Abstract
Adult bone marrow-derived mesenchymal stem cells (MSCs) are able to differentiate into myofibroblasts and be recruited into wound lesions and contribute to wound healing. The cellular and molecular mechanism responsible for MSC trafficking and differentiation, however, are poorly understood. Local resting resident fibroblasts are activated after injury and play a critical role in recruiting MSCs. We investigated the role of platelet derived growth factor-B-activated fibroblasts (PDGF-B-aFBs) in regulating recruitment, migration and differentiation of MSCs from GFP transgenic mice in an in vitro wound healing assay and a novel three-dimensional (3D) model. PDGF-B-aFBs caused significant increases in MSCs migration velocity compared to control as demonstrated by time-lapse photography in an in vitro wound healing assay. Consistently, invasion/migration of MSCs into 3D collagen gels was enhanced in the presence of PDGF-B-aFbs. In addition, PDGF-B-aFBs induced differentiation of MSCs into myofibroblast. The regulatory effects of PDGF-B-aFBs are likely to be mediated by basic fibroblast growth factor (bFGF) and epithelial neutrophil activating peptide-78 (ENA-78 or CXCL5) as protein array analysis indicated an elevated levels of these two soluble factors in culture supernatant of PDGF-B-aFBs. Blocking antibodies against bFGF and CXCL5 were able to inhibit both trafficking and differentiation of MSCs into 3D collagen gels while supplement of exogenous bFGF and/or CXCL5 promoted invasion/migration of MSCs into 3D collagen gels. Our results reveal that PDGF-B-aFBs play a key role in recruitment/migration and differentiation of MSCs and implicate a bFGF- and CXCL5-dependent mechanism in mediating these effects.
Keywords: Fibroblasts, Platelet derived growth factor-B (PDGF-B), basic fibroblast growth factor (bFGF), endothelial neutrophil activating peptide-78 (ENA-78) or CXCL5, bone marrow-derived mesenchymal stem cells, wound healing
Introduction
Fibroblasts play an integral role in the process of cutaneous wound healing. They coordinate keratinocyte and endothelial cell growth and differentiation and are responsible for collagen deposition and connective tissue remodeling[1]. They undergo transdifferentiation into myofibroblasts and generate wound tension to reestablish skin integrity and architecture[2]. Recent evidence suggests that bone marrow-derived mesenchymal stem cells (BM-MSCs) participate in and contribute to wound healing as well[3]. BM-MSCs are multipotent progenitor cells that are able to differentiate into bone, cartilage, tendon, adipose, myocytes and myofibroblasts. BM-MSCs have been isolated from the peripheral circulation and they move to inflammatory environments, such as implanted vascular grafts, and wounds[4]. Resting residential fibroblasts are activated after injury. Activated fibroblasts secrete a panel of soluble factors and some of them are involved in the control of migration, proliferation and differentiation of BM-MSCs. Although several chemokines have been implicated, specific mechanisms driving BM-MSCs differentiation into myofibroblasts and their recruitment and migration towards wound tissues, are not well defined. Identification of key growth factors driving recruitment and differentiation of BM-MSCs may reveal therapeutic targets that enhance participation of BM-MSCs in the repair of non-healing cutaneous wounds. This is particularly important in the context of lower extremity ulcer disease caused by venous or arterial insufficiency and diabetes. Venous disease alone is responsible for leg ulcerations in an estimated 500,000 to 2 million Americans[5]. Early senescence and dysmotility have been described in local skin fibroblasts from chronic wound biopsies[6, 7]. Regardless of ulcer etiology, local growth factor imbalance likely contributes to the impaired healing. Correcting this imbalance may accelerate skin repair and avoid complications such as amputation and lifelong disability.
Platelet derived growth factor (PDGF) is a polypeptide dimer released by platelets during degranulation, but is also expressed in endothelial cells, vascular smooth muscle cells and infiltrating inflammatory cells at the site of injury[8]. The polypeptides are connected by disulfide bonds and exist as isoforms A, B, C and D [9]. Human platelets contain PDGF-AB, PDGF-BB (PDGF-B) and PDGF-CC, which bind to both PDGF receptors, PDGFRα and PDGFR.[10, 11]. PDGF-B has a mitogenic effect on a variety of mature connective tissue cells, including fibroblasts, vascular smooth muscle cells, chondrocytes and osteoblasts[12–14]. Its chemotactic effect on fibroblasts and smooth muscle cells implicates PDGF-B as a key regulatory molecule in wound healing, neo-intimal formation and atherosclerosis[15]. PDGF-B is among the most potent stimuli for mesenchymal cell migration. PDGF-B-activates dermal fibroblasts and stimulates proliferation, matrix protein production, and matrix receptor expression[16, 17]. PDGF-B also stimulates fibroblast mitogen production for keratinocytes and endothelial cells[18]. There is evidence of deficient PDGF-B levels in non-healing diabetic leg ulcers and increased levels in ulcers that heal completely[19]. Thus, growth factors expressed in PDGF-B-aFBs during normal wound healing are potential candidates for wound therapy, but little is known about the role they play in controlling recruitment, migration of BM-MSCs, and/or differentiation into myofibroblasts.
To elucidate the molecular mechanism underlying the critical roles of both PDGF-B and fibroblasts in wound healing, we studied the effects of PDGF-B-aFBs on controlling migration/invasion and differentiation of BM-MSCs in a novel 3D model which has been developed to mimic the in vivo dermis-like environment and includes the major type of extracellular matrix in skin (type I collagen), fibroblasts and soluble factors. Our data demonstrated that PDGF-B-aFBs are chemotactic to BM-MSCs and influence their differentiation into myofibroblasts. Regulatory effects appear to be mediated by CXCL5 and bFGF secreted from PDGF-B-aFBs.
Methods
Animals and Cells
Dermal fibroblasts from human foreskin were obtained from ATCC (Manassas, VA) and cultured in Dulbecos Modified Eagle’s Medium (DMEM) with glutamine (Gibco/BRL, Gaithersburg, MD) and fetal bovine serum (FBS) (10%; Hyclone, Logan, UT). Bone marrow was harvested from femurs of anesthetized GFP+/FVB transgenic mice or Tie2-LacZ+/FVB transgenic mice (Jackson Laboratories, Bar Harbor, Maine) under protocol approved by the institutional animal care and use committee. Cells were incubated with Red Cell Lysis buffer® (Sigma, St. Louis, MO) for 90 seconds and rinsed with isolation buffer (phosphate buffered saline (PBS), 2% FBS, 40-µg/ml gentamycin). Total bone marrow cell population was cultured in fibronectin-coated flasks in Endothelial Basal Medium-2 (EBM2; Cambrex, East Rutherford, NJ) overnight. To exclude the mature endothelial cells within the isolated fresh bone marrow, cells attaching to fibronectin overnight were discarded and the non-adherent cell population was re-plated[20] for use in 3D assays as described below.
Adenoviral Vector Transduction
Recombinant adenoviruses carrying reporter gene beta galactosidase (LacZ/Ad5) or PDGF-B (PDGF-B/Ad5) were a gift from Dr. M Herlyn (Wistar Institute, Philadelphia, PA). The construction of these vectors has previously been described[21]. Fibroblasts were plated on 25-mm dishes (Falcon, BD Biosciences, San Jose, CA) and cultured in DMEM plus 10% FBS at 37°C with 5% CO2. After reaching 80% confluence, supernatant was removed and cells were washed with PBS. Serum free DMEM, containing 20 plaque-forming units (PFU) per cell of either PDGF-B/Ad5 or LacZ/Ad5, was added to cells. Fibroblasts were incubated at 37°C with 5% CO2, 4 hours before medium was aspirated and replaced with DMEM plus 10% FBS for overnight culture.
