Abstract
Axonal regeneration is normally limited after injuries to CNS white matter. Infusion of neurotrophins has been successful in promoting regenerative growth through injured white matter but this growth generally fails to extend beyond the infusion site. These observations are consistent with a chemotropic effect of these factors on axonal growth and support the prevailing view that neurotrophin-induced axonal regeneration requires the use of gradients, i.e., gradually increasing neurotrophin levels along the target fiber tract. To examine the potential of global overexpression of neurotrophins to promote, and/or modify the orientation of, regenerative axonal growth within white matter, we grafted nerve growth factor (NGF) responsive neurons into the corpus callosum of transgenic mice overexpressing NGF throughout the CNS under control of the promoter for glial fibrillary acidic protein. One week later, glial fibrillary acidic protein and chondroitin sulfate proteoglycan immunoreactivity increased within injured white matter around the grafts. NGF levels were significantly higher in the brains of transgenic compared with non-transgenic mice and further elevated within injury sites compared with the homotypic region of the non-injured side. Although there was minimal outgrowth from neurons grafted into non-transgenic mice, extensive parallel axonal regeneration had occurred within the corpus callosum up to 1.5 mm beyond the astrogliotic scar (the site of maximum NGF expression) in transgenic mice. These results demonstrate that global overexpression of neurotrophins does not override the constraints limiting regenerative growth to parallel orientations and suggest that such factors need not be presented as positive gradients to promote axonal regeneration within white matter.
Keywords: Transgenic, GFAP, Axonal guidance, Neurotropism, Chemotropism, Astrocyte, Axonal regeneration, Gradient, Geometry, Neurotrophin, Laminin
Introduction
Axonal regeneration generally fails after injury to adult CNS white matter (Ramón y Cajal, 1928), primarily due to its non-permissive environmental properties (Tello, 1911; Nathaniel and Clemente, 1959; Björklund and Stenevi, 1971; Svendgaard et al., 1976; Heinicke, 1978; David and Aguayo, 1981), although intrinsic factors likely also play a role (Plunet et al., 2002). The non-permissiveness of the CNS environment for axonal regeneration has been attributed to inhibitors associated with myelin and/or inhibitors such as chondroitin sulfate proteoglycans (CSPG) that form in astrogliotic scars after injury (reviewed by Sandvig et al., 2004). Insufficient neurotrophic support may also contribute to regeneration failure (Kromer and Cornbrooks, 1987; Berry et al., 1988; Tuszynski et al., 1990, 1994; Hagg et al., 1991; Kawaja et al., 1992; Houle and Ziegler, 1994; Varon and Conner, 1994; Xu et al., 1995; Nakahara et al., 1996; Bregman et al., 1997; Bradbury et al., 1999; Liu et al., 1999; Namiki et al., 2000; Ramer et al., 2000; Romero et al., 2000; Bamber et al., 2001; Zhou et al., 2003).
Salutary axonal regeneration may depend not only on regrowth but also on such growth being organized in parallel with the fiber tract. One approach that has been utilized successfully to promote parallel axonal regeneration is to take advantage of the chemotropic effect of neurotrophins such as nerve growth factor (NGF; Levi-Montalcini, 1976; Gundersen and Barrett, 1979; Scalapino et al., 1996). Although local infusion of neurotrophins has been shown to promote axonal regeneration within white matter (Oudega and Hagg, 1996, 1999; Lu et al., 2004), this regenerative growth failed to extend beyond the site of infusion, supporting the conclusion that gradients of such factors may be required (Lu et al., 2004). Consequently, it is not clear whether parallel, long-distance regenerative growth requires a neurotrophic gradient (Oudega and Hagg, 1996, 1999; Lu et al., 2004), increased neurotrophins at injury sites (Coumans et al., 2001), or could be accomplished by non-graded, global elevation of neurotrophins. In the latter case, global elevation may promote regenerative axonal growth but such growth may be induced to extend in non-parallel orientations or undergo undesirable branching within the fiber tract prior to reaching the target (Yasuda et al., 1990).
In the present study, we sought to determine whether axonal regeneration can be promoted through injured white matter, and subsequently constrained to a parallel orientation, in the presence of globally elevated NGF. Adult transgenic (GFAP-NGF-Tg) mice overexpressing NGF throughout the CNS under control of the promoter for glial fibrillary acid protein (GFAP) were utilized as a model of CNS injury. Because GFAP is invariably upregulated within astrogliotic sites of CNS injury, NGF levels in homogenized tissue were measured 1 week following parasagittal knife lesions of the brain using a sensitive 2-site ELISA. To assess the effects of global NGF overexpression on axonal regeneration, adult superior cervical ganglia (SCG) were grafted into the corpus callosum and outgrowth within the fiber tract was assessed histologically using glyoxylic acid catecholamine staining 1 week later.
Materials and methods
Surgical procedures
Three- to seven-month-old transgenic mice (n=10) expressing NGF under control of the GFAP promoter (GFAP-NGF-Tg mice; University of Cincinnati colony established by breeders kindly provided by Dr. Michael D. Kawaja, Queen's University, Kingston, ON, who also provided genotype data based on RT-PCR) and non-transgenic (either C57BL/6 mice or siblings that did not inherit the transgene as determined by RT-PCR) mice (n=7) received parasagittal knife lesions. Alternatively, superior cervical ganglia were transplanted from non-transgenic mice into non-transgenic mice (Wt/Wt; n=15), from non-transgenic mice into GFAP-NGF-Tg mice (Wt/NGF; n=13), from GFAP-NGF-Tg mice into non-transgenic mice (NGF/Wt; n=10) or from GFAP-NGF-Tg mice into GFAP-NGF-Tg mice (NGF/NGF; n=28). Prior to surgery, all mice were deeply anesthetized using isoflurane and administered buprenex (0.5 μg/g) subcutaneously to control pain. For both procedures, a midline scalp incision was made and the scalp was retracted. Lidocaine (0.08 cc) was applied to the calvaria and the periosteum was removed.
For the knife-lesion procedure, a parasagittal linear craniectomy was made using a Dremel tool, from bregma +1 mm to bregma -3 mm, 1 mm to the right of the midline. A modified scalpel blade (4 mm long segment of a #11 blade) was then slowly lowered into the brain to a depth of 3 mm below the skull surface using a stereotaxic frame. The blade was slowly withdrawn, hemostasis attained using sterile polyester fiber tipped applicators, the cavity filled with gelfoam and the incision closed with wound clips.
For grafting, the host mouse was placed in a stereotaxic frame in sternal recumbency. A 2-mm diameter craniectomy was then made using a Dremel tool, 1 mm anterior and 1 mm lateral to bregma. A 2.3-mm deep cavity was made in the brain by aspiration using a stereotactically controlled Pasteur pipet and vacuum pump. Hemostasis was attained using sterile polyester fiber tipped applicators. The donor mouse was placed in dorsal recumbency and a midline incision was made on the ventral side of the neck. Blunt dissection was used to expose the bifurcation of the common carotid artery and the superior cervical ganglion was removed using microscissors. The incision was closed with wound clips or sutures. The graft was placed within the prepared cavity in the host mouse at a depth of 2 mm below the pial surface using fine forceps or by using a modification of established procedures (Itakura et al., 1994; Breeze et al., 1995). In the latter case, the graft was placed in the end of a 2-mm-long glass cannula. The cannula was then stereotactically guided to the target and the graft ejected by inserting an obdurator into the cannula. The graft was allowed to settle for 2 min and then the guide cannula was slowly withdrawn while the obdurator was held in place. The cavity was subsequently filled with gelfoam and the incision closed with wound clips. For allografting procedures, the donor and host mice were anesthetized concurrently throughout the duration of the procedure (45 to 60 min).
All mice were administered buprenex immediately after surgery and for 2 days BID. One week after surgery, all mice were again deeply anesthetized with isoflurane and sacrificed by decapitation. Brains were rapidly removed, frozen on dry ice and stored at -70 °C. All mice were handled in accordance with the guidelines of the University of Cincinnati Institutional Animal Care and Use Committee.
GFAP immunohistofluorescence
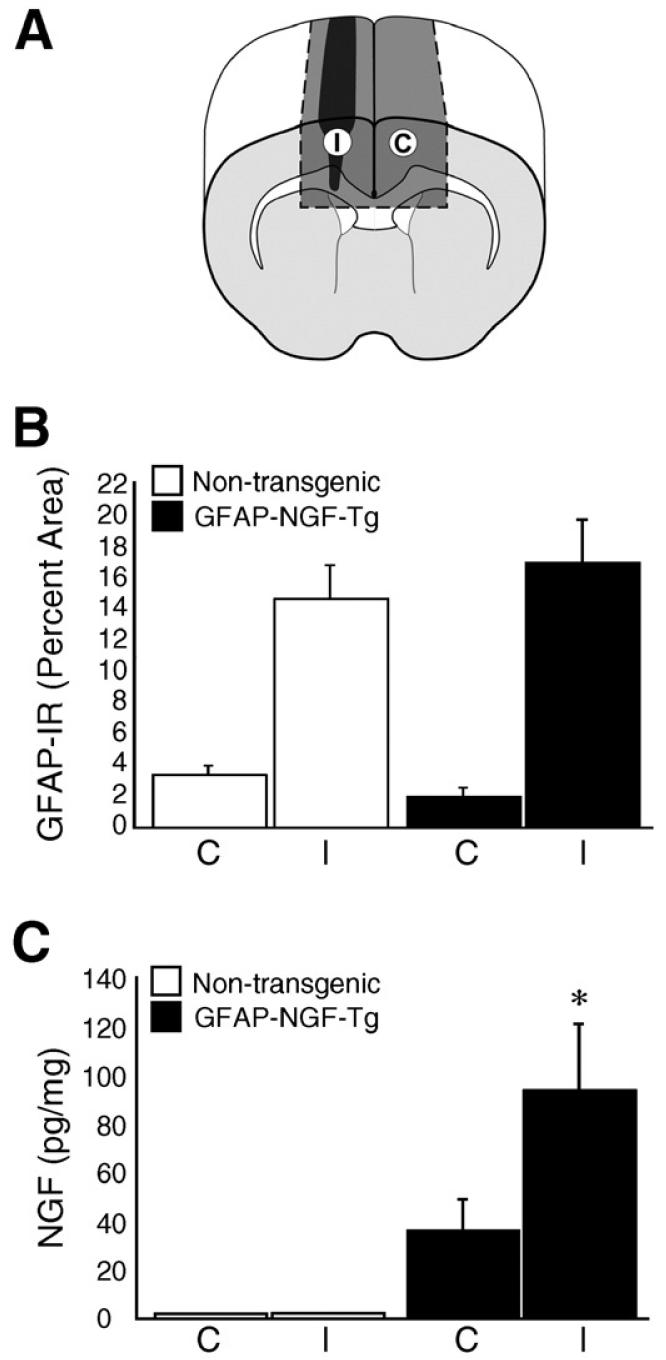
Sixteen-micrometer-thick cryostat sections were thaw-mounted on superfrost slides and fixed using 4% paraformaldehyde for 1 h at 4 °C. Sections were rinsed three times with 0.01 M PBS and incubated with blocking solution (0.01 M PBS containing 10% goat serum and 0.3% Triton X-100) for 1 h at room temperature (RT) in a humidified chamber. The sections were rinsed twice and incubated with Cy3-conjugated monoclonal anti-GFAP (catalog #C9205; Sigma, St. Louis, MO; 1:500 in blocking solution) overnight at RT. Images were captured using a Nikon Microphot microscope, Nikon DM580 fluorescent filter and a MicroPublisher 5.0 RTV digital camera with uniform settings across sections. To quantify GFAP-immunofluorescence in knife-lesioned mice, 4 to 5 sections (spaced ~80 μm apart) through the posterior 400 μm of the lesion were selected. In thresholded regions of interest, corresponding to the dark gray areas enclosed in dashed lines shown in cross section in Fig. 2A, the number of GFAP-immunoreactive pixels was counted using NIH ImageJ software. The results are expressed as percent GFAP-immunohistofluorescent tissue area and averaged across sections for each animal.
