Abstract
We have previously described differential effects of physiologic (5%) and pathologic (18%) cyclic stretch (CS) on agonist-induced pulmonary endothelial permeability. This study examined acute and chronic effects of CS on agonist-induced intracellular signaling and cell morphology in the human lung macro- and microvascular endothelial cell (EC) monolayers. Endothelial permeability was assessed by analysis of morphological changes, parameters of cell contraction and measurements of transendothelial electrical resistance. Exposure of both microvascular and macrovascular EC to 18% CS for 2 – 96 hrs increased thrombin-induced permeability and monolayer disruption. Interestingly, the ability to promote thrombin responses was present in EC cultures exposed to 48–96 hrs of CS even after replating onto non-elastic substrates. In turn, physiologic CS preconditioning (72 hrs) attenuated thrombin-induced paracellular gap formation and MLC phosphorylation in replated EC cultures. Long term preconditioning at 18% CS (72 hrs) increased the content of signaling and contractile proteins including Rho GTPase, MLC, MLC kinase, ZIP kinase, PAR1, caldesmon and HSP27 in the pulmonary microvascular and macrovascular cells. We conclude that short term CS regulates EC permeability via modulation of agonist-induced signaling, whereas long term CS controls endothelial barrier at both post-translational level and via magnitude-dependent regulation of pulmonary EC phenotype, signaling and contractile protein expression.
Keywords: cyclic stretch, cytoskeleton, pulmonary endothelium, permeability
INTRODUCTION
Pathologic lung over-distention caused by mechanical ventilation at high tidal volumes compromises the blood-gas barrier, increases lung permeability, and may culminate in ventilator-induced lung injury (VILI) and pulmonary edema [1, 2]. Vascular leak observed in VILI patients is also associated with increased levels of edemagenic agents and inflammatory cytokines such as thrombin, histamine, TNF-alpha, IL-8, and IL-1 in the lung [1, 3–5]. The significance of the interactions between the edemagenic agents and pathological mechanical distension of the lung tissue in progression of VILI-associated vascular leak and pulmonary edema becomes increasingly recognized. Therefore, two-hit animal models, which combine lung inflammation induced by pro-inflammatory agents and mechanical ventilation at high tidal volumes reflect more appropriately common co-morbidities and risk factors present in patients with acute lung injury [6]. Importantly, in vitro models of pulmonary cells exposed to pathologic or physiologic mechanical stimulation and edemagenic agonists may provide vital information about molecular mechanisms regulating lung endothelial or epithelial permeability in VILI patients.
Biomechanical forces acting on vascular endothelium stimulate a variety of signaling pathways including MAP kinase cascades (Erk-1,2, JNK, p38), non-receptor tyrosine kinases (p60Src, FAK), integrin-mediated signaling, and ion channels [7–16]. Small GTPases Rac and Rho directly regulate cytoskeletal reorganization and endothelial permeability and can be activated by mechanical stimuli of different origin and magnitude [17, 18]. We have recently shown the differential effects of physiologic and pathologic magnitudes of cyclic stretch (CS) applied to pulmonary endothelial cell (EC) monolayers on the agonist-induced EC barrier disruption [19, 20]. Consistent with differential effects on monolayer integrity, 18% CS enhanced thrombin-induced Rho activation, whereas 5% CS promoted Rac activation critical for EC recovery phase. These studies revealed critical roles of the amplitude of applied cyclic stretch on the Rac/Rho GTPase balance and mechano-chemical regulation of the lung EC barrier.
However, the role of long term exposure to physiologic or pathologic levels of cyclic stretch on pulmonary EC permeability responses, phenotypic expression and cell signaling relevant to more prolonged periods of mechanical ventilation in vivo remains to be investigated. Microarray analysis of mRNA expression profiles in endothelial cells exposed to various levels of cyclic stretch and shear stress reported by our group and others indicates significant effects of different types of mechanical stimulation on gene expression patterns [21–24].
This study directly tested effects of chronic CS preconditioning at physiologic and pathologic amplitudes on sustained changes in the pulmonary EC signaling, thrombin-induced permeability responses, and expression of contractile and signaling proteins.
MATERIALS AND METHODS
Cell culture and reagents
Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Rho, Rac, PAR2, and MLCK antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); di-phospho-MLC and HSP27 antibodies were obtained from Cell Signaling (Beverly, MA); β-catenin, ZIP kinase, and PAR1 antibodies were obtained from BD Transduction Laboratories (San Diego, CA). All reagents for immunofluorescence staining were purchased from Molecular Probes (Eugene, OR). Human pulmonary macro- and micro-vascular endothelial cells (HPAEC and HLMVEC, respectively) were obtained from Lonza Inc (Allendale, NJ), maintained according to the vendor’s protocol, and used at passages 5–8 for cyclic stretch experiments, as previously described [19, 21].
Cell culture under cyclic stretch
All cyclic stretch (CS) experiments were performed as previously described [19, 21] using FX-4000T Flexcell Tension Plus system (Flexcell International, McKeesport, PA) equipped with 25 mm BioFlex Loading station designed to provide uniform radial and circumferential strain across a membrane surface along all radii. BioFlex Loading station is composed of a single plate and six planar 25 mm cylinders per plate centered beneath each well of the BioFlex plate, and the top surface is just below the BioFlex membrane surface. Each BioFlex membrane is stretched over the post when under vacuum pressure, creating a single-plane uniformly stretched circle. The radial and circumferential strain was experimentally determined by vendor (Flexcell International) by stamping the BioFlex membrane with a dot pattern followed by labeling the distance between each pair of dots and measuring their change relative to vacuum levels. Experimental measurements demonstrated that the part of the membrane stretching over the post receives uniform strain in the radial direction that was proportional to vacuum level. In immunofluorescence experiments, microscopic fields in the range of one half - two thirds of radius distance from the center of the membrane were used for analysis of cytoskeletal changes. Experiments were performed in the presence of culture medium containing 2% fetal bovine serum. Briefly, endothelial cells (EC) were seeded at standard densities (8×105 cells/well) onto collagen I-coated flexible bottom BioFlex plates. After 48 hrs of culturing, each plate received fresh medium, was mounted onto the Flexcell system and exposed to either low magnitude (5% elongation) or high magnitude (18% elongation) cyclic stretch with frequency 15 cycles/min for various periods of time (2 to 96 hrs) to recapitulate the mechanical stresses experienced by the alveolar endothelium during normal respiration and high tidal volume mechanical ventilation, respectively [21, 25, 26]. At the end of CS preconditioning, cells were treated with thrombin with continuous exposure to cyclic stretch. Alternatively, to examine sustained changes in pulmonary EC phenotype and thrombin-induced signaling caused by long-term preconditioning at pathologic or physiologic CS, BioFlex plates with CS-preconditioned pulmonary macrovascular or microvascular EC were treated with trypsin, and cells were replated onto plastic dishes, microelectrodes, or glass coverslips. After 16 hrs in static culture to ensure cell spreading and monolayer formation, analysis of cell signaling, cytoskeletal rearrangements or changes in transendothelial resistance in response to thrombin were monitored as described below. Control BioFlex plates with static EC culture were placed in the same cell culture incubator and processed similarly to CS-preconditioned cells. The volumes of cell lysates obtained from the individual stretch plate well were equalized. Equal volumes of cell lysates from different experimental conditions were used for western blot analysis.
