Abstract
Oxidized HDL has been proposed to play a key role in atherogenesis. A wide range of reactive intermediates oxidizes methionine residues to methionine sulfoxide (MetO) in apolipoprotein A-I (apoA-I), the major HDL protein. These reactive species include those produced by myeloperoxidase, an enzyme implicated in atherogenesis. The aim of the present study was to develop a sensitive and specific ELISA for detecting MetO residues in HDL. We therefore immunized mice with HPLC-purified human apoA-I containing MetO86 and MetO112 (termed apoA-I+32) to generate a monoclonal antibody termed MOA-I. An ELISA using MOA-I detected lipid-free apoA-I+32, apoA-I modified by 2e-oxidants (hydrogen peroxide, hypochlorous acid, peroxynitrite), and HDL oxidized by 1e- or 2e-oxidants and present in buffer or human plasma. Detection was concentration dependent, reproducible, and exhibited a linear response over a physiologically plausible range of concentrations of oxidized HDL. In contrast, MOA-I failed to recognize native apoA-I, native apoA-II, apoA-I modified by hydroxyl radical or metal ions, or LDL and methionine-containing proteins other than apoA-I modified by 2e-oxidants. Because the ELISA we have developed specifically detects apoA-I containing MetO in HDL and plasma, it should provide a useful tool for investigating the relationship between oxidized HDL and coronary artery disease.
Keywords: protein oxidation, lipid hydroperoxides, dysfunctional HDL, myeloperoxidase, oxidative stress, reactive nitrogen species
Atherosclerosis is characterized by heightened oxidative damage to lipids and proteins in the affected arterial wall (1), and the extent of this damage is comparable for different classes of lipoproteins including LDL and HDL (2). Recent interest has focused on the functional consequences of HDL oxidation. Oxidation could conceivably contribute to the formation of dysfunctional HDL, proposed to be present in humans with cardiovascular disease (3). One potentially important pathway for generating dysfunctional HDL via oxidation involves myeloperoxidase, an enzyme that converts hydrogen peroxide (H2O2) and chloride ion to hypochlorous acid (HOCl). Myeloperoxidase is expressed in human atherosclerotic tissue (4), and HOCl-modified proteins are present in such lesions (5).
HOCl converts protein tyrosine residues to 3-chlorotyrosine, and methionine residues to methionine sulfoxide (MetO). In addition, MetO can also be formed from exposure of HDL's major protein, apolipoprotein A-I (apoA-I) to H2O2 (6) or lipid hydroperoxides (7, 8), the latter generated during the oxidation of HDL lipids. Oxidation of tyrosine and methionine residues can have dramatic consequences on the functions of apoA-I/HDL, including reverse cholesterol transport, wherein HDL accepts cholesterol from macrophage foam cells in the artery wall and transports it back to the liver for excretion (9). Specifically, apoA-I containing 3-chlorotyrosine and MetO has an impaired ability to promote cholesterol efflux by the ABCA1 pathway (10–13). Similarly, oxidation of Met148 impairs apoA-I's ability to activate lecithin-cholesterol acyltransferase (14).
There is evidence that oxidized apoA-I is present in human blood plasma. Thus, levels of 3-chlorotyrosine are higher in circulating HDL isolated from patients with cardiovascular disease than in HDL isolated from controls (10, 11, 15), suggesting that myeloperoxidase may target HDL for oxidation in humans during atherogenesis. Similarly, apoA-I containing methionine residues 86 and 112 as MetO (hereafter referred to as apoA-I+32) has been detected in circulating HDL, and is present at increased concentrations in subjects with a genotype associated with increased coronary artery disease (16). Moreover, it was recently shown that the MetO content of apoA-I is elevated in human type 1 diabetes (17), a disorder commonly associated with increased oxidative stress (18).
The above findings suggest that oxidized forms of apoA-I containing 3-chlorotyrosine and/or MetO could be clinically relevant. However, testing this possibility is limited by current methods for oxidized apoA-I detection that require time-consuming HDL isolation and HPLC- or mass spectroscopy-based detection steps. We therefore sought to develop a simple method for the specific detection of apoA-I containing MetO. Here we describe a sensitive and specific ELISA that detects MetO-containing apoA-I in HDL and plasma.
MATERIALS AND METHODS
Materials
Cell culture media and reagents including DMEM, fetal bovine serum, and hybridoma-SFM were purchased from Invitrogen (Carlsbad, CA). EZ-Link Sulfo- NHS-LC-Biotin was obtained from Pierce (Rockford, IL). 2,2'-Azobis(2-amidinopropane) dihydrochloride (AAPH) was purchased from Wako Pure Chemical Ind. (Osaka, Japan), and 3,3′,5,5′-tetramethylbenzidine, potassium bromide, diethylene triamine pentaacetic acid, ascorbic acid, sydnonimine-1, H2O2, BSA (fraction V), myoglobin, ferritin, and fibrinogen were obtained from Sigma (Saint Louis, MO). Sodium hypochlorite (4% solution) was obtained from Septone (Hemmant, Australia), while PBS pH 7.4 (10 mM) was prepared from tablets (Oxoid, Basingstoke, England). Trifluoroacetic acid (TFA) and HPLC-grade water were obtained from British Drug House (Poole, England). Other HPLC grade solvents were obtained from Merck (Darmstadt, Germany). All other reagents used were of analytical grade. Water was purified by a Milli-Q Ultrapure water system (Millipore, Sydney, Australia).
Isolation of HDL
Fresh blood (200 ml) from healthy and overnight fasted subjects (male, age 30–50 years) was collected into heparinized vacutainers, and plasma obtained by centrifugation at 1,430 g for 20 min at 4°C. HDL was isolated from the plasma by sequential ultracentrifugation (19). Briefly, the density of pooled plasma was adjusted to 1.24 g/ml with potassium bromide and the plasma then centrifuged for 3 h at 206,360 g and 10°C (Beckman Optima L-90K, VTi50 rotor). The resulting HDL band was aspirated, added to a new quick seal tube, and topped up with 0.9% saline of 1.24 g/ml density (adjusted with potassium bromide, 381.6 mg/ml), and then recentrifuged overnight at 206,360 g and 10°C. HDL (top band) was removed and dialyzed generously against PBS containing 0.1% EDTA and 0.01% chloramphenicol. The obtained HDL was used within 24 h.
Oxidation of HDL
Native HDL (1–1.5 mg protein/ml) was oxidized in 20 mM PBS containing 100 μM diethylene triamine pentaacetic acid under air and at 37°C by exposure to either the peroxyl radical generator AAPH (2 mM, 3 h) (7), H2O2 (100 mM, 24 h), or HOCl (500 μM, 1 h). The reactions were terminated by addition of butylated hydroxytoluene (100 μM), catalase (200 nM), or methionine (2.5 mM), respectively. The reaction mixture (1 ml) was then passed through a gel filtration column (3 ml, NAP-10, GE Healthcare, Uppsala, Sweden) eluted with 1.5 ml of PBS. The oxidized HDL was analyzed within 24 h.
HPLC analysis of native and oxidized HDL
Freshly isolated HDL (0.06 mg protein) or differently oxidized HDL (1 mg protein) was subjected to a C18 column (250 × 4.6 mm, 5 μm, Vydac) with guard (5 μm, 4.6 ID, Vydac) eluted at 50°C and 0.5 ml/min, with the eluant monitored at 214 nm. The instrument settings were modified slightly from the previously described method (16). Briefly, after initial equilibration in 25% solvent A (acetonitrile containing 0.1 vol % TFA) and 75% solvent B (water containing 0.1 vol % TFA) for 10 min, the concentration of solvent A was increased linearly to 45% over 5 min, then to 55% over 32 min, to 95% over 10 min, and finally to 100% in 1 min, after which solvent A was decreased to 25% for column reequilibration. For the purpose of preparation of apolipoprotein standards, a semipreparative RP C18 column (250 × 10 mm, 5 μm, Vydac, flow rate 2 ml/min) was used to allow injection of HDL and differently oxidized HDL (up to 5 mg protein).