Fig. 2.
Quantification of GFAP-IR and NGF protein in knife-lesioned mice. (A) The illustration shows regions in cross-section (dark gray areas enclosed in dashed lines) in which GFAP-IR was quantified on the side ipsilateral (I) and contralateral (C) to the knife lesion (black). This same illustration shows the blocks of tissue (dark gray volumes enclosed in dashed lines) sampled for measurement of NGF protein using ELISA. (B) GFAP-IR is quantified as percent of tissue area that is GFAP-immunoreactive. GFAP-IR was significantly higher on the injured side (I) of both non-transgenic (white) and GFAP-NGF-Tg (black) mice compared with the homotypic region on the contralateral side (C). GFAP-IR was not significantly different between non-transgenic and GFAPNGF-Tg mice. (C) NGF protein was significantly higher in tissue sampled from GFAP-NGF-Tg mouse brains compared with non-transgenic mouse brains irrespective of injury. NGF was further elevated (*p<0.05) on the injured side (I) compared with the uninjured side (C) in GFAP-NGF-Tg mice. Bars represent mean±SEM.
CSPG immunohistofluorescence
Sixteen-micrometer-thick cryostat sections were thaw-mounted on superfrost slides and fixed using cold acetone for 10 min at 4 °C. Sections were then incubated for 1 h at RT in blocking solution (10% normal goat serum in 10 mM PBS), then excess blocking solution was removed and sections were incubated with mouse monoclonal anti-chondroitin sulfate (CS56; catalog #C-8035; Sigma; 1:200 in blocking solution) overnight at RT. Sections were then rinsed three times with 10 mM PBS (each rinse, 5 min), incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (catalog #A-11029; Molecular Probes, Eugene, OR; 1:1000 in blocking solution) for 1 h at RT in a humidified chamber. Sections were rinsed again, air-dried and cover-slipped using Vectashield medium (catalog #H-1400; Vector Laboratories, Buringame, CA). Images were captured using a Nikon Microphot microscope, Chroma 41001 HQ:F fluorescent filter (Rockingham, VT) and a MicroPublisher 5.0 RTV digital camera with uniform settings across sections.
Laminin immunohistofluorescence
Sixteen-micrometer-thick cryostat sections were thaw-mounted on superfrost slides and fixed with Carnoy solution for 30 min at RT. Sections were rinsed 3 times with 10 mM PBS and incubated for 30 min at RT in blocking solution (3% BSA, 3% normal goat serum and 0.1% DMSO in 10 mM PBS). Sections were then incubated overnight at RT in a humidified chamber with rabbit anti-mouse laminin (catalog BT-594; Biomedical Technologies, Inc.; 1:1000 in blocking solution). Sections were rinsed, incubated with Alexa Fluor 546-conjugated goat anti-rabbit IgG (catalog #A-11035; Molecular Probes, Eugene, OR; 1:1000 in blocking solution) for 1 h at RT in a humidified chamber. Sections were then rinsed, air-dried and cover-slipped using Vectashield medium (catalog #H-1400; Vector Laboratories, Buringame, CA). Images were captured using a Nikon Microphot microscope, Nikon DM580 fluorescence filter and a MicroPublisher 5.0 RTV digital camera.
NGF ELISA
From knife-lesioned brains, blocks of tissue were dissected from the injured and non-injured sides, corresponding to the dark gray volumes enclosed in dashed lines shown in Fig. 2A. In all cases, sampled tissue consisted primarily of dorsal neocortex, deep white matter and corpus callosum. All tissue was weighed and stored at -70 °C. Wells in 96-well plates were coated with either goat non-immune serum or goat anti-mouse NGF antiserum in 50 mM sodium carbonate/bicarbonate buffer, pH 9.6, overnight at 4 °C. The following morning, the plates were washed (10 mM PBS with 0.05% Tween 20) and the wells blocked with 1% FBS in PBS for 1 h at room temperature. Tissue samples were homogenized on ice by sonication in 10 mM PBS buffer containing 0.1% Tween, 0.5% bovine serum albumin, 0.0001% aprotinin, 0.1 mM benzethonium chloride and 0.1 mM phenylmethyl sulfonyl fluoride. Samples were then centrifuged at 15,000×g for 30 min. Plates were washed and tissue homogenate supernatants added. A series of NGF standards, ranging from 0 to 100 pg/100 μl, were included on each plate. The plate was incubated overnight at 4 °C, and after washing, monoclonal anti-NGF (1G3, 1:200 in 10 mM PBS with 1% FBS and 0.02% Tween) was added and allowed to bind overnight at 4 °C. Affinity-purified biotinylated goat anti-rat IgG (1:5000 in PBS with 1% FBS) was then added for 2.5 h at room temperature, followed by streptavidin-horseradish peroxidase (1:2000 in 10 mM PBS with 1% FBS) for 1 h. The plate was developed using o-phenylenediamine as a chromogen and the reaction allowed to proceed for 7 min in the dark at room temperature. The reaction was stopped with 2 M H2SO4. Plates were read using a Bio-Rad 2550 EIA reader at 492 nm. Non-specific binding was controlled by subtracting the optical densities of wells containing non-immune serum from those coated with goat anti-mouse antiserum. NGF protein levels were estimated by linear interpolation between the two nearest standards and normalized to tissue weight.
Catecholamine histofluorescence
Transplanted neurons and their axons were detected using the sucrose phosphate glyoxylic acid (SPG) histofluorescence method for detecting catecholamines. Sixteen-micrometer-thick cryostat sections of fresh-frozen brain were thaw-mounted on frosted slides and dipped 3 times in SPG buffer (aqueous solution containing 6.8% sucrose, 3.2% KH2PO4, 1% glyoxylic acid, pH 7.4). Sections were dried under a cool blower for 5 min, covered with heated mineral oil (80 °C) and placed in a 95 °C oven for 2.5 min. Sections were then cover-slipped and imaged using a Nikon Microphot microscope, Nikon DM430 fluorescent filter and a MicroPublisher 5.0 RTV digital camera with uniform settings across sections.
Quantification of neuronal viability
Additional 16-μm-thick sections were collected and stained with cresyl violet. All cases where grafts were placed within the corpus callosum were assessed, including those that were not stained using the SPG method. The number of neurons within the grafts was counted by an individual who was blinded with respect to the donor and host genotypes. Neurons were identified based on morphology, particularly size and the presence of a neuron-like profile. The area (pixels) of the graft in each section was also measured using ImageJ software (NIH) and converted to square millimeters using a known standard. The neuronal density was computed for each section by dividing the neuron count by the graft area (expressed as neurons per square millimeters). For each case, an average of 3 to 4 sections through the middle of the graft was measured (some sections were excluded based on poor histology; neither host nor donor type was a significant factor in the number of sections excluded, Wilcoxon Rank Sum, p=0.81) and averaged for each of the 3 indices: raw neuron count, graft area and neuronal density.
Quantification of axonal length
Axonal outgrowth within the corpus callosum was quantified from SPG+ grafts (cases were excluded if they showed no SPG staining) placed within the corpus callosum. In each case, the lengths of the five longest sympathetic axons in the corpus callosum were measured (in pixels) using ImageJ by drawing a perpendicular line from the distal end of each axon to a vertical line through the proximal border of the graft. The length of each line was then converted to mm using a known standard. Each mouse was ultimately represented by the average of these five axons for the purpose of statistical analysis (i.e., each mouse was treated as one independent observation).
Statistical analysis
Comparisons of GFAP-IR were made using two-way mixed design ANOVA (factors: GFAP-NGF-Tg vs. non-transgenic; ipsilateral vs. contralateral to injury). Comparisons of NGF levels from ELISAwere made between GFAP-NGF-Tg and non-transgenic groups and between injured and non-injured sides using two-way mixed design ANOVA (one-tailed, a priori hypotheses were that NGF in GFAP-NGF-Tg mice>NGF in non-transgenic mice and that NGF ipsilateral to injury>contralateral NGF in GFAP-NGF-Tg mice). Comparisons of graft area, raw neuron counts, neuronal density and axonal length were made between the groups (NGF/NGF, Wt/NGF, Wt/Wt, NGF/Wt) using two-way factorial design ANOVA.