Three-Dimensional (3D) Assays
3D models were designed to include BM-MSCs, Fibroblasts, type I collagen and soluble factors. Briefly, 5 × 105 BM-MSCs harvested from GFP+/FVB transgenic mice were plated on fibronectin-coated 24-well plates and overlaid with 150-µl cell-free collagen [type I bovine collagen (1-mg/ml, Organogenesis Inc, Canton, MA), 50 mM sodium bicarbonate (Cambrex), 100 mM L-glutamine (BioWhitaker Molecular Applications, Rockland, ME) and M199 medium (Cambrex) supplemented with 100-U/ml heparin (American Pharmaceutical Partners Inc, Schaumburg, IL), 50-µg/ml Vitamin C (Sigma) and 1% FBS], followed by a second 450-µl layer of collagen (equally prepared) including various experimental conditions with and without additional cells (as detailed below). The 3D constructs were incubated for 5 minutes at room temperature to allow for collagen polymerization and cultured in EBM2 medium that was changed every 48 hours. We evaluated the effects of the following study conditions on BM-MSC invasion/migration and differentiation by removing the gel at the completion of experiment and quantifying cell number and quantifying cell lineage that invaded/migrated into the gel.
Collagen alone;
40-ng/ml human recombinant PDGF-B (hrPDGF-B, R&D Systems, Minneapolis, MN);
hrPDGF-B activated fibroblasts (2.5 × 105 cells/ml plus 40-ng/ml hrPDGF-B);
Control fibroblasts (2.5 × 105 cells/ml untransduced);
Human microvascular endothelial cells (HMVEC), control cell line (2.5 × 105 cells/ml untransduced);
PDGF-B/Ad5-aBFs activated fibroblasts (2.5 × 105 cells/ml transduced @ 20 PFU PDGF-B/Ad5);
Control LacZ/Ad5 fibroblasts (2.5 × 105 cells/ml transduced @ 20 PFU LacZ/Ad5);
PDGF-B/Ad-5 activated fibroblasts plus 0.15-µg/ml anti-PDGF-B blocking antibody;
PDGF-B/Ad-5 activated fibroblasts plus 3.75-µg/ml anti-ENA78 blocking antibody;
PDGF-B/Ad-5 activated fibroblasts plus 6.25-µg/ml anti-bFGF blocking antibody
In addition, we added recombinant human CXCL5 and bFGF (R&D Systems) into the medium in 3D assay to examine the effects of exogenous CXCL5 and/or bFGF on invasion/migration of BM-MSCs into 3D collagen gels.
Anti-αSMA and anti-CD31 antibody stains were performed to assess BM-MSC differentiation into either myofibroblasts or endothelial cells, by co-localizing GFP expression and either αSMA or CD31 expression, respectively. Anti-Ki-67 stain and TUNEL assay (terminal deoxynucleotidyl transferase mediated dUTP nick end labeling) were used to assess BM-MSC proliferation and apoptosis, respectively, in cells where they co-localized with GFP expression. In experiments using Tie2-lacZ+ BM-MSCs, endothelial lineage cells were quantified by β-gal staining.
Protein Array
Standardized 1-ml aliquots from culture supernatants collected from 3D model were analyzed with RayBio® Human Angiogenesis Antibody Array I kit (Norcross, GA) following manufacturer’s protocol, to characterize differential expression of potential secondary growth factors secreted by PDGF-B-aFBs versus control fibroblasts. This array employs antibodies to simultaneously detect protein expression levels for 20 pro-angiogenic molecules. Antibody membranes were exposed to Kodak X-ray film (Rochester, NY). Scanned images were analyzed with Image J software (NIH) and relative densities ratios were calculated.
Antibodies
Goat anti-human PDGF-B antibody, goat anti-human CXCL5 (ENA-78) antibody, rabbit anti-human bFGF, and isotype matched control antibodies were purchased from R&D Systems. Goat anti-mouse CD31 antibody was obtained from Santa Cruz Biotechnology. Murine anti-human α-SMA antibody was obtained from Sigma. TUNEL assay was performed with TACS™ T&T kit from R&D Systems to detect apoptotic cells. Cell proliferation was assessed with murine anti-Ki-67 antibody (Sigma, St. Louis, MO). When anti-human antibodies were utilized, murine cross-reactivity and blocking efficiency were confirmed with manufacturer. Horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG and rhodamine-conjugated goat anti-mouse secondary antibodies were purchased from Dako (Carpintaria, CA) and Biomeda (Foster City, CA) respectively.
Immunohistochemistry
Harvested collagen gels were fixed with Prefer® (Anatech LTD, Battle Creek, MI), containing glyoxal, ethanol and buffer, for 4 hours at room temperature, proceeded as whole-mount or embedded in paraffin and serially sectioned for staining. Immunohistochemistry was performed after sections were deparaffinized and rehydrated. For α-SMA stains, sections were subsequently incubated with Target Retrieval buffer (Dako) and Dual Endogenous Enzyme Block (Dako), followed by an overnight incubation with primary antibody at 4°C. Secondary antibody and Hoechst (Sigma) stain were added the next morning. For CD31 stains, tissue sections were treated as above, except following the secondary antibody incubation, DAB (3,3’-diaminobenzidine tetrahydrochloride)/Metal Concentrate (Pierce) diluted in Stable Peroxide Substrate Buffer (Pierce) was added to each section. Harris Modified Hematoxylin (Fisher Scientific, Pittsburg, PA) was used for nuclear identification.
Staining for β-galactosidase
After medium was removed, harvested collagen gels were fixed in 0.5% glutaraldehyde for 10 minutes at room temperature, washed with PBS/MgCl solution twice for 10 minutes and incubated with X-gal at 37°C overnight. The following day, gels were fixed with Prefer® before embedding in paraffin. Nuclear Fast Red (Sigma) was used for nuclear identification.
In vitro Wound Healing Assay
In vitro wound healing assay was used to measure cell traffic. Direction and velocity of BM-MSC migration toward fibroblasts were studied using co-culture and time-lapse photography. Glass coverslips placed upright into fibronectin-coated 35 mm culture dish wells were secured with paraffin to create a partition for each well. 2.5 × 105 GFP+-BM-MSCs harvested from GFP+ transgenic mice were cultured on one side and incubated for 4 hours at 37°C with 5% CO2 for cell adherence. 2.5 × 105 PDGF-B-aFBs (transduced PDGF-B/Ad5 @ 20 PFU) were cultured for 4 hours, on the other side. After 24 hour-incubation at 37°C with 5% CO2, partitions were removed creating a gap between cell populations. Fibroblasts, transduced with LacZ/Ad5 @ 20 PFU, were used as control. Co-cultures were observed using contrast phase and fluorescence inverted microscopy and time-lapse photography at 10-minute intervals, for 20 hours. ImagePro 5.0 software (MediaCybernetics, Silver Spring, MD) tracked 15 randomly selected individual cells per dish. This program has 3D measurement features and analysis tools specifically designed for in vivo studies.