Measurement of transendothelial electrical resistance
Measurements of transendothelial electrical resistance (TER) across confluent HPAEC monolayers were performed using electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as previously described [27, 28].
Endothelial cell imaging
EC monolayers grown on glass coverslips were stimulated with agonist of interest, then washed with room temperature PBS twice and fixed in 3.7% paraformaldehyde solution in PBS for 10 min at room temperature followed by immunofluorescence staining with Texas Red-conjugated phalloidin to visualize actin filaments or/and β-Catenin antibody to visualize EC adherens junctions. After immunostaining, the glass slides were analyzed using a Nikon video-imaging system (Nikon Instech Co., Japan) consisting of a phase contrast inverted microscope Nikon Eclipse TE2000 connected to Hamamatsu (Hamamatsu Photonics K.K., Japan) digital camera and image processor. The images were recorded and processed using Adobe Photoshop 6.0 software.
Determination of intracellular ROS production
ROS generation in pulmonary EC was measured using Image-iTTM LIVE Green Reactive Oxygen Species Detection Kit (Molecular Probes, Inc. Eugene, OR) according to manufacturer’s recommendations. In brief, HPAEC grown on BioFlex plates were starved with EBM-2 medium containing 2% FBS for 2 h. Cells were pretreated with 10 μM 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) for 15 min. Next, cells were subjected to 18% cyclic stretch or left static as control for 2 hrs followed by thrombin stimulation (0.1 U/ml, 10 min). Then, cells were washed with medium once and subjected to fluorescence microscopy using Nikon video-imaging system (Nikon Inc, Tokyo, Japan). The 16-bit images were analyzed using Image J software (National Institute of Health, Washington, DC). At least 20 microscopic fields for each experimental condition were analyzed. The extent of ROS formation is expressed in arbitrary units of fluorescence intensity.
Immunoblotting
After stimulation cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane and probed with specific antibodies as previously described [21, 29]. The relative intensities of immunoreactive protein bands were quantified by scanning densitometry using Image Quant software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
Results are expressed as means ± SD of three to five independent experiments. Stimulated samples were compared with controls by unpaired Student’s t-test. For multiple-group comparisons, one-way analysis of variance (ANOVA) followed by the post hoc Fisher’s test were used. P<0.05 was considered statistically significant.
RESULTS
Effects of high magnitude cyclic stretch and thrombin on pulmonary EC remodeling and ROS production
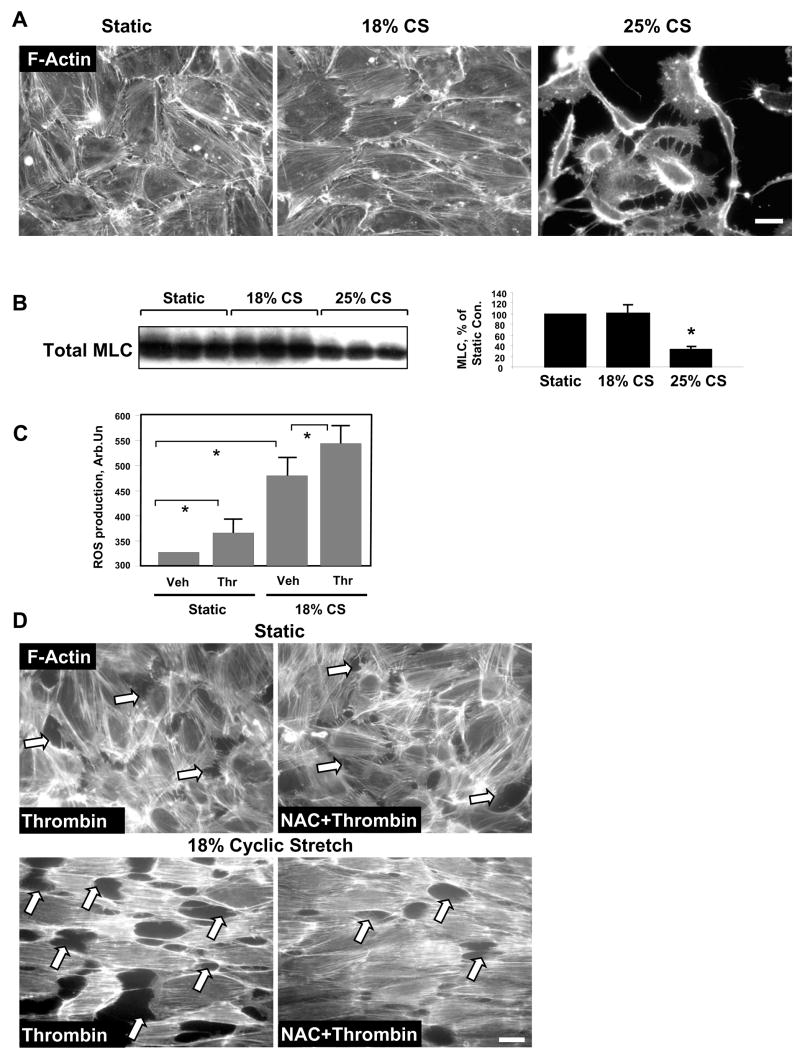
We and others have previously defined cyclic stretch of pulmonary EC at 18% elongation or 5% elongation as pathologically or physiologically relevant amplitudes, respectively [20, 21, 25, 26]. EC preconditioning at 18% CS neither considerably increased cell apoptosis rates, nor affected EC monolayer integrity [21]. We next tested effects of higher CS amplitude on EC remodeling and monolayer integrity. HPAEC were grown to confluence followed by exposure to 18% or 25% CS for 72 hrs. Elevation of CS amplitude to 25% caused disruption of cell-cell contacts and significant cell detachment (Figure 1A). These observations were confirmed by decreases in the total myosin light chain (MLC) content in cell lysates indicating increased cell detachment caused by 25% CS (Figure 1B). Based on these findings the following experiments were performed at 18% CS.
Figure 1. Effects of 18% and 25% CS on EC culture.
