Abstract
Polycomb-group (Pc-G) proteins ensure late maintenance of transcriptional repression outside the expression domain of target genes in flies and vertebrates. They act in complexes, presumably by modulating chromatin structure. In Drosophila, they have been found to be associated with transcriptionally inactive loci but seem to be present in association with actively transcribed promoters as well, a feature which is not yet understood. In the mouse, mutations in several Pc-G genes result in an often subtle, local derepression of only a subset of the Hox genes rostral to their expression domains. We report here that Hox/reporter fusion genes, either randomly integrated as transgenes or as insertions within endogenous loci, are transcriptionally silenced in two mouse Pc-G-null mutants, Mel18 and rae28. Transcriptional silencing of Hox/reporter transgenes in Pc-G mutants was accompanied by increased DNA methylation in the promoter region. Gene silencing was observed at early developmental stages, long before Pc-G and trithorax-group proteins exert their function in maintenance of the Hox patterns. Although all five Hox genes tested as Hox/reporter fusions were silenced in the Pc-G mutants, transcription of the endogenous loci was mildly decreased in a subset of these Hox genes, and Hoxb1 was the most strongly affected. We discuss the possibilities that the observed negative effect of Pc-G mutations on Hox and Hox/reporter expression may reflect a positive involvement of the Pc-G epigenetic repressors in initial Hox gene transcription and that this requirement is exacerbated by the reporter insertion.
The spatially restricted expression domains of the Hox genes, key regulators of anteroposterior patterning in all bilaterians, are crucial for gene function. Mouse Hox genes have been found to be regulated by a hierarchy of control mechanisms at different stages of the ontogeny of the expression patterns (1, 2). After sequential transcriptional initiation of 3′-5′ genes in the clusters, the definitive expression domains are established under the influence of a complex series of events modulating gene expression differentially in mesoderm and neurectoderm (3). Late maintenance of the anteroposteriorly restricted expression patterns is operated by the epigenetic system of Polycomb-group (Pc-G) repressors (acting outside the expression domains) and trithorax-group (trx-G) activators (maintaining gene transcription within the expression domains). Both the Hox genetic system and its late epigenetic maintenance seem to have been evolutionarily conserved, because homologs of Drosophila Pc-G and trx-G genes (4) have been identified and shown to affect Hox gene expression similarly in the mouse, albeit often in a more subtle way.
The mouse Pc-G gene Mel18 is a homolog of Drosophila posterior sex comb. Its inactivation was shown to give rise to posterior homeotic-like transformations in mouse embryos, as expected from a mutation knocking out a Hox repressor (5). The rostral expression boundaries of a subset of Hox genes were shown to be slightly anteriorly shifted in Mel18 mutant mouse embryos (5), in the mesoderm exclusively. Among the target Hox genes is Hoxb8. The Pc-G gene rae28 (also called mph1) (6) is a homolog of Drosophila polyhomeotic. No effect on Hox gene expression has been reported so far in rae28-null embryos (6), and Hoxb8 was found not to be affected by this mutation (D.T., Y.T., and H.K., unpublished observations).
Regulation of eukaryotic gene expression is known to depend both on a direct interaction between control sequences and DNA-binding activators and silencers and on the physical accessibility of the chromatin to complexes between these regulatory proteins and their cofactors and to the transcription machinery. The interactions between the effector molecules at these different regulatory levels are only beginning to be understood. Among the crucial data revealing the extent and versatility of these interactions is the discovery that proteins acting at these different levels are endowed with catalytic activity [histone acetylation (7), histone methylation (8), and histone phosphorylation (9)] able to modify the behavior of interacting proteins and to alter gene transcription.
It is an increasingly well accepted idea that the concept of epigenetic maintenance of repression by Pc-G genes and activation by trx-G genes is less clear-cut than originally thought. Several Pc-G and trx-G proteins seem to have a dual role in both activation and repression of transcriptional activity, depending on the locus/genetic context (10). Very recently, actively transcribing promoters have been found to be key targets of Pc-G function (11), and several Drosophila Pc-G proteins have been found in robust association with a DNA-binding transcription factor and with components of the general transcription complex TFIID (12). A molecular link between transcriptional control and chromatin remodeling has been uncovered by the mechanistic coupling of histone deacetylation and transcriptional silencing by Pc-G proteins (13). In addition, transcriptional silencing has been linked to histone deacetylation by the discovery of an association between histone deacetylases and the methyl CpG-binding protein MeCP2 (14). This couples DNA methylation and transcription to chromatin remodeling and histone deacetylation. Given the growing degree of complexity of eukaryotic gene control mechanisms discovered so far, it is clear that essential molecular interactions between the transcriptional machinery and the effector complexes involved in enhancement or silencing of gene expression still have to be better understood.