Results
The chemotactic effect of PDGF-B-aFBs on BM-MSCs
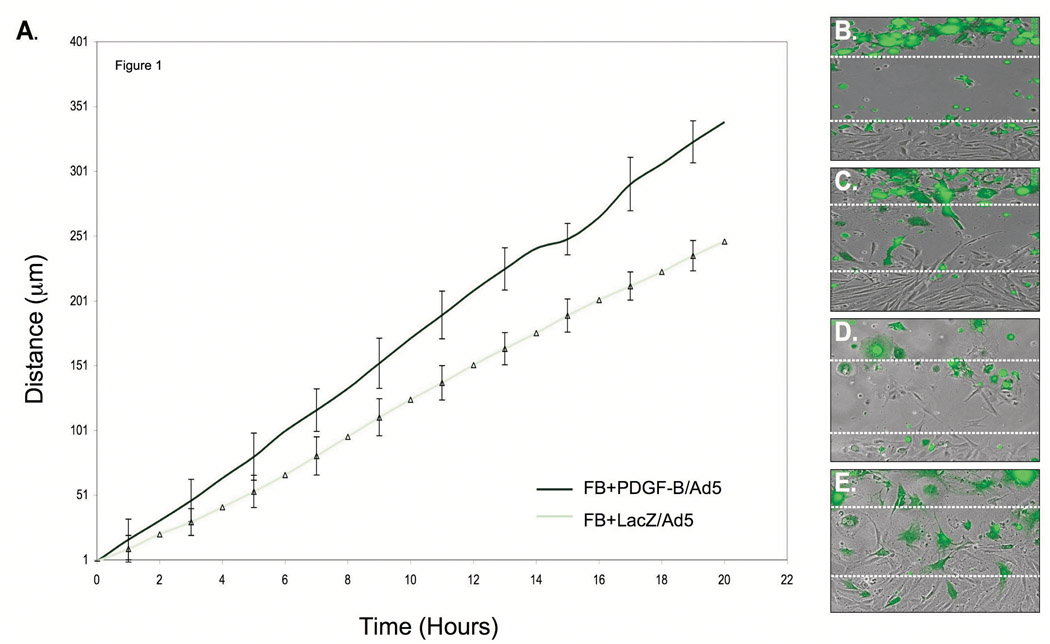
To determine if PDGF-B-aFBs influence BM-MSC migration velocity and direction, we used time-lapse photography to track and measure cell trafficking in a modified scratch assay (in vitro wound healing assay) where two cell types were co-cultured, but separated by a standardized cell-free gap. GFP+-BM-MSCs were co-cultured with PDGF-B/Ad5-transdued fibroblasts or LacZ/Ad5-transduced fibroblasts (control). Direction and velocity of individual BM-MSC crossing the gap were tracked, recorded and analyzed by computer software. The average distance traveled of BM-MSC toward the fibroblast population was greater at all time points when co-cultured with PDGF-B-aFBs versus control (Fig 1A). Direction of individual BM-MSC migration (as measured by serial motion vectors) was not significantly different between groups and indicated a motion vector from the BM-MSC side to the fibroblast side (quantitative data not shown), representative photos shown in Fig 1B–E.
Figure 1. BM-MSC velocity toward co-cultured PDGF-B-aFBs.
(A) BM-MSC migration was faster at all time points when co-cultured with FB-PDGF-B/Ad5 versus FB-LacZ/Ad5 (P<0.05). The migration of 15 individual cells were tracked over time in each experiment and presented in (A) as mean ± standard deviations (SD) of three independently performed experiments. Representative 40X magnification still pictures of GFP+-BM-MSC migration in the presence of FB-LacZ/Ad5 at time zero (B), FB-LacZ/Ad5 at hour 20 (C), FB-PDGF-B/Ad5 at time zero (D), and FB-PDGF-B/Ad5 at hour 20 (E). The open spaces between the dash lines represent gaps. Experiments were repeated three times, independently. Differences in mean BM-MSC velocity toward the two different fibroblasts groups (PDGF-B/Ad5 vs. LacZ/Ad5) at various time points were calculated using ANOVA and student t-test (Excel, Microsoft).
PDGF-B-aFBs promote invasion/migration of BM-MSCs into 3D collagen gels
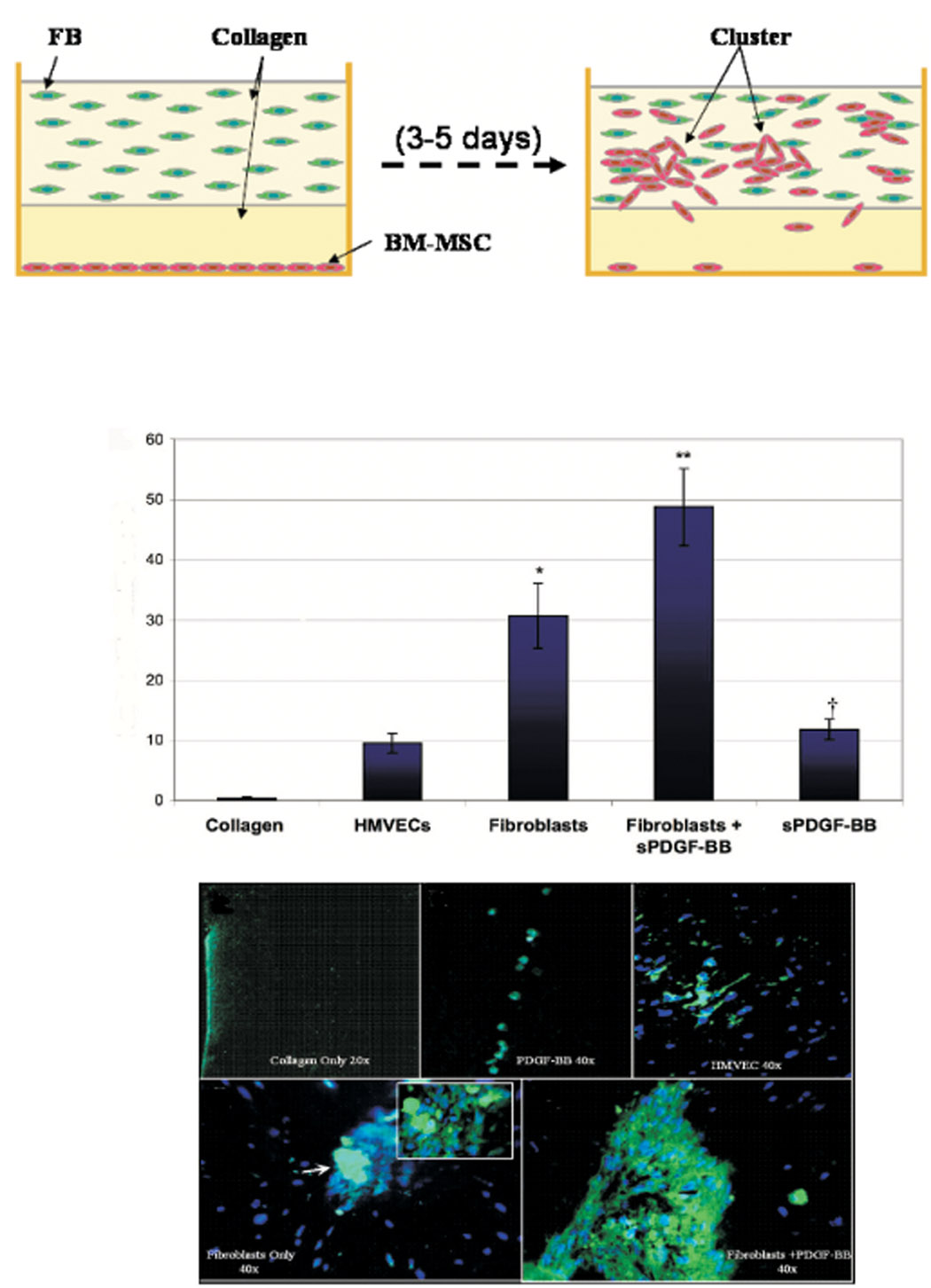
A 3D model was developed to study invasion and migration of BM-MSCs in response to fibroblasts embedded within an in vitro dermis-like environment (Fig 2A). The presence of fibroblasts within the construct was enough to cause significant increases in BM-MSC invasion/migration into type I collagen, as compared to collagen alone, hrPDGF-B or HMVEC. BM-MSC invasion/migration was most significantly increased in the PDGF-B-aFBs group versus controls (Fig 2B). Formation of large BM-MSC clusters occurred in the presence of PDGF-B-aFBs (Fig 2G), to a lesser extent in fibroblast control groups (Fig 2F) but was absent in the other control groups (Fig 2C–2E). To determine if increases of BM-MSCs in the collagen gel were caused by proliferation in response to PDGF-B-aFBs rather then cell invasion/migration, tissue sections were assessed with anti-Ki-67 antibody. Additionaly, a TUNEL assay was conducted to detect potential cell apoptosis in the collagen gel. BM-MSC proliferation and apoptosis were consistent between groups and lacked statistically significant differences (data not shown), implicating that the increased number of BM-MSCs observed in the 3D collagen gels was due to enhanced invasion/migration of BM-MSCs from the bottom monolayer into the gel.
