Abstract
The dynamics of invasive species may depend on their abilities to compete for resources and exploit disturbances relative to the abilities of native species. We test this hypothesis and explore its implications for the restoration of native ecosystems in one of the most dramatic ecological invasions worldwide, the replacement of native perennial grasses by exotic annual grasses and forbs in 9.2 million hectares of California grasslands. The long-term persistence of these exotic annuals has been thought to imply that the exotics are superior competitors. However, seed-addition experiments in a southern California grassland revealed that native perennial species, which had lower requirements for deep soil water, soil nitrate, and light, were strong competitors, and they markedly depressed the abundance and fecundity of exotic annuals after overcoming recruitment limitations. Native species reinvaded exotic grasslands across experimentally imposed nitrogen, water, and disturbance gradients. Thus, exotic annuals are not superior competitors but rather may dominate because of prior disturbance and the low dispersal abilities and extreme current rarity of native perennials. If our results prove to be general, it may be feasible to restore native California grassland flora to at least parts of its former range.
Invasions by exotic species can change ecosystem functioning and threaten ecosystem biodiversity (1-5), but the mechanisms determining why, when, and where some species are invasive and what impacts they have are poorly understood. A better understanding of these mechanisms could lead to strategies to identify potentially invasive species, control existing exotic species, restore native species, and also provide insight into the controls of community assembly.
The mechanisms governing community assembly, exotic invasions, and native restorations are conceptually similar (6). To be successful, a new species (whether native or exotic) must both survive and attain a positive rate of increase while living on the resources left unconsumed by the resident species (7-10). The ability to attain positive population growth rates under these conditions would allow the invading species to increase in abundance while in the presence of numerically superior, well established residents. Furthermore, two species should coexist if each has a positive growth rate when the other species is at or near its maximal (equilibrial) abundance (11, 12). This coexistence can happen, for instance, if an interspecific tradeoff occurs such that each is a superior competitor for a different limiting resource, or if tradeoffs occur between growth rate, ability to compete for resources, and dispersal ability (13, 14). In contrast, one species will displace the other if it can increase when rare but the other species cannot. Exclusion could occur if only one limiting resource exists or if a single species is a superior competitor for several resources. If neither species can invade an equilibrial population of the other species, priority effects would lead to multiple stable equilibria (MSE) (11, 12). MSE can result from resource preemption. Thus, the mechanisms governing community assembly, exotic invasion, and restoration may depend on the levels to which various species can reduce limiting resources.
The spread and long-term dominance of an invader might result from any one of three alternative mechanisms related to competitive ability. First, such dominance might be indicative of competitive superiority relative to native species, a hypothesis that has rarely been tested experimentally (15). Competitive superiority could occur because an invader had a competitively unique trait, (e.g., a deep tap root or high N fixation capacity) (16), was from a biogeographic realm in which species had evolved a superior tradeoff surface for limiting resource and factor requirements (7, 8, 10), or had gained increased competitive ability by escape from natural enemies (17, 18). If an invader is competitively superior, long-term prospects for restoration of native species rely on biological control of the exotic or the difficult process of eradication and quarantine (1, 4, 19).
Second, exotic dominance might result from anthropogenic disturbance (e.g., farming, grazing, clear-cutting, nitrogen deposition, or changes in fire regimes) that favor the exotic. After the cessation of disturbance, such exotic dominance could persist as a transient, although potentially long-lasting, state if competitively superior native species were rare and recruitment limited. Succession to a native-dominated state would be slowed further if a tradeoff occurred between competitive ability and colonization ability (14, 20). Recruitment limitation is common in plant communities (21) and may result from low abundance or fecundity of reproductive adults, poor seed dispersal, seed predation, or low seedling establishment. In such cases, native species could be restored by introducing seeds of native species, even in the face of competition from established exotic species (6).
Third, exotic domination could also occur if MSE existed, such that the outcome of competition is determined by priority effects and initial species abundances. If MSE exist, an abundant species or suite of functionally similar species could maintain dominance by competitively preventing invasion and growth of a different species or suite of species and vice versa. As in the preceding example, a strong intervention (disturbance) would have been needed to create an exotic-dominated state. However, in contrast to the recruitment-limitation mechanism, a similarly strong intervention would be needed to switch the system from exotics back to a native-dominated state (22, 23). Thus, MSE can create a stable native-dominated state. These three alternative scenarios mean that restoration of native species could range from highly possible to futile, depending on the mechanism maintaining exotic dominance.
Here, we investigate the mechanisms underlying the invasion of a native perennial California grassland by annual grasses and forbs introduced from the Mediterranean region (3, 24, 25). Overgrazing and drought during the 19th century are thought to have caused 9.2 million hectares (ha) of California grasslands to become dominated by exotic species (25-29). Although this conversion is often attributed to grazing, exotic annuals have maintained their dominance in many areas that have now been excluded from livestock grazing for decades (30). Along with the displacement of the native perennial flora, this invasion has potentially increased nitrate leaching, altered fire regimes, and decreased carbon storage (29, 31-33).