A: Endothelial monolayers grown on BioFlex plates were exposed to 18% or 25% CS for 72 hrs, or left under static conditions followed by immunofluorescene staining for F-actin. Scale bar = 10 μm. B: Total MLC protein levels were detected in static EC or in cells subjected to 18% or 25% CS for 72 hrs. Equal lysate volumes from each well were used for analysis. Results are representative of three independent experiments. C: ROS production was assessed in EC monolayers preconditioned under static conditions or exposed to 18% CS for 2 hrs followed by thrombin treatment (0.1 U/ml, 10 min). D: Pulmonary EC were pretreated with NAC (1 mM, 45 min) followed by CS preconditioning (18%, 2 hrs) and thrombin stimulation (0.1 U/ml, 10 min). Control cells were left under static conditions. Paracellular gap formation was analyzed by immunofluorescence staining for F-actin using Texas-Red phalloidin. Paracellular gaps are marked by arrows. Scale bar = 10 μm. Results are representative of three independent experiments.
Since reactive oxygen species (ROS) as second messengers may contribute to the activation of signal transduction cascades including Rho pathway [30, 31], we first investigated the potential synergistic mechanism of EC barrier disruption induced by thrombin and pathologic CS via ROS production. EC treatment with thrombin or preconditioning at 18% CS for 2 hrs significantly increased intracellular ROS levels monitored by DCFDA fluorescence. The combination of thrombin and 18% CS further increased ROS production (Figure 1C). Pretreatment with ROS scavenger N-acetyl cysteine (NAC) had no significant effects on thrombin-induced stress fiber and paracellular gap formation in static EC. In contrast, NAC pretreatment markedly attenuated enhancing effect of 18% CS preconditioning (2 hrs) on thrombin-induced paracellular gap formation in the pulmonary EC monolayers (Figure 1D). These results strongly suggest the involvement of ROS mechanism in synergistic effects of pathologic CS on the pulmonary EC barrier dysfunction induced by edemagenic agonists.
Effects of long term low and high magnitude cyclic stretch on thrombin-induced endothelial barrier disruption
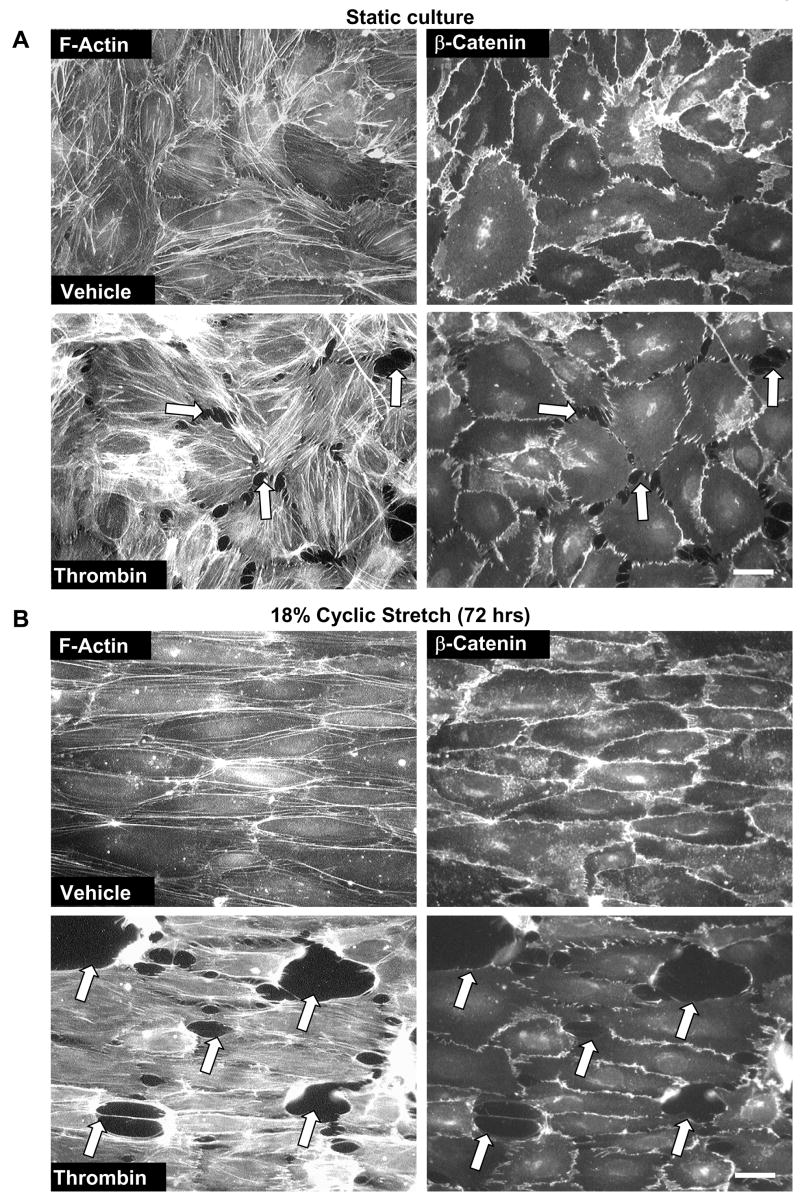
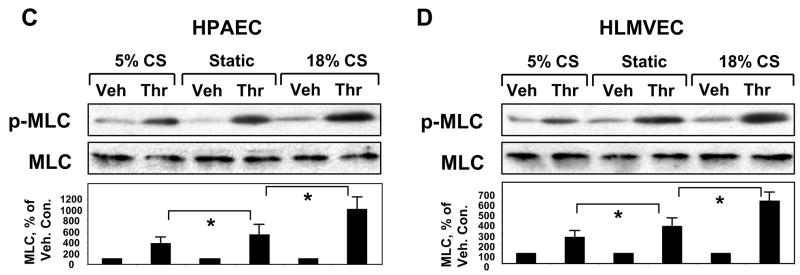
Our previous studies have shown promoting effects of acute (2 hrs) and long-term (24 hrs) pathologic CS on thrombin-induced EC barrier disruption [20, 21]. To further characterize a model of chronic lung overdistension, we tested effects of 18% CS preconditioning at a later time point (72 hrs). In agreement with previous studies, thrombin-induced cell retraction, formation of actin stress fibers and paracellular gaps observed in static EC cultures (Figure 2A) was further enhanced by 72-hr EC preconditioning at 18% CS (Figure 2B). Barrier-disruptive effects of thrombin on static and 18% CS-preconditioned pulmonary EC were also accompanied by increased loss of adherens junction continuity between the cells (Figure 2, right panels), which was monitored by immunofluorescence staining of EC monolayers with β-catenin antibody. Biochemical analysis of human pulmonary artery EC contractile response to thrombin showed increased levels of MLC phosphorylation, a marker of cell contraction, in CS-preconditioned cells (Figure 2C). In contrast, EC preconditioning at physiologic CS (5% elongation) for 72 hrs noticeably decreased levels of thrombin-induced MLC phosphorylation (Figure 2C). Because lung microvessels represent the major site of transvascular flux, and phenotypic differences between macro- and microvascular endothelium are well recognized [32], we next used human lung microvascular EC to characterize effects of CS on endothelial barrier regulation (Figure 2D). Similarly to experiments with pulmonary artery EC, CS preconditioning of lung microvascular EC at pathologic amplitude (18%) for 72 hrs significantly increased thrombin-induced MLC phosphorylation indicating elevated EC barrier compromise. In contrast, physiologic CS attenuated these effects in both, pulmonary macrovascular and microvascular endothelial cells.