Apolipoprotein standards
Appropriate protein fractions of HDL and oxidized HDL eluting from the HPLC column were collected on ice, dried under vacuum (AES1010 speedyvac system, Thermo Savant, Waltham, MA) and reconstituted in PBS. The identity of nonoxidized apoA-I and different forms of oxidized apoA-I, containing MetO instead of methionine residues as the only modification(s), was confirmed by mass spectroscopy, as described previously (16, 20). Reconstituted native and oxidized forms of apoA-I and apoA-II were overlaid with argon and stored at −20°C for up to 12 months prior to use. Such storage did not change the nature of the various apoA-I species, as verified by HPLC (data not shown). HPLC-purified apolipoproteins are subsequently referred to as lipid-free forms of apolipoproteins. Concentrations of proteins in each standard were determined using the bicinchoninic acid protein assay with BSA as the standard (Pierce).
Anti-human apoA-I+32 monoclonal antibodies (mAb)
The generation of anti-human apoA-I+32 monoclonal antibodies was performed by the Centre for Animal Biotechnology (University of Melbourne, Melbourne, Australia) with approval from the local animal ethics committee. Briefly, six Balb/c mice were immunized three times, 4 weeks apart by subcutaneous and intravenous injection of 10 μg HPLC-purified human apoA-I+32 in Freund's adjuvant. The strongest responding mice, as shown by serum Ig levels on an ELISA, were chosen for the fusion. Five days before the fusion and at least 4 weeks after the previous boost immunization, a final boost apoA-I+32 (10 μg in saline) was administered by intravenous injection. On the day of fusion, spleen cells taken from the immunized mice were added to NS-1 cells in serum-free DMEM and the fusion carried out using 50% polyethyleneglycol. Fused cells were plated in 96-well plates with rat thymocyte feeder cells. Seven to 10 days after the fusion, hybridoma supernate was screened for antibodies with solid-phase ELISA using apoA-I+32 as the immunogen. Hybridomas from high producing cell pools were subsequently cloned by limited dilution. Three rounds of clonal isolation were performed to obtain stable cell lines producing monoclonal antibodies. Two hybridoma cell lines (6.4.4.26 and 6.4.4.17.2.2) were selected based on preferential recognition of apoA-I+32 versus apoA-I. Hybridomas initially cultured in DMEM medium containing 10% FBS, were adapted to serum-free medium (Hybridoma-SFM, Invitrogen, Carlsbad, CA). Supernates of the selected hybridoma cell lines were harvested and IgG purified by FPLC using a Hitrap protein G HP affinity column (Amersham Biosciences). To determine the isotype, the mouse immunoglobulin screening/isotyping kit (Zymed Laboratories Inc., San Francisco, CA) was used following the manufacturer's protocol. Purified monoclonal antibodies (mAbs) were biotinylated by mixing with a 20-fold molar excess of Sulfo-NHS-LC-Biotin and incubation at RT for >30 min. The reaction was stopped and biotinylated mAb (B-mAb) dialyzed extensively against PBS at 4°C for 2 days. Finally, the concentration of B-mAb was determined by absorbance at 280 nm.
Development of ELISA
Ninety-six-well microplates were coated overnight at 4°C with 100 μl mouse anti-human apoA-I mAb (Chemicon, MAB010-A/11, 1:500 dilution in PBS), plates washed with 0.1% Tween 20 in PBS, followed by incubation for 1 h at 37°C with 2% BSA in PBS as blocking buffer. Plates were washed, samples (lipid-free and lipid-associated apolipoproteins, differently oxidized HDL or other proteins, and plasma) added, and plates incubated for 1 h at 37°C. Following washing, 100 μl B-mAb was added (1:400 dilution with 2% BSA in PBS) before further incubation for 1 h at 37°C. Plates were washed again and streptavidin horseradish peroxidase (Dako, 1:2,500 dilution with 2% BSA in PBS) added and the plate incubated for a further 1 h at 37°C. Following four washes, 100 μl 3,3′,5,5′-tetramethylbenzidine (liquid substrate system solution for ELISA, Sigma) was added and color developed for 5–10 min at RT. The reaction was stopped by addition of 100 μl 20% H2SO4, and the absorbance read at 450 nm in a plate reader.
For competition assay, plates were coated with 100 μL goat anti-human apoA-I polyclonal antibody (Rockland, 1:10,000 dilution in PBS), incubated at 4°C overnight, washed and blocked, and 100 μL apoA-I+32 (400 ng/ml) then added. After incubation (37°C) and washing (0.1% Tween 20 in PBS), 100 μL B-mAb17 (see later discussion) and the competing antibody were added, the plate was incubated for 1 h at 37°C, and the color was developed. For detection of oxidized forms of lipid-free apoA-I, respective apolipoproteins were used at serial dilutions (0-500 ng/ml PBS containing 2% BSA), and the sensitivity of the ELISA compared with the HPLC assay. For detection of oxidized apoA-I associated with HDL, 100 μL of AAPH-oxidized HDL (0–15 μg protein/ml) was added either in PBS containing 2% BSA, or in 40% acetonitrile. Signals were compared with readings obtained with lipid-free apoA-I+32, with the amount of apoA-I+32 contained in AAPH-oxidized HDL determined by HPLC. Where indicated, native HDL (0–15 μg/ml) or LDL (0–15 μg/ml) was used.
To apply the ELISA to human plasma, plasma samples (10 μl) were filtered (0.22 μm), supplemented with 90 μl oxidized HDL (0–15 μg), and then applied to a hydrated SwellGel Blue column (SwellGel Blue albumin removal kit, Pierce). After 2 min incubation at RT, columns were centrifuged (12,000 g, 1 min). The sample flow-through (100 μl) was reapplied to the column, the column recentrifuged, and the second sample flow-through (100 μl) collected. The column was then washed with 400 μl optimized buffer (25 mM Tris, 300 mM NaCl, pH 7.4), the wash flow-through (400 μl) collected and combined with the above second sample flow-through prior to ELISA.
SDS-PAGE
SDS-PAGE was performed using 10% bis-TRIS Nupage gels (Invitrogen) under reducing conditions. Samples loaded consisted of 1 μl of 10 × diluted plasma without and with an aliquot (15 μg protein) of the oxidized HDL loaded onto the SwellGel Blue, or 5 μl of the combined sample and wash flow-through prepared as previously described. After electrophoresis, proteins were transferred to nitrocellulose membranes (Hybond ECL, Amersham Biosciences) and immunoblotted using polyclonal goat anti-human apoA-I (Rockland) antibody as the primary and anti-goat horseradish peroxidase (Sigma) as the secondary Ab.
Preparation of differently oxidized lipid-free apoA-I
Solutions of lipid-free apoA-I (5 μM) in 20 mM PBS containing 100 μM diethylene triamine pentaacetic acid were oxidized under air at 37°C in the presence of different 1e- or 2e-oxidants. Specifically, for the Fenton reaction, 2 h incubation with FeCl3 (10 μM) plus H2O2 (5 mM), or 1 h incubation with FeSO4 (10 μM) plus ascorbic acid (1 mM) was used. Alternatively, apoA-I was oxidized with CuSO4.5H2O (5 μM) or FeCl3 (10 μM) for 2 h. In each case, oxidation was stopped by the addition of EDTA (5 mM final concentration). For 2e-oxidants, H2O2 (5 mM, 24 h incubation stopped with 200 nM catalase), HOCl (50 μM, 1 h incubation stopped with 2.5 mM methionine), or H2O2 (5 mM) plus HOCl (50 μM) (1 h incubation stopped with methionine and catalase) were used. Alternatively, apoA-I was oxidized by 2 h exposure to sydnonimine-1 (1 mM), a generator of nitric oxide plus superoxide anion radical, with the reaction stopped by 100 μM butylated hydroxytoluene. For controls, incubations were carried out in the absence of the respective oxidant. Differently oxidized forms of apoA-I were then subjected to ELISA (250 ng protein/ml) and, after gel filtration, to HPLC (5 μg protein).