We used seed-addition experiments and measurements of likely limiting resources (nitrogen, water, and light) to test three possible mechanisms that may maintain the dominance of exotic annuals in a southern California grassland. (i) Exotic annual species could be superior resource competitors; if species compete for a single limiting resource, theory predicts that a competitively dominant species should be able to deplete the limiting resource to a lower level (13), as has been demonstrated in a midwestern grassland (34). (ii) Native perennial grasses could be superior resource competitors but be recruitment-limited; the addition of native seeds should lead to their dominance (21). (iii) Two multiple stable states, annual domination or perennial domination, could exist. Identification of MSE requires mutual invasibility trials in which seeds of the native species are introduced into established stands of the exotic competitors and vice versa (11). We also test whether the relative importance of these mechanisms varies across experimentally imposed gradients of disturbance and resource availability.
Methods
Site Description. Sedgwick Reserve is in the Santa Ynez valley (separated from the Pacific Ocean by the Santa Ynez Mountains). Its hot dry summer climate more closely resembles the Central Valley of California than nearby coastal areas. Typical summer high temperatures range from 32° and 34°C (summer high temperatures are between 25° and 26° in coastal areas). As is the case throughout California, the Santa Ynez valley is typified by extremely variable winter rainfall. Total precipitation at Sedgwick Reserve in 1997-1998, 1998-1999, 1999-2000, 2000-2001, and 2001-2002 was 797, 297, 326, 493, and 263 mm. A rain year begins on Julian day 184. In the grassland communities we studied, all exotic species are annual and all native species are perennial. The soil in our study area is a sandy clay loam with lower total C (1.97%) and total N (0.18%) than nearby stands of native perennial grasses (C = 3.37%, N = 0.30%; P < 0.0001, for both comparisons). Nutrient-addition experiments at Sedgwick show nitrogen, phosphorus, cations, and water to be potentially limiting (unpublished data) consistent with other California sites (33, 35).
Restoration Experiment. The goal of this experiment was to determine whether native perennial grasses would colonize and grow in an agricultural field (that would be dominated by exotic species in the absence of addition of seed of native species), and how disturbance (burning and gopher digging) and N addition affected the dominance of annual versus perennial grasses. In January 1998, we plowed a 2.5-ha exotic-annual-dominated field, then seeded it with 500 seeds m-2 of each of five native perennial grasses, (Bromus carinatus, Elymus glaucus, Nassella cernua, Nassella pulchra, and Poa secunda). This seeding rate ensured that few microsites remained unoccupied at the start of the experiment, thus eliminating seed limitation. In 1999, we established 36 plots (20 × 20 m) within the field. Plots were surrounded by fencing (1-cm mesh) extending 1.5 m belowground and 0.5 m aboveground to exclude or contain pocket gophers (Thomomys bottae), a potentially significant cause of disturbance (36). Plots received a factorial combination of two treatments: gopher addition (zero or four gophers) and nitrogen/fire (control, summer burn in 2000, or addition of 4 g of N per m2 per yr added quarterly as NH4NO3) for a total of six treatment combinations. The incomplete factorial combination of N addition and fire was due to the limited number of fenced plots we could construct. These nitrogen-addition rates are similar to rates from anthropogenic sources in urbanized areas of southern California. The experiment had six replicates in a completely randomized block design.
We estimated plant species abundances at peak biomass (mid-April to mid-May) by using a pinframe (we recorded each species touching a vertical wire placed at each of 100 uniformly spaced points in three randomly placed permanent 1-m2 subplots in each 20 × 20-m plot) annually from 1999 to 2002. We conducted additional presence/absence surveys in each plot at 64 uniformly spaced points in a 14 × 14-m area. Thus, cover estimates represent the mean of 364 presence/absence samples.
Mutual Invasibility Experiments. The goal of these experiments was to determine the mutual invasibility of native and exotic grasslands. To increase the generality of the results, we conducted invasibility trials under a range of abiotic conditions (burning, nitrogen addition, and water addition). In the summer of 2000 (when native perennials dominated the 2.5-ha experimental field) we established three experiments. Each experiment included plots that had an experimentally determined initial composition (exotic annual or native perennial) and included a single addition of perennial seed to annual communities and of annual seed to the perennial communities in the fall of 2000. Seeding rates were 1,000 live seeds per m2 (average seed production of perennial grasses in our experiment). To establish exotic-annual communities, we killed perennial grasses with a short-lived herbicide (Roundup) 1 year before the seed-addition treatments. To ensure the establishment of a dense stand of exotics, we added all seeds collected from a nearby stand of exotic annuals of equal area to each annual plot. The three most common species in the annual plots were Bromus hordeaceus, Bromus diandris, and Brassica nigra.
The initial composition and seeding treatments were overlain with one of three additional treatments to create three fully randomized mutual invasibility experiments:
Seed addition by fire experiment. Half of the plots were burned in the summer of 2000 before seed addition (four replicates for a total of 32 plots, 4 × 5 m).
Seed addition by nitrogen experiment. Half of the plots had 4 g of N per m2 per yr added as NH4NO3 (five replicates for a total of 40 plots, 3 × 3 m).
Seed addition by water experiment. Half of the plots received a supplemental weekly watering to match the 50-year mean rainfall + 2 SD, 854 mm·yr-1 (eight replicates for a total of 64 plots, 5 × 5 m).