In an attempt to analyze the regulatory interactions between Pc-G and Hox genes at the molecular level and with the intention to map Polycomb-response elements (4) on the genomic surrounding of Hoxb8 and other Hoxb genes, we crossed the Mel18 and rae28 Pc-G heterozygous mutant mice with a series of HoxlacZ/reporter transgenic lines. We report here that instead of the expected rostral shift in HoxlacZ expression boundaries in Pc-G homozygous mutants, a considerable reduction in β-galactosidase (β-gal) activity was observed. Not only did the HoxlacZ transgenes lose their expression in these Pc-G-null mutants, but a HoxAlkPhos transgene and HoxlacZ “knock-in” alleles (targeted fusion of lacZ within a Hox locus) also did. We found that this silencing effect occurred at stages considerably earlier than when Pc-G and trx-G genes were reported to first affect the Hox patterns. The effects of the Pc-G mutations on endogenous Hox transcription were more subtle, but a subset of the endogenous Hox counterparts exhibited a lower transcription level within their expression domain in Pc-G mutants, Hoxb1 being the most strongly affected. We discuss the possibility that these data reflect a positive involvement of at least some of the Pc-G gene products at the initial stages of Hox gene expression and that loss of specific Pc-G proteins, in combination with alteration of the chromatin structure by reporter insertion, severely compromises early Hox transcription.
Materials and Methods
Animals. The Hoxb8lacZ (15) and Hoxc8lacZ (16) knock-in mouse lines were described earlier, as were transgenic Hoxb8lacZ (17), Hoxb7lacZ (18), and Hoxb1lacZ (19). The Hoxb9AlkPhos/Hoxb8lacZ transgene was generated by recombining a Hoxb9AlkPhos construct made by J. Sharpe (National Institute for Medical Research, London) and R. Krumlauf (The Stowers Institute for Medical Research, Kansas City, MO) (see ref. 20 for the use of AlkPhos as a Hox reporter) with the Hoxb8lacZ transgenic construct mentioned above (17). Transgenic mice were generated by pronuclear injection as described (17). Prx2lacZ knock-in (21) and Rosa26lacZ (22) mice have been described.
All reporter and Pc-G mutant mice were in the F1 C57BL6 × CBA-J hybrid background. Only Hoxb1lacZ, Prx2lacZ, and Rosa26 mice were bred as homozygotes, and all of the other strains used were bred as heterozygotes or hemizygotes. Mel18-null mice do not survive more than a few days after birth, so those crosses were always with heterozygotes.
Detection of lacZ and AlkPhos Activity. β-gal activity was detected on 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal) staining in whole-mount embryos as described by Charité et al. (17). Determination of AlkPhos activity was according to Sharpe et al. (20).
Whole-Mount in Situ Hybridization. Transcript distribution was determined in whole-mount embryos by using riboprobes, as described by van den Akker et al. (23).
Analysis of RNA Accumulation. Embryonic day (E) 11.5 HoxlacZ transgenic embryos from a cross between HoxlacZ/Mel18 double heterozygotes and Mel18 heterozygous mutants were genotyped by PCR on yolk sac DNA. Total RNA was purified from each individual embryo, fractionated on a gel, blotted on a filter, and hybridized with a Hoxb8 and a lacZ probe. GAPDH hybridization of the same filters after melting the first hybrids served as an internal control. Signals were recorded by using a PhosphorImager (Amersham).
Analysis of DNA Methylation. The E11.5 Hoxb8lacZ and E10.5 Hoxb1lacZ embryos in different Mel18 backgrounds were generated as described above. After genotyping, DNA was extracted and aliquots were digested with infrequently cutting restriction endonucleases (EcoRI × HindIII for Hoxb8 and EcoRI × EagI for Hoxb1). A subpart of the reaction was then treated with the methylation-sensitive restriction enzymes HpaII for Hoxb8 and HpaII and HhaI for Hoxb1. The digestion products were separated on gel, blotted, and hybridized with a Hoxb8 and a Hoxb1 probe, respectively. Signals were recorded by using a PhosphorImager.
Results
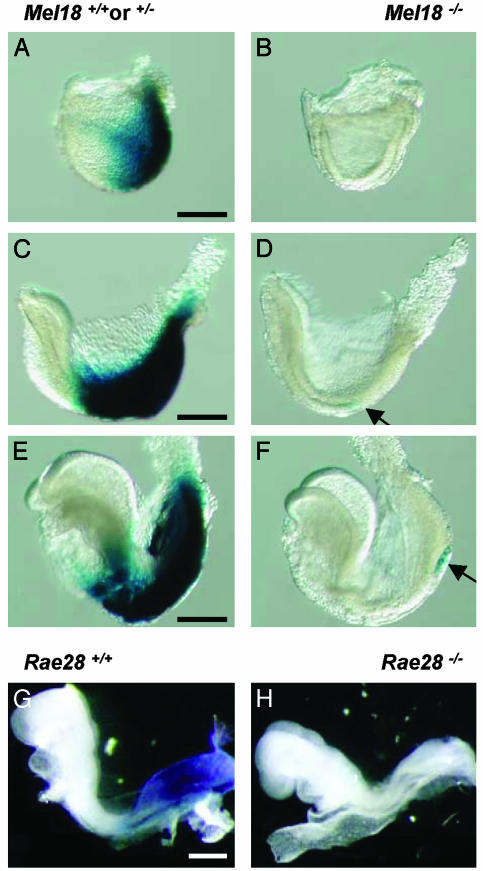
HoxlacZ Transgenes Are Transcriptionally Inactive in Mel18 Pc-G-Null Mutant Embryos. We introduced a series of Hoxb8lacZ (17) in the Mel18-null mutant background (5). Instead of the slight rostral shift in reporter expression boundaries expected from the effect on the endogenous gene (5), we observed a dramatic drop in gene activity in homozygous null mutant embryos. Some embryos exhibited no β-gal activity at all (data not shown), whereas other embryos (Fig. 1 A and B) revealed remnant stripes of staining reminiscent of the variegated pattern obtained after parental imprinting. However, there was no correlation between cases in which the transgene was inherited from the mother or the father (data not shown). The Hoxb8lacZ transgenic lines tested, comprising between 11 and 3 kb of upstream sequences, were found, in at least two cases, to carry only one or two copies of the transgene (data not shown). The silencing effect was therefore independent of the transgene integration site and did not depend on the presence of a high copy number of the transgene.