Results
GFAP immunoreactivity is increased at injury sites
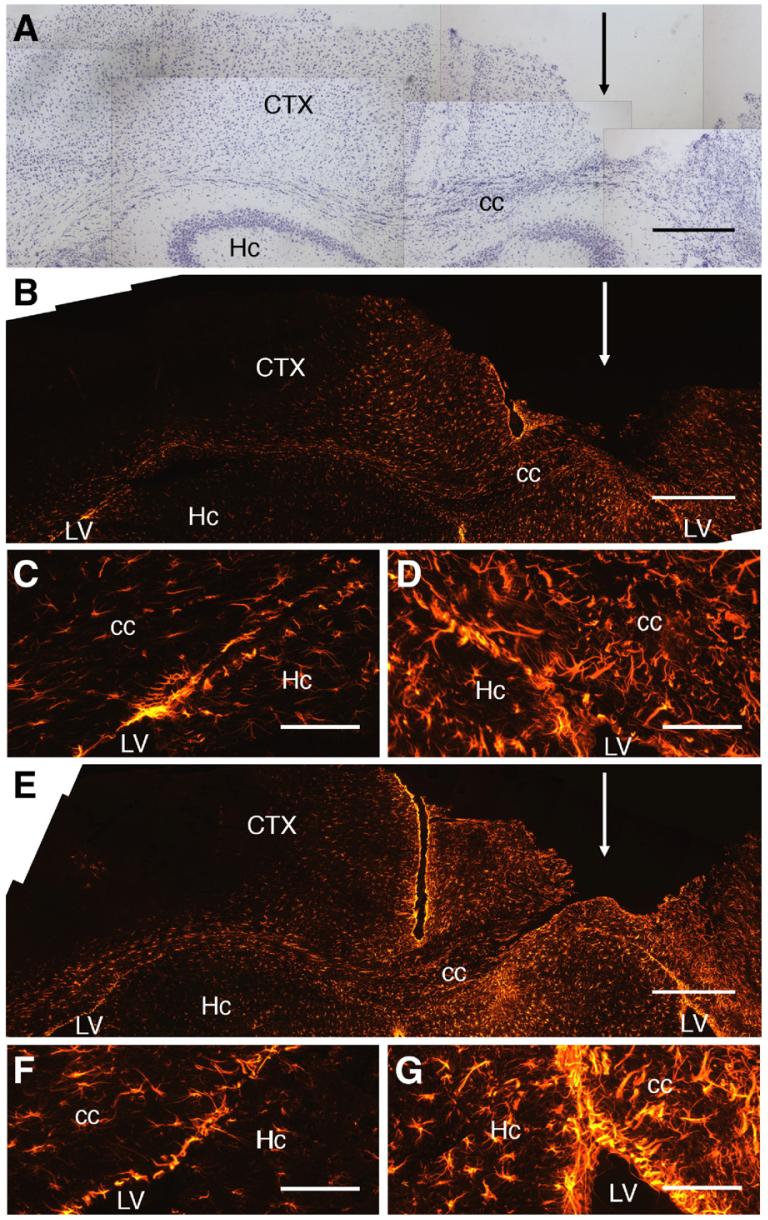
One week after parasagittal knife lesion, furrows in the dorsal surface of the cortex, coinciding with the plane of knife entry, indicated significant tissue loss at the injury site. This furrow could be easily identified in cross-section by staining with cresyl violet (Fig. 1A) and immunostaining for GFAP (Figs. 1B and E). Cresyl violet staining showed loss of neuronal perikarya for several hundred micrometers on either side of the plane of knife entry (Fig. 1A). The extent of injury to the corpus callosum varied between mice. In some cases, the corpus callosum was completely transected at the ventral margin of the lesion. In other cases, the corpus callosum was densely infiltrated by small cells, which were presumably phagocytes. No obvious systematic differences in cresyl violet staining were observed between GFAP-NGF-Tg and non-transgenic mice.
Fig. 1.
GFAP-IR is increased near parasagittal knife lesions. (A) Cresyl violet-stained section of brain from a GFAP-NGF-Tg mouse having undergone a parasagittal knife lesion (the plane of knife entry is indicated by the arrow) 1 mm to the right of midline. The injured hemisphere (right side of micrograph) exhibited a furrow in the neocortex (CTX) encroaching on the corpus callosum (cc). Extensive neuronal loss occurred in the 300-μm wide cortical zone medial to the injury. Small cells, likely phagocytes, infiltrated the corpus callosum within the zone of injury. (B) Low power micrograph of a brain section from a non-transgenic mouse. The injured hemisphere (right) showed markedly increased GFAP-IR in all regions, including the corpus callosum and the overlying neocortex, compared with the uninjured (left) side. (C, D) High power micrographs centered at the apices of the lateral ventricles (LV) of the injured non-transgenic mouse shown in panel B. Astrocytes appeared hypertrophic in all areas on the injured side (panel D) compared with the corresponding contralateral field (panel C). (E) Low power micrograph showing a section from a GFAP-NGF-Tg mouse that had undergone knife lesion in the same manner. Increased GFAP-IR was evident on the injured side (right) similar to that in the non-transgenic mouse. (F, G) High power images of the injured (panel G) and uninjured (panel F) sides of the GFAP-NGF-Tg mouse section shown in panel E. Hc, hippocampus. Scale bars=500 μm in panels A, B, E; 150 μm in panels C, D, F, G.
GFAP immunoreactivity (GFAP-IR) was enhanced in the region of the knife lesion compared with the contralateral side in all non-transgenic (n=7; Fig. 1B) and GFAP-NGF-Tg (n=10; Fig. 1E) mice. High power images showed marked hypertrophy of astrocytes within the corpus callosum and subependymal layers at the site of injury in both non-transgenic (Fig. 1D) and GFAP-NGF-Tg (Fig. 1G) mice compared with the homotypic fields of the uninjured side (Figs. 1C and F, respectively). There were no obvious qualitative differences in the intensity or distribution of GFAP-IR between GFAP-NGF-Tg and non-transgenic mice.
The percentage of tissue area that is GFAP-immunoreactive was assessed in both non-transgenic and GFAP-NGF-Tg mice on the sides ipsi- and contralateral to the knife lesion. Regions of interest, indicated by the dark gray shaded fields (enclosed in dashed lines) shown in cross-section in Fig. 2A, were quantified in serial sections. Two-way mixed design ANOVA revealed a significant effect of knife lesion (I) compared with the non-injured side (C) on percent GFAP-immunoreactive area (Fig. 2B; p<0.0001). However, there was no significant GFAP-NGF-Tg/non-transgenic group effect (p=0.83) or interaction between group and side (p=0.38) on GFAP-IR. Thus, injury appears to significantly enhance GFAP-IR in both GFAP-NGF-Tg and non-transgenic mice and the astrogliotic response to injury, at least to the extent reflected by changes in GFAP-IR, is not significantly affected by the overexpression of NGF.
NGF is elevated near CNS injuries in GFAP-NGF-Tg mice
To determine if NGF protein is increased after injury, tissue samples were dissected from fresh-frozen brains and NGF measured using a sensitive two-site ELISA. Ipsilateral to knife lesions, blocks of brain (dark gray volume, labeled I, enclosed by dashed lines in Fig. 2A) were collected that included the lesion. Each block was roughly 3.5 mm long, extending from ~1 mm anterior to ~2.5 mm posterior to bregma, and contained mostly dorsal neocortex, cingulum and corpus callosum. For comparison, an anatomically corresponding block was collected from the contralateral side (dark gray volume, labeled C, enclosed by dashed lines in Fig. 2A).
NGF protein ranged from 0 to 6 pg per mg wet weight of tissue from knife-lesioned non-transgenic mice with no significant difference between sides. In knife-lesioned GFAP-NGF-Tg mice, NGF protein ranged from 20 to 280 pg per mg on the injured side and from 6 to 115 pg per mg of tissue on the non-injured side. Two-way mixed-design ANOVA revealed significantly higher NGF levels in the GFAP-NGF-Tg (n=10) mice compared with non-transgenic (n=7) mice (Fig. 2C; p<0.01), consistent with previous studies (Kawaja and Crutcher, 1997; Krol et al., 2000; Hannila and Kawaja, 2003). Moreover, there was a significant injury vs. non-injury effect (p<0.05) as well as a significant interaction between genotype group and side (p<0.05). The effect of injury was highly robust: NGF levels were higher on the injured side compared with the non-injured side in all 10 GFAP-NGF-Tg cases examined. Thus, NGF appears to be significantly elevated near CNS injuries in GFAP-NGF-Tg mice but not in non-transgenic mice. Because the knife lesions did not involve transplantation, this elevation was not dependent on the placement of grafted tissue.
Graft viability
One week after grafting of superior cervical ganglia into the brains of GFAP-NGF-Tg and non-transgenic mice, all mice were sacrificed and brains sectioned for histological analysis. The SPG catecholamine histofluorescence technique was used to visualize grafts and associated axonal outgrowth. Within the corpus callosum, native fibers labeled with this technique are limited to rare central noradrenergic fibers that can be easily distinguished from sympathetic fibers by morphology and intensity of labeling (Crutcher, 1982). Therefore, within the corpus callosum, the SPG technique provides a fairly specific means of identifying grafted sympathetic ganglia and their axons.