Figure 2. GFP+-BM-MSC invasion/migration into 3D collagen gel is increased by PDGF-B-aFBs.
(A) Schematic illustration of 3D collagen model. (B) Average number of BM-MSC invasion/migration into 3D collagen gels per high power field (HPF). Increase in BM-MSC migration was greater in the PDGF-B-aFB group versus fibroblast control (**P<0.05) and hrPDGF-B groups (†P<0.05). Representative sections of GFP+-BM-MSC 3D gels at 40X magnification under various conditions; collagen only (C), rhPDGF-B (D), HMVEC (E), fibroblasts (100X magnification insert of BM-MSC cluster indicated by white arrow) (F) and rhPDGF-B-aFBs (G). Nuclei were stained with Hoesht. All experiments were performed in duplicate and repeated three times, independently. Means ± SD were compared by ANOVA or student t-test.
PDGF-B-aFBs induce BM-MSC differentiation into myofibroblast in 3D model
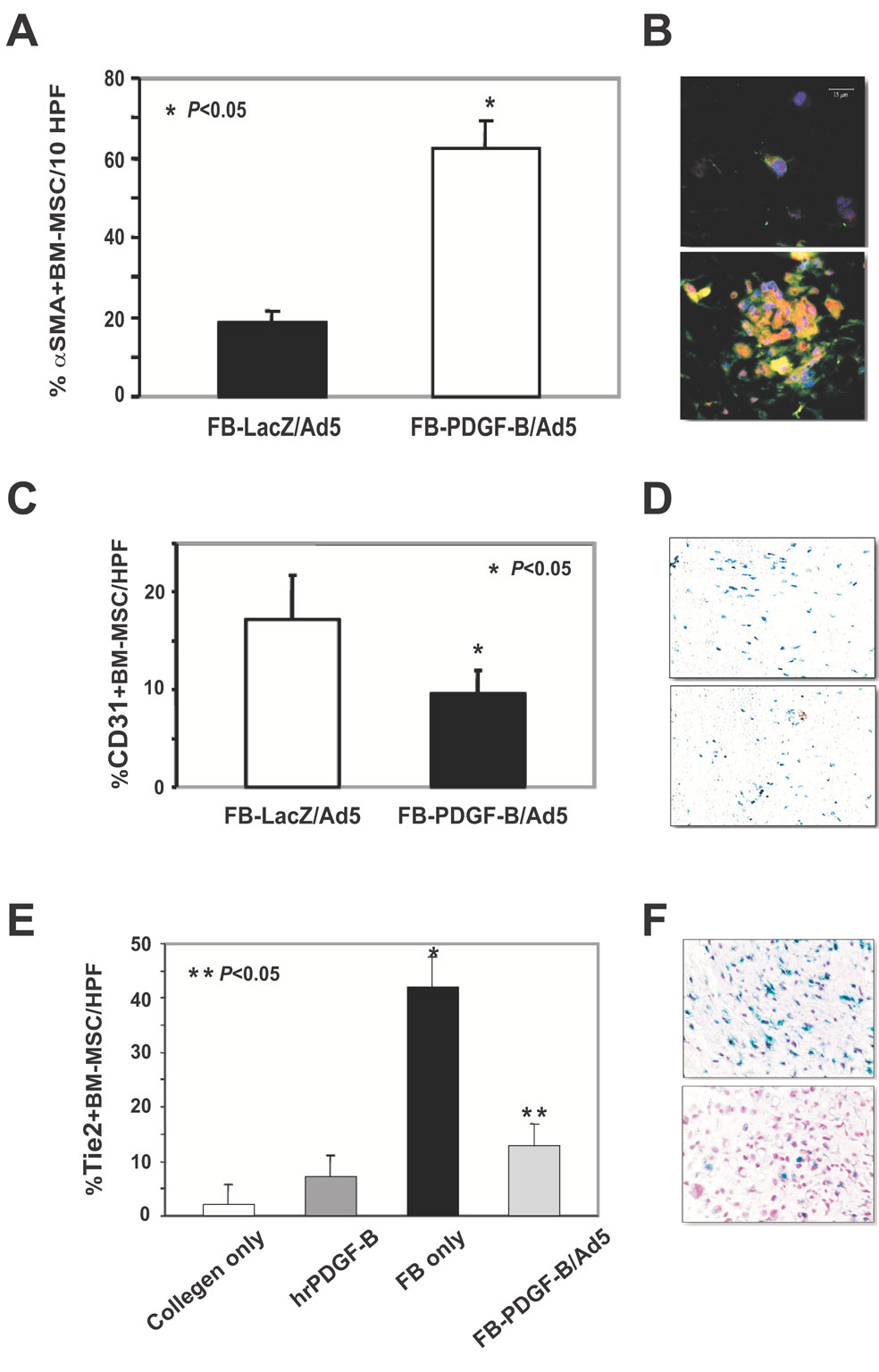
To examine the effect of PDGF-B-aFBs on regulating differentiation of BM-MSCs, we probed for αSMA expression co-localizing to GFP+ cells to identify BM-MSCs that had differentiated into myofibroblast. We also probed for CD31 co-localizing to GFP+ cells to identify BM-MSCs that differentiated into endothelial cell (EC) lineage (including endothelial progenitor cell (EPC)). Additional experiments were conducted to confirm EC lineage differentiation by testing BM-MSCs from transgenic Tie-2/LacZ mice (in which the LacZ reporter gene is linked to Tie2 promoter expression, labeling all cells of endothelial lineage[22]). 3D assays were performed using BM-MSCs harvested from Tie2-LacZ+/FVB transgenic mice, instead of that from GFP+/FVB transgenic mice. Collagen gels were stained for β-galactosidase (β-gal) activity and EPC/EC were identified as β-gal+ cells. BM-MSCs that differentiated into myofibroblasts (GFP+/αSMA+) were significantly increased in the presence of PDGF-B-aFBs (Fig 3A). In contrast, endothelial lineage differentiation was decreased under the same conditions (Fig 3B and 3C), indicated by reduced numbers of endothelial lineage cells within the collagen gels containing PDGF-B-aFBs versus control fibroblasts. These results indicated that PDGF-B-aFBs favorably induce BM-MSC differentiation into myofibroblast, but not endothelial cell lineage, in the 3D collagen model.
Figure 3. Preferential enhancement of differentiation of BM-MSCs into myofibroblast, but not endothelial lineage, by PDGF-B-aFBs.
(A) Average percent α-SMA+-BM-MSCs per HPF in the presence of FB-LacZ/Ad5 versus FB/PDGF-B/Ad5 in 3D assay. Mesenchymal differentiation was increased in the presence of FB-PDGF-B/Ad5 versus FB-LacZ/Ad5. (B) Representative sections at 40X magnification of α-SMA+-BM-MSCs co-cultured with FB-LacZ/ Ad5 (upper) and FB-PDGF-B/Ad5 (lower). (C) Average percent CD31+-BM-MSCs per HPF in the presence of FB-LacZ/Ad5 versus FB-PDGF-B/Ad5 in 3D assay. (D) Representative sections at 100X magnification of CD31+-BM-MSCs co-cultured with FB/LacZ/Ad5 (upper) and FB-PDGF-B/Ad5 (lower). Endothelial differentiation of BMDPC was diminished in the presence of FB-PDGF-B/Ad5 versus FB-LacZ/Ad5 [BM in A–D from GFP+ mice]. (E) Inhibition of endothelial differentiation of Tie2-LacZ+-BM-MSCs when co-cultured with PDGF-B-aFBs. Average percent of endothelial lineage cells (β-gal+ cells) within 3D model. Endothelial lineage differentiation is diminished in FB-PDGF-B/Ad5 versus FB only [untransduced FBs used as control]. (F) Representative sections of Tie2+-BM-MSCs co-cultured with FB (upper) versus FB-PDGF-B/Ad5 (lower) in 40X magnification. All experiments were performed in duplicate and repeated three times, independently. Means ± SD were analyzed by ANOVA or student t-test.