Fig. 1.
Strong decrease in expression level of Hoxb8lacZ and Hoxb1lacZ transgenes in homozygous Mel18 Pc-G mutants and milder effects on endogenous Hox genes. (A and B) X-gal-stained Hoxb8lacZ embryos at E11.5 in the WT (A) and Mel18 homozygous null mutant (B) backgrounds. (C and D) Expression of endogenous Hoxb8 in E9.6 WT (C) and Mel18-null mutant (D) embryos; both embryos are also null mutants for Bmi1 (Bmi1-null mutants show the same Hoxb8 expression as WT). Marked are expression within (*) and outside (o) the normal Hoxb8 expression domain. (E) lacZ and Hoxb8 transcript accumulation in E11.5 Hoxb8lacZ transgenic embryos of different Mel18 genotypes. (Left) lacZ expression in embryos WT (1), heterozygous (3), and homozygous (4 and 5) for Mel18. (Right) Hoxb8 expression in the same samples as at Left. Position of the 28S and 18S RNAs is indicated, and hybridization of the blots with GAPDH is shown in Lower. (F and G) X-gal staining of E8.5 Hoxb1lacZ embryos WT (F) or homozygous (G) for the Mel18-null mutation. (H and I) Endogenous Hoxb1 expression in an E8.5 Mel18 heterozygous (H) or homozygous (I) mutant embryo. (J and K) Hoxb1lacZ expression via lacZ RNA detection in E9 embryos WT (J) and homozygous null (K) for Mel18. X-gal staining was performed overnight, and enzyme reaction time in the whole-mount in situ hybridizations was 7 h in all cases.
In situ hybridization with a lacZ probe (data not shown) and analysis of E11.5 RNA levels (Fig. 1E) revealed a drop in Hoxb8lacZ transcript accumulation in Mel18-null embryos (Fig. 1E, lacZ probe, lanes 4 and 5 vs. 1 and 3). We conclude that silencing of the HoxlacZ gene occurred at the transcriptional level.
In addition to the different Hoxb8lacZ transgenes (Fig. 1 A and B and data not shown), we tested the expression of Hoxb1lacZ (19) in Mel18-null and control embryos. The transgene was silenced in the Pc-G mutant context (Fig. 1 F and G). Silencing of Hoxb1lacZ also occurred at the transcriptional level, as shown by the whole-mount assay of lacZ transcript distribution in Mel18-null embryos compared with controls (Fig. 1 K vs. J).
Negative Effect of Pc-G Mutations on the Transcription Level of Endogenous Hox Genes. We analyzed the expression of endogenous Hoxb8 and Hoxb1 in Mel18-null embryos. Hoxb8 RNA accumulation was slightly higher in Mel18-null embryos than in controls, probably as a result of local derepression rostral to the expression domain (5) (Fig. 1E, Hoxb8 probe, lanes 4 and 5 vs. 1 and 3). The transcription level of Hoxb8 within its expression domain was, however, clearly decreased in Mel18/Bmi1 double Pc-G mutants (Fig. 1 C and D), despite the local anterior derepression, rostrally shifting the Hoxb8 expression boundary in the neural tube (Fig. 1 C and D). Accumulation of endogenous Hoxb1 transcripts was significantly lower in Mel18 single Pc-G mutants than in controls (Fig. 1H and I). These data showed that the transcriptional inhibition by Pc-G mutations of the transcription of Hox/reporter fusions is also exerted on endogenous Hox loci, although to a lesser extent.
Different Reporters Coupled to the Promoter Region of Four Different Hox Genes Are Silenced in Several Pc-G-Null Mutants. We tested other Hox/reporter transgenes in Mel18-null mutants. Expression of Hoxb7lacZ (18) turned out to be completely silenced (Fig. 2 A and B) in Mel18 mutant embryos. Assay of RNA accumulation by RNase protection made it clear that the decrease in lacZ expression was transcriptional for this HoxlacZ transgene (data not shown), as in the two cases described above.
