Abstract
Current sexual selection theory proposes several potential mechanisms driving the evolution of female mating preferences, few of which involve social interactions. Although vertebrate examples of socially influenced mating preferences do exist, the invertebrate examples are virtually nonexistent. Here I demonstrate that the mating preferences of female wolf spiders can be acquired through exposure as subadults to unrelated, sexually active adult males. I first conducted exposure trials during which subadult females of the wolf spider Schizocosa uetzi were allowed to interact with mature males of an experimentally manipulated phenotype (either black or brown forelegs). After maturation, these previously exposed females were paired with a male of either a familiar or unfamiliar manipulated phenotype for mate-choice trials. Subadult females that were exposed to directed courtship by mature males of a particular morphological phenotype were subsequently more likely to mate with a male of a familiar phenotype as adults. Furthermore, females that were exposed as subadults were more likely, as adults, to cannibalize a courting male with an unfamiliar phenotype. Unexposed females did not distinguish between phenotypes in either mate choice or cannibalism frequency. These results suggest a previously uncharacterized mechanism influencing the origin of female mating preferences and ultimately the evolution of male traits: subadult experience. This study also stresses the potential importance of learning and memory on adult mate choice in an arthropod.
Female preference is known to be a strong driving force behind the evolution of inter- and intraspecific divergence of male signaling traits. Currently, sexual selection theory proposes Fisherian self-reinforcing selection, genetic indicator mechanisms, species recognition, and direct benefits as possible mechanisms driving the evolution of these female preferences (1). Although the existence of socially and environmentally influenced preferences and their subsequent impact on male trait evolution has received comparatively little attention (2), several field and laboratory studies exist that demonstrate that female mate choice can be affected by previous experience with males. All examples of “previous male effects” thus far (3), however, have been documented in vertebrates, and the acquisition of the experience is generally described as occurring during the female's adult life [fish (refs. 4-6), birds (ref. 7), reptiles and amphibians (ref. 8), and mammals (ref. 9 and references therein)]. A recent study involving Japanese quail, for example, has demonstrated the importance of cultural transmission (specifically mate-choice copying) on the establishment of female preferences. White and Galef (10) found that female Japanese quail prefer males possessing traits that were shared with males they had previously seen copulating. By extending the mechanism of mate-choice copying beyond a preference for a focal male to a preference for a male phenotype, this study clearly illustrates the plausibility of cultural transmission influencing the evolution of male phenotypes. In addition to empirical studies, theory has also been developed to explore the influence of mate-preference acquisition through cultural transmission on the evolution of sexually selected traits (11, 12).
Sexual imprinting, although different from cultural transmission such as mate-choice copying, has also been demonstrated clearly in birds (13, 14) as well as mammals (15, 16) and is a process that is thought to occur primarily in taxa with extensive parental care. This type of socially influenced mate choice generally involves imprinting on parental phenotypes and is thought to be important in species recognition later in life (but see ref. 17). Again, in addition to empirical studies, theory has also been developed to look at the influence of sexual imprinting on the evolution of secondary sexual traits (18, 19). Although examples of cultural transmission of female preferences demonstrate the effect of prior adult experience on subsequent adult behavior, studies of sexual imprinting demonstrate the effect of immature experience, often during a critical period, on subsequent adult behavior. In many animal taxa including humans, however, subadult females are often exposed to courtship advances of unrelated sexually active males. In this study, by using the wolf spider Schizocosa uetzi, I test the hypothesis that a subadult female's experience with a courting adult male can subsequently influence her adult mate choice.
Wolf spiders in the genus Schizocosa exhibit tremendous variation in the secondary sexual traits involved in male courtship displays, providing an ideal system for studies of male trait evolution and female preference (20-23). S. uetzi males possess visual ornamentation consisting of black pigmentation on a portion of the tibia of their forelegs. Within a single local population, males exhibit variation in both the darkness and the total area of pigmentation (24). The courtship display of S. uetzi is multimodal, consisting of a visual arching movement of the foreleg as well as a substrate-borne vibration (24). Previous studies using visual video playbacks of phenotypically manipulated males in this species revealed no evidence that male foreleg ornamentation exerts a significant influence on female mate choice, calling into question the function of this presumed secondary sexual trait (21). However, although prior experiments did control for female mating experience and female age, they did not control for subadult female experience. All previous studies used virgin females that were collected from the field as immatures and housed in isolation until maturation (21). However, isolating subadult females before adult mate-choice trials is not necessarily realistic. Under natural conditions, males tend to mature a few weeks before females, and, as such, in the field it is likely that subadult females encounter mature, sexually active males before their own maturation.