Figure 2. Effects of long term CS on thrombin-induced cytoskeletal and adherens junction remodeling and paracellular gap formation.
Pulmonary EC grown to confluence on BioFlex plates were left under static conditions (A) or exposed to pathologic 18% CS for 72 hrs (B) followed by stimulation with thrombin (0.1 U/ml, 10 min). Immunofluorescence double staining was performed using Texas-Red phalloidin to detect F-actin and β-catenin to detect adherens junctions. Paracellular gaps are marked by arrows. Results are representative of three independent experiments. Scale bar = 10 μm. HPAEC (C) or HLMVEC (D) were subjected to 5% or 18% CS for 72 hrs or left static. Phosphorylated MLC was detected by immunoblotting with di-phospho-MLC specific antibodies. Quantitative analysis depicts relative levels of phosphorylated target proteins expressed as ratio of phospho-protein to the total protein in the same lysate. In some experiments, the levels of phosphorylated and total target proteins were detected by immunoblotting of two or more identically loaded membranes with corresponding antibody. Results are representative of three independent experiments.
Analysis of acute and long term effects of CS preconditioning on the modulation of EC response to thrombin
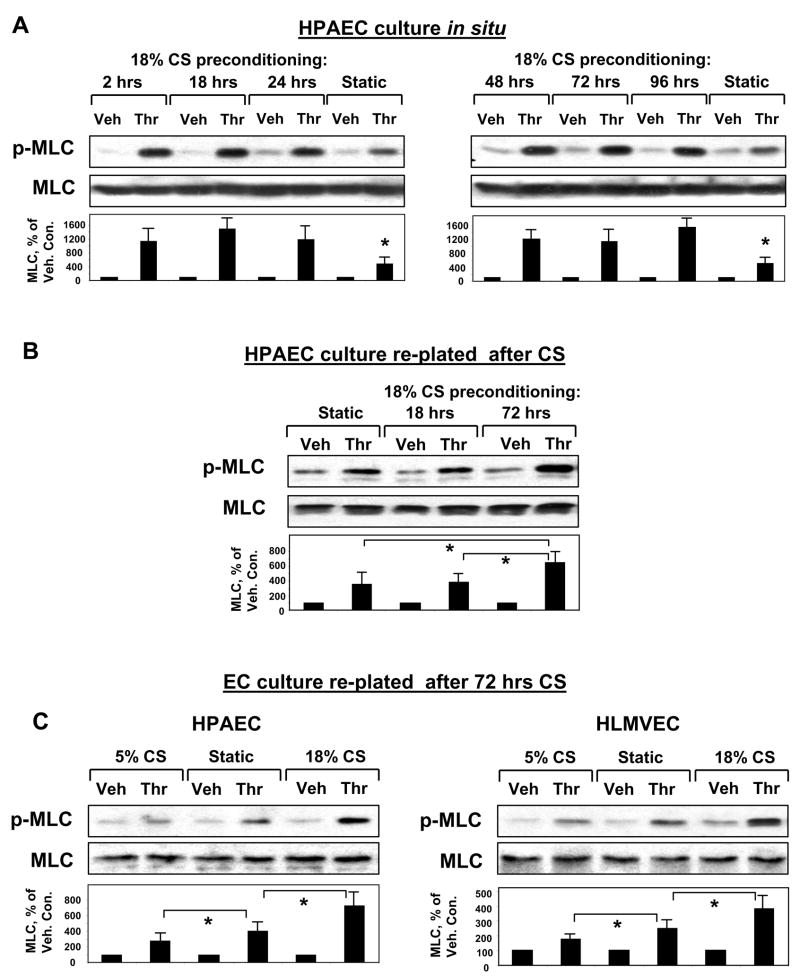
In the following experiments we investigated effects of duration of pathologic CS on its ability to modulate thrombin-induced MLC phosphorylation, which is an indicator of EC contraction and barrier compromise. First, pulmonary EC cultures were subjected to 18% CS for 2 hrs, 18 hrs, 24 hrs, 48 hrs, 72 hrs, and 96 hrs followed by thrombin challenge under continuing CS stimulation (Figure 3A). Enhancement of thrombin-induced MLC phosphorylation by 18% CS in EC cultures was detected at all time points of stretch preconditioning, as compared to static EC cultures. We next evaluated whether CS preconditioning may induce sustained changes in cellular signaling or EC contractile phenotype and therefore contribute to the mechanism of CS-induced potentiation of thrombin-induced EC permeability. In these experiments, after 72-hr exposure to 18% CS, pulmonary EC were detached from BioFlex plates by trypsinization and replated onto plastic culture dishes for further analysis (Figure 3B). Control static cells were grown in BioFlex plates without stretching and processed in a similar fashion. CS exposure to relatively short times (up to 18 hours) did not affect thrombin-induced MLC phosphorylation in replated EC cultures, as compared to non-stretched EC cultures. However, CS preconditioning for 72 hrs significantly increased MLC phosphorylation response to thrombin challenge (Figure 3B). To examine if the same trend exists for EC exposed to physiologic CS, we performed similar experiments in EC cultures preconditioned at 5% CS for 72 hrs (Figure 3C, left panels). Long term preconditioning of pulmonary EC at physiologic CS levels reduced the levels of phosphorylated MLC after thrombin stimulation, as compared to static replated cultures and EC subjected to high magnitude CS. Long term CS preconditioning of the microvascular endothelium revealed similar results (Figure 3C, right panels). Thrombin-induced MLC phosphorylation in HLMVEC replated after 72-hr exposure to 18% CS was increased, whereas in HLMVEC replated after exposure to 5% CS it was reduced.
Figure 3. Time-dependent effects of CS on thrombin-induced MLC phosphorylation in stretch-exposed EC and in replated CS-preconditioned EC cultures.
A: Human pulmonary EC were subjected to 18% CS for various periods of time followed by thrombin stimulation (0.1 U/ml, 10 min) with continuing CS stimulation. Analysis of MLC phosphorylation was performed using di-phospho-MLC specific antibodies. B: HPAEC exposed to 18 hrs or 72 hrs of 18% CS were replated onto plastic dishes, and thrombin-induced MLC phosphorylation was measured 16 hrs after replating. Quantitative analysis depicts relative levels of phosphorylated target proteins expressed as ratio of phospho-protein to the total protein in the same lysate. In some experiments, the levels of phosphorylated and total target proteins were detected by immunoblotting of two or more identically loaded membranes with corresponding antibody. Results are representative of three independent experiments. C: Comparison of thrombin-induced MLC phosphorylation in static or CS-preconditioned (5% or 18%, 72 hrs) macro- and micro-vascular pulmonary EC. Results are representative of three independent experiments.