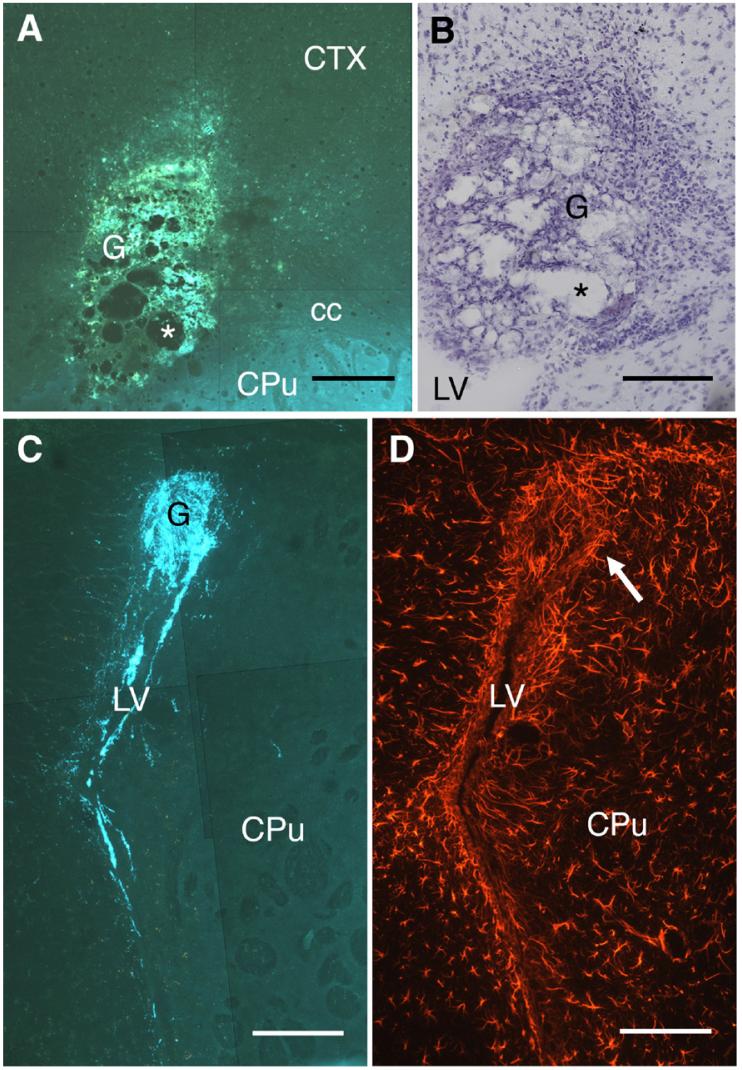
A total of 66 mice underwent grafting (NGF/NGF, 28; Wt/NGF, 13; Wt/Wt, 15; NGF/Wt, 10). In 32 cases, the grafts did not settle within the corpus callosum and were excluded from further analysis. The remaining cases (NGF/NGF, 11; Wt/NGF, 10; Wt/Wt, 7; NGF/Wt, 6) were evaluated for graft viability based on both SPG and cresyl violet staining. Using the SPG technique, non-viable sympathetic grafts were easily identified by the predominance of yellow autofluorescence, in contrast to the blue fluorescence induced by SPG staining of catecholamines such as in the caudate-putamen (Fig. 3A). Stained with cresyl violet, non-viable grafts were characterized by the presence of cystic cavities and small, densely packed cells, presumably phagocytes, and by the absence of neuronal perikarya (Fig. 3B). In 4 cases (1 from each group), the grafts were clearly dead based on negative SPG staining within the graft (with positive staining within the caudate-putamen indicating that the SPG method was working) and the absence of cresyl violet-stained neurons. In contrast, viable grafts stained with SPG generally contained a dense plexus of blue fluorescent fibers and, when outgrowth occurred, similar fibers could be clearly visualized within the host brain. For example, Fig. 3C shows a viable, SPG+ graft that settled within the lateral ventricle, rather than the corpus callosum, of a GFAP-NGF-Tg mouse with outgrowth occurring along the ventricular wall. Similar outgrowth along the ventricular wall has been observed when sympathetic ganglia were grafted into the brains of normal rats receiving intraventricular infusions of NGF (Saffran and Crutcher, unpublished observations). The periventricular outgrowth appeared to follow the relatively intense GFAP-IR within the subependymal layers (Fig. 3D).
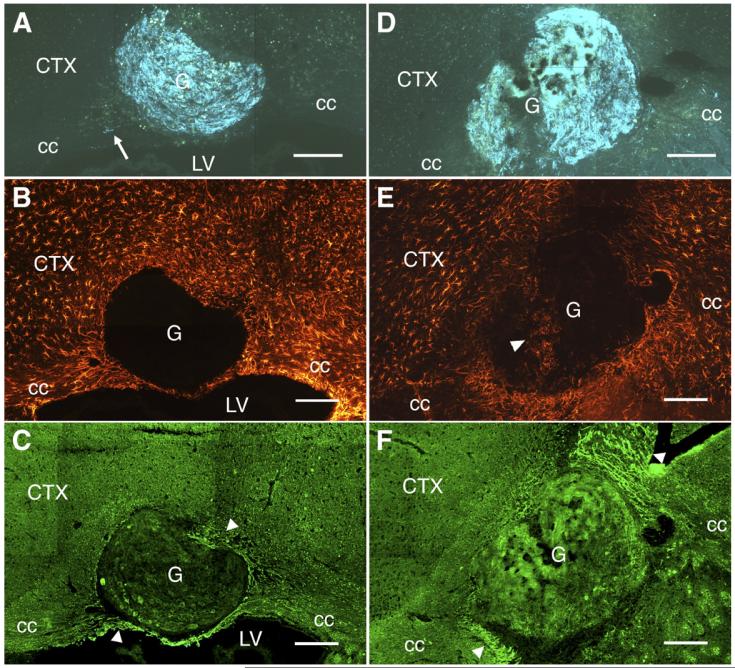
Fig. 3.
Viable grafts are clearly distinguishable from non-viable grafts. (A) A non-viable sympathetic graft (G) is shown placed within the corpus callosum (cc) stained using the SPG method. The graft showed predominantly yellow autofluorescence and contained several cystic cavities (*). In contrast, the caudate-putamen (CPu) was stained blue due to dense dopaminergic innervation. (B) A section adjacent to panel A stained with cresyl violet. As is the case with SPG staining, cystic cavities (*) were evident but no neurons could be seen. Instead, the graft was replete with small cells, likely to be phagocytes. (C) A viable sympathetic graft (G), stained blue using the SPG method, located within the lateral ventricle (LV) of a GFAP-NGF-Tg mouse (NGF/NGF). A dense plexus of blue fibers was found within the graft as well as along both ventricular walls. (D) A section adjacent to panel C showing the typically intense GFAP-IR within the subependyma, as well as elevated GFAP-IR anterior to the graft (arrow). CTX, neocortex. Scale bars=200 μm in panel A; 150 μm in panels B-D.
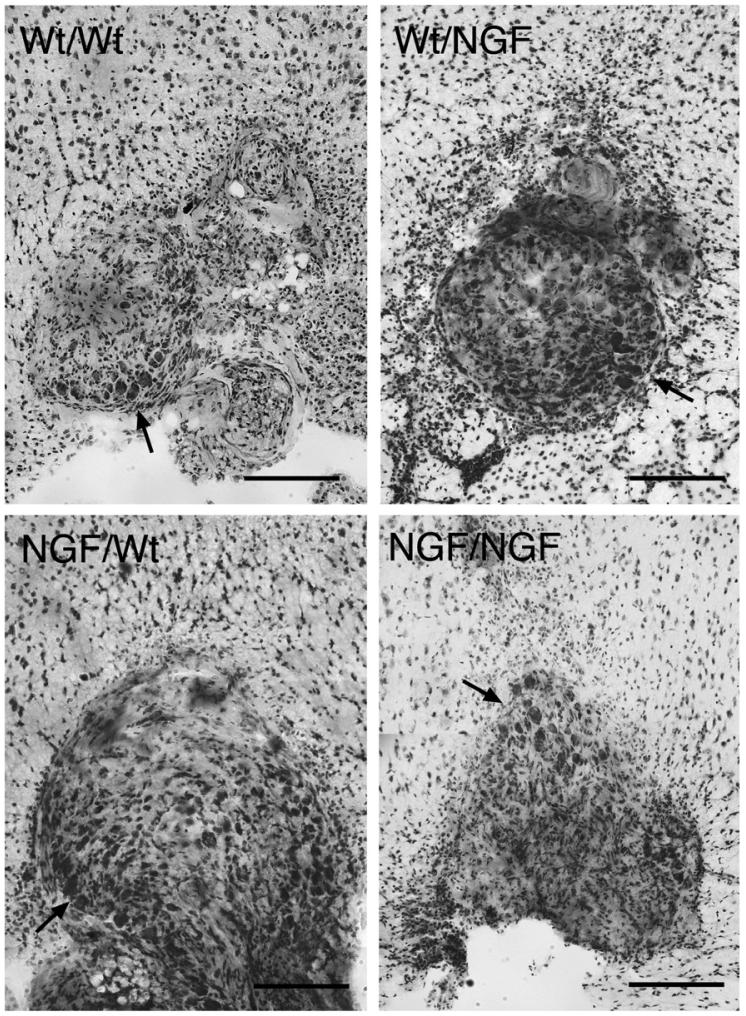
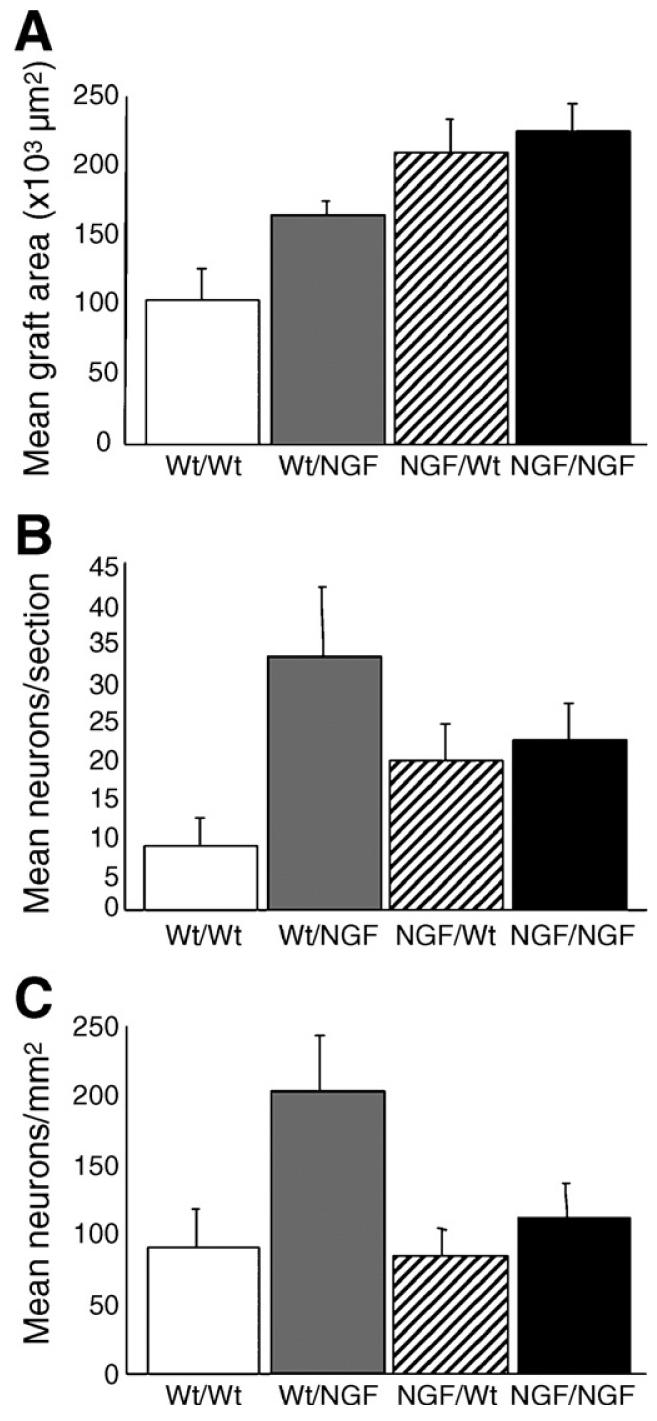
Representative examples of viable grafts stained with cresyl violet placed within the corpus callosum in each of the 4 transplantation paradigms are shown in Fig. 4 (compare with the non-viable graft stained with cresyl violet in Fig. 3B). Neurons usually appeared in clusters, generally along the perimeter of viable grafts. Neurons were counted and graft areas measured to assess the relative viability of the grafts among the transplantation paradigms. Grafts harvested from GFAP-NGF-Tg donors had significantly greater areas than those harvested from non-transgenic donors (Fig. 5A; two-way ANOVA, p=0.001), consistent with previously documented differences in superior cervical ganglion size in situ (Coome and Kawaja, 1999). Two-way ANOVA detected a significant host effect on the average neuronal count per section (Fig. 5B, p=0.02). When the counts were expressed in terms of neuronal density (Fig. 5C; neurons per square millimeters), a significant host effect was also detected (p=0.02) as well as a nearly significant interaction between the donor and host variables (p=0.06). Examination of the summary data in Fig. 5C suggests that greater survival is promoted specifically in the cases where non-transgenic ganglia have been grafted to GFAP-NGF-Tg hosts.