Wild-type apoA-I and mutants with one, two, or three of the methionine residues replaced with leucine residues were expressed in E. coli as described (14, 21). Individual substitution mutations within human apoA-I cDNA were introduced by primer-directed PCR mutagenesis or mega-primer PCR. All mutations were verified by dideoxy automated fluorescent sequencing of cDNA, and confirmed by MS analysis of the protein (13, 22). These mutants (5 μM) were then exposed to 20 mM phosphate buffer pH 7.4 containing 100 μM diethylene triamine pentaacetic acid without (control) and with H2O2 (50 mM) at 37°C for 24 h to convert the remaining methionine residue(s) to the corresponding MetO. The reaction was terminated by addition of 200 nM catalase (to scavenge remaining H2O2) and 10 mM methionine (to inhibit further oxidation). The formation of MetO was confirmed by LC-ESI-MS/MS analysis of tryptic or Glu-C digests of oxidized apoA-I protein and the product yield of individual MetO residues was determined with reconstructed ion chromatograms of product and precursor peptides as described (14). Wild-type and mutant native and oxidized apoA-I were then shipped to Sydney for ELISA (500 ng protein/ml) and HPLC analyses (5 μg protein).
Preparation of oxidized, methionine residue-containing proteins
Native HDL (1 mg/ml) or 1 mg/ml BSA, myoglobin, ferritin, or fibrinogen were oxidized at 37°C in 20 mM PBS containing 100 μM diethylene triamine pentaacetic acid with either H2O2 (100 mM) or HOCl (500 μM). Samples were incubated for 24 h (H2O2) or 1 h (HOCl) and oxidation terminated by addition of catalase (200 nM) or methionine (2.5 mM), respectively. Oxidized proteins were then subjected to ELISA.
Statistical analysis
Data are shown as mean ± SEM unless otherwise stated. Intra- and interassay reproducibility was determined in four independent analyses each in triplicate.
RESULTS
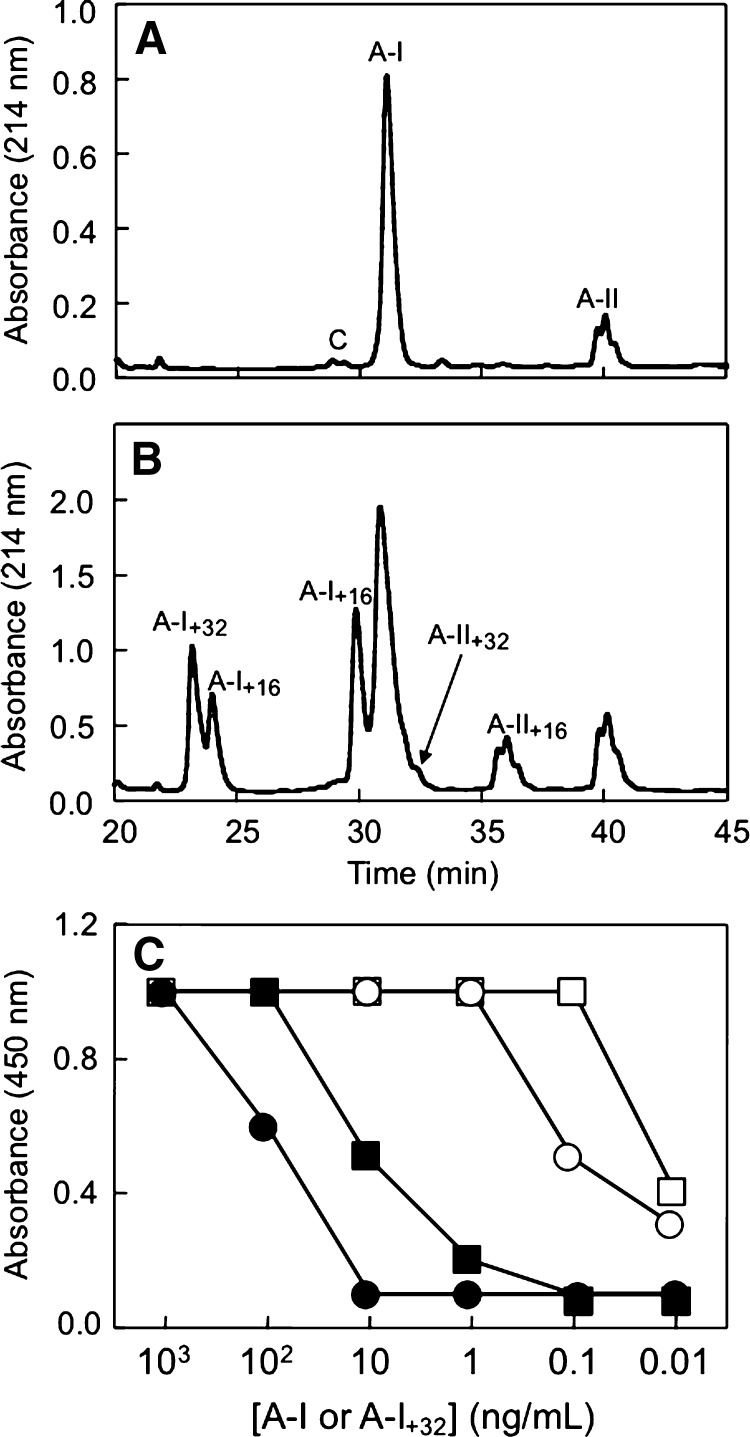
Native HDL isolated from freshly obtained human plasma contains apolipoproteins apoA-I and apoA-II as the major proteins, which can be separated by HPLC (Fig. 1A). AAPH-oxidized HDL contained oxidized forms of apoA-I and apoA-II, which exhibit shorter retention times compared with their respective native forms (Fig. 1B). These oxidized forms of apoA-I were designated apoA-I+32 (containing MetO86 plus MetO112 and eluting at ∼23 min) or apoA-I+16, containing MetO112 (∼24 min) or MetO86 (∼30 min). Methionine modifications were the only modifications present in these oxidized forms of apoA-I, as verified by LC-MS/MS (16, 20). Native apoA-II and its oxidized form (apoA-II+16) eluted as three distinct, albeit incompletely resolved species (Fig. 1B), and were characterized recently (20).
Fig. 1.
Typical HPLC traces of native and peroxyl radical-oxidized HDL. HDL was isolated and then subjected to HPLC analysis without and with prior oxidation with 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) (2 mM, 37°C, 3 h) as described in Materials and Methods. A: Native HDL (0.06 mg protein injected) contains apolipoprotein A-I (apoA-I) (A-I) and apoA-II (A-II) as major proteins. B: Oxidized HDL (1 mg protein injected) contains oxidized forms of apoA-I and apoA-II in addition to A-I and A-II. The oxidized forms detected include (in order of elution) apoA-I+32 [A-I+32, containing methionine sulfoxide (MetO)86 and MetO112], A-I+16 (MetO112), A-I+16 (MetO86), A-II+32 (containing both Met26 of apoA-II dimer as MetO) and A-II+16 (containing one of the two Met26 of apoA-II dimer as MetO). For assignment of the different peaks see Refs (7, 8, 16, 20). C: Representative result from the original screen of mouse serum. Of six mice immunized with purified apoA-I+32, serum from mouse 1 (circles) and mouse 6 (squares) showed preferred recognition of apoA-I+32 (open symbols) over apoA-I (closed symbols).