From 2000 to 2002, we clipped, sorted to species, dried, and weighed aboveground biomass at peak production (April or May) in two 0.1 × 1 m strips in each of the 136 plots of the mutual invasibility experiments. At the same time, we measured photosynthetically active radiation with a ceptometer (Decagon Devices, Pullman, WA) at ground level and above vegetation to calculate the amount of light captured by vegetation (one interception). Photosynthetically active radiation readings were conducted within 2 h of solar noon. We collected 5-10 individuals of each of three common exotic annual grass species (B. hordeaceus, Bromus madritensis, and Hordeum murinum), dried and weighed each individual, and counted and weighed the seeds.
We measured soil moisture in the annual and perennial unseeded controls (i.e., no seed or resource addition) in four replicates of the seed addition by nitrogen experiment and four replicates of the seed addition by water experiment (n = 16). Soil moisture was measured weekly by using two 20-cm time-domain reflectometry probes (SoilMoisture, Santa Barbara, CA) buried at 15 and 60 cm in these plots. We measured soil nitrate levels in the annual and perennial unseeded plots in four replicates of the seed addition by nitrogen experiment and four replicates of the seed addition by water experiment (n = 32). Extractable soil nitrate was measured in a 0.5 M K2SO4 extract of a composite of four 2.5-cm-diameter × 15-cm soil cores per plot taken on April 9, May 5, and June 1, 2001. Samples at 15- to 30-cm and 30- to 45-cm depths were taken only on May 5. We estimated root biomass in the four pairs of annual and perennial control plots in the seed addition by water experiment in which we monitored soil moisture. We collected two 4.8-cm-diameter × 30-cm soil cores in each of these eight plots, dissolved the cores in water, and removed the live roots, which we dried and weighed.
Mowing Experiment. The goal of this experiment was to determine whether continued disturbance (mowing) would maintain annual dominance in the restored experimental grasslands. We mowed 17 plots in the spring, 1998-2001 (plot size ranged from 8 to 25 m2). A subset of the plots was removed from the mowing treatment in each successive year. Seven of the plots in this experiment are unseeded perennial plots from the mutual invasibility experiment.
Results
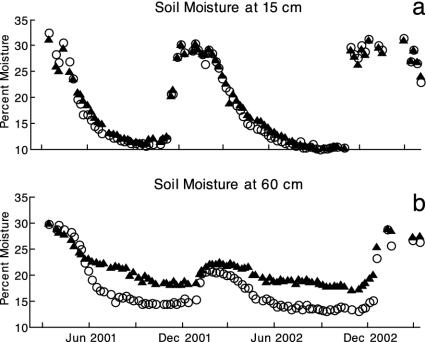
Evaluation of Resource-Reduction Abilities: Mutual Invasibility Experiments. Perennial-dominated communities reduced levels of light (photosynthetically active radiation) at the soil surface, soil water, and extractable soil nitrate to significantly lower levels than those of annual-dominated communities (Figs. 1 and 2). The proportion of photosynthetically active radiation reaching the soil surface in the perennial plots (0.061 ± 0.02) was significantly lower than in the annual plots (0.115 ± 0.02; P = 0.002). Soil moisture was always significantly lower in perennial plots at 60 cm (P = 0.05, repeated measures multiple ANOVA). Strong yearly and monthly interactions occurred such that differences in soil moisture were greatest in the summer at the 60-cm depth (e.g., P = 0.038 in June 2001, univariate ANOVA; Fig. 1).
Fig. 1.
Exotic annual species (▴) are less effective at depleting soil water at the 60-cm depth (b) than are native perennial species (○) (P = 0.050) across all dates. Water use at the 15-cm depth (a) is similar between community types (n = 16).
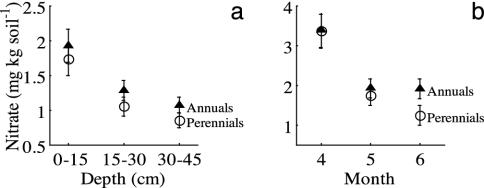
Fig. 2.
Exotic annual species (▴) are less effective than native perennial grasses (○) at extracting soil nitrate at all depths in May (a) and at 0-15 cm in June (b). Nitrate concentration by depth was recorded in May (a). Monthly sampling was conducted at the 0- to 15-cm depth (b). (Error bars = 1 SEM; n = 32.)
Soil nitrate patterns were similar to those of soil moisture: perennials reduced soil nitrate to lower levels than did annuals at all depths in May (P = 0.045, mixed-model repeated measures; Fig. 2b), and the differences were greatest in June (P = 0.035, univariate ANOVA; Fig. 2b). Nitrate differences in May were significant only when all three depths were analyzed simultaneously. No significant difference occurred between annuals and perennials in soil nitrate in April. The greater ability of native perennials to extract nitrate and water may result from their greater root/shoot ratios (0.62 ± 0.06) relative to those of exotic annuals (0.09 ± 0.03) and their longer growing season.
The per capita seed production of exotic annual species was 2-3.5 times lower in stands of native perennials than in stands of exotic annuals (Table 1).
Table 1. Fecundity of three common exotic annual grasses is lower in perennial than in annual plots.
| Seed production, seeds per plant
|
|||
|---|---|---|---|
| Species | Annual plots | Perennial plots | P |
| B. hordeaceus | 111.33 (15.13) | 32.05 (4.77) | <0.001 |
| B. madritensis | 176.34 (28.19) | 57.07 (7.25) | 0.034 |
| H. murinum | 95.3 (10.04) | 45.82 (7.65) | 0.002 |
Standard errors (n = 14) are shown in parentheses.