Fig. 2.
Decrease in expression of different Hox/reporter transgenes and HoxlacZ knock-in genes compared with other genes in Pc-G-null mutants and controls. (A and B) X-gal-revealed expression of the Hoxb7lacZ transgene in E11.5 WT (A) and Mel18-null (B) backgrounds. (C and D) Alkaline phosphatase activity of Hoxb9AlkPhos-reporter expression in E11.5 WT (C) and Mel18 homozygous null mutant (D) embryos. (E and F) Endogenous Hoxb9 expression in a WT for both Mel18 and Bmi1 (E) and in a double homozygous mutant embryo (F). (G and H) X-gal-revealed expression of the Hoxb8lacZ transgene in E11.5 WT (G) and rae28-null embryos (H). (I and J) β-gal activity of the Hoxc8lacZ knock-in in the Mel18-null context and control at E9.5. (K and L) β-gal activity of the Hoxb8lacZ knock-in in the Mel18-null context (L) and control (K) at E9.5. (M and N) Prx2lacZ knock-in expression in Mel18-null embryo (N) and control (M) at E10.5. (O and P) Rosa26lacZ transgene expression in a WT (O) and homozygous Mel18 mutant (P) at E11.5. k.i., Knock-in.
AlkPhos used as reporter also induced silencing of Hox transgene expression. Transgenic Hoxb9AlkPhos expression was lower in Mel18-null mutants than in controls and was completely prevented when Hoxb9AlkPhos was present together with a Hoxb8lacZ reporter 10 kb further on the same transgene (Fig. 2 C and D). The level of expression of endogenous Hoxb9 was unchanged in single Mel18 and Bmi1 Pc-G mutants (data not shown) and in double Mel18/Bmi1 mutants (Fig. 2 E and F). The anterior Hoxb9 expression boundary in the double Pc-G-null mutants was, as expected, slightly shifted rostrally in both the neurectoderm (level of somite 5 instead of 6) and in the mesoderm (level of somite 12 instead of 13) (not visible in Fig. 2 F vs. E).
Loss of HoxlacZ expression was observed in homozygous null mutants for another Pc-G gene, rae28 (6) (Fig. 2 G and H) but was not observed in embryos homozygous for a null mutation in the Pc-G genes Bmi1 (24) (data not shown) and M33 (25) (data not shown), despite the fact that Bmi1 and Mel18 are very closely related (5).
The silencing effect seems to show specificity for the Hox promoters because two non-Hox promoters/lacZ reporters, Prx2lacZ (ref. 21; Fig. 2 M and N) and ROSA26 (ref. 22; Fig. 2 O and P), were not affected by the Mel18-null mutation.
Hox-Targeted lacZ Knock-In Reporters Are also Silenced in Mel18-Null Mutants. Hoxc8 is a target of Mel18 (5). In Mel18-null mutants, the Hoxc8 expression boundary is slightly shifted anteriorly in the mesoderm, but the expression level of the gene within its expression domain is not affected (5). Genetic recombination of Hoxc8lacZ (16) with the Mel18-null allele led to a loss of Hoxc8lacZ expression (Fig. 2 I and J). We attempted to recombine the Hoxb8lacZ knock-in allele (15) with the Mel18 mutation. Because both loci are genetically linked, we had to test ≈400 lacZ DNA-positive descendants from a cross between Mel18/Hoxb8lacZ double heterozygotes and Mel18 heterozygotes to find one Mel18 homozygous null/Hoxb8lacZ genotype. This recombinant was the only one of the 400 Hoxb8lacZ embryos that did not express the transgene (Fig. 2 K and L). Reporter insertion within the endogenous Hox genes therefore also leads to silencing of these Hoxc8 and Hoxb8 loci. To check whether silencing of a HoxlacZ knock-in extended its transcriptional inhibitory influence into neighboring genomic regions in the Hox clusters, we tested the expression of the endogenous Hox genes flanking the targeted Hoxc8lacZ/reporter fusion. We did not detect any decrease in Hoxc6 or Hoxc9 expression in the Hoxc8lacZ Mel18 double homozygous null embryos (data not shown).