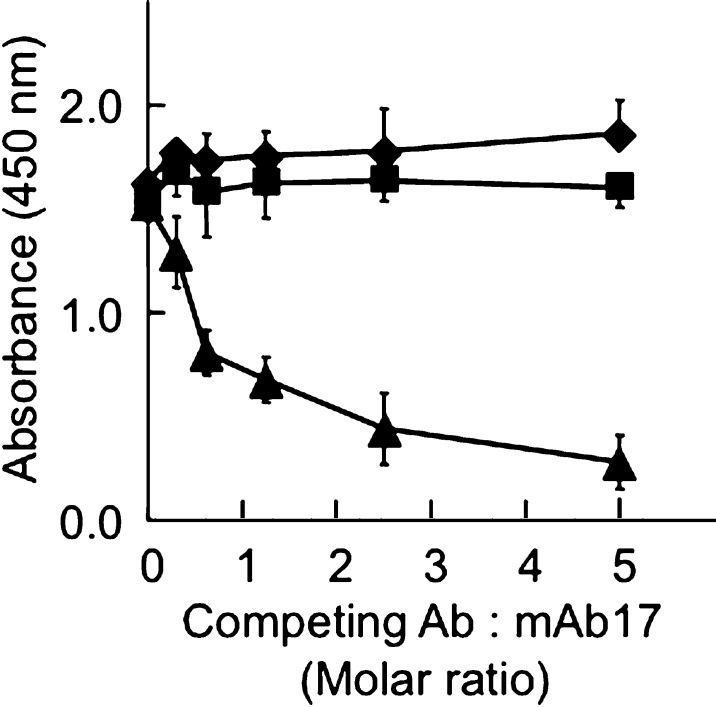
To develop an ELISA for MetO-containing apoA-I, we immunized six mice with HPLC-purified human apoA-I+32 (16). Of the sera from these mice, two showed increased recognition of apoA-I+32 over apoA-I (Fig. 1C). The spleens of these mice were used to generate hybridomas, yielding several different clones, of which two, 6.4.4.26 and 6.4.4.17.2.2 (yielding mAb17) were chosen for isolation of mAbs and subsequent experiments described herein. Of these, 6.4.4.26 (hereafter referred to as MOA-I) demonstrated the highest response to apoA-I+32 (data not shown). Competition experiments revealed that MOA-I and mAb17 raised against apoA-I+32 recognized the same epitope (Fig. 2), as did several commercial polyclonal anti-human apoA-I antibodies (i.e., 600−101−109 from Rockland, A95120H from Biodesign, and AB740 from Chemicon) (data not shown). In contrast, mAb010-A/11 (from Chemicon) and MOA-I bound mutually exclusive epitopes, as absorbance remained unchanged (Fig. 2). Therefore, we used mAb010-A/11 for capture and B-MOA-I for secondary detection in all subsequent experiments.
Fig. 2.
Mouse monoclonal anti-human apoA-I (MAb010-A/11) and anti apoA-I+32 (MOA-I) antibodies recognize different epitopes. Goat polyclonal anti human apoA-I (1:10,000 dilutions) was used as the capture antibody. ApoA-I+32 (400 ng/ml) was then added, followed by competitive ELISA as described in Materials and Methods and using biotinylated mAb17 (600 ng/ml) together with either MAb010-A/11 (squares), MOA-I (triangles) or control antibody (diamonds, anti-human smooth muscle actin) as competing antibody at the molar ratio indicated. The results show mean ± SEM of four separate experiments, each carried out in triplicates.
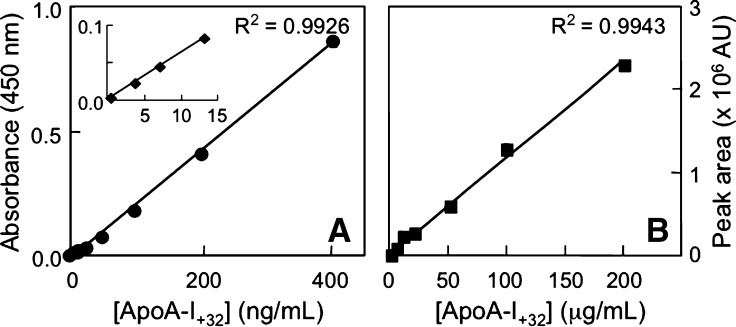
We first tested the specificity of MOA-I for various HPLC-purified, oxidized forms of lipid-free apoA-I. As can be seen, MOA-I detected apoA-I+32 in a concentration-dependent manner and yielded a linear response (Fig. 3A). There was no cross-reactivity with apoA-I and apoA-II (Fig. 3A), and comparatively modest recognition of apoA-I+16 (MetO112 or Met86) (Fig. 3B). Intra- and interassay variation for apoA-I+32 was 3.8 ± 2.7 and 6.3 ± 2.9%, respectively. The sensitivity of the ELISA for apoA-I+32 was nearly three orders of magnitude greater than that of the HPLC method, with detection limits of 3 ng/ml (Fig. 4A) and 1 μg/ml (Fig. 4B), respectively.
Fig. 3.
Specificity of MOA-I for MetO-containing, lipid-free apoA-I. Serial dilutions (0–500 ng/ml) of apoA-I+32 (diamonds), apoA-I (squares) or apoA-II (triangles) (A); or apoA-I+32 (diamonds), apoA-I+16 (MetO86, triangles) or apoA-I+16 (MetO112, squares) (B) were added, followed by the addition of detection antibody (MOA-I) as detailed in Materials and Methods. The results show mean ± SEM three separate experiments, each carried out in triplicates.
Fig. 4.
Sensitivity comparison between ELISA and HPLC for the detection of apoA-I+32. A and B show standard curves for apoA-I+32 in the ELISA and HPLC assay, respectively. ELISA and HPLC were carried out as described in Materials and Methods, using HPLC-purified apoA-I+32. The results show mean ± SEM of a representative comparison carried out in triplicates. Similar results were obtained in four separate experiments.
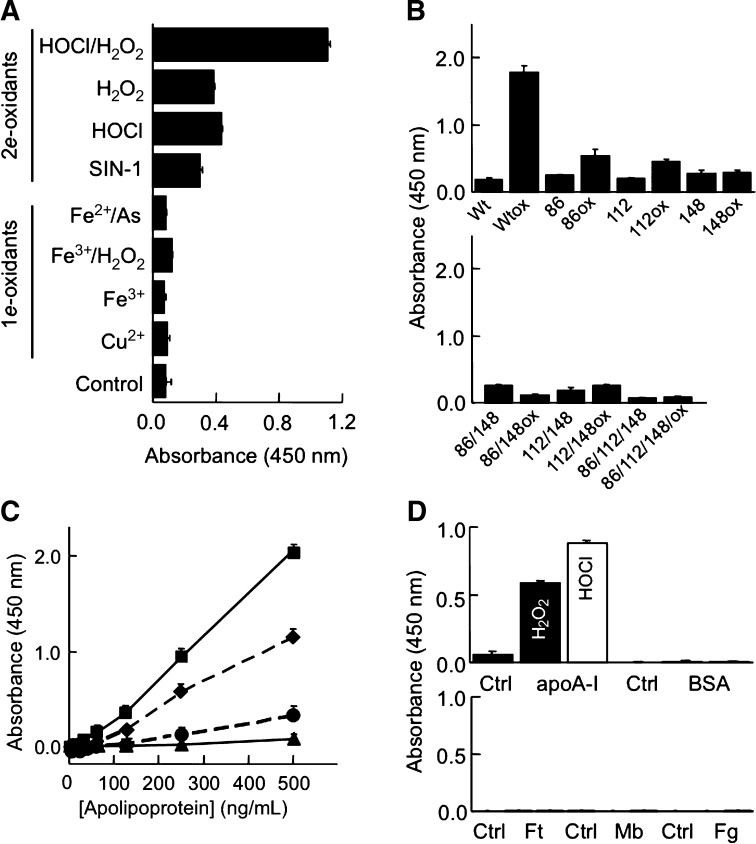
We next applied the ELISA to lipid-free apoA-I oxidized in vitro by different radical (i.e., 1e-oxidants) or nonradical (2e-) oxidants. The results show that MOA-I recognized apoA-I oxidized with the 2e-oxidants HOCl, H2O2 and peroxynitrite (derived from sydnonimine-1) (Fig. 5A). These 2e-oxidants are well known to oxidize methionine residues in proteins to MetO. In the case of apoA-I, this results in formation of apoA-I+32 (7, 8), as verified by HPLC for HOCl, H2O2 and peroxynitrite (data not shown). In contrast, lipid-free apoA-I oxidized by 1e-oxidants (i.e., hydroxyl radical and metal ions) was poorly recognized by MOA-I (Fig. 5A) and did not contain substantial amounts of apoA-I+32 or apoA-I+16, as verified by HPLC (data not shown). Together these observations suggest that MOA-I recognizes MetO-containing apoA-I.