Amplitude-dependent effects of chronic cyclic stretch preconditioning on thrombin-mediated cytoskeletal remodeling in replated endothelial cells
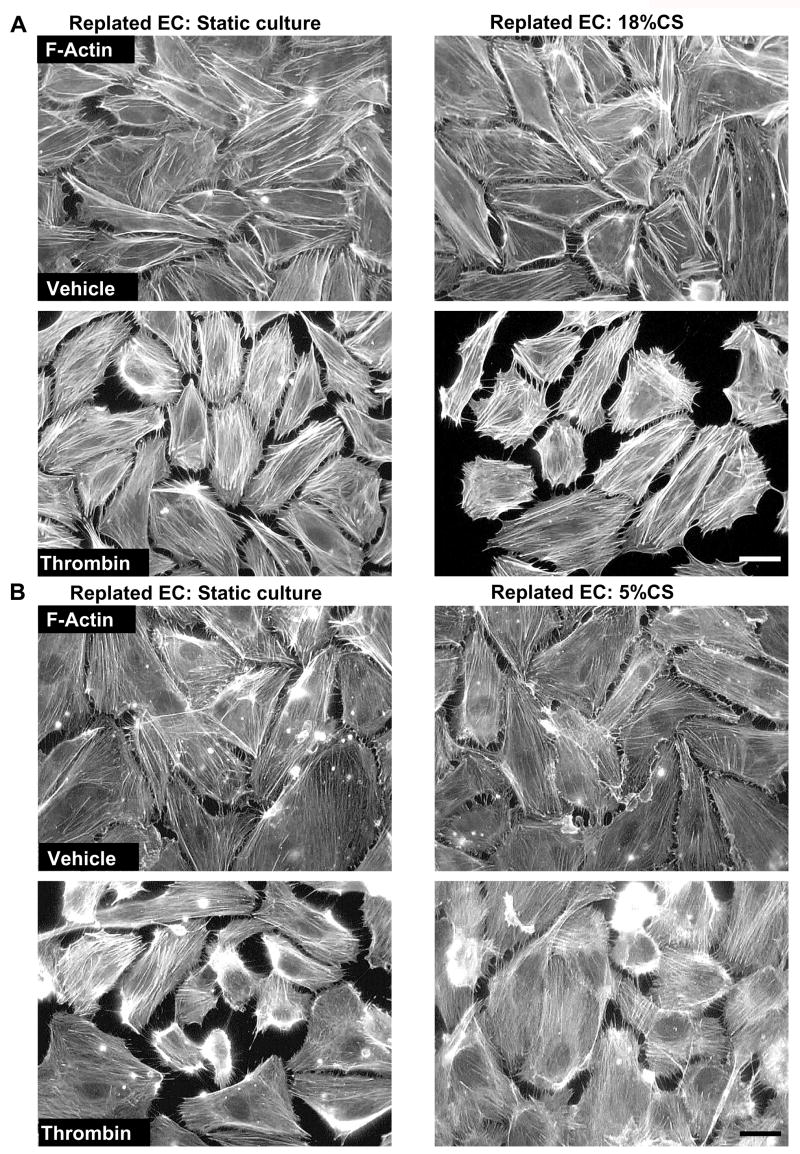
To further evaluate the ability of long term CS preconditioning to induce sustained changes in cellular physiological responses to agonists, we studied effects of chronic CS preconditioning on thrombin-induced cytoskeletal remodeling in replated HPAEC cultures. Cells were exposed to 18% CS, 72 hrs or left under static conditions followed by replating onto plastic dishes with glass coverslips. Although both, static EC cultures and cells preconditioned at 18%CS showed similar morphology and actin cytoskeletal arrangement 16 hrs after replating (Figure 4A, top panels), thrombin challenge caused much higher levels of stress fiber formation, cell retraction and even cell detachment in EC preconditioned at 18% CS, as compared to replated static controls (Figure 4A, bottom panels). In contrast, in EC replated after physiologic CS preconditioning (5% CS, 72 hrs), disruptive effects of thrombin on endothelial monolayer and stress fiber formation were markedly reduced (Figure 4B).
Figure 4. Effect of CS preconditioning on thrombin-induced cytoskeletal remodeling in replated EC.
Human pulmonary EC monolayers were subjected to CS preconditioning at 18% (A) or 5% (B) for 72 hrs followed by replating onto glass coverslips. Cells were stimulated with thrombin (0.1 U/ml, 10 min). Immunofluorescence staining using Texas-Red phalloidin was performed to detect F-actin. Scale bar = 10 μm. Results are representative of three to five independent experiments.
Amplitude-dependent effects of chronic cyclic stretch preconditioning on thrombin-induced permeability in replated EC
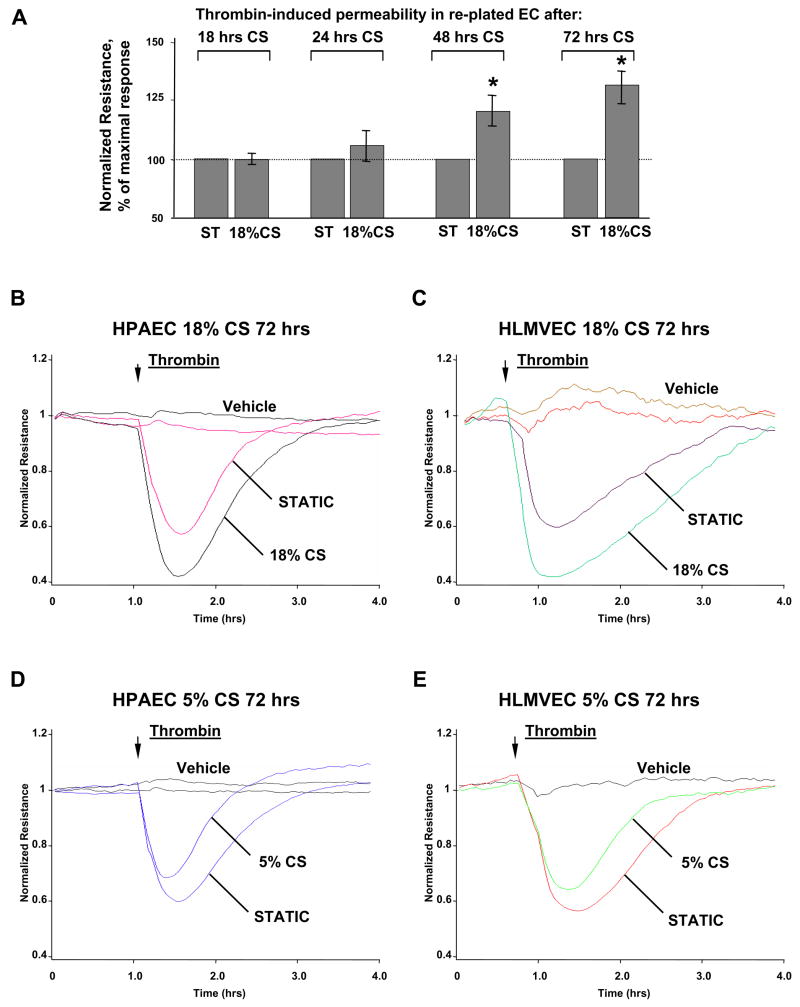
To test sustained effects of CS preconditioning on thrombin-induced permeability responses, pulmonary EC grown on BioFlex plates were subjected to CS for various periods of time followed by replating onto gold microelectrodes for permeability measurements. In the first series of experiments, permeability responses to thrombin were analyzed in EC replated after preconditioning at 18% CS for 18, 24, 48, or 72 hrs (Figure 5A). CS preconditioning during 18–24 hrs did not change permeability response to thrombin in replated cells, as compared to non-stretched controls. Remarkably, CS preconditioning during 48 and 72 hrs caused significant enhancement of thrombin-induced EC permeability response. The next experiments compared effects of chronic (72 hrs) preconditioning of macro- and microvascular EC monolayers at physiologically and pathologically relevant CS amplitudes on thrombin-induced permeability. Our results show that chronic 18% CS preconditioning enhanced thrombin-induced drop in TER reflecting increased permeability in replated HPAEC and HLMVEC and also delayed barrier recovery phase (Figure 5BC). In contrast, 5% CS preconditioning attenuated acute phase of thrombin-induced permeability response and accelerated barrier recovery phase in replated EC monolayers (Figure 5DE).