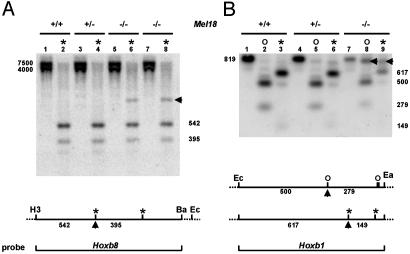
Silencing of Hox/Reporter Transcription Is Accompanied by DNA Hypermethylation of the Promoter Region. Hoxb8lacZ and Hoxb1lacZ are transcriptionally silenced in Mel18 Pc-G mutants. The methylation status of the HoxlacZ promoter region was compared to the endogenous situation by using methylation-sensitive restriction endonuclease (HpaII and HhaI) digestion. Hypermethylation at an HpaII site in the Hoxb8lacZ promoter region led to resistance of endonuclease digestion (arrow below the map) in Mel18-null Hoxb8lacZ transgenic embryos (Fig. 3A, lanes 6 and 8, and arrow) compared with Hoxb8lacZ transgenic embryos heterozygous (lane 4) and WT (lane 2) for Mel18. Hypermethylation was clearly observed as well in the promoter region of Hoxb1lacZ embryos homozygous for the Mel18 mutation (Fig. 3B, lanes 8 and 9, showing resistance to digestion with HhaI and HpaII, respectively, at the sites indicated by an arrow below the map) compared with Hoxb1lacZ embryos heterozygous (Fig. 3B, lanes 5 and 6) and WT (lanes 2 and 3) for Mel18. The EagI site defining the 3′ end of the promoter region analyzed is also methylation-sensitive and is more resistant to digestion in Mel18-null Hoxb1lacZ transgenic embryos than in Mel18 heterozygotes and WTs (Fig. 3B, lane 7 vs. 1 and 4).
Fig. 3.
Methylation status in the Hoxb8lacZ and Hoxb1lacZ promoter regions. (A) Increased methylation of the Hoxb8lacZ promoter region in Mel18-null embryos compared with heterozygotes and WTs. The Hoxb8 probe, depicted in Lower, detects the 7.5- and 4-kb HindIII-EcoRI endogenous and transgenic fragments (lanes 1, 3, 5, and 7), respectively. On further HpaII digestion (lanes 2, 4, 6, and 8), one site is resistant in Mel18 homozygous mutants (arrow, lanes 6 and 8) but not in WT (2) or Mel18 heterozygotes (4). (B) Similar analysis of the Hoxb1lacZ promoter region. The Hoxb1 probe detects the 819-bp EcoRI-EagI fragment present on both transgenic and WT alleles. Note that EagI is methylation-sensitive and partially inhibited (weaker 819-bp band, lane 7 vs. lanes 1 and 4) in Mel18-null embryos. Further digestion with the methylation-sensitive HpaII (*) or HhaI (○) revealed an endonuclease-resistant site in each case (arrows in both Lower and Upper) in Mel18-null embryos specifically (compare lane 8 with lanes 5 and 2 for HhaI, and lane 9 with lanes 3 and 6 for HpaII). H3, HindIII; Ba, BamHI; Ec, EcoRI; Ea, EagI; *, HpaII; and o, HhaI.
HoxlacZ Genes Are Transcriptionally Silent from Early Embryonic Stages and Thereafter. To evaluate the likelihood that the strong decrease in transcription of the Hox/reporter genes in Pc-G-null mutants might be a consequence of derepressing a Pc-G target that would negatively regulate the Hox genes, we examined the expression of HoxlacZ reporters in Pc-G mutants and controls from early stages on. At the late primitive streak stage (E7.2), Hoxb1lacZ was not expressed at all in Mel18 embryos, whereas it was expressed in posteriormost embryonic tissues in WT controls (data not shown). At the neural plate stage (E7.5), the earliest stage when Hoxb1lacZ is expressed rostrally to the node, in the region laying down the axial territories corresponding to the Hoxb1 expression domain (3), Hoxb1lacZ was completely silent throughout the embryo (Fig. 4 B vs. A). The reporter gene was not expressed either at the subsequent head fold (E7.75, Fig. 4D) or early somite (E8, Fig. 4F) stage, whereas it was expressed very strongly in heterozygous Mel18 mutants and WTs at the same stages (Fig. 4 A, C, and E). These experiments show that silencing occurs at a developmental stage long before the maintenance of Hox gene expression patterns by Pc-G and trx-G gene products is believed to take place (around E9.5) (26, 27).
Fig. 4.
Transcriptional inhibition of HoxlacZ expression occurs at early developmental stages. (A-F) Hoxb1lacZ expression in Mel18-null and control embryos at the neural plate stage (E7.5) (A and B), at the early head fold stage (C and D), and at the early somite stage (E8) (E and F). Arrows point to the area where a few distinct cells express the transgene, in the node region. (G and H) Hoxb8lacZ expression in the rae28-null mutants and controls at the early somite stage. Posterior is to the right. (Scale bar, 200 μm.)
Hoxb8 is initially transcribed later than Hoxb1, and its transcription domain spreads anterior to the node, where proliferating cells will make up the extending axis at early somite stages (E8) (3). Hoxb8lacZ at E8 was not expressed in rae28 homozygous null embryos at this, for Hoxb8, relatively early stage (Fig. 4 G and H), 1.5 days earlier than when epigenetic maintenance of the Hox patterns has been documented to occur (26, 27).