Fig. 5.
Specificity of ELISA. A: Recognition of lipid-free apoA-I oxidized by 2e-, but not 1e-oxidants. Native apoA-I (5 μM) isolated from human HDL and purified by HPLC was oxidized with the 1e- or 2e-oxidant indicated before being applied to the ELISA at 250 ng/ml. B: Recognition of recombinant human wild-type and mutant apoA-I before and after H2O2-mediated oxidation. Native and H2O2-oxidized wild-type and single methionine apoA-I mutants (upper panel) or native and H2O2-oxidized double and triple methionine apoA-I mutants (lower panel) were subjected to ELISA at 500 ng/ml. C: Recognition of apoA-I containing 0, 1, 2, or 3 MetO. Recombinant apoA-I without (triangles) and with H2O2 treatment (50 mM H2O2, 5 μM apoA-I, 37°C, 24 h) and containing all three methionine residues as MetO (Table 1) (squares), or HDL-derived, HPLC-purified apoA-I containing MetO86 (circles) or MetO86 plus MetO112 (diamonds) were subjected to ELISA at the serial dilutions indicated. D: ApoA-I or other Met-containing proteins (1 mg/ml) were oxidized with H2O2 (100 mM, filled bar) or hypochlorous acid (HOCl) (250 μM, open bar) and each oxidized protein (0.5 μg for apoA-I and 10 μg for all other proteins) then applied to ELISA. A–D, Oxidized apolipoproteins and proteins were generated as described in Materials and Methods. The results show mean ± SEM of three separate experiments, each carried out in triplicates. Mb, myoglobin; Ft, ferritin; Fg, fibrinogen.
To examine the relative contribution of the three methionine residues of apoA-I (i.e., Met86, Met112, and Met148) to recognition by MOA-I, we employed mutant apoA-I in which a single, two, or all three methionine residues were replaced with leucine. The sequences of wild-type and mutant apoA-I were confirmed by LC-ESI-MS/MS (not shown). These proteins were then oxidized by exposure to H2O2, the resulting formation of MetO confirmed by LC-ESI-MS/MS (Table 1) and the proteins then subjected to ELISA. As expected, H2O2-oxidized wild-type apoA-I yielded a high signal compared with the corresponding nonoxidized apoA-I (Fig. 5B). Compared with oxidized wild-type apoA-I, oxidized single methionine to leucine substituted apoA-I gave smaller signals, although they were still recognized to a greater extent than the corresponding nonoxidized counterparts, except for M148L apoA-I (Fig. 5B, upper panel). Consistently smaller signals were recorded with the oxidized double mutants (M86L/M148L and M112L/M148L) and the oxidized triple mutant (Fig. 5B, lower panel). HPLC analyses of the respective wild-type and mutant apoA-I revealed that high ELISA reactivity was associated with substantially shorter retention time of the oxidized compared with the nonoxidized protein (data not shown). Fig. 5C compares the immunoreactivity of recombinant and naturally occurring MetO-containing apoA-I, with the extent of recognition by MOA-I increasing with increasing numbers of MetO.
Table 1.
Yield of methionine sulfoxide (MetO) in H2O2-oxidized recombinant wild-type and mutant apolipoprotein A-I (apoA-I)
| ApoA-I | MetO86 | MetO112 | MetO148 |
|---|---|---|---|
| Wild-type | 0.6 | 0.8 | 0.5 |
| Wild-type ox | 94 | 100 | 99 |
| M86L | n/a | 0.3 | 0.3 |
| M86Lox | n/a | 100 | 98 |
| M112L | 0 | n/a | 0.5 |
| M112Lox | 88 | n/a | 95 |
| M148L | 0 | 0.2 | n/a |
| M148Lox | 67 | 100 | n/a |
| M86L/M148L | n/a | 0.3 | n/a |
| M86L/M148Lox | n/a | 100 | n/a |
| M112L/M148L | 0 | n/a | n/a |
| M112L/M148Lox | 60 | n/a | n/a |
| M86L/M112L/M148L | n/a | n/a | n/a |
| M86L/M112L/M148Lox | n/a | n/a | n/a |
Wild-type and mutant forms of apoA-I were expressed, purified, and then oxidized with reagent H2O2 as described in Materials and Methods. Oxidized proteins were then analyzed by LC-ESI-MS/MS to verify the extent of oxidation of methionine residues to MetO. Yield of MetO (%) = peak area of product ion / sum (peak area of precursor ion + peak area of product ion) × 100. The data represent averages of two sets of independent analyses, with the variation between the respective two values <3%.
Finally, we assessed the specificity of MOA-I for MetO contained in apoA-I versus other proteins. For this we exposed lipid-free apoA-I, BSA, ferritin, myoglobin, and fibrinogen to H2O2 or HOCl to convert their methionine residues to MetO prior to ELISA. As can be seen, of these proteins only oxidized apoA-I yielded positive ELISA readings (Fig. 5D). Together, these results indicate that MOA-I recognizes MetO specifically in apoA-I, and that the extent of this recognition increases with increasing MetO content.
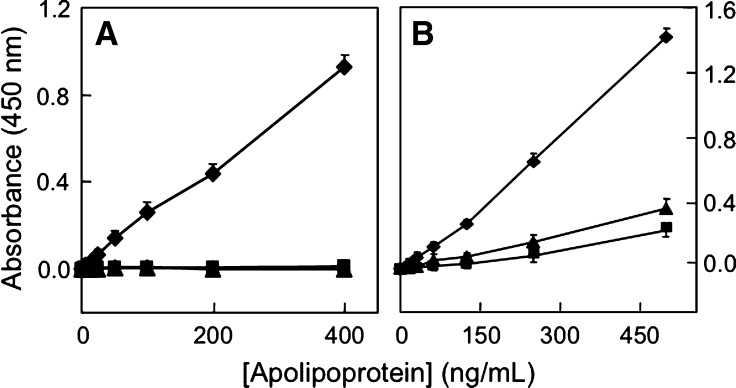
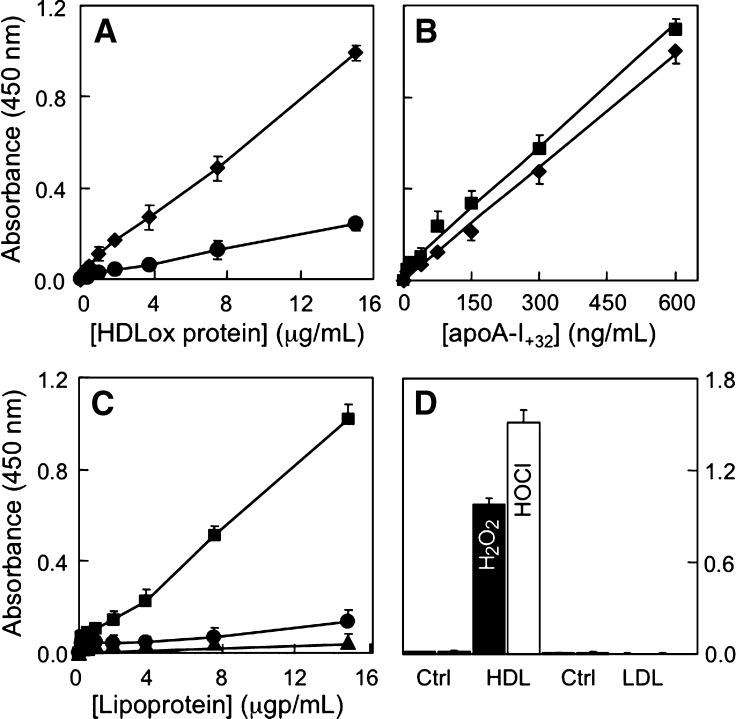
In a second part of our studies, we examined the suitability of the ELISA to detect MetO-containing apoA-I in HDL and in plasma. For this we utilized AAPH-oxidized HDL. As can be seen, the ability of MOA-I to detect such oxidized HDL was modest (Fig. 6A). However, sensitivity was enhanced substantially when acetonitrile was added to the oxidized HDL prior to its application to the ELISA (Fig. 6A). We chose acetonitrile to “delipidate” MetO-containing apoA-I in HDL because of its proven efficacy for this purpose in the HPLC assay. Optimization experiments revealed that acetonitrile at 40% final concentration gave best results (data not shown). At this concentration, the ELISA response for delipidated apoA-I+32 contained in oxidized HDL was similar to that observed with lipid-free apoA-I+32 (Fig. 6B). The slightly higher response seen with oxidized HDL was likely due to the presence of some apoA-I+16 in oxidized HDL but not purified, lipid-free apoA-I+32. Compared with AAPH-oxidized HDL, the ELISA did not detect native LDL, and reactivity with freshly isolated, native HDL was low (Fig. 6C). Detection of AAPH-oxidized HDL was reproducible, with intra- and interassay variation of 4.1 ± 3.2 and 9.3 ± 4.6%, respectively (n = 4 separate experiments, each carried out in triplicate).
Fig. 6.
Specific detection of MetO-containing apoA-I associated with HDL by ELISA. A: Standard curves of AAPH-oxidized HDL (0–15 μg protein/ml) in PBS (circles) or 40% acetonitrile (diamonds). B: Comparison of ELISA response to HDL-associated apoA-I+32 (squares) versus isolated, lipid-free apoA-I+32 (diamonds). The amount of apoA-I+32 contained in AAPH-oxidized HDL was determined by HPLC as described in Materials and Methods. C: Standard curves of AAPH-oxidized HDL in 40% acetonitrile (squares) versus native HDL (circles) and LDL (triangles) in 40% acetonitrile. D: Comparison of ELISA reactivity for H2O2- and HOCl-oxidized HDL and LDL. Results show mean ± SEM of four (A–C) and three separate experiments (D), each performed in triplicates.
We next examined whether the ELISA was suitable to detect oxidized HDL other than AAPH-oxidized HDL. For this, we used HDL oxidized with H2O2 or HOCl (i.e., 2e-oxidants that directly convert methionine in apoA-I to MetO). H2O2- and HOCl-oxidized HDL were clearly recognized by MOA-I (Fig. 6D). Interestingly, and in contrast to the situation with AAPH-oxidized HDL, delipidation with acetonitrile was not required to detect H2O2- and HOCl-oxidized HDL. Importantly, the ELISA did not detect LDL oxidized with either H2O2 or HOCl (Fig. 6D), indicating specificity of the assay for HDL.
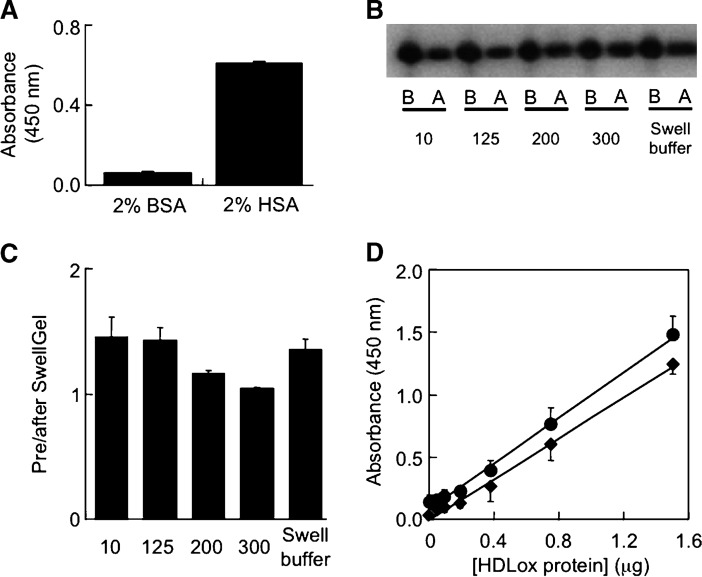
We next applied the ELISA to freshly obtained human plasma samples. Initial experiments revealed high background readings in diluted and lipid-extracted plasma samples (data not shown), and albumin to be responsible for this (Fig. 7A). Efficient (i.e., >95%) removal of albumin was achieved using the SwellGel Blue Albumin Removal Kit. However, such removal was not specific for albumin, as it also removed ∼50–60% of apoA-I (data not shown). Specificity for albumin removal (i.e., >80% removal of albumin with essentially 100% apoA-I retained) was achieved with the Swellgel Blue approach using plasma volumes <20 μl and 300 mM NaCl, 25 mM Tris, pH 7.4 as the washing buffer (Fig. 7B, C). This method was then used to examine the ELISA response to plasma containing increasing amounts of AAPH-oxidized HDL. As can be seen, the ELISA response to plasma containing increasing amounts of oxidized HDL was linear (Fig. 7D). Importantly, the extent of the response was comparable for isolated oxidized HDL (diamonds in Fig. 7D) and oxidized HDL added to plasma (circles in Fig. 7D). The intra- and interassay variation for oxidized HDL in plasma was 7.6 ± 3.6 and 13.3 ± 5.3%, respectively. Together, these results indicate the usefulness of the ELISA for the determination of MetO-containing, HDL-associated apoA-I in human plasma.
Fig. 7.
Determination of MetO-containing apoA-I in HDL added to plasma by ELISA. A: Effect of BSA and human serum albumin (HSA) on background absorbance in ELISA. BSA or HSA (100 μl of a 2% solution each) was applied to the ELISA in the absence of apolipoproteins, and the background readings recorded. B: Recovery of apoA-I after albumin removal by SwellGel Blue using 25 mM Tris, pH 7.4 and the NaCl concentration indicated. Filtered and 1:10 diluted plasma (total volume 100 μl) before (“B”) or after (“A”) SwellGel Blue column filtration was subjected to SDS-PAGE/Western as described in Materials. Representative Western blots are shown. C: Quantification of the Western data shown in B. Results are expressed as the ratio of apoA-I before to after SwellGel Blue albumin removal process. D, ELISA detection of MetO-containing apoA-I in HDL and added to human plasma. Fresh plasma (10 μl) was spiked with increasing concentrations of AAPH-oxidized HDL (0–15 μg/ml) and then passed through SwellGel Blue column, washed with optimized buffer (300 mM NaCl, 25 mM Tris pH 7.4) and the combined flow-through and wash sample (total volume 100 μl) applied to ELISA as described in Materials and Methods. For comparison, the response to AAPH-oxidized HDL added to plasma and passed through the SwellGel Blue column (circles) was plotted against a standard curve obtained with AAPH-oxidized HDL alone (triangles). Results show mean ± SEM of a representative of four separate experiments each carried out in triplicates.
DISCUSSION
It has been proposed that oxidized HDL plays a major role in atherogenesis, yet most presently available methods to detect oxidized HDL are not readily applicable to large numbers of clinical samples because they are time consuming and require specialized and expensive equipment. The present work describes a simple and robust ELISA for the specific detection of MetO-containing apoA-I in HDL as a novel tool potentially useful for evaluating the relationship between oxidized HDL, atherosclerosis and coronary artery disease.
The ELISA described here is based on MOA-I, a monoclonal antibody that we raised against HPLC-purified, lipid-free human apoA-I+32 containing two (Met86 and Met112) of its three methionine residues as MetO. Several independent lines of evidence support the specificity of MOA-I for MetO-containing apoA-I. First, MOA-I readily recognized apoA-I+32 but not native apoA-I (Fig. 3A) purified from oxidized and native human HDL by HPLC. Second, the experiments with recombinant human wild-type and mutant apoA-Is (Fig. 5) showed that methionine residues were required for MOA-I to recognize H2O2-oxidized apoA-I. In the absence of cysteine residues in apoA-I, H2O2 selectively oxidizes methionine to MetO. Importantly, we used MS-based methods to unambiguously identify and confirm MetO as the only modified chemical moiety in the different forms of oxidized apoA-Is used in these experiments. In addition to H2O2-oxidized apoA-I, MOA-I also recognized lipid-free apoA-I oxidized by other 2e-oxidants (HOCl and peroxynitrite), whereas the antibody was nonreactive toward apoA-I modified by 1e-oxidants (Fig. 5). It is well established that 2e-oxidants, but not 1e-oxidants, effectively convert methionine residues into MetO (7, 8). Finally, specificity of MOA-I for MetO-containing apoA-I is indicated by the fact that the ELISA did not detect H2O2- or HOCl-treated, methionine-containing proteins other than apoA-I.
In contrast to isolated, lipid-free apoA-I, MOA-I recognized HDL oxidized by 2e-oxidants and 1e-oxidants (i.e., peroxyl radicals). This observation is explained readily by the fact that 1e-oxidants, like AAPH-derived peroxyl radicals, preferentially react with the lipoprotein's lipids. This causes lipid peroxidation with the resulting accumulation of hydroperoxides of phospholipids and cholesterylesters (23) that then oxidize apoA-I's methionine to MetO (7, 8, 24, 25). The fact that our ELISA recognizes HDL oxidized by a range of different oxidants increases its applicability to biological samples, at least in the context of cardiovascular diseases. This is because various oxidants contribute to oxidative modifications taking place in the affected arterial wall during atherogenesis (1). Thus, while “oxidized HDL” is not a chemically defined term, the presence of MetO-containing apoA-I in such modified lipoproteins likely represents a feature of oxidative damage required for MOA-I recognition.
We raised MOA-I using apoA-I+32 as the immunogen because this specifically oxidized apoA-I is formed as a major and relatively stable oxidation product when HDL is exposed to radical oxidants in vitro (16). Also, apoA-I+32 has physical properties clearly distinct from native, nonoxidized apoA-I that allows the two forms of the protein to be separated by HPLC (Fig. 1), and that we hypothesized would generate antigen(s) suitable for the generation of mAbs. Indeed, sera from two of the six animals immunized with apoA-I+32 showed increased recognition of apoA-I+32 over apoA-I (Fig. 1C). Perhaps more surprisingly, we noticed that most commercially available monoclonal and polyclonal anti-human apoA-I antibodies also preferentially recognized apoA-I+32 over native apoA-I (not shown). We believe this is likely due to the lipid hydroperoxide-mediated conversion of methionine residues to MetO when apoA-I is associated with lipids as in HDL, and samples are worked up or stored under conditions that do not effectively prevent inadvertent lipid oxidation. Our findings may also explain why antibody-based assays are not commonly employed in clinical biochemistry laboratories for the determination of HDL, if the antibodies used preferentially recognize oxidized apoA-I and samples are stored in the absence of added antioxidants for varying times (see also Ref. (26). That most commercial antibodies recognize oxidized apoA-I in preference to nonoxidized apoA-I also suggests that caution is needed when interpreting data based on antibody-dependent quantification of apoA-I in biological samples. In atherosclerosis research, for example, samples often vary in terms of lipid content and are processed (e.g., fixed or homogenized) and/or stored without controlling for ex vivo oxidation events.
We are aware of only two previously reported antibodies against oxidized apoA-I or HDL: mAb C311 raised against AAPH-oxidized apoA-I (27), and mAb 9F5-3a raised against HDL oxidized by CuSO4 (28). Both these mAbs likely recognize epitopes not directly related to MetO, as MetO is not a major product in apoA-I oxidized with AAPH (7), and it is not responsible for cross-linked apoA-I that is recognized by mAb 9F5-3a (29). Irrespective, Nakajima et al. (29) reported increased levels of oxidized HDL, defined by immunoreactivity with mAb 9F5-3a, in plasma from patients with coronary artery disease compared with healthy subjects and patients with noninsulin-dependent diabetes mellitus. It remains to be established how this finding relates to the presence of ‘oxidized HDL’ with MetO-containing apoA-I.
Our results with isolated mutants (Fig. 5) indicate that the extent of recognition by MOA-I is higher for apoA-I with all three methionine residues present as MetO than for apoA-I+32, the immunogen used in the present study. Thus, rather than directly recognizing MetO, MOA-I appears to bind a conformational epitope of apoA-I that is generated as its three methionine residues become converted to MetO. This interpretation is consistent with the lack of ELISA response to methionine-containing proteins other than apoA-I, oxidized by H2O2 or HOCl. In apoA-I, the hydrophobic methionine residues are located at the boundary between polar and nonpolar faces of amphipathic helices, such that introduction of the more polar MetO could alter the hydrophobic moment and hence secondary structure of the protein (30).
The ELISA described in the present manuscript is likely relevant to clinical samples, as judged by its limits of detection of MetO-containing apoA-I and our previous HPLC-based study, indicating that in such samples, up to 15% of endogenous apoA-I may be present as apoA-I+32 (16). Thus, the amount of MetO-containing apoA-I (MetO86, MetO112, or MetO86 plus MetO112) added as “HDLox” to the 1/10 diluted plasma in Fig. 7D ranged from 0–0.525 μg, as determined by HPLC analysis of the AAPH-oxidized HDL used. This corresponds to 0–7% of the endogenous apoA-I contained in the plasma, given that apoA-I constitutes ∼70% of HDL's protein and the concentration of apoA-I in human plasma is ∼1.5 mg/ml (31).
While feasible (see the introduction to this article), the biological or pathological role of MetO-containing apoA-I is presently not established. This could limit the importance of the ELISA described herein. Also, we exclusively used in vitro generated oxidized forms of apoA-I and HDL, whereas the oxidative modifications occurring to apoA-I in the diseased artery wall are conceivably more complex (1). For example, we do not know whether, and if so to what extent, oxidative modifications in addition to MetO formation, such as cross-linking of apoA-I, affect the response of the ELISA described in the present study. An additional potential limitation of the present assay is that HDL is subject to continuous remodeling in vivo. This includes dissociation of apoA-I from the lipoprotein particle, a process that could be increased by oxidation (32). Clearly, future studies are required to assess these various aspects, as well as the utility of the ELISA described for clinical studies and/or the use of MOA-I to immunoprecipitate modified apoA-I from biological materials for subsequent characterization.
Acknowledgments
We thank Mr Peter Anderson-Stewart for initial experiments.
Published, JLR Papers in Press, October 2, 2008.
Footnotes
This research was supported by Psiron Pty Ltd and a University of Sydney Post-graduate Award Scholarship (to X.S.W.), the National Health & Medical Research Council of Australia (Program grant and Fellowship to R.S.), the University of Sydney Medical Foundation (to R.S.), and by grants from the National Institutes of Health (K99HL091055, HL086798, P30DK017047, and PO1HL030086). Mass spectrometry analyses of recombinant apoA-Is were supported by the Mass Spectrometry Core, Diabetes, and Endocrinology Research Center, University of Washington.
References
- 1.Stocker R., and J. F. Keaney, Jr. 2004. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 84 1381–1478. [DOI] [PubMed] [Google Scholar]
- 2.Niu X., V. Zammit, J. M. Upston, R. T. Dean, and R. Stocker. 1999. Co-existence of oxidized lipids and α-tocopherol in all lipoprotein fractions isolated from advanced human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 19 1708–1718. [DOI] [PubMed] [Google Scholar]
- 3.Barter P. J., S. Nicholls, K. A. Rye, G. M. Anantharamaiah, M. Navab, and A. M. Fogelman. 2004. Antiinflammatory properties of HDL. Circ. Res. 95 764–772. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty A., J. L. Dunn, D. L. Rateri, and J. W. Heinecke. 1994. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest. 94 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazell L. J., L. Arnold, D. Flowers, G. Waeg, E. Malle, and R. Stocker. 1996. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J. Clin. Invest. 97 1535–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anantharamaiah G. M., T. A. Hughes, M. Iqbal, A. Gawish, P. J. Neame, M. F. Medley, and J. P. Segrest. 1988. Effect of oxidation on the properties of apolipoproteins A-I and A-II. J. Lipid Res. 29 309–318. [PubMed] [Google Scholar]
- 7.Garner B., P. K. Witting, A. R. Waldeck, J. K. Christison, M. Raftery, and R. Stocker. 1998. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that correlates with lipid peroxidation and can be enhanced by α-tocopherol. J. Biol. Chem. 273 6080–6087. [DOI] [PubMed] [Google Scholar]
- 8.Garner B., A. R. Waldeck, P. K. Witting, K-A. Rye, and R. Stocker. 1998. Oxidation of high density lipoproteins. II. Evidence for direct reduction of HDL lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 273 6088–6095. [DOI] [PubMed] [Google Scholar]
- 9.Oram J. F., and J. W. Heinecke. 2005. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85 1343–1372. [DOI] [PubMed] [Google Scholar]
- 10.Bergt C., S. Pennathur, X. Fu, J. Byun, K. O'Brien, T. O. McDonald, P. Singh, G. M. Anantharamaiah, A. Chait, J. Brunzell, et al. 2004. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA. 101 13032–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L., B. Nukuna, M. L. Brennan, M. Sun, M. Goormastic, M. Settle, D. Schmitt, X. Fu, L. Thomson, P. L. Fox, et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest. 114 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng D. Q., Z. Wu, G. Brubaker, L. Zheng, M. Settle, E. Gross, M. Kinter, S. L. Hazen, and J. D. Smith. 2005. Tyrosine modification is not required for myeloperoxidase-induced loss of apolipoprotein A-I functional activities. J. Biol. Chem. 280 33775–33784. [DOI] [PubMed] [Google Scholar]
- 13.Shao B., M. N. Oda, C. Bergt, X. Fu, P. S. Green, N. Brot, J. F. Oram, and J. W. Heinecke. 2006. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J. Biol. Chem. 14 9001–9004. [DOI] [PubMed] [Google Scholar]
- 14.Shao B., G. Cavigiolio, N. Brot, M. N. Oda, and J. W. Heinecke. 2008. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA. 105 12224–12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennathur S., C. Bergt, B. Shao, J. Byun, S. Y. Kassim, P. Singh, P. S. Green, T. O. McDonald, J. Brunzell, A. Chait, et al. 2004. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 279 42977–42983. [DOI] [PubMed] [Google Scholar]
- 16.Pankhurst G., X. L. Wang, D. E. Wilcken, G. Baernthaler, U. Panzenböck, M. Raftery, and R. Stocker. 2003. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J. Lipid Res. 44 349–355. [DOI] [PubMed] [Google Scholar]
- 17.Brock J. W., A. J. Jenkins, T. J. Lyons, R. L. Klein, E. Yim, M. Lopes-Virella, R. E. Carter, S. R. Thorpe, and J. W. Baynes. 2008. Increased methionine sulfoxide content of apoA-I in type 1 diabetes. J. Lipid Res. 49 847–855. [DOI] [PubMed] [Google Scholar]
- 18.Baynes J. W., and S. R. Thorpe. 1999. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 48 1–9. [DOI] [PubMed] [Google Scholar]
- 19.Sattler W., D. Mohr, and R. Stocker. 1994. Rapid isolation of lipoproteins and assessment of their peroxidation by HPLC postcolumn chemiluminescence. Methods Enzymol. 233 469–489. [DOI] [PubMed] [Google Scholar]
- 20.Woodward M., K. D. Croft, T. A. Mori, H. Headlam, X. S. Wang, C. Suarna, M. J. Raftery, S. W. Macmahon, and R. Stocker. 2009. The association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clin. Sci. 116 53–60. [DOI] [PubMed]
- 21.Ryan R. O., T. M. Forte, and M. N. Oda. 2003. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr. Purif. 27 98–103. [DOI] [PubMed] [Google Scholar]
- 22.Shao B., and J. W. Heinecke. 2008. Using tandem mass spectrometry to quantify site-specific chlorination and nitration of proteins: model system studies with high-density lipoprotein oxidized by myeloperoxidase. Methods Enzymol. 440 33–63. [DOI] [PubMed] [Google Scholar]
- 23.Bowry V. W., K. K. Stanley, and R. Stocker. 1992. High density lipoprotein is the major carrier of lipid hydroperoxides in fasted human plasma. Proc. Natl. Acad. Sci. USA. 89 10316–10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sattler W., J. K. Christison, and R. Stocker. 1995. Cholesterylester hydroperoxide reducing activity associated with isolated high- and low-density lipoproteins. Free Radic. Biol. Med. 18 421–429. [DOI] [PubMed] [Google Scholar]
- 25.Mashima R., Y. Yamamoto, and S. Yoshimura. 1998. Reduction of phosphatidylcholine hydroperoxide by apolipoprotein A-I: purification of the hydroperoxide-reducing proteins from human blood plasma. J. Lipid Res. 39 1133–1140. [PubMed] [Google Scholar]
- 26.Nakano T., and A. Nagata. 2005. Oxidative susceptibility of apolipoprotein AI in serum. Clin. Chim. Acta. 362 119–124. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T., and A. Nagata. 2003. Immunochemical detection of circulating oxidized high-density lipoprotein with antioxidized apolipoprotein A-I monoclonal antibody. J. Lab. Clin. Med. 141 378–384. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima T., Y. Sakagishi, T. Katahira, A. Nagata, T. Kuwae, H. Nakamura, I. Inoue, K. Takahashi, S. Katayama, and T. Komoda. 1995. Characterization of a specific monoclonal antibody 9F5–3a and the development of assay system for oxidized HDL. Biochem. Biophys. Res. Commun. 217 407–411. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T., T. Matsunaga, S. Kawai, S. Hokari, I. Inoue, S. Katayama, A. Nagata, and T. Komoda. 2004. Characterization of the epitopes specific for the monoclonal antibody 9F5–3a and quantification of oxidized HDL in human plasma. Ann. Clin. Biochem. 41 309–315. [DOI] [PubMed] [Google Scholar]
- 30.Panzenbock U., and R. Stocker. 2005. Formation of methionine sulfoxide-containing specific forms of oxidized high-density lipoproteins. Biochim. Biophys. Acta. 1703 171–181. [DOI] [PubMed] [Google Scholar]
- 31.Barter P., A. M. Gotto, J. C. LaRosa, J. Maroni, M. Szarek, S. M. Grundy, J. J. Kastelein, V. Bittner, and J. C. Fruchart. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357 1301–1310. [DOI] [PubMed] [Google Scholar]
- 32.Panzenböck U., L. Kritharides, M. Raftery, K. A. Rye, and R. Stocker. 2000. Oxidation of methionine residues to methionine sulfoxides does not decrease potential anti-atherogenic properties of apolipoprotein A-I. J. Biol. Chem. 275 19536–19544. [DOI] [PubMed] [Google Scholar]