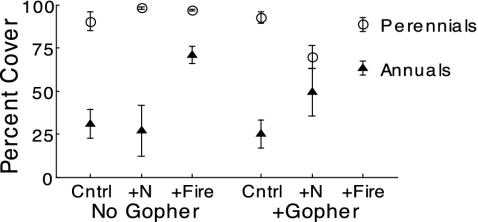
Evaluation of Seed Limitation of Perennial Grasses: Restoration Experiment. We found that native perennial grasses were seed-limited. Native perennial grasses were dominant (99-84% cover) in all treatments during all years sampled (1999-2002; Fig. 3). The success of the perennials cannot be attributed to a depauperate annual seed bank. Although perennial grasses eventually dominated the restoration experiment, annual species initially were abundant (40.4 ± 12.8% cover in 1999).
Fig. 3.
Cover of native perennial grasses (○) was higher than exotic annual species (▴) 5 years (2002) after adding native perennial seed in the restoration experiment. Plots were subjected to factorial combination of pocket gophers (No Gopher; +Gopher) and nitrogen and burning (Control, +N, and +Fire). (Error bars = 1 SEM; n = 36.)
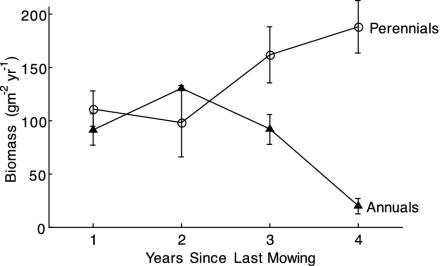
Evaluation of Disturbance: Restoration and Mowing Experiments. The abundance of annual species relative to perennial grasses depended on disturbance. A single burn increased cover of annuals from 27.9 ± 5.9% to 70.6 ± 4.7% (Fig. 3). Similarly, in the nitrogen-addition plots, pocket gopher disturbance increased annual abundance from 25.1 ± 8.1% to 49.5 ± 13.8% (Fig. 3). Annuals decreased and perennials increased in abundance once mowing ceased (Fig. 4).
Fig. 4.
Production of exotic annual (▴) and native perennial (○) species is nearly identical 1 year after mowing. Dominance of perennial grasses increased after cessation of mowing. (Error bars = 1 SEM; n = 17.)
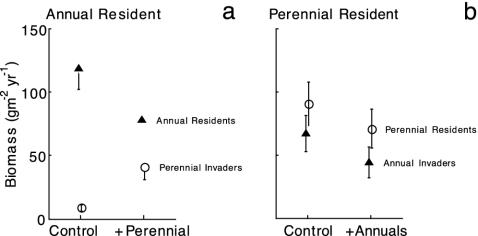
Evaluation of MSE: Mutual Invasibility Experiments. Native perennials, added as seed, successfully invaded communities dominated by exotic annuals and caused a significant decline in annual abundance (Fig. 5a). In contrast, addition of seed of exotic annuals to established perennial-dominated communities did not increase the abundance of annuals and did not lead to a significant decrease in perennials. Thus, we did not find priority effects that could lead to MSE; no cases occurred in which annual communities were resistant to invasion by native perennial grasses.
Fig. 5.
(a) The addition of seeds of perennial species to an annual community resulted in higher production of perennials (P < 0.001) and a corresponding decline in annuals (P = 0.021) 2 years after the initial seed introductions. (b) The addition of annual seeds to a perennial-dominated community had no significant effect on perennial (P = 0.167) or annual (P = 0.358) species. Means are shown from plots receiving no resource additions. (Error bars = 1 SEM; n = 17.)
As in the restoration experiment, the native perennial invasion did not result from low densities of annuals. The seed production from just three exotic annual grasses amounted to 19,297 ± 6,766 seeds m-2·yr-1 (Table 1). Furthermore, the annual-dominated plots had 2.6 times more aboveground production than the perennial plots in the year the perennials were introduced (2001; data not shown). Production in annual and perennial plots was similar in the second year, 2002 (Fig. 5).
Native perennial grasses invaded exotic annual plots equally well in the ambient (493 mm·yr-1) and high (854 mm·yr-1) rainfall plots. Water addition significantly increased perennial grass establishment in the first year, but this effect did not persist into the second year; the seeding by water interaction was not significant for the biomass response of the seeded perennials added to the exotic annual community (Table 2). Furthermore, native perennial grasses maintained their dominance in the mutual invasibility and restoration experiments, although the years after the initial sowings (1999 and 2002) had precipitation levels that were in the lowest 10% of the long-term precipitation pattern for this region (37). Plant productivity is limited by water (Table 2; perennial communities increase significantly in response to water addition; annuals increase but not significantly).