Failure to Rescue HoxlacZ Expression in Whole Embryos in Vivo and in Vitro and in Primary Cultures of Embryonic Cells. The inhibitor of histone deacetylation (trichostatin A) (40 nM) was capable of inhibiting histone deacetylation in whole WT embryos cultured in vitro from primitive streak stages (E7.5) until somite stages (E8.5) (data not shown), but it did not rescue normal expression of the transgene in Mel18 homozygous null embryos (data not shown). In utero retinoic acid exposure of Hoxb8lacZ Mel18 homozygous embryos between E8.5 and E10.5 did not rescue expression of the transgene within the Hoxb8 expression domain either. Neither trichostatin A (40 nM), combined or not with an inhibitor of DNA methylation (5-AZA-2′-deoxycytidine, 1 μM), nor retinoic acid (10-6 M) could counteract inhibition of HoxlacZ transcription in primary cultures of either neural tube or fibroblast cells from E9.5 and E11.5 Hoxb8lacZ Mel18 homozygous embryos compared with controls (data not shown).
Discussion
Hox/Reporter Silencing in Pc-G Mutants Is a Much Earlier Event than Epigenetic Maintenance of the Hox Patterns. Physical association of Drosophila Pc-G components with actively transcribed promoters (11) and with elements of the transcriptional machinery (12) is compatible with the possibility that the repressive Pc-G complexes might participate in the molecular realization of transcriptional activation. Mechanistic connections between positive and negative maintenance of transcription recently have been strikingly demonstrated in Drosophila (28). Pc-G and trithorax (TRX) complexes share histone H3 methyltransferase activity, strengthening the argument of a community of action between these keepers of positive and negative transcriptional memory at the chromatin level (28).
The observation by Akasaka et al. (27) of a decreased transcription of mouse Hoxb3 and Hoxb6 in E9.5 double Pc-G-null mutants was compatible with the possibility that Pc-G products may directly or indirectly play a positive role in the transcription of mouse endogenous Hox loci. However, an alternative possibility was that a derepressed Hox repressor subsequently caused the decrease in Hox gene transcription in the Pc-G-null mutants (27). We now show that transcriptional inhibition of Hox genes in Pc-G mutants occurs at much earlier embryonic stages than when Pc-G and trx-G are thought to start maintaining the Hox patterns. These data therefore establish that transcriptional inhibition of Hox genes in Pc-G mutants is not a consequence of the relief of the maintenance function of Pc-G complexes due to the Pc-G mutation but is an early event.
The complete absence of Hoxb1lacZ transcripts in Mel18-null mutants at the late streak (E7.2) (data not shown) and neural plate (E7.5) stages (Fig. 4) indicates that transcription of the transgene was not initiated in the primitive streak as it is normally in the WT context (3). A few widespread β-gal-positive cells were observed around the node at the head fold and early somite stages (just visible and indicated by arrows in Fig. 4 D and F). This observation, together with the variegated appearance of the very low HoxlacZ staining in some silenced HoxlacZ/Mel18 homozygous mutants (e.g., see Fig. 1B), suggests that this considerably delayed and reduced expression results from a stochastic event turning on the transgene within the normal expression domain of the corresponding Hox gene much later than when the endogenous counterpart is initiated. One hypothesis is that the reporter insertion generates an altered chromatin structure of the locus, which, in combination with the absence of specific Pc-G gene products normally present at the promoters (11), is incompatible with transcription. An involvement of chromatin structure in the relief of Hox clusterwide repression leading to sequential initiation of 3′-5′ Hox genes has been proposed (1, 29). Whether locus interruption by reporter insertion and loss of Pc-G proteins interfere with this initiation process will have to be investigated more extensively.
Differential Sensitivity of Endogenous Hox Genes to the Pc-G Mutations. Hox/reporter transgenes and knock-in reporters analyzed in this work are all dramatically silenced in specific Pc-G mutant contexts, and the transcription level of a subset of the corresponding endogenous Hox genes is decreased as well, although to a lesser extent, in single or double Pc-G mutants. The endogenous Hox genes seem to be differentially sensitive to this inhibitory effect of Pc-G mutations, some being partially transcriptionally inhibited in single Pc-G mutants [e.g., Hoxb1 (this work)], others being only affected in double Pc-G mutants [e.g., Hoxb3 and Hoxb6 (27) and Hoxb8 (this work)], and others appearing not to be affected at all in single and in double mutants [e.g., Hoxb9 (this work)]. Hoxb1, the Hoxb gene closest to the 3′ end, is more affected than the other endogenous Hoxb genes we tested because it is the only one for which the expression level is clearly lower in single Mel18-null mutants than in controls. It is possible that a positive requirement of Pc-G gene products in the transcription of Hox target loci is stronger for the 3′ extremity of the Hox clusters, in line with polarized opening of chromatin structure (29, 30).
The much stronger silencing effect of Pc-G mutations on Hox/reporter fusions than on intact endogenous Hox loci may reflect a perturbing character of the insertion on the mechanistic interactions between the locus and the transcriptional machinery, involving Pc-G gene products. The reporter interruption of Hox loci may exacerbate an existing dependence of initial Hox transcription on the full complement of Pc-G modulators that was proposed earlier to modulate Hox gene initiation (ref. 31, regarding endogenous Hoxd11 in the M33, Polycomb-like, null mutant). The Hox-specificity of the Pc-G silencing suggested in our experiments strengthens this hypothesis of a positive role of Pc-G proteins in Hox gene transcription. The promoter configuration of the Rosa26 (22) and Prx2 (21) loci, not known to be Pc-G targets, would not be sensitive to altered Pc-G complexes and may reside in a reporter-insensitive chromatin structure.
