryl LA with an additional PEA, respectively, the relative abundances of the peaks vary considerably in the spectra of the different strains. In addition to these three peaks, several other LA peaks can be observed in the spectra such as m/z 1,738, which is likely due to prompt fragmentation with loss of H3PO4 (−98 Da) from the ion observed at m/z 1,836.2. Peaks for B-type fragment ions for the OS can also be seen in the spectra that are consistent with masses for intact LOS ions observed at higher masses. Data are representative of three or more independent MS analyses.
In addition to the prominent LA fragment ions at m/z 1,836, 1,713, and 1,515, less abundant peaks were observed, which are consistent with the additional loss of P or H3PO4 (98 Da), for example, in Fig. 5A at m/z 1,634.1 (m/z 1,713.2–80.0 Da) and 1,417.2 (m/z 1,514.9–98 Da) and in Fig. 5B at m/z 1,737.9 (m/z 1,836.2–98 Da). Notably, the relative abundances of the Y-type fragment ions for LA at m/z 1,515, 1,713, and 1,836 in the spectra of strains GC56, DOV, 7880, and 7889 differed dramatically for the four strains as shown in Fig. 5. The peaks for the diphosphoryl hexaacyl (m/z 1,713) and pentaacyl (m/z 1,515) LA fragment ions are the most prominent peaks in the spectrum of the gonococcal GC56 LOS as shown in Fig. 5A, whereas these peaks are much less prominent in the spectra of the meningococcal 7880 and 7889 LOS in which the ions at m/z 1,836 for the diphosphoryl LA with a single PEA group are the most abundant.
Cytokine induction by THP-1 cells
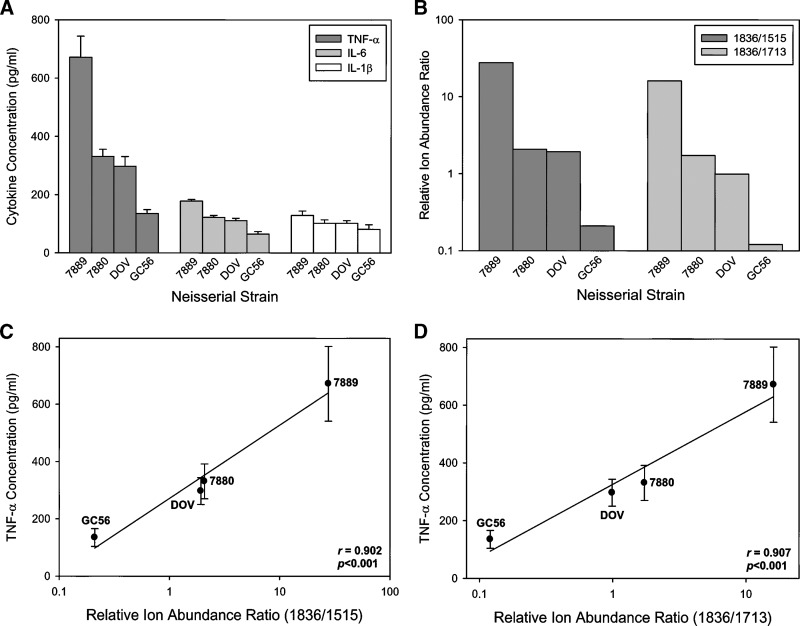
Given the variability of the relative ion abundances of the LA fragment ions, we next determined whether this variability affected the bioactivity of the LOS by measuring the proinflammatory cytokine response of THP-1 monocytic cells to LOS from the four strains. As shown in Fig. 6A, LOS from the strains induced the production of significantly different concentrations of TNF-α, IL-1β, and IL-6 from THP-1 cells (P < 0.01 for all cytokines). The highest concentration of all three cytokines was in response to 7889 LOS, which was the LOS with the greatest relative ion abundance ratios for both 1,836/1,515, and 1,836/1,713 (Fig. 6B). In contrast, the weakest inducer of all three cytokines was GC56 LOS, which also had the lowest relative ion abundance ratios. LOS from strains 7880 and DOV were intermediate between 7889 and GC56 in cytokine induction and relative abundance ratios. Fig. 6C, D illustrate the linear relationship between the relative ion abundance ratios and the level of TNF-α production by THP-1 cells. Linear regression analyses of these data revealed a significant positive correlation between the two values (r = 0.902, P < 0.001 for 1,836/1,515; r = 0.907, P < 0.001 for 1,836/1,713). Similar significant positive correlations were found for IL-1β and IL-6 (results not shown).