To explore the possibility that subadult female experience influences subsequent adult mate choice, I exposed subadult females to males of a given phenotype and subsequently examined their adult mate choice. Here I report that subadult female exposure does indeed influence subsequent adult mate choice and that exposed females prefer to mate with males of a familiar phenotype and prefer to cannibalize males of an unfamiliar phenotype.
Materials and Methods
Species. Subadult S. uetzi of both sexes were collected from sites near Oxford, MS, in May 2002 and brought back to the laboratory at Cornell University, where they were housed individually in 6 × 6 × 8-cm AMAC (Westbrook, ME) plastic product boxes. Individuals were fed two to three crickets once a week and were kept on a 12-h light/12-h dark cycle. All cages had opaque sides so that individual spiders could not see one another. Maturation dates of all individuals were recorded.
Phenotype Manipulation. To control male phenotype, males were randomly assigned as they matured to one of two experimental foreleg manipulations: The tibia and patella were painted either black or brown, representing the extreme ends of the natural variation in this species (24). Once males were >5 days past their maturation molt, they were placed in a small Ziploc bag with a hole cut out of one corner. Each male was maneuvered so that each of his forelegs extruded through the hole while his body remained restrained in the bag. By using a small paintbrush, the dorsal and ventral surface of the tibia and patella of the first pair of legs of each male were painted with either black (NailSlicks, midnight metal, 551; CoverGirl) or brown (NailSlicks, bronze ice, 150; CoverGirl) nail polish under a dissecting scope. The nail polish dried quickly, and males were not able to groom it off. Immediately after painting, males engaged in persistent grooming that lasted only a few minutes. All males in this experiment were painted, and there was no further observable effect of painting on behavior.
Exposure Trials. Subadult females were assigned randomly to males with either black or brown foreleg phenotypes. During their penultimate stage (i.e., the last molt before maturation), subadult females were placed in an arena with a mature male for 30 min every other day until their final maturation molt. All experimental arenas were 8.73 × 8.73 × 11.27-cm AMAC plastic product clear boxes. All arenas had a single piece of filter paper lining the bottom. The night before both exposure trials and mate-choice trials, a mature female was placed on the filter paper and left overnight, allowing for the accumulation of mature female silk and associated pheromones (25, 26). Mature female pheromone elicits male courtship displays and thus, because of the presence of the pheromone-laden silk on the filter paper lining the arena, courtship was elicited in the mature males. In between trials, all arenas were swabbed with alcohol. Courting males typically directed their courtship displays toward the present subadult female, providing subadult females had experience with courting adult males. Individual males were used multiple times for the exposure trials (black, n = 14; brown, n = 13) but never with the same female; thus females that were exposed more than once were exposed to different males of the same phenotype.
Mate-Choice Trials. Once the exposed females molted to maturity, they remained isolated in their individual cages until the mate-choice trials. Mate choice of exposed females was tested 11-20 days after their final maturation molt and thus at least 11 days after their last exposure treatment. In adult mate-choice trials, females were assigned either to males with the same phenotype to which they had been originally exposed (“familiar” males) or to males with the alternative phenotype (“unfamiliar” males). Each female was paired with only one test male, resulting in four female treatment types of two classes of familiar males (black exposure/black mate choice and brown exposure/brown mate choice) and two classes of unfamiliar males (black exposure/brown mate choice and brown exposure/black mate choice). Females were placed in the same arenas as were used in the exposure trials. Mate-choice trials also lasted 30 min, and trials were scored for the presence/absence of copulation, the latency to copulation when present, and the presence/absence of sexual cannibalism. Seventy-five new males were used for the mate-choice trials and only three were used more than once.
Unexposed Females. The mate choice of unexposed females was also assessed in this study. Subadult females collected from the field remained isolated in their individual containers until 11-20 days after their final maturation molt. Unexposed females were assigned randomly either a black or brown male phenotype and were subjected to mate-choice trials in the same manner as the exposed females.