Figure 5. Effect of CS preconditioning on thrombin-induced permeability in replated EC.
A: HPAEC were subjected to CS preconditioning at 18% for indicated periods of time followed by replating onto gold microelectrodes. EC were treated with either vehicle or thrombin (0.1 U/ml) and used for measurements of transendothelial electrical resistance. HPAEC (B) or HLMVEC (C) were subjected to CS preconditioning at 18% for 72 hrs followed by replating onto microelectrodes. At the time indicated by the arrow EC were treated with either vehicle or thrombin, and transendothelial electrical resistance was monitored over the time. HPAEC (D) or HLMVEC (E) were subjected to CS preconditioning at 5% for 72 hrs followed by replating onto micro electrodes. At the time indicated by the arrow, EC were treated with either vehicle or thrombin, and transendothelial electrical resistance was monitored over the time. Results are representative of three to seven independent experiments.
Effects of long term cyclic stretch on protein expression
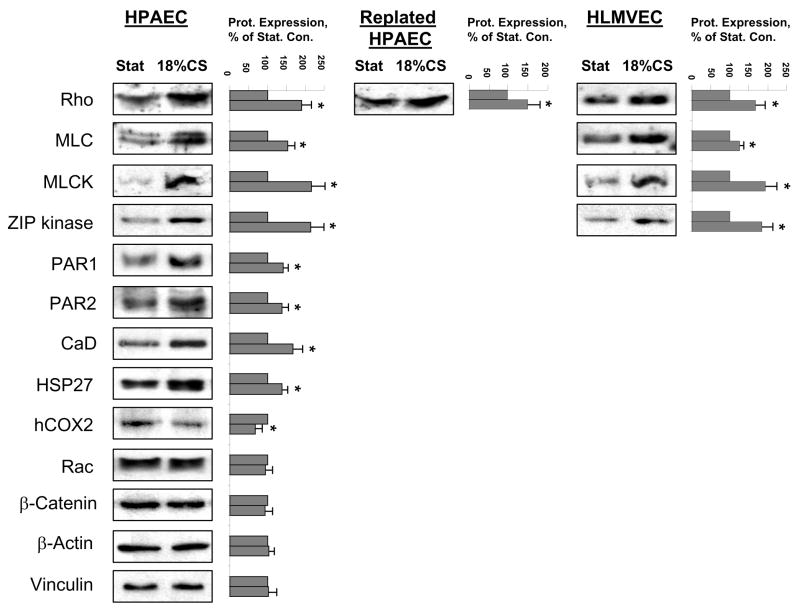
Mechanical stimulation is an important regulator of gene expression in many cell types including vascular endothelium. To link the ability of 18% CS to induce stable changes in cellular signaling and cytoskeletal response to thrombin with changes in EC phenotype, we analyzed expression of potential signaling and cytoskeletal targets involved in EC barrier regulation at protein level. After EC exposure to 72 hrs of 18% CS, expression of target proteins was analyzed by western blot (Figure 6). Long-term 18% CS caused increased expression of proteins involved in cytoskeletal regulation and responsible for contractile responses, such as small GTPase Rho, MLC, MLC kinase, ZIP kinase, thrombin membrane-associated receptors PAR1 and PAR2 and actin-binding proteins caldesmon, and HSP27 associated with EC cytoskeletal remodeling and barrier regulation. At the same time, expression of proteins not involved in the direct regulation of cell contraction (hCOX2, Rac, β-catenin, β-actin, and vinculin) was not affected or was even decreased. Importantly, analysis of CS-induced specific protein expression in the lung microvascular EC as well as in the HPAEC 16 hrs after replating showed the same trend and indicated increased expression of Rho GTPase, MLC, MLC kinase, and ZIP kinase. These results strongly suggest that, increased expression of proteins involved in specific signal transduction pathways and EC contractile responses may represent an important mechanism of alternative endothelial cell barrier regulation resulted from chronic exposure of pulmonary endothelium to physiologic or pathologic mechanical stimulation associated with ventilator-induced lung injury.
Figure 6. Effect of CS preconditioning on protein expression.
Human pulmonary EC monolayers were subjected to 18% CS for 72 hrs. Specific protein expression was detected in situ or in replated EC cultures by western blot analysis with corresponding antibodies. Results are representative of three independent experiments.
DISCUSSION
The main finding of this study is time- and magnitude-dependent changes in pulmonary endothelial signaling and specific protein expression caused by cyclic stretch, which may contribute to the altered responsiveness of pulmonary endothelium to edemagenic agents and exacerbation of lung vascular barrier dysfunction caused by mechanical ventilation at high tidal volumes. Our data show direct effects of pathologic CS on endothelial remodeling and increased permeability in acute conditions. Our experiments show that short term CS modulates agonist-induced EC signaling via post-translational mechanisms (such as effects on MLC phosphorylation), and these effects were only observed if agonist stimulation occurred in the presence of CS. However, chronic CS preconditioning (over 24 hrs) regulated EC permeability responses to thrombin at translational (increased expression of specific signaling and cytoskeletal proteins shown in this study) and post-translational levels. To the best of our knowledge this is the first demonstration of CS-induced phenotypic changes in endothelial cells which contribute to the modulation of agonist-induced contractile responses.