PDGF-B induces fibroblasts to produce CXCL5 and bFGF
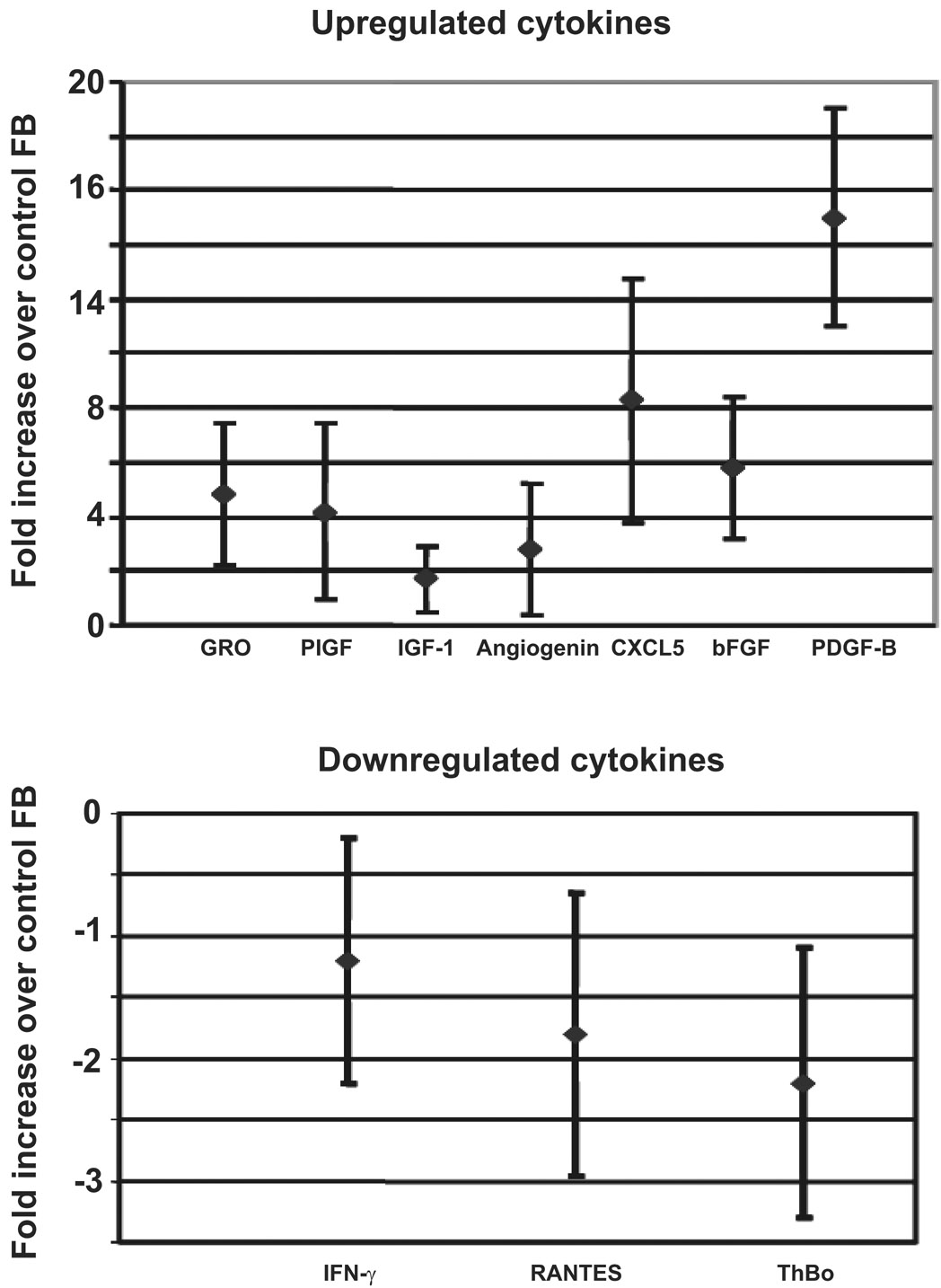
Based on the fact that only PDGF-B-aFBs were able to enhance migration/invasion of BM-MSCs into collagen gels and to induce differentiation into myofibroblasts, while application of PDGF-B alone failed to exert the same effects, we hypothesized that the regulatory effects of PDGF-B-aFBs on BM-MSCs are likely mediated through secondary factor(s) produced by fibroblasts following PDGF-B stimulation. To test this hypothesis, cell culture supernatants from PDGF-B-aFBs and control fibroblasts were collected and assessed with a protein array to detect potential cytokines and chemokines involved in the wound healing cascade. Of twenty candidates in the protein array membrane, CXCL5 (ENA-78) and bFGF were elevated 8-fold and 5-fold in the PDGF-B-aFBs group, respectively, compared to the control fibroblast group (Fig 4). CXCL5 is an inflammatory chemokine belonging to the CXC subfamily and bFGF is a well-known mitogen[23, 24]. Our data demonstrated that PDGF-B induces fibroblasts to secrete CXCL5 and bFGF.
Figure 4. PDGF-B induces fibroblasts to produce CXCL5 and bFGF.
Protein array analyses were conduced by testing the cell culture supernatants of FB-PDGF-B/Ad5 versus FB-LacZ/Ad5 using a protein array set of RayBio® Human Angiogenesis Antibody Array I kit. CXCL5 (ENA-78) and bFGF were elevated 8-fold and 5-fold in the PDGF-B-aFB group, respectively, compared to the control fibroblast group. Expression of IFN-γ, RANTES and thrombopoietin (ThBo) was slightly decreased. Experiments were repeated three times, independently. Means ± SD were analyzed by ANOVA or student t-test.
In contrast, the expression of interferon-γ, RANTES (regulated upon activation, normal T-cell expressed, and presumably secreted) and thrombopoietin were marginally decreased by 1 to 2-fold in the culture supernatant of PDGF-B-aFBs. All three factors are proinflammatory chemokines[25–27]. We elected to investigate CXCL5 and bFGF further, as they showed the most pronounced change in expression.