Table 2. Mutual invasibility experiments testing the ability of native perennial grass seedlings to invade exotic annual communities and vice versa.
| Biomass response to treatment
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Perennials added to annual community
|
Annuals added to perennial community
|
|||||||||
| Resident annuals
|
Seeded perennials
|
Resident perennials
|
Seeded annuals
|
|||||||
| Experiment | Source | df | S.S. | P | S.S. | P | S.S. | P | S.S. | P |
| Water | Block | 7 | 144.37 | 0.332 | 388.44 | <0.001 | 122.43 | 0.087 | 205.11 | 0.033 |
| Seeding | 1 | 48.46 | 0.104 | 86.05 | 0.002 | 22.60 | 0.113 | 3.84 | 0.553 | |
| Water | 1 | 56.22 | 0.082 | 137.92 | <0.001 | 140.82 | <0.001 | 8.45 | 0.381 | |
| Seed with water | 1 | 1.92 | 0.739 | 7.45 | 0.314 | 11.21 | 0.257 | 14.65 | 0.252 | |
| Total | 31 | 604.04 | 767.054 | 470.47 | 453.80 | |||||
| Nitrogen | Block | 4 | 59.77 | 0.305 | 68.62 | 0.005 | 32.13 | 0.673 | 34.11 | 0.763 |
| Seeding | 1 | 87.31 | 0.016 | 58.34 | <0.001 | 3.48 | 0.622 | 4.26 | 0.641 | |
| Nitrogen | 1 | 1.33 | 0.734 | 1.73 | 0.438 | 1.56 | 0.740 | 16.96 | 0.361 | |
| Seed with N | 1 | 4.42 | 0.538 | 0.01 | 0.962 | 2.27 | 0.690 | 0.32 | 0.899 | |
| Total | 19 | 284.67 | 160.94 | 173.87 | 240.34 | |||||
| Fire | Block | 3 | 47.46 | 0.041 | 20.41 | 0.193 | 15.54 | 0.136 | 37.53 | 0.292 |
| Seeding | 1 | 17.06 | 0.063 | 91.35 | <0.001 | 4.34 | 0.190 | 31.31 | 0.089 | |
| Fire | 1 | 2.34 | 0.452 | 2.30 | 0.430 | 0.83 | 0.551 | 0.65 | 0.790 | |
| Seed with fire | 1 | 3.31 | 0.374 | 0.02 | 0.946 | 2.90 | 0.277 | 17.53 | 0.189 | |
| Total | 15 | 104.19 | 145.68 | 43.07 | 164.56 | |||||
Biomass of resident and seeded species are analyzed separately for two scenarios: perennials seeded into an annual community and annuals seeded into a perennial community.
We tested for the effects of nitrogen supply in our study site by including a nitrogen-addition treatment in all of our mutual invasibility and restoration experiments. As for water addition, nitrogen addition significantly increased the invasion rate of the native perennial grasses the first year, although this effect did not persist into the second year; the seeding by nitrogen interaction was not significant for the biomass response of the seeded perennials added to the exotic annual community (Table 2). This experiment did not reveal consistent N limitation, although other studies at Sedgwick have demonstrated N limitation (unpublished data).
Discussion
We found that native perennials reduced soil moisture, soil nitrogen, and light to lower levels than did exotic annuals. This differential pattern of resource reduction means that native perennials should be able to increase in abundance on the levels of nitrate, water, and light left unconsumed by established stands of exotic annuals and, in so doing, competitively suppress the exotic annuals. Our invasion experiments supported these predictions, thus suggesting that the current rarity of native perennials at our site is caused by natives being recruitment-limited and not by exotic annuals being superior resource competitors.
In general, perennials are thought to be competitively superior to annuals in undisturbed habitats. Other studies in California have shown that native perennial grasses can be more effective competitors for deep soil water and for light than annual grasses (33, 38) and may invade stands of exotic annuals and reduce their density (39, 40). Furthermore, undisturbed relict or restored stands of native perennial grasses are resistant to exotic annual invasion (30, 38). In contrast, annual species tend to allocate fewer resources to roots and more resources to leaf and seed production (41-44), a tradeoff that should make annuals faster growers and better exploiters of disturbances but weaker competitors for belowground resources (43, 45) than perennials.
In addition to nitrate concentrations (our measure), the nitrogen environment experienced by plants is a function of multiple components of the nitrogen cycle (e.g., ammonium concentrations and mineralization and nitrification rates). Plants may affect these components of the nitrogen cycle either directly or indirectly by way of effects on soil moisture. Further work is necessary to isolate the direct effects of plant uptake on soil nitrogen from the indirect effects mediated by plant-generated changes in soil moisture.
Regardless of the treatment, exotic annuals and native perennials were able to coexist; neither extirpated the other in this study. In particular, exotic annuals persisted in plots to which native perennials had been added and vice versa. The abundance of annual species increased with increasing levels of disturbance (burning, gopher disturbance, or mowing). The response to disturbance suggests that annual-perennial coexistence may be maintained by a competition-colonization tradeoff or by a tradeoff between competitive ability and maximal rates of vegetative growth (46). Coexistence might also be maintained through spatial and temporal differences in resource use. Perennials reduced limiting soil moisture and nitrate to lower levels deeper in the soil and later in the season than did annuals. In this way, perennials garnered resources that were unavailable to the annual species. We did not find conditions under which exotic annual species resisted invasion by native perennials, a requirement for the existence of MSE.
The qualitative ability of native perennial grasses to invade stands of exotic annuals did not depend on low abundance of exotic annuals, soil nitrogen levels, rainfall, or burning. However, our results might have been influenced by the agricultural history, including recent plowing, of this field. The long-term tilling that our experimental fields experienced before our study left soils lower in carbon and nitrogen than adjacent areas that had been less heavily used for agriculture. This tilling could also have modified or disrupted feedback between the soil biota and plant communities that could increase or decrease invasibility, depending on the nature of the interactions (e.g., symbiotic or pathogenic) (47-49).