Previous studies have described a role of Rac and Rho GTPases in the modulation of agonist-induced pulmonary endothelial permeability by physiologic and pathologic magnitudes of cyclic stretch [20, 21]. However, mechanisms of synergy between edemagenic agonists and pathologic CS in EC barrier regulation remain to be investigated. In the clinical settings, excessive ROS production caused by mechanical ventilation at high tidal volumes is a well recognized pathologic factor of VILI and ALI/ARDS [6, 33, 34]. Pathologic CS triggers generation of ROS [35], which may function as second messengers in the signal transduction cascades leading to additional activation of Rho. This study demonstrates additive effects of 18% CS and thrombin on ROS production. Importantly, barrier-disruptive effects of 18% CS and thrombin on EC monolayers were markedly attenuated by ROS inhibitor NAC. Consistent with results of this study showing different effects of 5% CS and 18% CS on thrombin-induced barrier dysfunction and enhanced ROS production by 18% CS, other studies showed that strain levels of 10% did not increase superoxide anion production in pulmonary epithelial cells, whereas 15, 20, and 30% significantly increased generation of superoxide anion [36]. Thus, enhanced ROS production caused by superposition of pathologic mechanical stimulation and elevated inflammatory mediators may reflect an additional pathogenic mechanism associated with VILI and leading to lung injury and vascular leak. Another possible mechanism of synergy between pathologic CS and agonist-induced Rho signaling may involve CS-induced activation of signaling serine/threonine- and tyrosine-specific protein kinases [35, 37–39] leading to activation of Rho-specific guanine nucleotide exchange factors and further stimulation of Rho pathway of barrier dysfunction. These mechanisms await further investigation.
In contrast to pathologic CS, acute exposure of pulmonary EC to physiologic CS levels decreased thrombin-induced MLC phosphorylation (Figures 2,3) and promoted EC barrier recovery [20]. Other study shows that physiologic CS inhibited EC apoptosis, and this effect was dependent on the activation of phosphatidylinositol 3-kinase and activation of Akt [40]. Because Akt activation is also important for regulation of Rac activity, these findings indicate a potential mechanism of PI3K-Akt-mediated stimulation of Rac pathway of EC barrier enhancement by physiologic CS. In turn, pathologic level of cyclic stretch (18–20%) stimulated EC apoptosis and MLC phosphorylation reflecting increased EC contractility [21, 40]. In agreement with these reports, further elevation of CS magnitude to 25% caused progressive cell detachment (Figure 1).
Another major finding of this study is sustained effects of CS preconditioning on cell responses to edemagenic stimulation, which suggested phenotypic changes in pulmonary EC exposed to chronic CS. The current study shows that sustained changes in EC cytoskeletal and permeability responses to thrombin caused by chronic CS developed after 48 hrs of stimulation. This study also provides for the first time a comprehensive analysis of CS-induced phenotypic changes leading to altered pulmonary EC permeability response to thrombin. These changes retained even 16–24 hrs after cell replating from stretched plates. Along with increased expression of endothelial MLCK, MLC, and Rho, chronic CS preconditioning increased expression of actin binding proteins (caldesmon and HSP27) and ZIP-kinase, another Ca2+-independent enzyme capable of phosphorylating MLC [41]. Stretch-induced expression of PAR1 and PAR2 receptors that bind thrombin may also increase pulmonary EC sensitivity to thrombin and enhance the magnitude of thrombin-induced EC cytoskeletal and permeability response in pulmonary endothelium chronically exposed to high magnitude cyclic stretch stimulation.
Microarray analysis of genes highly upregulated in the in vivo models of ventilator induced lung injury indicates a number of immediate-early response genes Nur77, Egr1, Btg2, and c-Jun, heat shock proteins and inflammatory cytokines indicating general lung stress response to excessive mechanical stimulation [42]. Analysis of high magnitude CS effects on phenotypic expression in cultured endothelial cells revealed elevation of the mRNA levels of five specific smooth muscle markers, SM22-α, α-smooth muscle actin (α-SMA), caldesmon-1, smooth muscle myosin heavy chain (SMMHC), and calponin-1 [43]. These authors proposed that excessive hemodynamic forces may play an important role in modulating endothelial phenotype and even induce a possible endothelial cell to SMC transdifferentiation in response to cyclic strain. DNA microarray analysis reported in our previous study further extended a number of signaling and cytoskeletal genes potentially induced by pathologic CS [21]. Thus, the results of this study and data from microarray gene profiling strongly suggest that long term high magnitude CS stimulation of pulmonary microvascular endothelium associated with mechanical ventilation at high tidal volumes may induce phenotypic changes in pulmonary EC leading to enhanced permeability response to circulating edemagenic agents.
It is also important to note that enhancement of thrombin-induced permeability and EC monolayer disruption was observed in macrovascular and microvascular pulmonary EC preconditioned at 18% CS (up to 72 hrs) both, under continuing CS and after replating. These results indicate a lack of EC adaptation to chronic pathologic mechanochemical stimulation associated with lung mechanical ventilation at high tidal volumes.
In summary, this study shows that exposure of pulmonary endothelium to excessive mechanical stretch associated with HVT may augment barrier disruptive effects of circulating edemagenic and inflammatory mediators and thus contribute to pathogenesis of VILI. Moreover, more prolonged high amplitude lung mechanical strain associated with chronic HVT may induce gene expression changes leading to increased expression of regulatory and signaling proteins in the pulmonary endothelium, which may further accentuate barrier-disruptive response to edemagenic agents. These findings may explain higher sensitivity of pulmonary endothelium to edemagenic stimuli even after HVT was discontinued. Our results indicate that HVT for up to 24 hrs does not induce sustained changes in the contractile or signaling protein expression and sensitivity to edemagenic agents by pulmonary EC. Thus, short term mechanical ventilation may affect pulmonary EC sensitivity and responses to agonists at the level of modulation of intracellular signaling, whereas longer periods of HVT (48 hrs and more) may cause phenotypic EC changes leading to sustained alteration of EC permeability responses to edemagenic stimulation. These findings should be considered in interpretation of the mechanisms of pulmonary vascular hyperreactivity in patients exposed to chronic mechanical ventilation.