Fig. 6.
Proinflammatory cytokine production by THP-1 cells in response to LOS from N. meningitidis and N. gonorrheae. A: Cells were challenged with 100 ng/ml of LOS for 18 h after, which TNF-α, IL-6, and IL-1β concentrations in the supernatants were determined using a bead-based multiplex cytokine assay. The data are representative of three independent experiments. The bars represent the mean of triplicate data points and the error bars are ± the standard deviation. B: Relative ion abundance ratios for LOS from N. meningitidis and N. gonorrheae. C, D: Linear regression analyses of the relative ion abundance ratios of either 1,836/1,515 (C) or 1,836/1,713 (D) compared with the production of TNF-α by THP-1 cells. The data used in these analyses derive from the TNF-α concentrations in A and the relative ion abundance ratios in B.
DISCUSSION
Among the recognized virulence factors involved in the pathogenesis of Neisserial infections, LOS is a major inducer of the proinflammatory cytokine response to the organisms (6). Our previous work and that of others has shown that the LA portion of Neisserial LOS is the bioactive component (6, 10–13). Several studies have shown with the use of mutational or enzymatic alterations of LOS that structural modifications of Neisserial LA result in substantial differences in biological potency (11–14). In this report, we demonstrate that MALDI mass spectrometry of native LOS provides a more thorough analysis of LA structural variability than previous approaches. We found that this strategy provides rapid profiling of the structural heterogeneity of native LOS and that the heterogeneity profile correlates with the induction of proinflammatory cytokines TNF-α, IL-1β, and IL-6 in THP-1 monocytic cells.
We found that the relative ion abundances for LA at m/z 1,515, 1,713, and 1,836 differed dramatically among strains. Thus, the LA expressed by these organisms exhibits heterogeneity in acylation and phosphorylation, both of which have been shown to influence the endotoxicity in E. coli LPS (3). In particular, the spectrum of the LA from GC56 contained a predominance of diphosphoryl hexaacyl (1,713) and pentaacyl (1,515) ions, whereas the most abundant ions from 7880 and 7889 LOS were the diphosphoryl hexaacyl LA with a single PEA group (1,836). When the data were analyzed in terms of ion abundance ratios of 1,836/1,515 or 1,836/1,713, a significant correlation was observed between both ratios and the degree of proinflammatory cytokine induction. This suggests that the abundance of these three structures is related to LOS bioactivity; however, the individual contribution of each structure to the cytokine responsiveness of THP-1 cells is yet to be determined.
Studies of the physiochemical characteristics of LA from the LPS of Gram-negative bacteria have concluded that there is a relationship between the three-dimensional shape of LA and its bioactivity (34). This is most likely the result of differences in the direct binding of different shaped LA molecules to TLR4, MD2, CD14, and LBP (35, 36). The structural variables reported to influence the three-dimensional conformation of LA are the length and number of acyl chains, asymmetry of acyl groups, and the number and distribution of negative charges owing to the P groups. It is likely that the heterogeneity in acylation and phosphorylation of Neisserial LA effects three-dimensional conformation and bioactivity similarly.
Herein is the first report that indicates that the predominant form of the LA in some gonococcal LOS, and more specifically that from the wild-type strain 1291, is a diphosphoryl moiety that expresses a single PEA. In addition, the MALDI analysis suggests that there could be a third P group on the 1291 lipoidal moiety. The only peak observed for loss of a fatty acyl group was that for loss of hydroxylaurate, suggesting that this is evidence of heterogeneity rather than a product of prompt fragmentation occurring in the mass spectrometer when the laser hits the sample.
Originally, an extensive analysis of the LA of N. gonorrheae was performed on monophosphoryl molecules (25). Our previous MALDI and electrospray analyses of O-deacylated LOS from other strains of N. gonorrheae indicated that the LA was diphosphoryl (33), and a more recent report on the 1291 LOS structure supported the presence of both a mono- and diphosphoryl LA along with a less abundant component, which was monophosphoryl and contained a single PEA (23). However, analysis of a mutant strain of 1291 produced peaks for diphosphoryl LA Y-type fragment ions with and without PEA on the reducing terminal P (23).
Previous electrospray analysis of O-deacylated LOS from N. meningitidis 7889 was consistent with a composition containing 2 Hex, 1 HexNAc, 2 Hep, 2 Kdo, and a diphosphoryl lipoidal moiety. The data were in agreement with localization of PEA groups on either the OS or lipoidal portion of the molecule (20). The prototypical LA from meningococcal LOS is thought to be a diphosphoryl moiety with nonstoichiometric substitution by PEA on the phosphates on both the reducing and nonreducing terminal ends (13, 27).
The MALDI TOF analyses of the native N. meningitidis 7880 and 7889 LOS provide further evidence of a primary diphosphoryl LA with a single PEA substituent. Interestingly, in the spectrum of 7889 there are several molecular ion and fragment ion peaks that provide evidence of a triphosphoryl LA that has two PEA groups. In addition, the spectrum is consistent with the presence of two additional PEA groups in the OS. Phosphorylation of the LOS is thought to play a role in its biological activity. For example, meningococcal strains with greater predominance of PEA substituent on the O-6 of HepII were found to be more efficient in binding complement component C4b than those containing O-3 PEA groups and were more susceptible to complement-mediated killing in serum bactericidal assays (37).
In addition to permitting the detection of groups, such as the O-linked Gly or Ac, which could be lost in O-deacylation, the spectrum of the native compared with the O-deacylated 7889 LOS produced a different profile of the phosphorylation. In the spectrum of the native 7889 LOS shown in Fig. 2A, the majority of the molecular ions and the Y-type LA fragment ions are consistent with a diphosphoryl LA with a PEA substituent. In contrast, in the spectrum shown in Fig. 4A of the O-deacylated LOS the majority of the molecular ions and the Y-type LA fragment ions are consistent with the presence of only the diphosphoryl LA. Only the small molecular ion peak at m/z 2,672.4 and the Y-type fragment ions at m/z 1,075.8 provide evidence of the presence of the LA PEA in the O-deacylated spectrum. Comparisons of the spectra of the native, HF-treated, and the O-deacylated 7889 LOS provide more complete structural information than would be available from any one of them individually.
For our analyses, we used a sample preparation method that was originally developed for negative-ion MALDI analysis of acidic molecules and adapted for application to MALDI of R-type LPS (16, 38). This technique employs a thin-layer method for deposition of the matrix, which is composed of a mixture of THAP with nitrocellulose (16). The advantages of using methodology enabling the mass spectral analysis of native LOS are illustrated by the data presented, indicating that this procedure provides systematic profiling of naturally occurring mixtures of native LOS from N. gonorrheae and N. meningitidis, and direct detection of their significant structural heterogeneity (25, 39, 40). The technique enabled the detection of structural heterogeneity in the LOS from different strains, which correlated with differences in biological activity. There have been several reports of MALDI analysis whereby we (40) and others (41) analyzed native R-type LPS using 2,5-dihyroxybenzoic acid (DHB) as a matrix. However, we have observed that MALDI analysis of intact LOS with the THAP/nitrocellulose thin-layer matrix (16) has greater sensitivity, thus permitting the analysis of smaller quantities of sample. In addition, this method is significantly more reproducible with less spot-to-spot variation in ion production.
LOS presents a variety of challenges for structural elucidation being a complex, amphipathic, and highly charged molecule, which is poorly soluble. In most analytical strategies including our own, the LOS has been hydrolyzed or chemically degraded in some manner (42). A potential disadvantage of this type of approach is the loss of more labile substituents, for example, sialic acid, which can be cleaved by mild acid hydrolysis procedures used to cleave the Kdo moiety linking the OS and LA. In the past, we have O-deacylated the LOS to prevent losses from acid hydrolysis and to increase solubility and detection in liquid secondary ion (19, 43), electrospray, and MALDI mass spectrometry (33).
Challenges in mass spectrometric analysis of mixtures include the relationship between detection sensitivity and molecular structure, and the occurrence of ion suppression (44). Differences in mass, charge, and polarity can result in suppression of the signal of certain compounds. In general, less polar molecules will suppress the signal of more polar ones, and molecules of higher mass tend to suppress the signal of those with lower mass, and the degree of suppression can vary with concentration (44). Using the THAP/nitrocellulose system, variation in the sensitivity of detection of individual components of the LOS or ion suppression is apparent. In a heterogenous mixture of LOS molecules, the presence of additional phosphorylation would increase polarity and, therefore, the susceptibility of those LOS molecules to ion suppression. For example, the most prominent peaks in the spectra in Figs. 1–3 represent the LA fragment ions, whereas the peaks for the OS-containing B-type fragment ions, which should be of similar abundance are much less prominent.
Previous quantitative studies of mixtures of biomolecules such as proteins and oligosaccharides have used internal standards (45, 46). In this study, we used the endogenous 1,836 ion peak present in all spectra as an internal calibrant to normalize the absolute peak abundances in order to compare their variability between the spectra of LOS deriving from different Neisserial strains. Using this approach, we found significant correlations between cytokine induction and the relative ion abundance ratios of the LA fragment ions for three ion peaks. The MALDI methodology used is simple to perform, enables analysis of the native LOS, and has good sensitivity. Analysis of the native LOS along with its chemically modified forms yields a type of fingerprinting that can be used to assess the impact of LOS structural heterogeneity on the induction of innate immune responses.
In conclusion, the data obtained suggests that some heretofore unrealized structural heterogeneity in Neisserial LOS exists and that the repertoire of heterogeneity in the LA correlates with bioactivity. A more complete characterization of the structural heterogeneity of Neisserial LOS is warranted, particularly in the context of understanding the innate immune responses to these molecules.
Abbreviations
Ac, acetyl
Gal, galactose
Glc, glucose
GlcN, glucosamine
GlcNAc, N-acetylglucosamine
Gly, glycine
Hep, L-glycero-D-manno-heptose
Hex, hexose
HexNAc, N-acetylhexosamine
Kdo, 3-deoxy-D-manno-oct-2-ulosonic acid
LA, lipid A
LOS, lipooligosaccharide
LPS, lipopolysaccharide
OS, oligosaccharide
P, phosphate
PEA, phosphoethanolamine
THAP, 2,4,6-trihydroxyacetophonone
TLR4, toll-like receptor 4
TOF, time-of-flight
TREM, triggering receptor expressed on myeloid cells
Published, JLR Papers in Press, October 2, 2008.
Footnotes
This work was supported by Public Health Service Grant AI063927 from the National Institute of Allergy and Infectious Diseases and by the Research Service of the US Department of Veterans Affairs. The authors would like to acknowledge the UCSF Mass Spectrometry Core Facility that is supported by an NCI Cancer Center Support Grant (P30 CA82103) and the Sandler Family Foundation, and the UCSF Mass Spectrometry Facility (A.L. Burlingame, Director), which is supported by the Biomedical Research Technology Program of the National Center for Research Resources, NIH, NCRR P41RR001614. This is paper number 103 from the Center for Immunochemistry.
Figures 2B, 3B, and 4B revised in press due to recent data (26).