Fig. 4.
Representative images of grafted ganglia stained with cresyl violet (panel labels: donor/host). Sympathetic neuronal profiles could be identified based on size and morphology, and generally appeared in isolated clusters (arrows) near the perimeters of grafts. Scale bars=200 μm.
Fig. 5.
Quantification of neurons within grafted sympathetic ganglia stained with cresyl violet. (A) Graft area was significantly greater in ganglia harvested from GFAP-NGF-Tg mice compared with those harvested from non-transgenic mice (p=0.001). No significant host effect on graft area was detected (p=0.11; host × donor, p=0.32). (B) Significantly more neurons were counted per section in GFAP-NGF-Tg hosts compared with non-transgenic hosts (p=0.02). The donor effect was not significant (p=0.99; host × donor, p=0.06). (C) Neuronal density was significantly greater in GFAP-NGF-Tg hosts (p=0.02). No significant donor effect on neuronal density was detected (p=0.09; host × donor, p=0.12). Bars represent mean±SEM.
GFAP and CSPG immunoreactivity are increased near grafts
Sympathetic ganglion grafts within the corpus callosum were readily visualized by SPG staining in both non-transgenic (Fig. 6A) and GFAP-NGF-Tg (Fig. 6D) mice. GFAP-IR was enhanced in brain tissue surrounding the grafts regardless of host genotype (Figs. 6B and E). The grafts were usually devoid of GFAP-IR but occasionally GFAP-immunoreactive processes or cells extended into the transplanted ganglia (Fig. 6E). However, there were no obvious systematic differences in GFAP-IR within or surrounding the grafts as a function of host or donor genotype or graft viability.
Fig. 6.
Sympathetic grafts (G) placed in both GFAP-NGF-Tg and non-transgenic brains were associated with increased GFAP- and CSPG-IR in the host. (A) An SPG-stained, non-transgenic sympathetic graft placed in the corpus callosum (cc) of a non-transgenic mouse. The graft contained many fibers but only one could be seen exiting the graft, extending a short distance into the corpus callosum (arrow). (B) An adjacent section through the graft shown in panel A immunostained for GFAP. As demonstrated here, GFAP-IR is rarely observed within grafts but increased GFAP-IR surrounding the graft was always evident. (C) An adjacent section through the graft shown in panel A immunostained for CSPG. CSPG-IR is generally greater in the cc compared with the overlying cortex (CTX) and the graft interior and is further elevated in patches within the cc on either side of the graft (arrowheads). (D) An SPG-stained graft harvested from a GFAP-NGF-Tg mouse placed in the corpus callosum of a GFAP-NGF-Tg mouse. (E) Section adjacent to panel D showing elevation of GFAP-IR around the graft. GFAP-immunoreactive cells (arrowhead) were seen within the graft in this case, though this observation did not generalize to this (NGF/NGF) or any experimental group. (F) As in panel C, CSPG-IR is elevated in patches on either side of the graft. LV, lateral ventricle. Scale bars=200 μm.
CSPG-IR was observed throughout the host brain, especially within white matter (Figs. 6C and F). The grafts also appeared to exhibit moderate levels of CSPG-IR, particularly around the neurons. CSPG-IR was circumferentially enhanced in brain tissue surrounding the grafts. More distally, enhanced CSPG-IR often appeared within the injured corpus callosum as fibrillar bands oriented in parallel with the longitudinal axis of the tract.
Global NGF overexpression promotes parallel axonal regeneration in white matter
The SPG technique was used to detect sympathetic axons within the grafts and host corpus callosum. Grafts were usually well integrated with the host tracts (Figs. 7 and 8). SPG staining was associated with sympathetic axons, rather than somata, and viable grafts placed in both GFAP-NGF-Tg and non-transgenic brains generally contained a dense plexus of SPG+ axons. Nevertheless, ganglia grafted into the corpus callosum of nontransgenic mice showed minimal outgrowth into the host brain (the most impressive examples of outgrowth in non-transgenic hosts are shown in Figs. 7A and B). When outgrowth did occur, it rarely, if ever, extended beyond injured (yellow autofluorescent) regions of the adjacent fiber tract (Figs. 7A and B). Within these regions, axonal growth was sometimes orthogonal to the fiber tract (Fig. 7B).
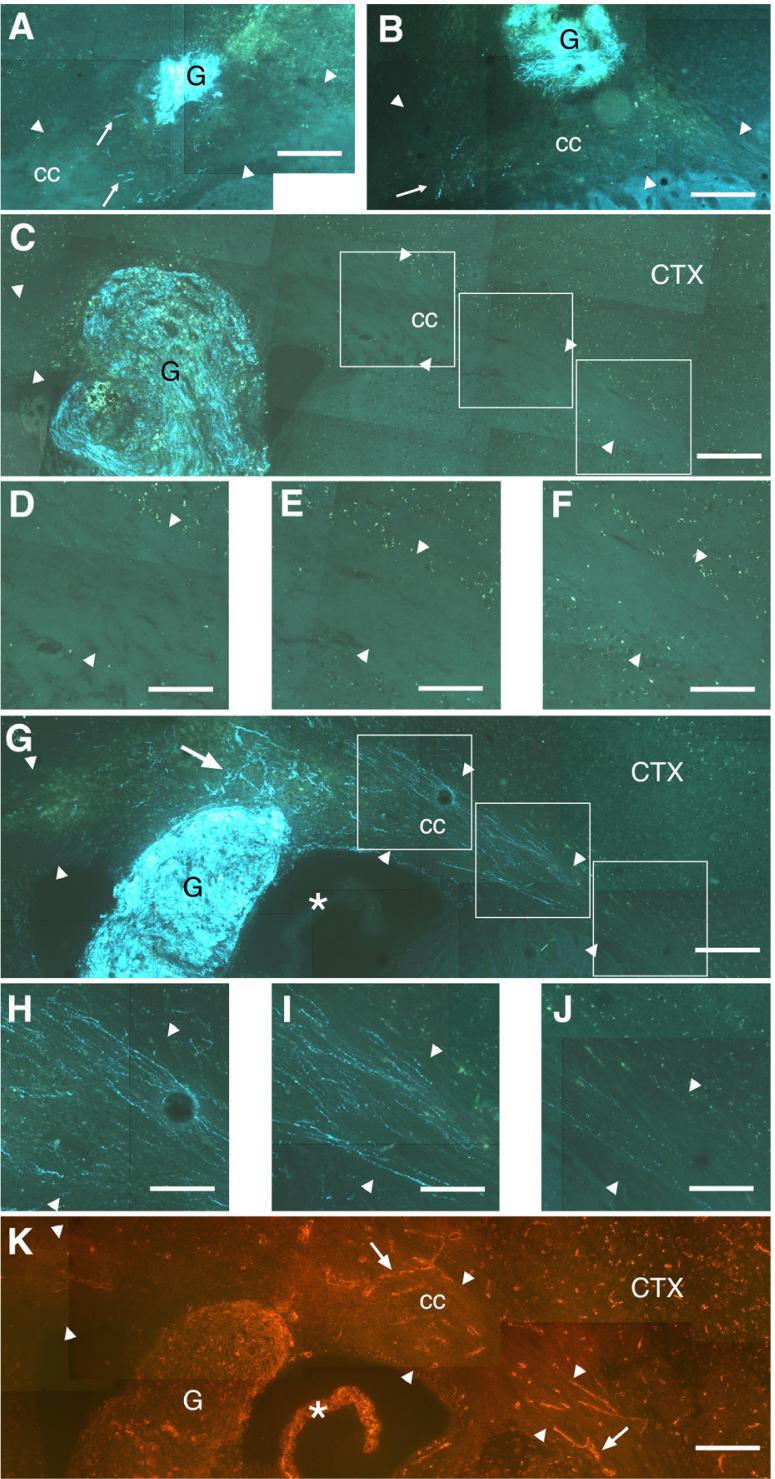
Fig. 7.