CXCL5 and bFGF were responsible for mediating the effect of PDGF-B-aFBs on BM-MSC trafficking
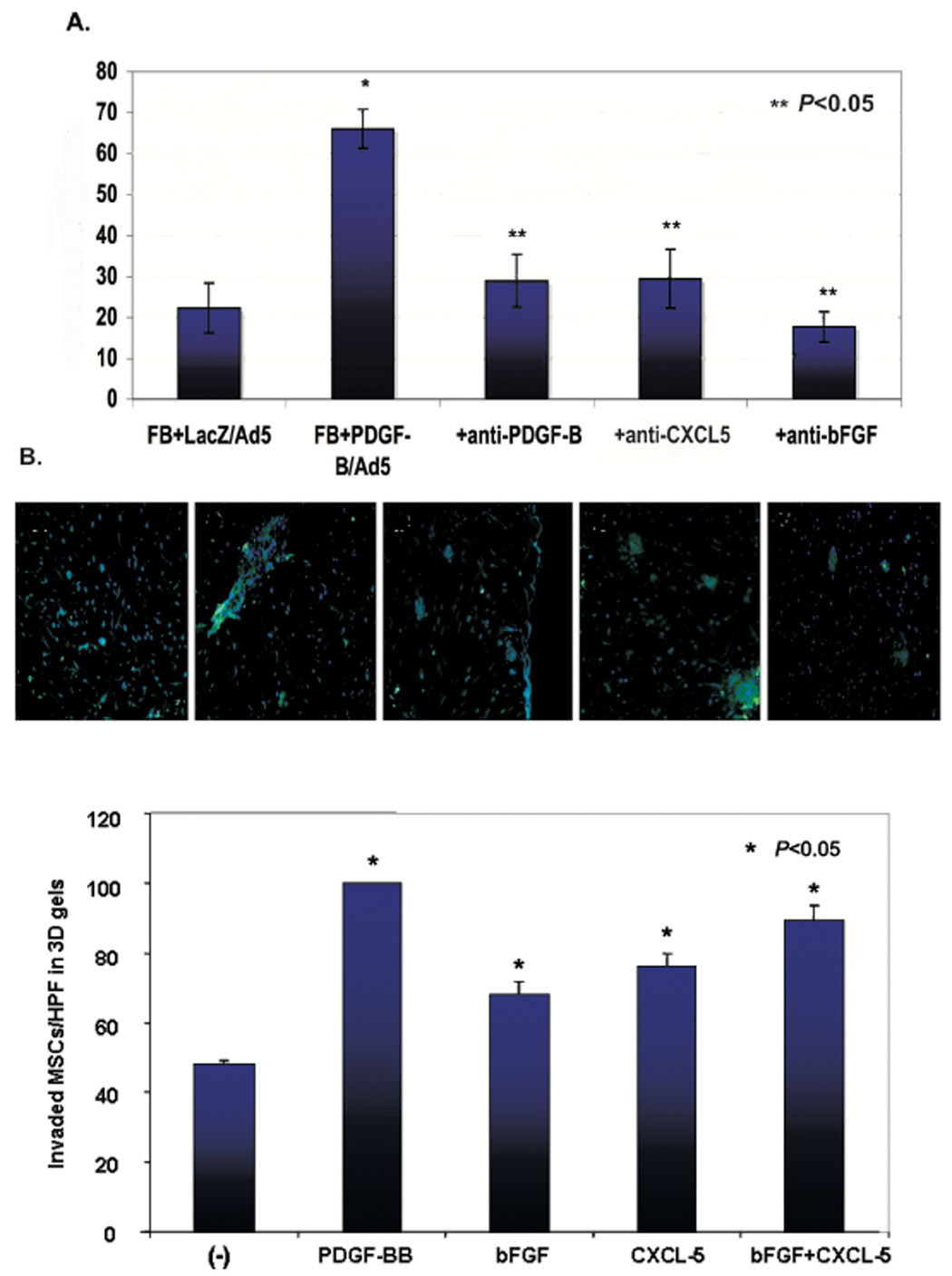
Protein array data suggested that CXCL5 and/or bFGF might function as a mediator to control invasion/migration of BM-MSCs observed in the 3D collagen model. To test whether the regulator effect of PDGF-B-aFBs on BM-MSC trafficking is mediated by CXCL5 and/or bFGF, we applied functional blocking antibodies against CXCL5 and bFGF into 3D assays. Additionally, blocking antibody against PDGF-B was also tested. Not surprisingly, addition of anti-PDGF-B blocking antibody to the PDGF-B-aFBs group resulted in complete inhibition of BM-MSC invasion/migration in 3D assays. Interestingly, application of either anti-CXCL5 or anti-bFGF blocking antibody, caused a very similar inhibitory effect on BM-MSC invasion/migration into collagen gels as that induced by anti-PDGF-B blocking antibody (Fig 5 A, B), while isotype matched control antibodies had no effect (data not shown). In contrast, we also tested the effects of exogenous CXCL5 and bFGF on invasion/migration of BM-MSCs into 3D collagen gels. Supplement of recombinant human CXCL5 (40 ng/ml) and/or bFGF (50 ng/ml) in a 3D setting, in which FBs were not activated by PDGF-B, significantly promoted invasion/migration of BM-MSCs into 3D collagen gels (Fig 5C). Taken together, these results suggested that the effect of PDGF-B-aFBs on BM-MSC trafficking in 3D assays is mediated by CXCL5 and bFGF.
Figure 5. Effect of PDGF-B-aFbs is mediated by CXCL5 and bFGF.
(A) Inhibition of BM-MSC invasion/migration by blocking antibodies against PDGF-B, CXCL5 and bFGF. Effects of blocking antibodies against PDGF-B, CXCL5 or bFGF on BM-MSC migration/invasion in 3D gel. Average percent of GFP+-BM-MSCs per HPF in the presence of FB-LacZ/Ad5 versus FB-PDGF-B/Ad5. Invasion/migration of BM-MSCs into collagen gels was significantly suppressed (**P<0.05). When recombinant PDGF-B or blocking antibodies were used in the collagen, they were added to the medium at the same concentration. Concentration of blocking antibody was within its neutralization dose (ND50), as titrated by the manufacturer, R&D Systems. All experiments were performed in duplicate and repeated three times, independently. Means ± SD were analyzed by ANOVA or student t-test. (B) Representative sections under 20X magnification were shown (order from left to right is the same as the bar graph). (C) Effect of exogenous CXCL5 and/or bFGF on BM-MSC invasion/migration into 3D gels. Invaded cell number of GFP+-BM-MSCs per HPF in the 3D gels in which FBs were not activated by PDGF-B was shown. CXCL5 and/or bFGF significantly promoted invasion/migration of BM-MSCs into 3D collagen gels compared with non-stimulated (−) group. Data were normalized by comparison with hrPDGF-B groups which were set as “100”. All experiments were performed in duplicate and repeated three times, independently. Means ± SD were analyzed by ANOVA or student t-test.