Our results suggest that recruitment limitation of native perennials is a factor maintaining dominance of exotic annual species in our area. Thus, restoration of the major native perennial grass species may be possible by means of direct seeding into stands of exotic annuals. However, California grasslands are extremely varied, and it is plausible that the means by which exotic annuals maintain dominance vary geographically. For example, the elimination of grazing alone can result in a return to perennial dominance in coastal grasslands (50, 51). In these areas, the precipitation and summer fog are ample for perennials to maintain green tissue year round (J. Corbin, personal communication), and this lengthened growing season may increase the competitive dominance of the native perennial species (38). The prevalence of exotic perennial grasses in coastal grasslands supports the supposition that coastal grasslands are more suitable for perennial grasses than inland areas (such as our study site), in which few exotic perennial grasses exist.
In contrast, in the Central Valley of California the plausibility of MSE is suggested by an apparent asymmetry in the competitive ability of the dominant native perennial grass in California, N. pulchra, and exotic annual species. Studies in the Central Valley show that dense exotic annual stands severely decrease the establishment of N. pulchra planted as seeds (52, 53), whereas N. pulchra seedlings and adults can be strong competitors with exotic annuals (30, 54, 55).
The difference between our results and these studies in the Central Valley may also be attributable to our use of multiple species in our seeding experiment. As in the Central Valley studies, we found that N. pulchra had low survival as a seedling when added to unplowed exotic annual communities, whereas E. glaucus and B. carinatus were able to rapidly colonize these areas (unpublished data). In contrast, N. pulchra rapidly became the competitive dominant when seeded into the plowed soils in the restoration experiment (unpublished data). If we had used only N. pulchra, our results might have suggested the presence of MSE. Further work is required to determine the long-term outcome of such seed-addition experiments and the applicability of our results and their implications for restoration of areas with different histories, soil types, climates, and disturbance regimes.
At our site, dominance by annual grasses seems best explained as a transient state with a long persistence due to recruitment limitation of locally rare native perennial grasses. Although little is known about the initial invasion and spread of exotic annuals in California grasslands, it has been attributed to heavy grazing and drought (28, 29). A century or more of heavy grazing may have either extirpated native perennials or have so greatly reduced their abundances from such a large proportion of their range that they no longer provide a significant seed source in many localities, especially when coupled with low rates of seed production, establishment, or dispersal. Although we cannot with certainty discern the original cause of the collapse of California's native perennial grasslands, our results indicate that the decline of the native perennials was not due to the introduction and spread of taxa that are competitively superior to native perennials in the absence of disturbance. Thus, it should be possible to restore viable populations of native perennials even in the face of competition from exotic annual species. In contrast, if exotic annuals were competitively dominant, reestablishment of the native flora would depend on the eradication and continued quarantine of exotics, two difficult and costly ventures (1, 4).
If a tradeoff between competitive ability and dispersal ability for California grassland plants is proven, superior competitors would be slow to recover from the disturbance once driven to low densities as has been shown elsewhere. For example, at Cedar Creek Natural History Area, the two dominant native perennial bunchgrasses require about 15-20 years before they are observed anywhere in a field abandoned from agriculture, and they require another 25-35 years before they obtain peak abundances, even in fields surrounded by intact native grasslands (20). Clearly, competitively dominant natives would attain numerical dominance even more slowly at Cedar Creek if they were as rare as native perennial grasses are throughout California.
Our results support the hypothesis that invasions occur when the invading species are able to survive and grow on the resources left unconsumed by existing species. In the current study, the existing species were exotic annuals. Native perennials, which our studies showed could live on lower levels of resources than those left unconsumed by exotic annuals, were able to successfully invade dense, established stands of exotic annuals and markedly decrease annual plant abundances. This successful invasion occurred even when the native perennial grasses were seeded into stands of exotic annuals producing tens of thousands of seeds per square meter. Moreover, exotic annuals were not recruitment-limited, which is consistent with their being superior colonists and/or exploiters of resource pulses.
Despite efforts to determine the general characteristics of communities vulnerable to invasion by exotic species and the characteristics of successful invaders (4, 56, 57), few cases exist in which this approach has shown much generality. For example, theoretical and empirical support exist for both a positive (58-60) or a negative (7, 10, 61-66) relationship between species richness and invasiveness, depending on the scale of the study (17, 67, 68). Ultimately predicting the outcome of competitive interactions between native and exotic species may depend on determining the causes of mutual invasibility patterns (17).
Our work has demonstrated that a resource-based, mechanistic approach to understanding invasion success or failure can provide insights for conservation and restoration efforts. Our experiments also suggest that historical disturbance events might lead to extreme recruitment limitation on the part of native species. Superior competitors may be particularly susceptible to seed limitation, because they often have low seed production and/or seed dispersal. As a result, it is possible to have transient, but long-term dominance by competitively inferior invaders. In areas where this mechanism is in effect, equating abundance of exotic species with competitive dominance would give an overly pessimistic assessment of the prospects for restoration.