Acknowledgments
This work was supported by HL076259, HL075349, HL58064 and the American Lung Association Career Investigator Award for K.G.B., and American Heart Association National Scientist Development Grant and the American Lung Association Biomedical Research Grant for A.A.B. The authors wish to thank Nurgul Moldobaeva for superb laboratory assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol. 2000;89:1645–55. doi: 10.1152/jappl.2000.89.4.1645. [DOI] [PubMed] [Google Scholar]
- 2.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–6. doi: 10.1152/ajplung.00124.2001. [DOI] [PubMed] [Google Scholar]
- 3.Narimanbekov IO, Rozycki HJ. Effect of IL-1 blockade on inflammatory manifestations of acute ventilator-induced lung injury in a rabbit model. Exp Lung Res. 1995;21:239–54. doi: 10.3109/01902149509068830. [DOI] [PubMed] [Google Scholar]
- 4.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998;275:L1040–50. doi: 10.1152/ajplung.1998.275.6.L1040. [DOI] [PubMed] [Google Scholar]
- 5.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol. 1999;277:L167–73. doi: 10.1152/ajplung.1999.277.1.L167. [DOI] [PubMed] [Google Scholar]
- 6.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–35. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 7.Bhullar IS, Li YS, Miao H, Zandi E, Kim M, Shyy JY, Chien S. Fluid shear stress activation of IkappaB kinase is integrin-dependent. J Biol Chem. 1998;273:30544–9. doi: 10.1074/jbc.273.46.30544. [DOI] [PubMed] [Google Scholar]
- 8.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 9.Correa-Meyer E, Pesce L, Guerrero C, Sznajder JI. Cyclic stretch activates ERK1/2 via G proteins and EGFR in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L883–91. doi: 10.1152/ajplung.00203.2001. [DOI] [PubMed] [Google Scholar]
- 10.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–60. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda M, Kito H, Sumpio BE. Phosphatidylinositol-3 kinase dependent MAP kinase activation via p21ras in endothelial cells exposed to cyclic strain. Biochem Biophys Res Commun. 1999;257:668–71. doi: 10.1006/bbrc.1999.0532. [DOI] [PubMed] [Google Scholar]
- 12.Ingram AJ, James L, Ly H, Thai K, Scholey JW. Stretch activation of jun N-terminal kinase/stress-activated protein kinase in mesangial cells. Kidney Int. 2000;58:1431–9. doi: 10.1046/j.1523-1755.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 13.Kano Y, Katoh K, Fujiwara K. Lateral Zone of Cell-Cell Adhesion as the Major Fluid Shear Stress- Related Signal Transduction Site. Circ Res. 2000;86:425–433. doi: 10.1161/01.res.86.4.425. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Hu Y, Sturm G, Wick G, Xu Q. Ras/Rac-Dependent Activation of p38 Mitogen-Activated Protein Kinases in Smooth Muscle Cells Stimulated by Cyclic Strain Stress. Arterioscler Thromb Vasc Biol. 2000;20:E1–E9. doi: 10.1161/01.atv.20.3.e1. [DOI] [PubMed] [Google Scholar]
- 15.Malek AM, Jiang L, Lee I, Sessa WC, Izumo S, Alper SL. Induction of nitric oxide synthase mRNA by shear stress requires intracellular calcium and G-protein signals and is modulated by PI 3 kinase. Biochem Biophys Res Commun. 1999;254:231–42. doi: 10.1006/bbrc.1998.9921. [DOI] [PubMed] [Google Scholar]
- 16.Naruse K, Sai X, Yokoyama N, Sokabe M. Uni-axial cyclic stretch induces c-src activation and translocation in human endothelial cells via SA channel activation. FEBS Lett. 1998;441:111–5. doi: 10.1016/s0014-5793(98)01528-2. [DOI] [PubMed] [Google Scholar]
- 17.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158:153–64. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res. 2005;304:40–9. doi: 10.1016/j.yexcr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006;168:1749–61. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol. 2003;285:L785–97. doi: 10.1152/ajplung.00336.2002. [DOI] [PubMed] [Google Scholar]
- 22.Frye SR, Yee A, Eskin SG, Guerra R, Cong X, McIntire LV. cDNA microarray analysis of endothelial cells subjected to cyclic mechanical strain: importance of motion control. Physiol Genomics. 2005;21:124–30. doi: 10.1152/physiolgenomics.00029.2003. [DOI] [PubMed] [Google Scholar]
- 23.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, Chittur KK. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci U S A. 2001;98:8955–60. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 25.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med. 2000;162:357–62. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 26.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol. 1999;86:2026–33. doi: 10.1152/jappl.1999.86.6.2026. [DOI] [PubMed] [Google Scholar]
- 27.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res. 2004;67:64–77. doi: 10.1016/j.mvr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95:892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- 29.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26:453–64. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- 30.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–83. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 31.Chiba Y, Ishii Y, Kitamura S, Sugiyama Y. Activation of rho is involved in the mechanism of hydrogen-peroxide-induced lung edema in isolated perfused rabbit lung. Microvasc Res. 2001;62:164–71. doi: 10.1006/mvre.2001.2329. [DOI] [PubMed] [Google Scholar]
- 32.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res. 2004;68:1–12. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology. 2004;9:43–53. doi: 10.1111/j.1440-1843.2003.00527.x. [DOI] [PubMed] [Google Scholar]
- 34.Waters CM. Reactive oxygen species in mechanotransduction. Am J Physiol Lung Cell Mol Physiol. 2004;287:L484–5. doi: 10.1152/ajplung.00161.2004. [DOI] [PubMed] [Google Scholar]
- 35.Ali MH, Mungai PT, Schumacker PT. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells: role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol. 2006;291:L38–45. doi: 10.1152/ajplung.00287.2004. [DOI] [PubMed] [Google Scholar]
- 36.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;289:L834–41. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 37.Wang JG, Miyazu M, Xiang P, Li SN, Sokabe M, Naruse K. Stretch-induced cell proliferation is mediated by FAK-MAPK pathway. Life Sci. 2005;76:2817–25. doi: 10.1016/j.lfs.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 38.Iwasaki H, Eguchi S, Ueno H, Marumo F, Hirata Y. Mechanical stretch stimulates growth of vascular smooth muscle cells via epidermal growth factor receptor. Am J Physiol Heart Circ Physiol. 2000;278:H521–9. doi: 10.1152/ajpheart.2000.278.2.H521. [DOI] [PubMed] [Google Scholar]
- 39.Sai X, Naruse K, Sokabe M. Activation of pp60(src) is critical for stretch-induced orienting response in fibroblasts. J Cell Sci. 1999;112:1365–73. doi: 10.1242/jcs.112.9.1365. [DOI] [PubMed] [Google Scholar]
- 40.Liu XM, Ensenat D, Wang H, Schafer AI, Durante W. Physiologic cyclic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 2003;541:52–6. doi: 10.1016/s0014-5793(03)00285-0. [DOI] [PubMed] [Google Scholar]
- 41.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–74. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 42.Wurfel MM. Microarray-based analysis of ventilator-induced lung injury. Proc Am Thorac Soc. 2007;4:77–84. doi: 10.1513/pats.200608-149JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cevallos M, Riha GM, Wang X, Yang H, Yan S, Li M, Chai H, Yao Q, Chen C. Cyclic strain induces expression of specific smooth muscle cell markers in human endothelial cells. Differentiation. 2006;74:552–61. doi: 10.1111/j.1432-0436.2006.00089.x. [DOI] [PubMed] [Google Scholar]