Sections stained using the SPG method showing axonal growth from grafts (G) placed within the corpus callosum (cc). In all panels, the direction lateral to the grafts is to the right. (A) Wt/Wt case showing a few fibers (arrows) extending within the cc (borders of the fiber tract are indicated by arrowheads) in parallel with the fiber tract. These fibers were mostly limited to a yellow autofluorescent portion of the tract, characteristic of injured tissue. (B) Wt/Wt case with fibers (arrow) shown extending primarily within a yellow autofluorescent portion of the cc in directions orthogonal to the fiber tract. Representative NGF/Wt and NGF/NGF cases with similar placement within the cc are shown in panels C and G. High power micrographs of the fiber tracts lateral to the grafts are shown in panels D-F and H-J, respectively. Though SPG+ axons are apparent within the NGF/Wt graft shown in panel C, no outgrowth was observed either medial to the graft or 0.4 mm (panel D), 0.8 mm (panel E) or 1.2 mm (panel F) lateral to the graft. In contrast, a dense plexus of fibers extended laterally within the cc from the NGF/NGF graft shown in panel G. Outgrowth dorsal to the graft (arrow) within an injured yellow autofluorescent region is mixed in orientation, whereas growth that is more distal was essentially parallel to the fiber tract at 0.4 mm (panel H), 0.8 mm (panel I) or 1.2 mm (panel J) lateral to the graft. (K) A section adjacent to that shown in panel G shows laminin immunoreactivity primarily associated with the choroid plexus (*), the graft itself and the vasculature (e.g., arrows). Comparison with the pattern of axonal outgrowth in panel G suggests that it is unlikely that this outgrowth occurs preferentially along laminin-rich vessels. Scale bars=200 μm in panels A-C, G, K; 100 μm in panels D-F, H-J.
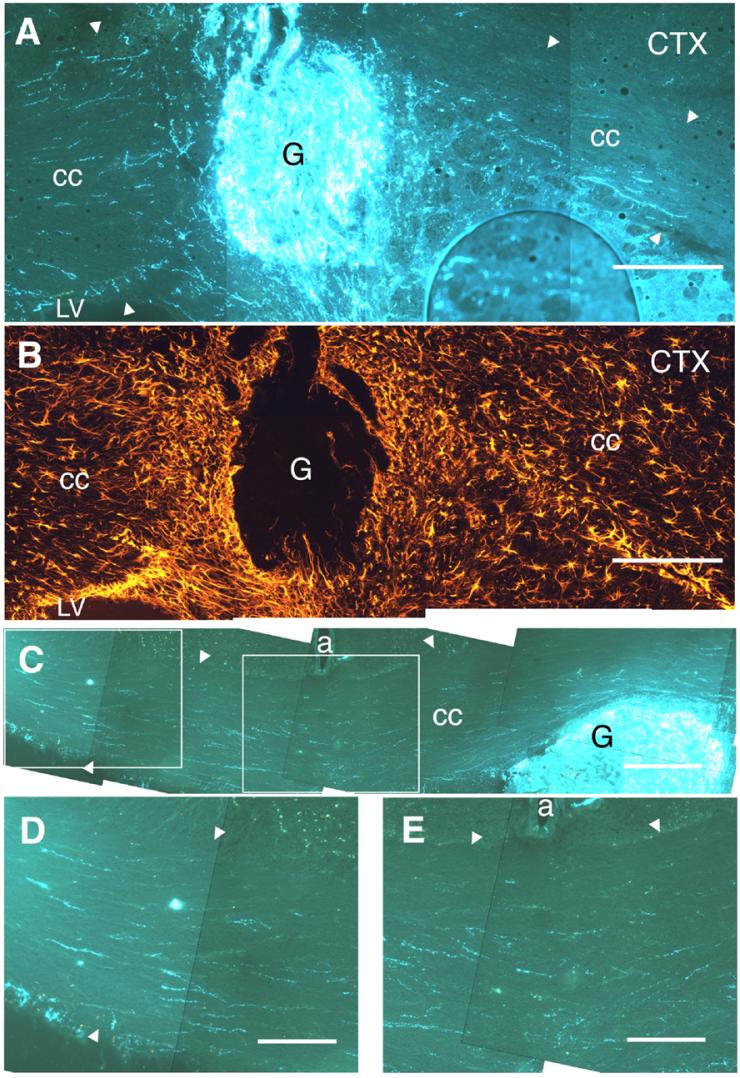
Fig. 8.
Sections stained using the SPG method showing axonal growth from grafts (G) placed within the corpus callosum (cc) of GFAP-NGF-Tg mice. In all panels, the direction lateral to the grafts is to the right. (A) NGF/NGF case with outgrowth extending within the cc (borders of the fiber tract indicated by arrowheads) in both medial and lateral directions. These axons extended beyond the zones of increased GFAP-IR surrounding the graft (cf. adjacent section shown in panel B). (C) Wt/NGF case with outgrowth that extended beyond the midline (note pericallosal artery, a). High power micrographs show axons that have extended 0.6 mm beyond the midline (panel D) and through the midline (panel E). These axons appear to be extending predominantly in parallel with the fiber tract. LV, lateral ventricle. Scale bars=200 μm in panels A-C; 100 μm in panels D, E.
Within GFAP-NGF-Tg host brains, axons exhibiting sympathetic morphology could often be seen in the septal nuclei and the anterior commissure on both sides of the midline. Sympathetic axons were not observed within the caudate-putamen or neocortex, except in association with vasculature. Sympathetic axons were not observed in the corpus callosum of either GFAP-NGF-Tg or non-transgenic mice containing dead grafts (e.g., Fig. 3A).
In contrast to non-transgenic hosts (e.g., Figs. 7C-F), in 10 of 19 cases (5 from each donor group) in which GFAPNGF-Tg mice were used as a host for viable transplanted ganglia, multiple SPG+ axons extended within the corpus callosum for distances of over 0.5 mm (e.g., Figs. 7G-J). Although axonal growth within the area of injury was often mixed in orientation or even orthogonal to the fiber tract (e.g., dorsal to the graft in Fig. 7G), axons acquired parallel orientations within the fiber tract further away from the graft site (Figs. 7H-J). In most of these cases, the axons could be traced directly to the grafted ganglia and extended well beyond the GFAP-immunoreactive astrogliotic halo surrounding the grafts (cf. Figs. 8A and B). In some instances, outgrowth extended beyond the midline to portions of the fiber tract in the contralateral hemisphere (Figs. 8C-E).
Laminin is a permissive substrate for sympathetic axons and sympathetic sprouting into other regions of the CNS appears to occur along penetrating blood vessels (McGinty et al., 1982; Crutcher and Marfurt, 1988). Consequently, adjacent sections were immunolabeled for laminin to determine if the pattern of axonal outgrowth from grafts coincided with the location of laminin-rich vessels. There was no obvious difference between GFAP-NGF-Tg and non-transgenic mice in the pattern, density or intensity of laminin immunofluorescence in the corpus callosum. As expected, laminin immunofluorescence was primarily associated with vessels, as well as the choroid plexus and grafts (Fig. 7K). Laminin-immunoreactive vessels within the corpus callosum were mixed in orientation and sparse in density, in contrast to axonal outgrowth, which was generally parallel and, in cases such as that shown in Fig. 7G, relatively dense. Consequently, it is unlikely that the axonal outgrowth occurred preferentially along laminin-rich vessels.
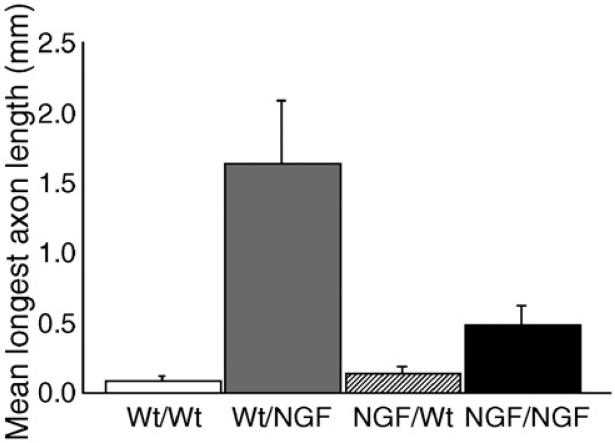
Measurements of axonal length and two-way ANOVA revealed significant host and donor effects (p<0.0001 and p=0.007, respectively) as well as a significant interaction between host and donor variables (p=0.003). Examination of the summary data showing the mean longest outgrowth for each transplantation paradigm (Fig. 9) suggests that the main effect on outgrowth is attributed to the host type, with GFAP-NGF-Tg hosts promoting more outgrowth than non-transgenic hosts. In addition, among the grafts placed within the corpus callosum of GFAP-NGF-Tg hosts, it appears that longer outgrowth was promoted from ganglia that had been harvested from non-transgenic mice compared with those harvested from GFAPNGF-Tg mice.
Fig. 9.
Axonal growth in the corpus callosum of GFAP-NGF-Tg mice is longer than that in non-transgenic mice (p<0.0001). The longest outgrowth was measured from non-transgenic grafts placed in the corpus callosum of GFAP-NGF-Tg mice (Wt/NGF), followed by grafts harvested from GFAP-NGF-Tg mice placed in GFAP-NGF-Tg mice (NGF/NGF). Both groups utilizing nontransgenic hosts showed minimal growth in the corpus callosum. Bars represent mean±SEM.
Discussion
NGF overexpression promotes axonal regeneration in white matter
Knife lesions were used to characterize the effects of injury on NGF levels within the brains of GFAP-NGF-Tg and nontransgenic mice. Expression of the endogenous NGF gene is normally restricted to the hippocampus and, to a lesser extent, the neocortex in mice (Ayer-LeLievre et al., 1988). In contrast, white matter tracts such as the corpus callosum show little evidence of endogenous NGF gene expression (Ayer-LeLievre et al., 1988). As previously documented, NGF levels were higher in the brains of GFAP-NGF-Tg compared with nontransgenic mice (Kawaja and Crutcher, 1997; Krol et al., 2000; Hannila and Kawaja, 2003). Analysis of tissue from the contralateral side indicates elevated baseline NGF levels throughout the fiber tract of GFAP-NGF-Tg mice (mean, 40 pg/mg), compared with non-transgenic mice due to constitutive activation in astrocytes of genes coupled to the GFAP promoter. Because NGF is expressed under control of the GFAP promoter, NGF was expected to be further elevated near sites of astrogliotic injury in GFAP-NGF-Tg mice, a prediction confirmed by ELISA analysis of tissue from all 10 knife-lesioned GFAP-NGF-Tg brains.