Acknowledgments
We thank E. Borer, J. Corbin, C. D'Antonio, A. Dyer, J. Gerlach, K. Rice, and three anonymous reviewers for comments on the manuscript, and A. Borcher, J. Quinn, and T. Yoshida for assistance with data collection. This work was supported by National Science Foundation Grants DEB 9806377, DEB 0080382, and DEB 0235624, Andrew W. Mellon Foundation grants (to E.W.S., O.J.R., and D.T.), the University of Minnesota's Distinguished McKnight Professorship (to D.T.), and the National Center for Ecological Analysis and Synthesis, a center funded by National Science Foundation Grant DEB-94-21535 and the University of California, Santa Barbara.
Abbreviation: MSE, multiple stable equilibria.
References
- 1.Lodge, D. M., Stein, R. A., Brown, K. M., Covich, A. P., Bronmark, C., Garvey, J. E. & Klosiewski, S. P. (1998) Aust. J. Ecol. 23, 53-67. [Google Scholar]
- 2.Mills, E. L., Leach, J. H., Carlton, J. T. & Secor, C. L. (1994) BioScience 44, 666-676. [Google Scholar]
- 3.Mooney, H. A., Hamburg, S. P. & Drake, J. A. (1986) in Ecology of Biological Invasions of North America and Hawaii, ed. Drake, J. A. (Springer, New York), pp. 250-272.
- 4.Hobbs, R. J. & Humphries, S. E. (1995) Conserv. Biol. 9, 761-770. [Google Scholar]
- 5.Drake, J. A., Mooney, H. A., di Castri, F., Groves, R. H., Kruger, F. J., Rejmanák, M. & Williamson, M., eds. (1989) Biological Invasions: A Global Perspective (Wiley, Chichester, U.K.).
- 6.Seabloom, E. W., Borer, E. T., Boucher, V. L., Burton, R. S., Cottingham, K. L., Goldwasser, L., Gram, W. K., Kendall, B. E. & Micheli, F. (2003) Ecol. Appl. 13, 575-592. [Google Scholar]
- 7.Knops, J. M. H., Tilman, D., Haddad, N. M., Naeem, S., Mitchell, C. E., Haarstad, J., Ritchie, M. E., Howe, K. M., Reich, P. B., Siemann, E. & Groth, J. (1999) Ecol. Lett. 2, 286-293. [DOI] [PubMed] [Google Scholar]
- 8.Huxel, G. R. & Hastings, A. (1999) Restor. Ecol. 7, 309-315. [Google Scholar]
- 9.Davis, M. A. & Pelsor, M. (2001) Ecol. Lett. 4, 421-428. [Google Scholar]
- 10.Tilman, D. (1999) Ecology 80, 1455-1474. [Google Scholar]
- 11.MacArthur, R. & Levins, R. (1967) Am. Nat. 101, 377-385. [Google Scholar]
- 12.Gurney, W. S. C. & Nisbet, R. M. (1998) Ecological Dynamics (Oxford Univ. Press, New York).
- 13.Tilman, D. (1982) Resource Competition and Community Structure (Princeton Univ. Press, Princeton). [PubMed]
- 14.Bolker, B. M. & Pacala, S. W. (1999) Am. Nat. 153, 575-602. [DOI] [PubMed] [Google Scholar]
- 15.Parker, I. M. & Reichard, S. H. (1998) in Conservation Biology, ed. Karieva, P. M. (Chapman & Hall, New York).
- 16.Vitousek, P. M., Wlaker, L. R., Whiteaker, L. D., Mueller-Dombois, D. & Matson, P. A. (1987) Science 238, 802-804. [DOI] [PubMed] [Google Scholar]
- 17.Shea, K. & Chesson, P. (2002) Trends Ecol. Evol. 17, 170-176. [Google Scholar]
- 18.Mitchell, C. E. & Power, A. G. (2003) Nature 421, 625-627. [DOI] [PubMed] [Google Scholar]
- 19.Seabloom, E. W., Borer, E. T., Boucher, V. L., Burton, R. S., Cottingham, K. L., Goldwasser, L., Gram, W. K., Kendall, B. E. & Micheli., F. (2003) Ecol. Appl. 13, 575-592. [Google Scholar]
- 20.Tilman, D. (1990) Oikos 58, 3-15. [Google Scholar]
- 21.Turnbull, L. A., Crawley, M. J. & Rees, M. (2000) Oikos 88, 225-238. [Google Scholar]
- 22.Noy-Meir, I. (1975) J. Ecol. 63, 459-481. [Google Scholar]
- 23.Laycock, W. A. (1991) J. Range Manage. 44, 427-433. [Google Scholar]
- 24.Armstrong, D. P. (1992) Behav. Ecol. Sociobiol. 30, 95-102. [Google Scholar]
- 25.Baker, H. G. (1978) in Plant Relations in Pastures, ed. Wilson, J. R. (Commonwealth Scientific and Industrial Research Organization, East Melbourne, Australia), pp. 368-384.
- 26.Burcham, L. T. (1957) California Range Land: An Historico-ecological Study of the Range of California (Division of Forestry, Department of Natural Resources, State of California, Sacramento).
- 27.Heady, H. F. (1977) in Terrestrial Vegetation of California, ed. Major, J. (Wiley, New York), pp. 491-514.