Results
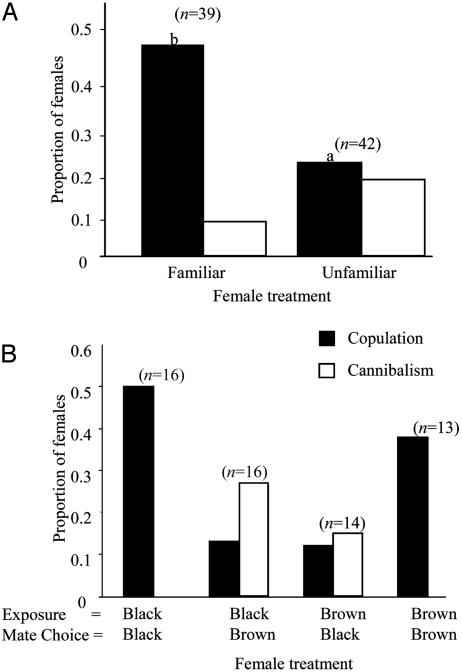
Eighty-one immature females gained experience with mature males of a given phenotype during the exposure trials. Of these females, 73% were exposed to more than one male, and no one single female was exposed to more than nine different males. I found that mate choice in adult females was affected by prior experience. Exposed females were more likely to mate with a male that had a familiar phenotype, and this mating preference was independent of initial exposure color [n = 21 black exposure/black mate choice; n = 20 black exposure/brown mate choice; n = 20 brown exposure/black mate choice; and n = 20 for brown exposure/brown mate choice; all trials, n = 81, χ2 = 5.64, P = 0.018 (Fig. 1A); only trials where females were exposed more than once: n = 59, χ2 = 6.0, P = 0.014 (Fig. 1B)]. With females that were exposed more than once, cannibalism also depended on prior experience (n = 59, χ2 = 8.11, P = 0.004; Fig. 1B). Females that were exposed more than once as subadults were more likely to cannibalize a male that had an unfamiliar phenotype. Although prior exposure influenced female mate choice, it did not influence the latency to copulation [F(3,23) = 0.38, P = 0.84; Table 1].
Fig. 1.
(A) Proportion of females that mated and cannibalized for all trials. Females were more likely to mate with a familiar male (n = 81: n = 21 black/black, n = 20 black/brown, n = 20, brown/black, and n = 20 brown/brown; χ2 = 5.64, P = 0.018). (B) Proportion of females that mated and cannibalized for all females exposed to more than one male (n = 59). Mating frequency depended on female treatment (χ2 = 7.8, P = 0.05). Females mated significantly more with males with a familiar phenotype than an unfamiliar phenotype (χ2 = 6.0, P = 0.014). Cannibalism frequency also depended on female treatment (χ2 = 9.6, P = 0.023). Females cannibalized males with unfamiliar phenotypes significantly more than familiar phenotypes (χ2 = 8.11, P = 0.004).
Table 1. Latency to copulation.
| Subadult exposure | Adult mate choice | n | Latency, min | SE |
|---|---|---|---|---|
| Black | Black | 21 | 10.69 | 2.4 |
| Black | Brown | 20 | 8.23 | 3.99 |
| Brown | Black | 20 | 7.15 | 3.56 |
| Brown | Brown | 20 | 10.32 | 3.01 |
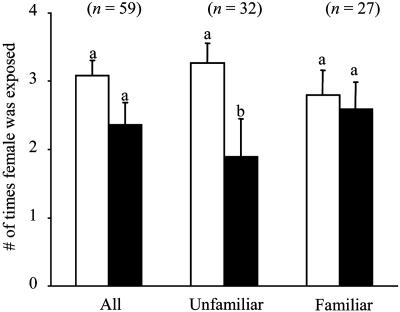
The amount of subadult exposure (i.e., the number of times a females was subjected to a 30-min exposure trial) did not affect the preference of a female for a familiar male, but it did affect the preference for, or rather the discrimination against, an unfamiliar male. Of the females that were paired with unfamiliar males, those that copulated were exposed fewer times than those that did not copulate [unfamiliar: F(1,40) = 4.76, P = 0.035; