Abstract
Host cells respond to viral infection by many mechanisms, including the production of type I interferons which act in a paracrine and autocrine manner to induce the expression of antiviral interferon-stimulated genes (ISGs). Viruses have evolved means to inhibit interferon signaling to avoid induction of the innate immune response. Herpes simplex virus 1 (HSV-1) has several mechanisms to inhibit type I interferon production, the activities of ISGs, and the interferon signaling pathway itself. We report that the inhibition of the Jak/STAT pathway by HSV-1 requires viral gene expression and that viral immediate early protein ICP27 plays a role in downregulating STAT-1 phosphorylation and in preventing the accumulation of STAT-1 in the nucleus. We also show that expression of ICP27 by transfection causes an inhibition of IFN-induced STAT-1 nuclear accumulation. Therefore, ICP27 is necessary and sufficient for at least some of the effects of HSV infection on STAT-1.
Introduction
Type I interferon (IFNα/β) signaling is an important antiviral response that results in the expression of antiviral, antiproliferative, and immunomodulatory proteins (Platanias and Fish, 1999). IFNα/β binds the type I interferon receptor subunits (IFNAR-1 and –2), causing heterodimerization and phosphorylation of the subunits. This activation leads to the phosphorylation of the Janus kinases, Jak-1 and Tyk-2, which in turn phosphorylate signal transducers and activators of transcription (STATs) 1 and 2. The STATs heterodimerize and translocate into the nucleus, where they associate with p48 (also known as IRF-9), bind a cis-acting DNA element called the interferon-stimulated response element, or ISRE (Platanias and Fish, 1999); reviewed in (Samuel, 2001) for the transactivation of interferon-stimulated genes (ISGs) (reviewed in (Garcia-Sastre and Biron, 2006).
Several viruses have evolved strategies to evade Type I IFN signaling at different stages of the pathway. Members of the family Paramyxoviridae block IFN signaling at various steps (Young et al., 2000), such as by degradation of STAT-1 by simian virus 5 (Didcock et al., 1999), or inhibition of Janus kinase activation by Sendai virus (Komatsu et al., 2000). Nipah virus proteins C, V, and W bind STAT-1 in the cytoplasm or nucleus and keep it from being activated (Park et al., 2003). The adenovirus E1A protein blocks signaling upstream of the formation of ISGF-3 (Leonard and Sen, 1997). Herpes viruses also have evolved anti-interferon signaling activities. Human herpesvirus 8 synthesizes an IRF homolog (vIRF) that inhibits transcription activation, and VZV inhibits expression of STAT-1 (reviewed in (Samuel, 2001). Herpes simplex virus 1 (HSV-1) proteins γ134.5 and ICP0 inhibit interferon signaling by binding and activating protein phosphatase 1, reversing the block in translation caused by of PKR (Chou et al., 1995; He et al., 1997), and by preventing IRF3 nuclear accumulation, thereby preventing production of IFNβ (Melroe, DeLuca, and Knipe, 2004; Melroe et al., 2007), respectively.
HSV-1 is a widespread human pathogen that causes a lytic infection in the mucosal epithelial cells and a life-long latent infection in neurons, from which it can be reactivated. During lytic infection, over 80 gene products are expressed in a highly regulated temporal cascade, immediate early (IE) proteins include ICP0, ICP4, ICP22, ICP27, and ICP47 and begin to be expressed as soon as viral DNA enters the nucleus (Honess and Roizman, 1974; Honess and Roizman, 1975). IE proteins stimulate expression of early (E) proteins, which are expressed at 5–7 hours post infection and are are largely involved in viral DNA replication (Honess and Roizman, 1974). Late genes are divided into two categories: those that can be expressed without viral DNA replication and those for which DNA replication is required. These include glycoproteins and other structural proteins and are expressed by 8 hours post infection (Honess and Roizman, 1974).
Because the innate immune response promotes viral clearance, it is important for HSV-1 to have processes that inhibit these pathways in order to productively infect the epithelial cells, establish latent infection, and persist in neurons throughout the life of the host. In addition to the mechanisms outlined above, recent studies have shown that HSV-1 infection causes a decrease in steady-state STAT-1 phosphorylation in response to IFNα treatment (Chee and Roizman, 2004; Yokota et al., 2001; Yokota et al., 2005). The virion host shutoff protein (VHS, also known as UL41) (Chee and Roizman, 2004) and UL13, both tegument proteins, have been implicated in the decreased sensitivity to IFNα (Yokota et al., 2005), and the mechanism of the decrease has been proposed to be either induction of suppressor of cytokine signaling 3 (SOCS-3) expression (Yokota et al., 2005) or degradation of the type I interferon receptor (Chee and Roizman, 2004). Here we show that ICP27 is necessary to decrease STAT-1 phosphorylation and sufficient for at least a partial block in STAT-1 translocation into the nucleus.
Results
Inhibition of IFNα-induced accumulation of phospho-STAT-1 by HSV-1 requires viral gene expression
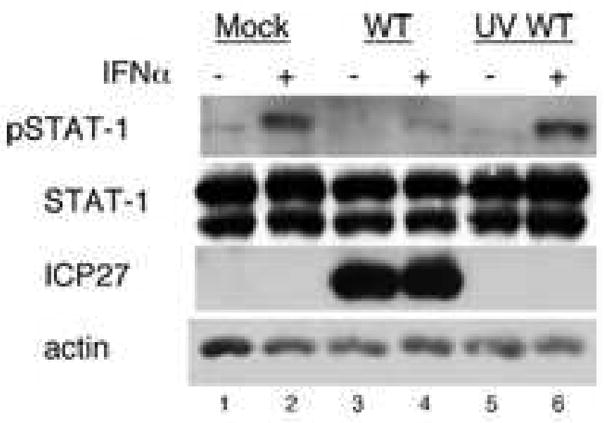
Some studies have reported that the virion tegument proteins UL13 and UL41 account for the HSV-induced inhibition of IFNα-induced STAT-1 phosphorylation (Chee and Roizman, 2004; Yokota et al., 2004), these data did not explain the requirement for gene expression (Yokota et al., 2001) and the early kinetics of the inhibition of IFNα-induced STAT-1 phosphorylation. To determine if viral tegument proteins were sufficient for the inhibition of IFNα signaling in our cell system, we mock-infected or infected Vero cells with WT HSV-1 or UV-treated HSV-1 at an MOI of 20 (titer of UV-treated virus before inactivation) for 10 hours. Cells were treated as indicated with 104 U/mL IFNα. Mock-infected cells showed a strong phospho-STAT-1 response to IFNα treatment (Figure 1, lane 2). HSV-1-infected cells showed expression of the immediate early protein ICP27 (Figure 1, lanes 3–4) and a very weak phospho-STAT-1 response to IFNα (Figure 1, lane 4). Cells infected with UV-inactivated WT HSV-1 did not express ICP27 (Figure 1, lanes 5–6) and had a strong phospho-STAT-1 response to IFNα treatment (Figure 1, lane 6), similar to that of mock infected cells. These results argued that though one or more tegument proteins may be involved in the inhibition of IFNα-induced STAT-1, they are not sufficient for this effect without viral gene expression.
Figure 1.
Viral gene expression is required for the inhibition of IFN induced STAT-1 phosphorylation by HSV-1. Vero cells were mock-infected or infected with WT or UV-inactivated WT virus at an MOI of 20 (MOI before UV treatment) for 10 hours and treated with IFNα as indicated. Western blot analysis was done with antibodies to pSTAT-1, STAT-1, ICP27, and actin.
HSV-1 infection inhibits phosphorylation of STAT-1 as early as 2 hours post infection
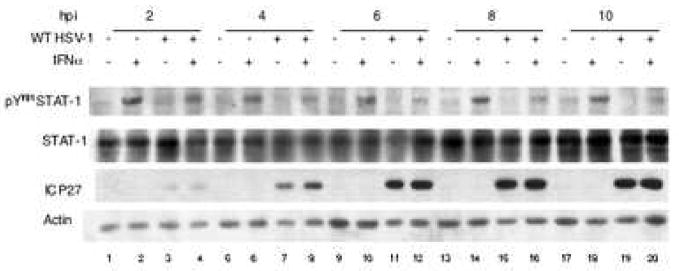
To determine the kinetics of the downregulation of the Jak/STAT pathway by HSV-1, we mock-infected or infected Vero cells with the WT strain of HSV-1 for 2, 4, 6, 8, or 10 hours and examined STAT-1 phosphorylation in response to IFNα treatment. Mock-infected cells consistently showed a strong STAT-1 phosphorylation response to IFNα treatment (Figure 2, lanes 2, 6, 10, 14, 18). However, STAT-1 phosphorylation in HSV-infected cells was decreased as compared to mock-infected cells at 2 hours post infection (Figure 2, lane 4) and continued to decrease further by 4 hours post infection (Figure 2, lane 8), after which it remained relatively constant.
Figure 2.
HSV-1 infection caused reduced phosphorylation of STAT-1 as early as 4 hours post infection. Cells were mock-infected or infected with KOS for 2, 4, 6, 8, or 10 hours, and treated with IFNα, as indicated. Western blot analysis was done with antibodies to pSTAT-1, STAT-1, ICP27, and actin.
From these results we concluded that the inhibition of the Jak/STAT pathway occurred at early times post infection, suggesting that the viral protein or proteins required for the inhibition of STAT-1 phosphorylation belong to the immediate-early or early temporal class.
ICP27 is required for inhibition of IFNα signaling
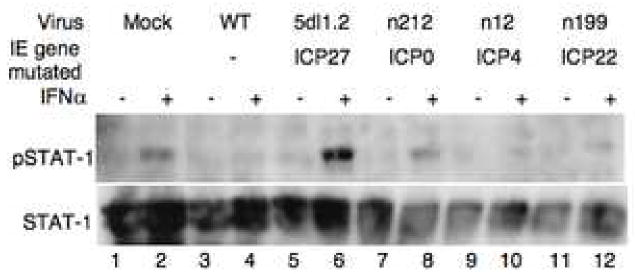
To determine which, if any, immediate-early protein was required for the inhibition of the Jak/STAT pathway by HSV-1, we infected cells with WT or mutant viruses defective for IE genes (Table 1). Mock-infected cells showed a robust STAT-1 phosphorylation response to IFNα treatment (Figure 3, lane 2) whereas cells infected with WT HSV-1 showed very little IFNα induced STAT-1 phosphorylation (Figure 3, lane 4). Cells infected with 5dl1.2, a virus defective for ICP27, showed a stronger induction of STAT-1 phosphorylation in response to IFNα treatment than mock-infected cells (Figure 3, lane 6), indicating that ICP27 was required for inhibition of STAT-1 phosphorylation. Cells infected with n12 or n199 (ICP4 null and ICP22 null, respectively) virus showed very little STAT-1 phosphorylation in response to IFNα (Figure 3, lanes 10, and 12, respectively), indicating that ICP4 and ICP22 were not required. In some experiments, cells infected with the ICP0 mutant virus, n212, showed an intermediate reduction of STAT-1 phosphorylation in response to IFNα (Figure 2, lane 8) when compared with mock-infected cells, indicating that ICP0 may play a partial role in the viral effect on STAT-1 phosphorylation.
Table 1.
HSV-1 Mutant Viruses Defective for Immediate-Early Genes
| Mutant Virus | IE gene mutated | Reference |
|---|---|---|
| 5dl1.2 | ICP27 | (McCarthy, McMahan, and Schaffer, 1989) |
| n12 | ICP4 | (DeLuca and Schaffer, 1988) |
| n212 | ICP0 | (Cai and Schaffer, 1989) |
| n199 | ICP22 | (Rice and Knipe, 1988) |
Figure 3.
ICP27 is necessary to block STAT-1 phosphorylaton in response to IFNα. Cells were infected with viruses with mutations in immediate early genes (see table 1) for 10 hours and treated with IFNα for 30 minutes prior to lysis. Western blot analysis was done with antibodies to pSTAT-1, and STAT-1.
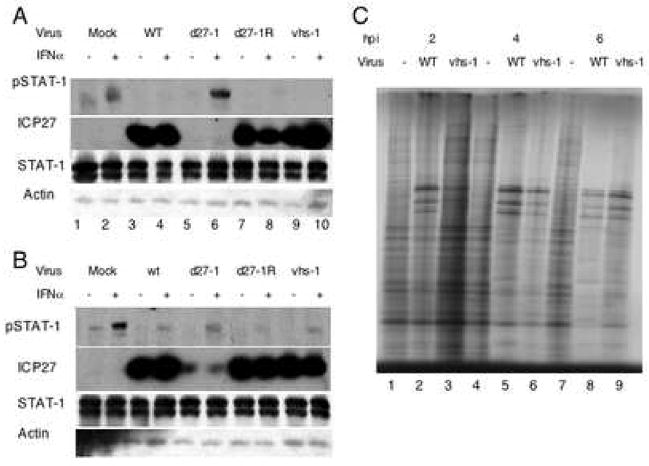
To confirm the involvement of ICP27, we mock-infected or infected Vero or V27 cells with the WT HSV-1 strain KOS1.1, d27-1, or the rescued virus d27-1R. Mock-infected Vero and V27 cells showed increased phosphorylation of STAT-1 after IFNα treatment (Figure 4A lane 2, and figure 4B lane 2). WT or d27-1R virus infection of either cell type showed a dramatic decrease in IFNα-induced STAT-1 phosphorylation (Figure 4A lanes 4 and 8, 4B lanes 4 and 8). Infection with d27-1 resulted in very strong induction of STAT-1 phosphorylation in Vero cells (Figure 4A lane 6) and a much weaker induction in V27 cells (Figure 4B lane 6). The intermediate phospho-STAT-1 signal in d27-1 infected V27 cells could be due to the relatively low levels of ICP27 expressed (Figure 4B lanes 5–6) when compared with the levels of ICP27 from WT and d27-1R infection in these cells (Figure 4B lanes 2–3, 7–8). These data indicated that ICP27 was required directly or indirectly for the downregulation of STAT-1 phosphorylation in response to IFNα treatment during HSV-1 infection.
Figure 4.
ICP27 but not the host shutoff function of vhs is required for HSV-1 inhibition of the Jak/STAT pathway. Vero (A) and V27 (B) cells were mock-infected or infected with with the WT HSV-1 KOS1.1 strain, d27-1, d27-1R, or VHS. 30 minutes before harvest, samples were treated with IFNα at 104U/mL (even lanes). Western blot analysis was done with antibodies to pSTAT-1, STAT-1, ICP27, and actin. To ensure the defect in host shut-off of vhs-1, cells were mock infected or infected with KOS (wt) or vhs-1 for 2, 4, or 6 hours, then incubated with [35S]-Met for 30 min. Shown (C) is an autoradiograph of the cell lysates separated on a 9.5% polyacrylamide gel.
A role for the vhs function in the block of the Jak/STAT pathway has been proposed (Chee and Roizman, 2004; Yokota et al., 2004). To determine if the host shut-off function of vhs was required for the inhibition of IFNα-induced STAT-1 phosphorylation, we mock-infected or infected Vero and V27 cells with WT virus or vhs-1 mutant virus. At 10 hpi, cells were treated with IFNα as indicated. In both Vero and V27 cells infected with the VHS mutant virus, IFNα-induced STAT-1 phosphorylation was blocked to WT-virus infection levels (Figure 4A lanes 3–4, 9–10, 3B lanes 3–4, 9–10). The vhs-1 mutant virus did exhibit a defect in host protein synthesis shut-off (Figure 4C, lanes 3, 6, 9), as shown originally (Read and Frenkel, 1983). These data indicated that host shutoff is likely not required for the inhibition of the Jak/STAT pathway for this strain of HSV-1 in Vero cells.
ICP27 is necessary for the inhibition of nuclear accumulation of STAT-1 in response to IFNα
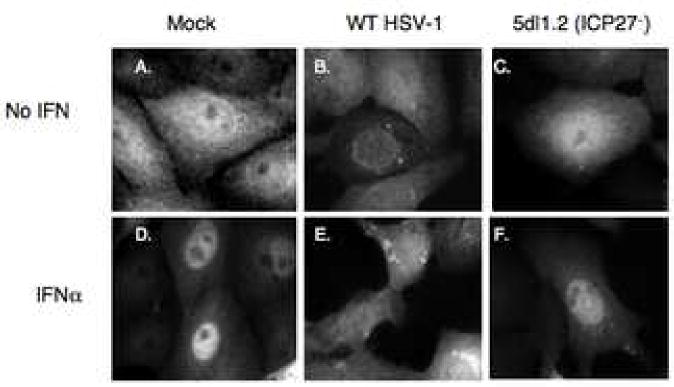
To determine the stage at which HSV-1 and ICP27 acted to inhibit IFNα-signaling, we examined the nuclear translocation of STAT-1. In response to IFNα treatment, STAT-1 translocated from a diffuse cytoplasmic and nuclear distribution to an almost completely nuclear localization in mock-infected cells (Figure 5, panels A, D). In WT virus-infected cells, STAT-1 remained cytoplasmic in both untreated and IFNα treated cells (Figure 5, panels B, E) and appeared to form perinuclear spots for both conditions. Cells infected with 5dl1.2 (ICP27−) virus showed nuclear translocation of STAT-1 (Figure 5, panels C and F) similar to that seen after mock infection. These data indicated that ICP27 was necessary not only for the decrease in IFNα-induced steady-state STAT-1 phosphorylation by HSV-1 but also for the inhibition of STAT-1 nuclear accumulation.
Figure 5.
ICP27 is necessary to block the nuclear translocation of STAT-1 in response to IFNα. Vero cells were mock-infected or infected with KOS or 5dl1.2 virus at an MOI of 20 for 10 hours. At 30 minutes prior to fixation, cells were treated with 104U/mL IFNα. Immunofluorescence was done with a polyclonal rabbit antibody to Stat-1 and alexafluor 488 anti-rabbit secondary antibodies. Immunofluorescence images are shown.
ICP27 is sufficient to block nuclear translocation of STAT-1 in response to IFNα
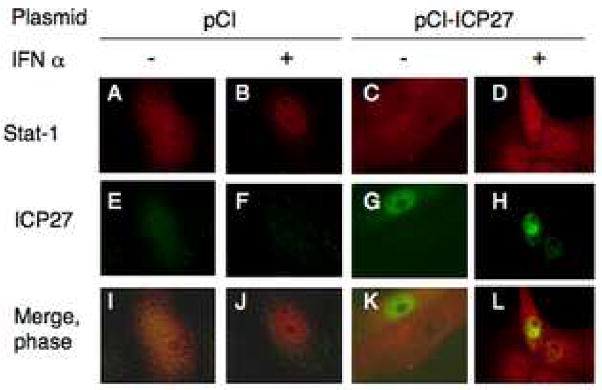
We observed that ICP27 was necessary for the HSV-1-induced block in STAT-1 activation and nuclear translocation. To determine if ICP27 was sufficient to block nuclear translocation of STAT-1, we transfected Vero cells with empty vector plasmid (pCI) or pCI-ICP27 plasmid and then treated the cells with IFNα. In cells transfected with pCI, IFNα treatment caused STAT-1 to redistribute from diffuse localization throughout the cell (Figure 6, panels A, I) to almost exclusively nuclear localization (Figure 6, panels B, J). In cells that expressed ICP27, ICP27 staining was mostly nuclear (Figure 6, panels G, H, K, L), as in previous reports (Rice and Knipe, 1990), and STAT-1 staining was diffuse throughout the cell regardless of IFNα treatment (Figure 6, panels C, D, K, L) in 100% of cells expressing transfected ICP27 (n=236). Many cells that did not express ICP27 showed nuclear staining for STAT-1 but several that did not obviously express ICP27 had more diffuse STAT-1 staining (Figure 6, panels D, H, and L). These data argued that ICP27 is sufficient for at least a partial block of the Jak/STAT pathway.
Figure 6.
ICP27 expression is sufficient to block nuclear translocation of STAT-1. Vero cells were transfected with pCI (A, B, E, F, I, J) or with pCI-ICP27 (C, D, G, H, K, L). After 24 hours, cells were treated with 104 U/mL human IFNα (B, D, F, H, J, L) for 30 minutes. Cells were immunostained with mouse monoclonal antibodies to ICP27 and rabbit polyclonal antibodies to STAT-1, and alexafluor 488 anti-mouse and alexafluor 594 anti-rabbit secondary antibodies. Immunofluorescenec images are shown.
Discussion
HSV-1 has several mechanisms to evade the effects of Interferon signaling, including inhibition of IFNβ production by blocking IRF-3 nuclear accumulation (Melroe, DeLuca, and Knipe, 2004), counteracting the effects of PKR activity through the activation of protein phosphatase 1 by γ134.5 (Chou et al., 1995; He et al., 1997), and inhibition of IFNα-induced STAT-1 phosphorylation (Yokota et al., 2001). In this study we show that the effects of HSV-1 on Jak/STAT signaling occur early in infection, that ICP27 is necessary for the inhibition of both the phosphorylation and nuclear accumulation of STAT-1, and that ICP27 is sufficient for at least a partial block in nuclear accumulation of STAT-1.
ICP27 is necessary for the inhibition of IFNα-induced STAT-1 phosphorylation by HSV-1
Consistent with previous reports (Yokota et al., 2001), our data show that IFNα-induced phosphorylation of STAT-1 is blocked as early as two hours post infection. Expression of HSV-1 proteins has been shown to be important to block Jak/STAT signaling (Yokota et al., 2001); therefore, we can conclude that the viral proteins involved in this inhibition belong to the immediate-early or early temporal classes. From the results of our tests of IE mutant viruses, ICP27 is required for the decrease in phosphorylated STAT-1, indicating a role for ICP27 in the inhibition of the Jak/STAT pathway by HSV-1. This is consistent with a recent study in which ICP27 was shown to be necessary for HSV-1 to cause a decrease in cytokine production by infected cells (Melchjorsen et al., 2006), as many cytokine genes are induced in response to IFNα signaling (Der et al., 1998). The possibility of a role for ICP27 in the inhibition of the Jak/STAT pathway was discussed by Chee and Roizman in conjunction with their studies using a vhs mutant virus (Chee and Roizman, 2004) as ICP27 has been shown to aid in the shut off of host cell protein expression (Hardwicke and Sandri-Goldin, 1994; Sacks et al., 1985). We propose that the role for ICP27 is independent of the role in host protein synthesis shut-off as the experiments we have done with a vhs mutant virus showed inhibition of IFNα induced STAT-1 phosphorylation to similar to that induced during WT-infection.
The reduced STAT-1 phosphorylation response to IFNα treatment in cells infected with the ICP0 mutant could indicate that ICP0 is also involved in the inhibition of the Jak/STAT pathway. ICP0 has been shown to have many activities to counteract various stages of IFN signaling (Halford et al., 2006; Melroe, DeLuca, and Knipe, 2004; Mossman, 2005; Sobol and Mossman, 2006) but because ICP27 expression is sufficient to inhibit IFNα-induced nuclear accumulation of STAT-1, it seems that any role ICP0 might play in this process is secondary to that of ICP27.
Inhibition of type I IFN signaling by HSV-1 occurs with very limited viral gene expression
Because ICP27 has several reported functions in viral replication and many binding partners, it was important to determine if ICP27 was sufficient for inhibition of type I IFN signaling. Our transfection studies with an ICP27 expression vector showed at least a partial block in STAT-1 translocation into the nucleus of ICP27 expressing cells. We were not able to determine the phosphorylation state of STAT-1 in cells transfected with ICP27 by Western blot because of the low frequency of transfected cells that expressed ICP27. During infection, ICP27 shuttles between the nucleus and cytoplasm but is predominantly nuclear (Ackermann et al., 1984; Knipe et al., 1987; Wilcox et al., 1980). Because ICP27 expression blocks nuclear accumulation of STAT-1, it seems unlikely that the mechanism by which ICP27 blocks Jak/STAT signaling occurs by a physical interaction between ICP27 and STAT-1. In transfected cells that showed cytoplasmic ICP27 localization, STAT-1 was also largely cytoplasmic, so it is unlikely that ICP27 is binding and sequestering in the nucleus a factor in the Jak/STAT pathway upstream of Stat activation.
ICP27 has been shown previously to affect the phosphorylation state of viral (Xia, DeLuca, and Knipe, 1996) and cellular proteins (Fraser and Rice, 2005; Kim and DeLuca, 2002; Koffa et al., 2003; Sciabica, Dai, and Sandri-Goldin, 2003), though few mechanisms have yet been determined. ICP27 inhibits splicing of cellular mRNAs by recruiting a normally cytoplasmic kinase, SRPK1 to the nucleus and causing the hypo-phosphorylation of the SR proteins, thus rendering them less active (Sciabica, Dai, and Sandri-Goldin, 2003). It is possible that ICP27 is recruiting one or both of the Janus kinases to the nucleus, which would result in the inhibition of the Jak/STAT pathway and, because correct Tyk-2 expression and localization is important for IFNAR localization to the plasma membrane (Ragimbeau et al., 2003), it is possible that this would result in the internalization of IFNAR.
In conclusion, we have shown that ICP27 is necessary for the inhibition of Type I interferon signaling by HSV-1 and is sufficient for at least a partial block in the nuclear accumulation of STAT-1. Further investigation of the role of ICP27 in the inhibition of IFNα signaling should provide additional insight into the mechanisms of innate immune system evasion by HSV-1.
Materials and Methods
Cells and Viruses
Vero and V27 cells (Vero cells stably transfected with the ICP27 gene) which is expressed following HSV-1 infection (Rice and Knipe, 1990) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco-BRL) supplemented with 5% heat-inactivated fetal calf serum (FCS) and 5% heat-inactivated newborn calf serum (BCS).
Viral stocks were grown in the appropriate complementing cell lines (the HSV-1 WT KOS and KOS1.1 strains, d27-1R, n199 and vhs-1 (Read and Frenkel, 1983) were grown on Vero cells, 5dl1.2 (McCarthy, McMahan, and Schaffer, 1989) and d27-1 (Rice and Knipe, 1990) on V827 cells, d106 and n12 on E11 cells (DeLuca and Schaffer, 1988; Samaniego, Wu, and DeLuca, 1997), and n212 on U2OS cells (Cai and Schaffer, 1989). Viral titers were determined by plaque assay in Vero cells or the indicated complementing cell line as described (Knipe and Spang, 1982).
For infection experiments, viruses were used at an MOI of 20 to infect Vero and V27 cells, which were incubated at 37°C in PBS with 1% BCS, 0.1% glucose for 1 h before removal of the inoculum and addition of DMEM supplemented with 1% FCS.
For UV inactivation, WT virus was diluted to 108 pfu/mL in PBS and exposed to shortwave ultraviolet light for 30 min prior to infection. Viral titer was reduced by 4 logs. Infections with UV-inactivated virus was based on titer before UV-irradiation.
Plasmids and transfections
Plasmid pCI was obtained from Promega. Plasmid pCI-ICP27 has been described previously (Olesky et al., 2005). At 24 hours before transfection, Vero cells were seeded into 6-well plates containing glass coverslips for fluorescence experiments. Transfections were carried out using Opti-MEM media (Gibco) and Genejuice reagent (Novagen) according to the manufacturer’s instructions.
Western blots
Mock-infected or infected cells were harvested from 25-cm2 flasks in 400 μL of SDS sample buffer (62.5mM Tris HCl pH 6.8, 20%Glycerol, 2% SDS, 0.1% bromophenol blue, 10mM β-glycerophosphate, 5 mM sodium fluoride, 1mM sodium vanadate, 0.5% β-mercaptoethanol) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in 12% bis-crosslinked polyacrylamide gels and electrically transferred to nitrocellulose in transfer buffer (25mM Tris, 192mM Glycine, 20% Methanol) overnight at 4°C with the Bio-Rad Transblot system. Membranes were probed with antibodies to STAT-1 (1:1000), pSTAT-1 (1:500), actin (1:200) from Santa Cruz Biotechnology, Inc, or ICP27 (H1119 at 1:10,000) from Virusys in PBST (0.5% Tween 20 in PBS from Gibco), washed twice for 5 min in PBST, and incubated with secondary antibodies conjugated to horseradish peroxidase (HRP) diluted 1:5000–1:20,000 from Santa Cruz. Biotechnology, Inc. HRP activity was detected using Western Lightening chemiluminescence reagent (PerkinElmer) and exposed on X-ray film (Kodak).
Antibodies and immunofluorescence
Secondary antibodies conjugated to Alexa 594 and Alexa 488 dyes were obtained from Molecular Probes Inc.
Vero cells were seeded for immunofluorescence at 5 × 105 cells/well on glass coverslips in 6-well plates and incubated overnight at 37°C before infection or transfection. Following incubation for the appropriate time, cells were fixed in 3.7% formaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature, and cells were permeabilized by incubation in methanol at −20°C for 2 min. Following several washes in PBS, cells were blocked overnight in IF buffer (PBS containing 2.5% goat serum [Sigma]). Primary antibodies were diluted appropriately (STAT-1 1:50, ICP27 1:750) and applied to cells in PBS, and the cells were incubated for 45 min at 37°C. Cells were washed twice for 5 min in PBS. Secondary antibodies were applied at 1:1,000 in PBS for 30 min at 37°C. Cells were then washed twice for 10 min in PBS at room temperature, and coverslips were mounted with Prolong antifade reagent (Molecular Probes, Inc.).
Slides were viewed with an Axioplan 2 microscope (Zeiss) with a 63x objective and a 10x ocular objective. Images were collected with the Axiovision 4.5 suite of programs (Zeiss) and a Hamamatsu C4742-95-12NR digital camera.
Labeling of proteins with [35S]-Methionine
Vero cells in 100 mm dishes were mock infected or infected with KOS or vhs-1 at an MOI of 20 for 2, 4, or 6 hours at which point the media was replaced with 3 mL cysteine/methionine-deficient media containing 300 μCi [35S]-Met per dish for 30 min. Cells were harvested, lysed in 500 μL of SDS sample buffer and 85 μL of each lysate was separated using SDS-PAGE on a 9.5% DATD-crosslinked polyacrylamide gel. The gel was dried and exposed on X-ray film for autoradiography.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackermann M, Braun DK, Pereira L, Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WZ, Schaffer PA. Herpes simplex virus type 1 ICP0 plays a critical role in the de novo synthesis of infectious virus following transfection of viral DNA. J Virol. 1989;63:4579–4589. doi: 10.1128/jvi.63.11.4579-4589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee VA, Roizman B. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at mutliple sites. J Virol. 2004;78:4185–4196. doi: 10.1128/JVI.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Chen JJ, Gross M, Roizman B. Association of a novel M190,000 phosphoprotein with PKR kinase in cells exhibiting enhanced phosphorylation of eIF-2alpha and premature shutoff of protein synthesis after infection with g-134.5− mutants of herpes simplex virus 1. Proc Nat Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca NA, Schaffer PA. Physical and functional domains of the herpes simplex virus transcriptional regulatory protein ICP4. J Virol. 1988;62:732–743. doi: 10.1128/jvi.62.3.732-743.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der SD, Zhou A, Williams BRG, Silverman RH. Idenification of genes differentially regulated by interferon alpha, beta or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95:15623–15628. doi: 10.1073/pnas.95.26.15623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didcock L, Young DF, Goodbourn S, Randall RE. Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser KA, Rice SA. Herpes simplex virus type 1 infection leads to loss of serine-2 phosphorylation on the carboxyl-terminal domain of RNA polymerase II. J Virol. 2005;79:11323–34. doi: 10.1128/JVI.79.17.11323-11334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–82. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Halford WP, Weisend C, Grace J, Soboleski M, Carr DJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol J. 2006;3(1):44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke MA, Sandri-Goldin RM. The herpes simplex virus regulatory protein ICP27 contributes to the decrease in cellular mRNA levels during infection. J Virol. 1994;68:4797–4810. doi: 10.1128/jvi.68.8.4797-4810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of gamma(1)34.5- herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol. 1997;71(8):6049–54. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macro-molecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis: Sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci U S A. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DB, DeLuca N. Phosphorylation of transcription factor Sp1 during herpes simplex virus type 1 infection. J Virol. 2002;76:6473–6479. doi: 10.1128/JVI.76.13.6473-6479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Senechek D, Rice SA, Smith JL. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987;61:276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Spang AE. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982;43:314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffa MD, Kean J, Zachos G, Rice SA, Clements JB. CK2 protein kinase is stimulated and redistributed by functional herpes simplex virus ICP27 protein. J Virol. 2003;77:4315–25. doi: 10.1128/JVI.77.7.4315-4325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T, Takeuchi K, Yokoo J, Tanaka Y, Gotoh B. Sendai virus blocks alpha interferon signaling to signal transducers and activators of transcription. J Virol. 2000;74:2477–80. doi: 10.1128/jvi.74.5.2477-2480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard GT, Sen GC. Restoration of interferon responses of adenovirus E1A-expressing HT1080 cell lines by overexpression of p48 protein. J Virol. 1997;71:5095–5101. doi: 10.1128/jvi.71.7.5095-5101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, McMahan L, Schaffer PA. Herpes simplex virus type 1 ICP27 deletion mutants exhibit altered patterns of transcription and are DNA deficient. J Virol. 1989;63:18–27. doi: 10.1128/jvi.63.1.18-27.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J, Siren J, Julkunen I, Paludan SR, Matikainen S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol. 2006;87(Pt 5):1099–108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- Melroe G, DeLuca N, Knipe DM. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. Journal of Virology. 2004;78:8411–20. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melroe GT, Silva L, Schaffer PA, Knipe DM. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: Potential role in blocking IFN-beta induction. Virology. 2007;360:305–21. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman K. Analysis of anti-interferon properties of the herpes simplex virus type I ICP0 protein. Methods Mol Med. 2005;116:195–205. doi: 10.1385/1-59259-939-7:195. [DOI] [PubMed] [Google Scholar]
- Olesky M, McNamee EE, Zhou C, Taylor TJ, Knipe DM. Evidence for a direct interaction between HSV-1 ICP27 and ICP8 proteins. Virology. 2005;331:94–105. doi: 10.1016/j.virol.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Park MS, Shaw ML, Munoz-Jordan J, Cros JF, Nakaya T, Bouvier N, Palese P, Garcia-Sastre A, Basler CF. Newcastle disease virus (NDV)-based assay demonstrates interferon-antagonist activity for the NDV V protein and the Nipah virus V, W, and C proteins. J Virol. 2003;77:1501–11. doi: 10.1128/JVI.77.2.1501-1511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC, Fish EN. Signaling pathways activated by interferons. Exp Hematol. 1999;27:1583–92. doi: 10.1016/s0301-472x(99)00109-5. [DOI] [PubMed] [Google Scholar]
- Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO Journal. 2003;22:537–47. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read GS, Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983;46:498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice SA, Knipe DM. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J Virol. 1988;62:3814–3823. doi: 10.1128/jvi.62.10.3814-3823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice SA, Knipe DM. Genetic evidence for two distinct transactivation functions of the herpes simplex virus alpha protein ICP27. J Virol. 1990;64:1704–1715. doi: 10.1128/jvi.64.4.1704-1715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks WR, Greene CC, Aschman DP, Schaffer PA. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J Virol. 1985;55:796–805. doi: 10.1128/jvi.55.3.796-805.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego LA, Wu N, DeLuca NA. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciabica KS, Dai QJ, Sandri-Goldin RM. ICP27 interacts with SRPK1 to mediate HSV splicing inhibition by altering SR protein phosphorylation. EMBO J. 2003;22:1608–19. doi: 10.1093/emboj/cdg166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol PT, Mossman KL. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J Virol. 2006;80:218–25. doi: 10.1128/JVI.80.1.218-225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KW, Kohn A, Sklyanskaya E, Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980;33:167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia K, DeLuca NA, Knipe DM. Analysis of phosphorylation sites of the herpes simplex virus 1 infected cell protein 4 (ICP4) J Virol. 1996;70:1061–1071. doi: 10.1128/jvi.70.2.1061-1071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Kubota T, Suzutani T, Yoshida I, Miura S, Jimbow K, Fujii N. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology. 2001;286:119–124. doi: 10.1006/viro.2001.0941. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virol. 2005;338:173–81. doi: 10.1016/j.virol.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Yokota S, Yokosawa N, Okabayashi T, Suzutani T, Miura S, Jimbow K, Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol. 2004;78:6282–6. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DF, Didcock L, Goodbourn S, Randall RE. Paramyxoviridae use distinct virus-specific mechanisms to circumvent the interferon response. Virology. 2000;269:383–90. doi: 10.1006/viro.2000.0240. [DOI] [PubMed] [Google Scholar]