Viable and non-viable grafts were easily distinguished. Non-viable grafts failed to stain with the SPG method, indicating a paucity of catecholamine content. Instead, under fluorescent conditions, non-viable grafts showed yellow autofluorescence, characteristic of damaged tissue. Furthermore, no neurons were seen in these grafts when stained with cresyl violet. Instead, the grafts contained cystic cavities and small cells that appeared to be phagocytes. Viable grafts, in contrast, stained intensely with SPG and neurons could be seen along the perimeter of these grafts when stained with cresyl violet. It was not obvious why some grafts survived and others did not. A likely explanation is that some grafts were injured more than others during the process of removing and/or implanting the grafts.
One week after grafting of adult sympathetic ganglia, most grafts were capable of producing extensive axonal growth as evidenced by the dense plexus of fibers generated within the grafts. However, little outgrowth occurred in non-transgenic hosts beyond the immediate region of injury surrounding the grafts. Consistent with previous studies utilizing local infusion of neurotrophins (Oudega and Hagg, 1996, 1999; Coumans et al. 2001; Lu et al., 2004), global elevation of NGF also promoted axonal regeneration in lesioned white matter. Within GFAP-NGF-Tg hosts, axonal outgrowth extended within the corpus callosum well beyond zones of astrogliotic injury, the site of maximum NGF levels. The longest outgrowth occurred from non-transgenic ganglia grafted to GFAP-NGF-Tg hosts, suggesting that elevated expression of NGF within the grafts from GFAP-NGF-Tg donors did not play a significant role in aiding axonal outgrowth.
Neuronal counts were greater in ganglia harvested from non-transgenic mice that had been grafted to GFAP-NGF-Tg mice compared with the other three groups. Thus, in these cases, it cannot be ruled out that the increased outgrowth may have been secondary to increased neuronal survival. However, greater outgrowth occurred in the NGF/NGF group than the NGF/Wt group, even though these groups exhibited similar graft viability (Fig. 5), making it unlikely that NGF-induced outgrowth can be attributed solely to greater neuronal survival.
That the axons observed in the corpus callosum originated in the grafted ganglia is supported by several lines of evidence. First, the SPG method generally does not stain axons in the corpus callosum, with the exception of occasional central noradrenergic fibers that are easily distinguished from sympathetic axons by morphology and intensity of staining (Crutcher, 1982). Moreover, the SPG method labeled no axons in the corpus callosum of cases in which grafts were clearly non-viable, including two GFAP-NGF-Tg host cases, indicating that the fibers observed in the presence of viable grafts were unlikely due to reactive sprouting of endogenous host fibers. Finally, many of these axons could be traced directly to the grafts. The reliability of SPG staining could be verified in all cases by staining of other structures in the same sections.
There were no obvious differences in GFAP-immunoreactivity between GFAP-NGF-Tg and non-transgenic brains, consistent with previous reports (Kawaja et al., 1992; Tuszynski et al., 1994; Nakahara et al., 1996). CSPG expression was also increased around grafts. Consistent with previous studies (Kawaja and Gage, 1991; Kawaja et al., 1992), astrogliosis did not appear to pose a barrier to outgrowth in the presence of elevated NGF. Axons extended through these astrogliotic regions, in some cases reaching the contralateral hemisphere. Possible substrates for growth in the corpus callosum include astrocytes (Kawaja and Gage, 1991) as well as Schwann cells (Kromer and Cornbrooks, 1985) from the graft. Regardless of the substrate, it is unlikely that the presence of conducive substrates alone is sufficient to permit regeneration (Kawaja and Gage, 1991; Kawaja et al., 1992).
The permissiveness of CNS white matter for axonal growth in this study is consistent with previous studies in which neurons were cultured on white matter (Pettigrew and Crutcher, 1999; Pettigrew et al., 2001) or grafted into fiber tracts (Davies et al., 1997, 1999). These earlier studies demonstrated extensive axonal growth within white matter without the need for exogenous neurotrophins. However, such growth occurred only in parallel with the longitudinal axis of the fiber tract indicating that white matter contains intrinsic cues that can constrain the orientation of axonal regeneration. Moreover, axonal growth in these studies occurred only if injury to the fiber tract was limited. In the present study, grafting was accompanied by significant injury (an intended experimental goal) and outgrowth was limited, as expected, in the absence of elevated neurotrophins.
NGF overexpression may have promoted axonal growth via any number of non-mutually exclusive mechanisms. First, because NGF is a well-documented promoter of sympathetic axonal growth, its overexpression may have tipped the balance of positive and negative growth regulators toward a net positive effect. NGF overexpression may also have accelerated growth such that the growth cones passed through the site of injury before a glial scar had time to form. NGF may also have promoted growth by disinhibiting growth cones from the effects of various factors associated with disrupted myelin sheaths and glial scars. For example, preincubation of NGF-sensitive neurons with large concentrations of NGF has been shown to reverse the inhibitory effects of myelin and myelin-associated glycoprotein (Cai et al., 1999). Moreover, it has been proposed that neurotrophins such as NGF and the microglial-derived plasminogen/plasmin cascade may form part of a positive feedback loop that can promote the degradation of CSPG core proteins (Davies et al., 2006). If this hypothesis is correct, the overexpression of NGF may have significantly reduced the inhibitory properties of the glial scar. Irrespective of the mechanism, global overexpression of NGF was sufficient to overcome barriers to axonal growth, at least for sympathetic neurons, within lesioned white matter.
Axonal regeneration is constrained to parallel orientations in the presence of global NGF
Although previous studies in which axonal growth occurred within white matter showed that this growth was oriented in parallel with the fiber tract (Davies et al., 1997, 1999), studies of mature NGF-responsive neurons in culture suggest that NGF induces branching rather than elongation of growth (Yasuda et al., 1990). Therefore, it was not certain if axonal growth would extend in parallel with white matter in the presence of global NGF overexpression. The design of therapeutic approaches to promote axonal regeneration in white matter would benefit from knowing whether it is necessary to provide both orientation cues that constrain growth to a parallel orientation (e.g., a neurotrophic gradient) as well as conditions that promote growth, or whether it is sufficient to promote growth because the fiber tract contains dominant orientation cues that constrain axons to a parallel orientation. The present data suggest that elevating neurotrophic support alone may be sufficient. Although axonal outgrowth sometimes had a mixed orientation within white matter that was disrupted by the grafting procedure, once axons extended away from the injury sites, they were constrained to a parallel orientation in white matter, despite global overexpression of NGF.
These results are consistent with in vitro studies in which axonal growth on longitudinal sections of white matter remained parallel when NGF was added to the culture medium (Pettigrew and Crutcher, 1999) and suggest that white matter contains substrate cues that are not overridden by global NGF expression. These substrate cues may include neurite inhibitors associated with CNS myelin and fibrous astrocytes, both of which are oriented in parallel with the tract. Such substrate cues may actually serve to enhance longitudinal regeneration within uninjured CNS fiber tracts by constraining growth to a parallel orientation (Pettigrew and Crutcher, 2001; Raisman, 2004). Irrespective of the constraint mechanism, it appears to dominate in determining the orientation of axonal growth.
NGF-induced axonal regeneration in injured white matter may not require a gradient
Previous studies that utilized local infusions of neurotrophins at a site distal to regenerating axons were successful in promoting parallel growth across a site of injury and into the distal white matter (Oudega and Hagg, 1996; Lu et al., 2004). Interestingly, this regenerative growth appeared to stop at the site of infusion, leading the authors to speculate that the axons were chemotropically attracted to this site (Oudega and Hagg, 1996) and that long-distance growth may require the establishment of an extended neurotrophic gradient (Lu et al., 2004).
Although previous studies demonstrated that NGF can act as a tropic factor both in vitro (Gundersen and Barrett, 1979) and in vivo (Levi-Montalcini, 1976; Scalapino et al., 1996), it is unlikely that the axonal growth in the present GFAP-NGF-Tg mice extended in the direction of a positive trophic factor gradient (i.e., increasing [NGF] with increasing distance from the graft site) because NGF levels appeared to be highest within astrogliotic tissue. In GFAP-NGF-Tg hosts, axons extended well beyond the region of astrogliosis surrounding the graft, in some cases, extending 0.5 mm beyond the midline along the fiber tract within the contralateral hemisphere. Although the presence of microgradients along the axis of the fiber tract cannot be ruled out, ELISA measurements indicated that less NGF is expressed in the hemisphere contralateral to injury, suggesting that if any NGF gradient was present within the corpus callosum of grafted GFAP-NGF-Tg mice, it was likely to be a negative (decreasing over distance) gradient over a distance of millimeters.
In any case, NGF levels were overexpressed throughout the fiber tract of GFAP-NGF-Tg mice, compared with non-transgenic mice. In cases in which local infusions of neurotrophins were used to promote regeneration that stopped at the infusion site, it is likely that the neurotrophic levels diminished asymptotically toward the normally low endogenous baseline with distance from the infusion site. Although the site of local neurotrophin infusion may have been a center of attraction for growing axons, the present data suggest that these axons nevertheless may have proceeded beyond the infusion site if sufficient levels of exogenous neurotrophins had been available along the extent of the fiber tract.
That a neurotrophic gradient may not be necessary for regenerative axonal growth in CNS white matter is consistent with similar conclusions reached about developmental growth of axons in peripheral nerves (Hoyle et al., 1993), although the authors in that study speculated that the developing growth cones had not yet acquired sensitivity to gradients, and reversal of age-related reductions in basal forebrain cholinergic innervation of the primate neocortex (Conner et al., 2001). The present results are also consistent with experiments in which cultures of explanted neural tissue are treated with exogenous neurotrophins that are well mixed in the culture medium. In these cases, outgrowth is significantly enhanced, in spite of the absence of an obvious neurotrophic gradient. A similar enhancement effect of globally elevated neurotrophic support may occur in vivo.
Conclusion
Extensive axonal regeneration was induced in white matter of mice in which NGF is overexpressed throughout the fiber tract but not as a positive gradient. This regenerative growth was aligned in parallel with the fiber tract, indicating that geometrically aligned guidance cues intrinsic to white matter dominate over the influence of NGF on the orientation of growth, even when NGF is globally overexpressed. Thus, in order to promote axonal regeneration through and beyond a white matter injury site, establishing a neurotrophic gradient may not be necessary. Global elevation of appropriate neurotrophins may be sufficient.