References
- 1.Kahler C. M., and D. S. Stephens. 1998. Genetic basis for biosynthesis, structure, and function of meningococcal lipooligosaccharide (endotoxin). Crit. Rev. Microbiol. 24 281–334. [DOI] [PubMed] [Google Scholar]
- 2.Cox A. D., J. C. Wright, J. Li, D. W. Hood, E. R. Moxon, and J. C. Richards. 2003. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 185 3270–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takayama K., N. Qureshi, C. R. Raetz, E. Ribi, J. Peterson, J. L. Cantrell, F. C. Pearson, J. Wiggins, and A. G. Johnson. 1984. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect. Immun. 45 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albiger B., S. Dahlberg, B. Henriques-Normark, and S. Normark. 2007. Role of the innate immune system in host defence against bacterial infections: focus on the toll-like receptors. J. Intern. Med. 261 511–528. [DOI] [PubMed] [Google Scholar]
- 5.Colonna M. 2003. TREMs in the immune system and beyond. Nat. Rev. Immunol. 3 445–453. [DOI] [PubMed] [Google Scholar]
- 6.Pridmore A. C., G. A. Jarvis, C. M. John, D. L. Jack, S. K. Dower, and R. C. Read. 2003. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect. Immun. 71 3901–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan D. N., M. D. Cooper, J. L. Potter, M. H. Roberts, H. Cheng, and G. A. Jarvis. 2008. TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 1 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erridge C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4 837–851. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., J. Gaekwad, M. A. Wolfert, and G. J. Boons. 2007. Modulation of innate immune responses with synthetic lipid A derivatives. J. Am. Chem. Soc. 129 5200–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth R. I., R. Yamasaki, R. E. Mandrell, and J. M. Griffiss. 1992. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect. Immun. 60 762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun J. M., C. C. Blackwell, I. R. Poxton, O. E. Ahmer, A. E. Gordon, O. M. Madani, D. M. Weir, S. Giersen, and J. Beuth. 2002. Proinflammatory responses to lipo-oligosaccharide of Neisseria meningitidis immunotype strains in relation to virulence and disease. J. Infect. Dis. 185 1431–1438. [DOI] [PubMed] [Google Scholar]
- 12.Erwin A. L., R. E. Mandrell, and R. S. Munford. 1991. Enzymatically deacylated Neisseria lipopolysaccharide (LPS) inhibits murine splenocyte mitogenesis induced by LPS. Infect. Immun. 59 1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Ley P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 69 5981–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis C. D., B. Lindner, C. M. Anjam Khan, U. Zähringer, and R. Demarco de Hormaeche. 2001. The Neisseria gonorrhoeae lpxLII gene encodes for a late-functioning lauroyl acyl transferase, and a null mutation within the gene has a significant effect on the induction of acute inflammatory responses. Mol. Microbiol. 42 167–181. [DOI] [PubMed] [Google Scholar]
- 15.Steeghs L., M. Berns, J. ten Hove, A. de Jong, P. Roholl, L. van Alphen, J. Tommassen, and P. van der Ley. 2002. Expression of foreign LpxA acyltransferases in Neisseria meningitidis results in modified lipid A with reduced toxicity and retained adjuvant activity. Cell. Microbiol. 4 599–611. [DOI] [PubMed] [Google Scholar]
- 16.Sturiale L., D. Garozzo, A. Silipo, R. Lanzetta, M. Parrilli, and A. Molinaro. 2005. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun. Mass Spectrom. 19 1829–1834. [DOI] [PubMed] [Google Scholar]
- 17.Apicella M. A., J. M. Griffiss, and H. Schneider. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235 242–252. [DOI] [PubMed] [Google Scholar]
- 18.Westphal, O., and K. Jann. 1965. Bacterial Lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. In Methods in Carbohydrate Chemistry. R. L. Whistler, editor. Academic Press. New York. 83–91.