Chromatin Structure, DNA Methylation, and Transcription. Examination of the DNA methylation patterns by restriction endonuclease analysis showed increased methylation of the promoter regions of Hoxb1lacZ and Hoxb8lacZ in Mel18-null embryos compared with heterozygotes and WTs. This is a feature often associated with transcriptional inhibition.
Our data are compatible with several models of molecular interactions possibly underlying gene silencing in Pc-G mutants.
Alteration of chromatin structure at Pc-G target loci has been demonstrated in Drosophila polyhomeotic (homolog of rae28) and posterior sex comb (homolog of Mel18) mutants (32). Mouse Pc-G mutations may similarly cause chromatin decompaction or provoke a decrease in Pc-G complex occupancy, which in turn may render target loci more accessible to DNA methylation. This effect may differ in intensity for different Hox genes and may be enhanced in double Pc-G mutants and in the presence of an inserted reporter. The presence of intact Pc-G complexes at target promoters would thus be required to prevent methylation from silencing the loci.
Alternatively, the primary effect of Pc-G mutations might be to alter physical interactions of Pc-G proteins with positive effectors of the transcriptional machinery, possibly to a greater extent when chromatin structure is altered by reporter insertion. Such physical interactions were recently shown in Drosophila, where Pc-G proteins directly interact with general transcription factors at target promoters (11) and with proteins of the general transcription machinery (12). The resulting Pc-G protein complexes are thought to prevent transcription outside the expression domains of target genes. It is possible that these Pc-G/general transcription factor complexes positively affect transcription from early stages within the expression domain of target genes, independently of their subsequent role in maintaining repression outside these domains. As evidenced in other instances (33), DNA methylation would be secondary, definitely locking transcription of loci that are already transcriptionally inactive. Such a dual role of Pc-G proteins on patterning genes may possibly explain a functional antagonism between two described Pc-G complexes observed in murine hemopoiesis (34).
The hypermethylated status of the Hox promoter in Pc-G-null mutants may thus result from independently elicited transcriptional inhibition or may cause loss of transcription altogether. Future investigations aimed at documenting the molecular interactions among the Hox genes, chromatin structure modulators, the Polycomb repressor and trithorax activator complexes, and the transcriptional machinery will undoubtedly help in better understanding the molecular events positively and negatively controlling transcription of Hox and other Pc-G target genes during development.
Acknowledgments
We thank Frits Meijlink for Prx2lacZ knock-in mice, helpful discussions, and critical reading of the manuscript; Robb Krumlauf for Hoxb1lacZ and Hoxb9AlkPhos constructs; and Laura Zeinstra for initial experiments. Y.T. was supported by the Leukemia Program Project of Hiroshima University. T.O. was supported by a grant from the Netherlands Organization for Scientific Research/Aard-en Levenswetenschappen (to J.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Pc-G, Polycomb-group; β-gal, β-galactosidase; X-gal, 5-bromo-4-chloro-3-indolyl β-d-galactoside; En, embryonic day n; trx-G, trithorax-group.
References
- 1.van der Hoeven, F., Zakany, J. & Duboule, D. (1996) Cell 85, 1025-1035. [DOI] [PubMed] [Google Scholar]
- 2.Deschamps, J., van den Akker, E., Forlani, S., de Graaff, W., Oosterveen, T., Roelen, B. & Roelfsema, J. (1999) Int. J. Dev. Biol. 43, 635-650. [PubMed] [Google Scholar]
- 3.Forlani, S., Lawson, K. & Deschamps, J. (2003) Development (Cambridge, U.K.) 130, 3807-3819. [DOI] [PubMed] [Google Scholar]
- 4.Pirrotta, V. (1998) Cell 93, 332-336. [Google Scholar]
- 5.Akasaka, T., Kanno, M., Balling, R., Mieza, M. A., Taniguchi, M. & Koseki, H. (1996) Development (Cambridge, U.K.) 122, 1513-1522. [DOI] [PubMed] [Google Scholar]