Regulatory effect of PDGF-B-aFBs on BM-MSC differentiation into myofibroblasts is also CXCL5- and bFGF-dependent
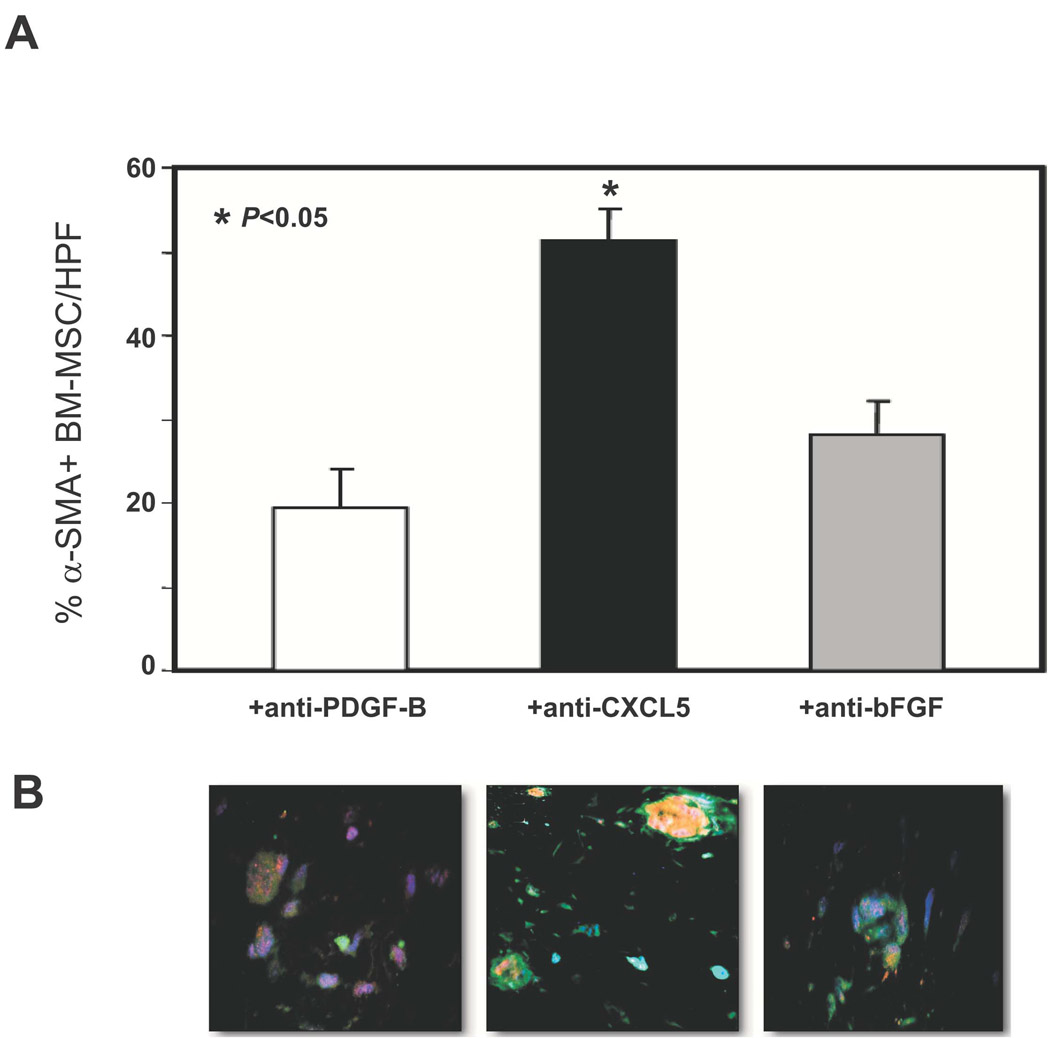
Similarly, to test whether the regulator effect of PDGF-B-aFBs on BM-MSC differentiation into myofibroblasts is mediated by CXCL5 and/or bFGF, we tested the effect of functional blocking antibodies against CXCL5 and bFGF in 3D assays. We observed that addition of anti-PDGF-B blocking antibody caused complete inhibition of mesenchymal differentiation, while anti-CXCL5 and anti-bFGF neutralizing antibodies resulted in partial, but significant inhibition of the PDGF-B-aFBs-induced pro-mesenchymal differentiation effect (Fig 6A and 6B). The inhibitory effects were specific as isotype matched control antibodies had no effect (data not shown). Thus, our data indicated that the regulatory effect of PDGF-B-aFBs on BM-MSC differentiation into myofibroblasts in 3D collagen assays is CXCL5- and bFGF-dependent.
Figure 6. Mesenchymal differentiation of BM-MSCs was partially inhibited by anti-PDGF-B, -CXCL5 or -bFGF neutralization antibodies.
(A) The effects of blocking antibodies against PDGF-B, CXCL5 or bFGF on myofibroblast differentiation of BM-MSCs in 3D gel. Average percentage of α-SMA+-BM-MSCs per HPF in 3D assays with FB-LacZ/Ad5 versus FB-PDGF-B/Ad5, respectively, under treatment with neutralization antibodies against PDGF-B, CXCL5 or bFGF. See Fig 3A for the control. A partial, but significant inhibition of differentiation was observed. Concentration of blocking antibody was within its neutralization dose (ND50), as titrated by the manufacturer, R&D Systems. All experiments were performed in duplicate and repeated three times, independently. Means ± SD were analyzed by ANOVA or student t-test. (B) Representative sections under 20X magnification were shown (see Fig 3B for the control).
Discussion
To our knowledge, this is the first report demonstrating that PDGF-B-aFBs are chemotactic to BM-MSCs in type I collagen and influence their differentiation into myofibroblasts, but not into endothelial cell lineage. Although PDGF receptors are present in BM-MSCs and can be upregulated by exogenous PDFG-B[28], we observed that the addition of soluble PDGF-B alone into collagen gel had a slight effect on induction of the observed effects. The presence of fibroblasts was a required component. The fact that PDGF-B-aFBs are requisite may be explained by secretion of secondary growth factors. Expression of CXCL5 (ENA-78) and bFGF was dramatically upregulated when fibroblasts were stimulated with PDGF-B. More importantly, CXCL5 and bFGF were shown to have significant and likely overlapping roles in promoting BM-MSC invasion/migration into collagen gel and in the induction of myofibroblast differentiation, as blocking antibodies against both CXCL5 and bFGF inhibited completely the BM-MSC invasion/migration into collagen gel and partially the differentiation into myofibroblasts. These observation strongly suggested that the regulatory effects of PDGF-BaFBs on BM-MSC trafficking and differentiation are mediated by CXCL5 and bFGF, although additional factor(s) or mechanism(s) may also be required in the control of myofibroblast differentiation. Additionally, since the addition of blocking antibody to PDGF-B resulted in complete inhibition of BM-MSC differentiation into myofibroblast, it is also possible that PDGF-B itself plays a specific and direct role in this event. Overall, this data indicates that a balanced collection of secondary soluble factors, secreted by PDGF-B-aFBs, may be necessary to induce BM-MSC invasion/migration into collagen, as well as specific differentiation cascades that favor myofibroblast differentiation.
It has been previously reported that resident skin fibroblasts are activated by PDGF-B and secrete secondary growth factors that recruit and organize keratinocyte and endothelial cell activity[17, 29]. Our findings extend the potential biological effects of PDGF-B-aFBs in cutaneous wound healing by unveiling a novel role of PDGF-B-aFBs in controlling BM-MSC recruitment and differentiation. Our findings are consistent with prior reports indicating PDGF-B as a potent mitogen for cells of mesenchymal origin, including fibroblasts, monocytes, and granulocytes[30]. However, our herein reported effects on BM-MSCs have not been previously studied. The importance of PDGF-B-activated resident wound fibroblasts is underscored by reports showing that decreased PDGF-B levels[19], incomplete fibroblast activation, dysmotility and early senescence occur in lower extremity chronic non-healing wounds, resulting in growth factor imbalance and non-healing[31–33]. Our findings may provide guidance for the development of effective therapeutic protocols in the treatment chronic and diabetic wounds as the effects of PDGF-B and its downstream mediators, CXCL5 and bFGF, identified in this study are highly relevant to the wound-healing cascade. The novel role of PDGF-B-aFBs in the induction of myofibroblast differentiation is particularly interesting, because myofibroblasts are key cells in wound contraction, which accounts for the rapid closure of full thickness wounds[2, 34]. Myofibroblasts are also major sources for the production of extracellular matrix, such as collagen, which determines tissue tension in wound tissues. Our findings open a new potential approach for developing a clinical regime which may include targeting the adult bone marrow stem cells for clinical therapy in wound healing.
Fibroblast progenitors have been identified in circulation and healing wounds[4]. Theoretically, poor recruitment of these remote myofibroblast progenitors may play a role in wound dysregulation, particularly in pathologic conditions where local mature cells are unable to effectively mediate healing. Identification and application of key growth factors involved in progenitor cell recruitment and differentiation may provide novel clues to potential targets for therapeutic intervention. Our study identifies three potential targets; PDGF-B-aFBs, CXCL5, and bFGF. We have demonstrated that BM-MSCs respond to PDGF-B-aFBs with enhanced invasion/migration into collagen and greater velocity toward the chemoattactant signal. Differentiation appears be preferentially stimulated toward the mesenchymal lineage, fibroblast, myofibroblast cascade with relative decrease in endothelial lineage differentiation. Our work suggests that PDGF-B acts directly and via subsequent secretion of multiple secondary growth factors by PDGF-B-activated dermal fibroblasts. It is likely, that the balance of these factors is as important as the presence of any one of them individually, with the exception of the major switch, PDGF-B. These findings suggest that independent manipulation of these factors in a fibroblast delivery system (PDGF-B, CXCL5, bFGF) could be utilized to affect not only recruitment of BM-MSCs, but their ability to contribute to wound contraction and healing.