- 19.Mandrell R. E., J. J. Kim, C. M. John, B. W. Gibson, J. V. Sugai, M. A. Apicella, J. M. Griffiss, and R. Yamasaki. 1991. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J. Bacteriol. 173 2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J. J., N. J. Phillips, B. W. Gibson, J. M. Griffiss, and R. Yamasaki. 1994. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect. Immun. 62 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai C. M., G. Kao, and P. Zhu. 2002. Influence of the length of the lipooligosaccharide alpha chain on its sialylation in Neisseria meningitidis. Infect. Immun. 70 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.John C. M., J. M. Griffiss, M. A. Apicella, R. E. Mandrell, and B. W. Gibson. 1991. The structural basis for pyocin resistance in Neisseria gonorrhoeae lipooligosaccharides. J. Biol. Chem. 266 19303–19311. [PubMed] [Google Scholar]
- 23.Post D. M., N. J. Phillips, J. Q. Shao, D. D. Entz, B. W. Gibson, and M. A. Apicella. 2002. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Domon B., and C. Costello. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5 397–409. [Google Scholar]
- 25.Takayama K., N. Qureshi, K. Hyver, J. Honovich, R. J. Cotter, P. Mascagni, and H. Schneider. 1986. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J. Biol. Chem. 261 10624–10631. [PubMed] [Google Scholar]
- 26.Mistretta, N., D. Seguin, P. Talaga, S. Vialle, F. Blanc, M. Brossaud, J. Thiebaud, L. Chapiron, G. Norheim, D. A. Caugant, et al. 2008. Genotyping and LOS structural characterization of immunotype L11 N. meningitidis strains. Abstracts 16th International Pathogenic Neisseria Conference, Rotterdam, The Netherlands. Abstract P194, p. 254.
- 27.Kulshin V. A., U. Zahringer, B. Lindner, C. E. Frasch, C. M. Tsai, B. A. Dmitriev, and E. T. Rietschel. 1992. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis.J. Bacteriol. 174 1793–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman M. M., D. S. Stephens, C. M. Kahler, J. Glushka, and R. W. Carlson. 1998. The lipooligosaccharide (LOS) of Neisseria meningitidis serogroup B strain NMB contains L2, L3, and novel oligosaccharides, and lacks the lipid-A 4'-phosphate substituent. Carbohydr. Res. 307 311–324. [DOI] [PubMed] [Google Scholar]
- 29.Kahler C. M., A. Datta, Y. L. Tzeng, R. W. Carlson, and D. S. Stephens. 2005. Inner core assembly and structure of the lipooligosaccharide of Neisseria meningitidis: capacity of strain NMB to express all known immunotype epitopes. Glycobiology. 15 409–419. [DOI] [PubMed] [Google Scholar]
- 30.Kahler C. M., S. Lyons-Schindler, B. Choudhury, J. Glushka, R. W. Carlson, and D. S. Stephens. 2006. O-Acetylation of the terminal N-acetylglucosamine of the lipooligosaccharide inner core in Neisseria meningitidis. Influence on inner core structure and assembly. J. Biol. Chem. 281 19939–19948. [DOI] [PubMed] [Google Scholar]
- 31.Kogan G., D. Uhrin, J. R. Brisson, and H. J. Jennings. 1997. Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr. Res. 298 191–199. [DOI] [PubMed] [Google Scholar]
- 32.Cox A. D., J. Li, and J. C. Richards. 2002. Identification and localization of glycine in the inner core lipopolysaccharide of Neisseria meningitidis. Eur. J. Biochem. 269 4169–4175. [DOI] [PubMed] [Google Scholar]