- 28.Jackson, L. E. (1985) J. Biogeogr. 12, 349-361. [Google Scholar]
- 29.D'Antonio, C. M. & Vitousek, P. M. (1992) Annu. Rev. Ecol. System. 23, 63-87. [Google Scholar]
- 30.Stromberg, M. R. & Griffin, J. R. (1996) Ecol. Appl. 6, 1189-1211. [Google Scholar]
- 31.Jackson, L. E., Strauss, R. B., Firestone, M. K. & Bartolome, J. W. (1988) Plant Soil 110, 9-18. [Google Scholar]
- 32.Christian, J. M. & Wilson, S. D. (1999) Ecology 80, 2397-2407. [Google Scholar]
- 33.Dyer, A. R. & Rice, K. J. (1999) Ecology 80, 2697-2710. [Google Scholar]
- 34.Tilman, D. & Wedin, D. (1991) Ecology 72, 1038-1049. [Google Scholar]
- 35.Huenneke, L. F., Hamburg, S. P., Koide, R., Mooney, H. A. & Vitousek, P. M. (1990) Ecology 71, 478-491. [Google Scholar]
- 36.Peart, D. R. (1989) J. Ecol. 77, 267-289. [Google Scholar]
- 37.Michaelsen, J., Haston, L. & Davis, F. (1987) Water Resourc. Bull. 23, 809-818. [Google Scholar]
- 38.Corbin, J. D. & D'Antonio, C. M., Ecology, in press.
- 39.Hamilton, J. G. (1997) Ph.D. dissertation (Univ. of California, Santa Barbara).
- 40.Bugg, R. L., Brown, C. S. & Anderson, J. H. (1997) Restor. Ecol. 5, 214-228. [Google Scholar]
- 41.Grime, J. P. & Hunt, R. (1975) J. Ecol. 63, 393-422. [Google Scholar]
- 42.Jackson, L. E. & Roy, J. (1986) Acta Oecol. 7, 191-212. [Google Scholar]
- 43.Garnier, E. (1991) Trends Ecol. Evol. 6, 126-131. [DOI] [PubMed] [Google Scholar]
- 44.Holmes, T. H. & Rice, K. J. (1996) Ann. Bot. (London) 78, 233-243. [Google Scholar]
- 45.Tilman, D. (1988) Dynamics and Structure of Plant Communities (Princeton Univ. Press, Princeton).
- 46.Pacala, S. W. & Rees, M. (1998) Am. Nat. 152, 729-737. [DOI] [PubMed] [Google Scholar]
- 47.Bever, J. D., Westover, K. M. & Antonovics, J. (1997) J. Ecol. 85, 561-573. [Google Scholar]
- 48.Bever, J. D. (1994) Ecology 75, 1965-1977. [Google Scholar]
- 49.Klironomos, J. N. (2002) Nature 417, 67-70. [DOI] [PubMed] [Google Scholar]
- 50.Heady, H. F. (1956) Ecology 37, 798-812. [Google Scholar]
- 51.Peart, D. R. (1989) J. Ecol. 77, 236-251. [Google Scholar]
- 52.Dyer, A. R. & Fossum, H. C. (1996) Madroño 43, 316-333. [Google Scholar]
- 53.Love, R. M. (1944) J. Am. Soc. Agron. 36, 699-703. [Google Scholar]
- 54.Robinson, R. H. (1971) Korean J. Bot. 14, 1-20. [Google Scholar]
- 55.Shoulders, C. L. (1994) M.S. thesis (Univ. of Wisconsin, Madison).
- 56.Sax, D. F. & Brown, J. H. (2000) Global Ecol. Biogeogr. 9, 363-371. [Google Scholar]
- 57.Lodge, D. M. (1993) Trends Ecol. Evol. 8, 133-137. [DOI] [PubMed] [Google Scholar]
- 58.Robinson, G. R., Quinn, J. F. & Stanton, M. L. (1995) Ecology 76, 786-794. [Google Scholar]
- 59.Smith, M. D. & Knapp, A. K. (1999) Oecologia 120, 605-612. [DOI] [PubMed] [Google Scholar]
- 60.Levine, J. M. & D'Antonio, C. M. (1999) Oikos 87, 15-26. [Google Scholar]
- 61.Fox, M. D. & Fox, B. J. (1986) in Ecology of Biological Invasions, ed. Groves, R. H. (Cambridge Univ. Press, New York), pp. ix, 166.
- 62.Case, T. J. (1990) Proc. Natl. Acad. Sci. USA 87, 9610-9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tilman, D. (1997) Ecology 78, 81-92. [Google Scholar]
- 64.Stachowicz, J. J., Whitlatch, R. B. & Osman, R. W. (1999) Science 286, 1577-1579. [DOI] [PubMed] [Google Scholar]
- 65.Elton, C. S. (1958) The Ecology of Invasions by Animals and Plants (Methuen, London).
- 66.Lyons, K. G. & Schwartz, M. W. (2001) Ecol. Lett. 4, 358-365. [Google Scholar]
- 67.Stohlgren, T. J., Binkley, D., Chong, G. W., Kalkhan, M. A., Schell, L. D., Bull, K. A., Otsuki, Y., Newman, G., Bashkin, M. & Son, Y. (1999) Ecol. Monogr. 69, 25-46. [Google Scholar]
- 68.Stohlgren, T. J., Barnett, D. T. & Kartesz, J. T. (2003) Frontiers Ecol. Environ. 1, 11-14. [Google Scholar]