Fibroblasts express both PDGFRα and β chains and PDGF-B is able to bind with both PDGFRα and PDGFRβ. It is unclear at present which receptor isoform mediates the observed effects of PDGF-B. It also remains unknown whether different isoforms of PDGF family members have the same effects as that of PDGF-B. Further studies are required to address these questions.
Cellular apoptosis and proliferation did not appear to contribute to the variance observed in the number of BM-MSC between experimental conditions. Therefore, the presence of large clusters of BM-MSCs that occurred in response to PDGF-B-aFBs suggests that these cells express adhesion molecules that promote the formation of cell clusters. The nature of these adhesion signals requires further study. It is known however that acute wound healing requires the upregulation of adhesion molecules in wound cells (e.g OB-cadherin in myofibroblasts)[35].
The nature of our in vitro 3D model is designed to allow for dissection of very complex and densely interconnected primary and secondary signals that impact BM-MSC invasion/migration and differentiation in type I collagen. It provides an alternative experimental model to study complicated biological process in which multiple elements, such as different types of cells and the interaction between them, soluble factors and extracellular matrix, are involved. This novel system is uniquely powerful by virtue of its ability to unravel otherwise highly complex processes, that contribute to wound healing. It bridges the complex in vivo and simple in vitro assays and can be applied to study cell invasion, migration, differentiation, proliferation, and survival in a variety of processes in which cell-cell communication, cell-matrix interaction and soluble factors are involved.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchese C, Felici A, Visco V, Lucania G, Igarashi M, Picardo M, Frati L, Torrisi MR. Fibroblast growth factor 10 induces proliferation and differentiation of human primary cultured keratinocytes. J Invest Dermatol. 2001;116:623–628. doi: 10.1046/j.0022-202x.2001.01280.x. [DOI] [PubMed] [Google Scholar]
- 2.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 3.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 4.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 5.Phillips TJ. Chronic cutaneous ulcers: etiology and epidemiology. J Invest Dermatol. 1994;102:38S–41S. doi: 10.1111/1523-1747.ep12388556. [DOI] [PubMed] [Google Scholar]
- 6.Harding KG, Moore K, Phillips TJ. Wound chronicity and fibroblast senescence--implications for treatment. Int Wound J. 2005;2:364–368. doi: 10.1111/j.1742-4801.2005.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanley AC, Fernandez NN, Lounsbury KM, Corrow K, Osler T, Healey C, Forgione P, Shackford SR, Ricci MA. Pressure-induced cellular senescence: a mechanism linking venous hypertension to venous ulcers. J Surg Res. 2005;124:112–117. doi: 10.1016/j.jss.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15:237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet-derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–1257. doi: 10.4065/81.9.1241. [DOI] [PubMed] [Google Scholar]
- 10.Hart CE, Forstrom JW, Kelly JD, Seifert RA, Smith RA, Ross R, Murray MJ, Bowen-Pope DF. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988;240:1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- 11.Fang L, Yan Y, Komuves LG, Yonkovich S, Sullivan CM, Stringer B, Galbraith S, Lokker NA, Hwang SS, Nurden P, Phillips DR, Giese NA. PDGF C is a selective alpha platelet-derived growth factor receptor agonist that is highly expressed in platelet alpha granules and vascular smooth muscle. Arterioscler Thromb Vasc Biol. 2004;24:787–792. doi: 10.1161/01.atv.0000120785.82268.8b. [DOI] [PubMed] [Google Scholar]
- 12.Kohler N, Lipton A. Platelets as a source of fibroblast growth-promoting activity. Exp Cell Res. 1974;87:297–301. doi: 10.1016/0014-4827(74)90484-4. [DOI] [PubMed] [Google Scholar]
- 13.Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71:1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilardetti RS, Chaibi MS, Stroumza J, Williams SR, Antoniades HN, Carnes DC, Graves DT. High-affinity binding of PDGF-AA and PDGF-BB to normal human osteoblastic cells and modulation by interleukin-1. Am J Physiol. 1991;261:C980–C985. doi: 10.1152/ajpcell.1991.261.6.C980. [DOI] [PubMed] [Google Scholar]
- 15.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 16.Pierce GF, Vande Berg J, Rudolph R, Tarpley J, Mustoe TA. Platelet-derived growth factor-BB and transforming growth factor beta 1 selectively modulate glycosaminoglycans, collagen, and myofibroblasts in excisional wounds. Am J Pathol. 1991;138:629–646. [PMC free article] [PubMed] [Google Scholar]
- 17.Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nesbit M, Schaider H, Berking C, Shih DT, Hsu MY, McBrian M, Crombleholme TM, Elenitsas R, Buck C, Herlyn M. Alpha5 and alpha2 integrin gene transfers mimic the PDGF-B-induced transformed phenotype of fibroblasts in human skin. Lab Invest. 2001;81:1263–1274. doi: 10.1038/labinvest.3780340. [DOI] [PubMed] [Google Scholar]
- 19.Pierce GF, Tarpley JE, Tseng J, Bready J, Chang D, Kenney WC, Rudolph R, Robson MC, Vande Berg J, Reid P, Kaufman S, Farrell CL. Detection of platelet-derived growth factor (PDGF)-AA in actively healing human wounds treated with recombinant PDGF-BB and absence of PDGF in chronic nonhealing wounds. J Clin Invest. 1995;96:1336–1350. doi: 10.1172/JCI118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 21.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 22.Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- 23.Gospodarowicz D, Moran JS. Stimulation of division of sparse and confluent 3T3 cell populations by a fibroblast growth factor, dexamethasone, and insulin. Proc Natl Acad Sci U S A. 1974;71:4584–4588. doi: 10.1073/pnas.71.11.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115:3339–3347. doi: 10.1172/JCI26674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall L, Burke F, Barton C, Smyth J, Balkwill F. IFN-gamma induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin Cancer Res. 2003;9:2487–2496. [PubMed] [Google Scholar]
- 27.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 28.Miyata T, Iizasa H, Sai Y, Fujii J, Terasaki T, Nakashima E. Platelet-derived growth factor-BB (PDGF-BB) induces differentiation of bone marrow endothelial progenitor cell-derived cell line TR-BME2 into mural cells, and changes the phenotype. J Cell Physiol. 2005;204:948–955. doi: 10.1002/jcp.20362. [DOI] [PubMed] [Google Scholar]
- 29.Mann A, Breuhahn K, Schirmacher P, Blessing M. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J Invest Dermatol. 2001;117:1382–1390. doi: 10.1046/j.0022-202x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 30.Siegbahn A, Hammacher A, Westermark B, Heldin CH. Differential effects of the various isoforms of platelet-derived growth factor on chemotaxis of fibroblasts, monocytes, and granulocytes. J Clin Invest. 1990;85:916–920. doi: 10.1172/JCI114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seah CC, Phillips TJ, Howard CE, Panova IP, Hayes CM, Asandra AS, Park HY. Chronic wound fluid suppresses proliferation of dermal fibroblasts through a Ras-mediated signaling pathway. J Invest Dermatol. 2005;124:466–474. doi: 10.1111/j.0022-202X.2004.23557.x. [DOI] [PubMed] [Google Scholar]
- 32.Loots MA, Lamme EN, Mekkes JR, Bos JD, Middelkoop E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch Dermatol Res. 1999;291:93–99. doi: 10.1007/s004030050389. [DOI] [PubMed] [Google Scholar]
- 33.Raffetto JD, Mendez MV, Marien BJ, Byers HR, Phillips TJ, Park HY, Menzoian JO. Changes in cellular motility and cytoskeletal actin in fibroblasts from patients with chronic venous insufficiency and in neonatal fibroblasts in the presence of chronic wound fluid. J Vasc Surg. 2001;33:1233–1241. doi: 10.1067/mva.2001.113297. [DOI] [PubMed] [Google Scholar]
- 34.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell. 2004;15:4310–4320. doi: 10.1091/mbc.E04-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]