- 33.John C. M., H. Schneider, and J. M. Griffiss. 1999. Neisseria gonorrhoeae that infect men have lipooligosaccharides with terminal N-acetyllactosamine repeats. J. Biol. Chem. 274 1017–1025. [DOI] [PubMed] [Google Scholar]
- 34.Schromm A. B., K. Brandenburg, H. Loppnow, A. P. Moran, M. H. Koch, E. T. Rietschel, and U. Seydel. 2000. Biological activities of lipopolysaccharides are determined by the shape of their lipid A portion. Eur. J. Biochem. 267 2008–2013. [DOI] [PubMed] [Google Scholar]
- 35.Shin H. J., H. Lee, J. D. Park, H. C. Hyun, H. O. Sohn, D. W. Lee, and Y. S. Kim. 2007. Kinetics of binding of LPS to recombinant CD14, TLR4, and MD-2 proteins. Mol. Cells. 24 119–124. [PubMed] [Google Scholar]
- 36.Thomas C. J., M. Kapoor, S. Sharma, H. Bausinger, U. Zyilan, D. Lipsker, D. Hanau, and A. Surolia. 2002. Evidence of a trimolecular complex involving LPS, LPS binding protein and soluble CD14 as an effector of LPS response. FEBS Lett. 531 184–188. [DOI] [PubMed] [Google Scholar]
- 37.Ram S., A. D. Cox, J. C. Wright, U. Vogel, S. Getzlaff, R. Boden, J. Li, J. S. Plested, S. Meri, S. Gulati, et al. 2003. Neisserial lipooligosaccharide is a target for complement component C4b. Inner core phosphoethanolamine residues define C4b linkage specificity. J. Biol. Chem. 278 50853–50862. [DOI] [PubMed] [Google Scholar]
- 38.Silipo A., S. Leone, R. Lanzetta, M. Parrilli, L. Sturiale, D. Garozzo, E. L. Nazarenko, R. P. Gorshkova, E. P. Ivanova, N. M. Gorshkova, et al. 2004. The complete structure of the lipooligosaccharide from the halophilic bacterium Pseudoalteromonas issachenkonii KMM 3549T. Carbohydr. Res. 339 1985–1993. [DOI] [PubMed] [Google Scholar]
- 39.Griffiss J. M., J. P. O'Brien, R. Yamasaki, G. D. Williams, P. A. Rice, and H. Schneider. 1987. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect. Immun. 55 1792–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson B. W., J. J. Engstrom, C. M. John, W. Hines, and A. M. Falick. 1997. Characterization of bacterial lipooligosaccharides by delayed extraction matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 8 645–658. [Google Scholar]
- 41.Therisod H., V. Labas, and M. Caroff. 2001. Direct microextraction and analysis of rough-type lipopolysaccharides by combined thin-layer chromatography and MALDI mass spectrometry. Anal. Chem. 73 3804–3807. [DOI] [PubMed] [Google Scholar]
- 42.van Baar B. L. 2000. Characterisation of bacteria by matrix-assisted laser desorption/ionisation and electrospray mass spectrometry. FEMS Microbiol. Rev. 24 193–219. [DOI] [PubMed] [Google Scholar]
- 43.Phillips N. J., C. M. John, L. G. Reinders, B. W. Gibson, M. A. Apicella, and J. M. Griffiss. 1990. Structural models for the cell surface lipooligosaccharides of Neisseria gonorrhoeae and Haemophilus influenzae. Biomed. Environ. Mass Spectrom. 19 731–745. [DOI] [PubMed] [Google Scholar]
- 44.Annesley T. M. 2003. Ion suppression in mass spectrometry. Clin. Chem. 49 1041–1044. [DOI] [PubMed] [Google Scholar]
- 45.Albrethsen J. 2007. Reproducibility in protein profiling by MALDI-TOF mass spectrometry. Clin. Chem. 53 852–858. [DOI] [PubMed] [Google Scholar]
- 46.Ninonuevo M. R., R. E. Ward, R. G. LoCascio, J. B. German, S. L. Freeman, M. Barboza, D. A. Mills, and C. B. Lebrilla. 2007. Methods for the quantitation of human milk oligosaccharides in bacterial fermentation by mass spectrometry. Anal. Biochem. 361 15–23. [DOI] [PubMed] [Google Scholar]