- 6.Takihara, Y., Tomotsune, D., Shirai, M., Katoh-Fukui, Y., Nishii, K., Motaleb, M. A., Nomura, M., Tsuchiya, R., Fujita, Y., Shibata, Y., Higashinakagawa, T., Shimada, K., et al. (1997) Development (Cambridge, U.K.) 124, 3673-3682. [DOI] [PubMed] [Google Scholar]
- 7.Imhof, A. & Wolffe, A. (1998) Curr. Biol. 8, R422-R424. [DOI] [PubMed] [Google Scholar]
- 8.Lachner, M. & Jenuwein, T. (2001) Curr. Opin. Cell Biol. 14, 286-298. [DOI] [PubMed] [Google Scholar]
- 9.Cheung, P., Allis, D. & Sassone-Corsi, P. (2000) Cell 103, 263-271. [DOI] [PubMed] [Google Scholar]
- 10.Brock, H. W. & van Lohuizen, M. (2001) Curr. Opin. Genet. Dev. 11, 175-181. [DOI] [PubMed] [Google Scholar]
- 11.Breiling, A., Turner, B. M., Bianci, M. E. & Orlando, V. (2001) Nature 412, 651-655. [DOI] [PubMed] [Google Scholar]
- 12.Saurin, A. J., Shao, Z., Erdjument-Bromage, H., Tempst, P. & Kingston, R. (2001) Nature 412, 655-660. [DOI] [PubMed] [Google Scholar]
- 13.van der Vlag, J. & Otte, A. P. (1999) Nat. Genet. 23, 474-478. [DOI] [PubMed] [Google Scholar]
- 14.Wade, P. A., Gegonne, A., Jones, P. L., Ballestar, E., Aubry, F. & Wolffe, A. (1999) Nat. Genet. 23, 62-66. [DOI] [PubMed] [Google Scholar]
- 15.van den Akker, E., Reijnen, M., Korving, J., Brouwer, A., Meijlink, F. & Deschamps, J. (1999) Mech. Dev. 89, 103-114. [DOI] [PubMed] [Google Scholar]
- 16.Le Mouellic, H., Lallemand, Y. & Brulet, P. (1992) Cell 69, 251-264. [DOI] [PubMed] [Google Scholar]
- 17.Charité, J., de Graaff, W., Vogels, R., Meijlink, F. & Deschamps, J. (1995) Dev. Biol. 171, 294-305. [DOI] [PubMed] [Google Scholar]
- 18.Vogels, R., Charité, J., de Graaff, W. & Deschamps, J. (1993) Development (Cambridge, U.K.) 118, 71-82. [DOI] [PubMed] [Google Scholar]
- 19.Marshall, H., Suder, M., Popperl, H., Aparicio, S., Kuroiwa, A., Brenner, S. & Krumlauf, R. (1994) Nature 370, 567-571. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe, J., Nonchev, S., Gould, A., Whiting, J. & Krumlauf, R. (1998) EMBO J. 17, 1788-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Berge, D., Brouwer, A., Korving, J., Martin, J. F. & Meijlink, F. (1998) Development (Cambridge, U.K.) 125, 3831-3842. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich, G. & Soriano, P. (1991) Genes Dev. 5, 1513-1523. [DOI] [PubMed] [Google Scholar]
- 23.van den Akker, E., Fromental-Ramain, C., de Graaff, W., Le Mouellic, H., Brûlet, P., Chambon, P. & Deschamps, J. (2001) Development (Cambridge, U.K.) 128, 1911-1921. [DOI] [PubMed] [Google Scholar]
- 24.van der Lugt, N. M., Domen, J., Linders, K., van Roon, M., Robanus-Maandag, E., te Riele, H., van der Valk, M., Deschamps, J., Sofroniew, M., van Lohuizen M., et al. (1994) Genes Dev. 8, 757-769. [DOI] [PubMed] [Google Scholar]
- 25.Coré, N., Bel., S., Gaunt, S., Aurrand-Lions, M., Pearce, M., Fisher, A. & Djabali, M. (1997) Development (Cambridge, U.K.) 124, 721-729. [DOI] [PubMed] [Google Scholar]
- 26.Yu, B., Hanson, R. D., Hess, J. L., Horning, S. E. & Korsmeyer, S. (1998) Proc. Natl. Acad. Sci. USA 95, 10632-10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akasaka, T., van Lohuizen, M., van der Lugt, N., Mizutani-Koseki, Y., Kanno, M., Taniguchi, M., Vidal, M., Alkema, M., Berns, A. & Koseki, H. (2001) Development (Cambridge, U.K.) 128, 1587-1597. [DOI] [PubMed] [Google Scholar]
- 28.Czermin, B., Melfi, R., McCabe, D., Seitz, V., Imhof, A. & Pirrotta, V. (2002) Cell 111, 185-196. [DOI] [PubMed] [Google Scholar]
- 29.Kondo, T. & Duboule, D. (1999) Cell 97, 407-417. [DOI] [PubMed] [Google Scholar]
- 30.Roelen, B., de Graaff, W., Forlani, S. & Deschamps, J. (2002) Mech. Dev. 119, 81-90. [DOI] [PubMed] [Google Scholar]
- 31.Bel-Vialar, S., Coré, N., Terranova, R., Goudot, V., Boned, A. & Djabali, M. (2000) Dev. Biol. 224, 238-249. [DOI] [PubMed] [Google Scholar]
- 32.Boivin, A. & Dura, J. M. (1998) Genetics 150, 1539-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bird, A. (2002) Genes Dev. 16, 6-21. [DOI] [PubMed] [Google Scholar]
- 34.Lessard, J., Schumacher, A., Thorsteinsdottir, U., van Lohuizen, M., Magnuson, T. & Sauvageau, G. (1999) Genes Dev. 13, 2691-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]