Acknowledgments
The authors gratefully acknowledge the assistance of Michelle A. Bunger (surgical procedures), Heather N. Lilley (histology), Kristina P. Bielewicz and Georgette L. Suidan (ELISA), Martha Headworth (illustration) and Sarah Carothers (quantitative analysis). Dr. Michael D. Kawaja (Queens Univ., Kingston, ON) kindly provided RT-PCR data confirming the genotype of GFAP-NGF transgenic mice. Grant sponsors: The National Institutes of Health (NS044972) and Research and Education Grants from Synthes Spine and Medtronic Sofamor Danek.
References
- Ayer-LeLievre C, Olson L, Ebendal T, seiger Å, Persson H. Expression of the β-nerve growth factor gene in hippocampal neurons. Science. 1988;240:1339–1341. doi: 10.1126/science.2897715. [DOI] [PubMed] [Google Scholar]
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 2001;13:257–268. [PubMed] [Google Scholar]
- Berry M, Rees L, hall S, Yiu P, Sievers J. Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Res. Bull. 1988;20:223–231. doi: 10.1016/0361-9230(88)90182-7. [DOI] [PubMed] [Google Scholar]
- Björklund A, Stenevi U. Growth of central catecholamine neurones into smooth muscle grafts in the rat mesencephalon. Brain Res. 1971;31:1–20. doi: 10.1016/0006-8993(71)90630-5. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur. J. Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Breeze RE, Wells TH, Jr., Reed CR. Implantation of fetal tissue for the management of Parkinson's disease: a technical note. Neurosurgery. 1995;36:1044–1048. doi: 10.1227/00006123-199505000-00027. [DOI] [PubMed] [Google Scholar]
- Bregman BS, McAtee M, Dai HN, Kuhn PL. Neurotrophic factors increase axonal growth after spinal cord injury and transplantation in the adult rat. Exp. Neurol. 1997;148:475–494. doi: 10.1006/exnr.1997.6705. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Conner JM, Darracq MA, Roberts J, Tuszynski MH. Nontropic actions of neurotrophins: subcortical nerve growth factor gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc. Natl. Acad. Sci. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coome GEA, Kawaja MD. Prolonged exposure to elevated levels of endogenous nerve growth factor affects the morphological and neurochemical features of sympathetic neurons of postnatal and adult mice. Neuroscience. 1999;90:941–955. doi: 10.1016/s0306-4522(98)00499-0. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT-S, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J. Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutcher KA. Histochemical studies of sympathetic sprouting: fluorescence morphology of noradrenergic axons. Brain Res. Bull. 1982;9:501–508. doi: 10.1016/0361-9230(82)90158-7. [DOI] [PubMed] [Google Scholar]
- Crutcher KA, Marfurt CF. Nonregenerative axonal growth within the mature mammalian brain: ultrastructural identification of sympathohippocampal sprouts. J. Neurosci. 1988;8:2289–2302. doi: 10.1523/JNEUROSCI.08-07-02289.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Davies SJA, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Tang X, Bournat JC, Davies SJA. Decorin promotes plasminogen/plasmin expression within acute spinal cord injuries and by adult microglia in vitro. J. Neurotrauma. 2006;23:397–408. doi: 10.1089/neu.2006.23.397. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, Barrett JN. Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science. 1979;206:1079–1080. doi: 10.1126/science.493992. [DOI] [PubMed] [Google Scholar]
- Hagg T, Gulati AK, Behzadian MA, Vahlsing HL, Varon S, Manthorpe M. Nerve growth factor promotes CNS cholinergic axonal regeneration into acellular peripheral nerve grafts. Exp. Neurol. 1991;112:79–88. doi: 10.1016/0014-4886(91)90116-t. [DOI] [PubMed] [Google Scholar]
- Hannila SS, Kawaja MD. Distribution of central sensory axons in transgenic mice overexpressing nerve growth factor and lacking functional p75 neurotrophin receptor expression. Eur. J. Neurosci. 2003;18:312–322. doi: 10.1046/j.1460-9568.2003.02752.x. [DOI] [PubMed] [Google Scholar]
- Heinicke EA. Vascular permeability and axonal regeneration into tissues autotransplanted into the brain. Proc. Can. Fed. Biol. Soc. 1978;21:40. doi: 10.1007/BF00707104. [DOI] [PubMed] [Google Scholar]
- Houle JD, Ziegler MK. Bridging a complete transection lesion of adult rat spinal cord with growth factor-treated nitrocellulose implants. J. Neural Transpl. Plast. 1994;5:115–124. doi: 10.1155/NP.1994.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle GW, Mercer EH, Palmiter RD, Brinster RL. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron. 1993;10:1019–1034. doi: 10.1016/0896-6273(93)90051-r. [DOI] [PubMed] [Google Scholar]
- Itakura T, Komai N, Ryujin Y, Ooiwa Y, Nakai M, Yasui M. Autologous transplantation of the cervical sympathetic ganglion into the Parkinsonian brain: case report. Neurosurgery. 1994;35:155–157. doi: 10.1227/00006123-199407000-00026. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Crutcher KA. Sympathetic axons invade the brains of mice overexpressing nerve growth factor. J. Comp. Neurol. 1997;383:60–72. [PubMed] [Google Scholar]
- Kawaja MD, Gage FH. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7:1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- Kawaja MD, Rosenberg MB, Yoshida K, Gage FH. Somatic gene transfer of nerve growth factor promotes the survival of axotomized septal neurons and the regeneration of their axons in adult rats. J. Neurosci. 1992;12:2849–2864. doi: 10.1523/JNEUROSCI.12-07-02849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krol KM, Crutcher KA, Kalisch BE, Rylett RJ, Kawaja MD. Absence of p75(NTR) expression reduces nerve growth factor immunolocalization in cholinergic septal neurons. J. Comp. Neurol. 2000;427:54–66. doi: 10.1002/1096-9861(20001106)427:1<54::aid-cne4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kromer LF, Cornbrooks CJ. Transplants of Schwann cell cultures promote axonal regeneration in the adult mammalian brain. Proc. Natl. Acad. Sci. 1985;82:6330–6334. doi: 10.1073/pnas.82.18.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer LF, Cornbrooks CJ. Identification of trophic factors and transplanted cellular environments that promote CNS axonal regeneration. Ann. N. Y. Acad. Sci. 1987;495:207–224. doi: 10.1111/j.1749-6632.1987.tb23676.x. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: its role in growth, differentiation and function of the sympathetic adrenergic neuron. Prog. Brain Res. 1976;45:235–258. doi: 10.1016/S0079-6123(08)60993-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kim D, Himes BT, Chow SY, Schallert T, Murray M, Tessler A, Fischer I. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forebrain function. J. Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J. Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Milner TA, Loy R. Association of sympathetic axons in denervated hippocampus to intracerebral vasculature. Anat. Embryol. 1982;164:95–100. doi: 10.1007/BF00301882. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5:191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]
- Namiki J, Kojima A, Tator C. Effect of brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 on functional recovery and regeneration after spinal cord injury in adult rats. J. Neurotrauma. 2000;17:1219–1231. doi: 10.1089/neu.2000.17.1219. [DOI] [PubMed] [Google Scholar]
- Nathaniel EJH, Clemente CD. Growth of nerve fibers into skin and muscle grafts in rat brains. Exp. Neurol. 1959;1:65–81. doi: 10.1016/0014-4886(59)90013-5. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp. Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Neurotrophins promote regeneration of sensory axons in the adult rat spinal cord. Brain Res. 1999;818:431–438. doi: 10.1016/s0006-8993(98)01314-6. [DOI] [PubMed] [Google Scholar]
- Pettigrew DB, Crutcher KA. White matter of the CNS supports or inhibits neurite outgrowth in vitro depending on geometry. J. Neurosci. 1999;19:8358–8366. doi: 10.1523/JNEUROSCI.19-19-08358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew DB, Crutcher KA. Myelin contributes to the parallel orientation of axonal growth on white matter in vitro. BMC Neurosci. 2001;2:9. doi: 10.1186/1471-2202-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew DB, Shockley KP, Crutcher KA. Disruption of spinal cord white matter and sciatic nerve geometry inhibits axonal growth in vitro in the absence of glial scarring. BMC Neurosci. 2001;2:8. doi: 10.1186/1471-2202-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plunet W, Kwon BK, Tetzlaff W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J. Neurosci. Res. 2002;68:1–6. doi: 10.1002/jnr.10176. [DOI] [PubMed] [Google Scholar]
- Raisman G. Myelin inhibitors: does NO mean GO? Nat. Rev., Neurosci. 2004;5:157–161. doi: 10.1038/nrn1328. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Degeneration and Regeneration of the Nervous System. Oxford Univ. Press; London: 1928. [Google Scholar]
- Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J. Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Scalapino K, Conner JM, Varon S. The role of NGF and afferent denervation in the process of sympathetic fiber ingrowth into the hippocampal formation. Exp. Neurol. 1996;141:310–317. doi: 10.1006/exnr.1996.0166. [DOI] [PubMed] [Google Scholar]
- Svendgaard N-A, Björklund A, Stenevi U. Regeneration of central cholinergic neurones in the adult rat brain. Brain Res. 1976;102:1–22. doi: 10.1016/0006-8993(76)90572-2. [DOI] [PubMed] [Google Scholar]
- Tello F. La influencia del neurotropismo en la regeneración de los centros nerviosos. Trab. Lab. Investig. Biol. 1911;9:123–159. [Google Scholar]
- Tuszynski MH, Buzsaki G, Gage FH. Nerve growth factor infusions combined with fetal hippocampal grafts enhance reconstruction of the lesioned septohippocampal projection. Neuroscience. 1990;36:33–44. doi: 10.1016/0306-4522(90)90349-9. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp. Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Varon S, Conner JM. Nerve growth factor in CNS repair. J. Neurotrauma. 1994;11:473–486. doi: 10.1089/neu.1994.11.473. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guénard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp. Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Sobue G, Ito T, Mitsuma T, Takahashi A. Nerve growth factor enhances neurite arborization of adult sensory neurons; a study in single-cell culture. Brain Res. 1990;524:54–63. doi: 10.1016/0006-8993(90)90491-s. [DOI] [PubMed] [Google Scholar]
- Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J. Neurosci. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]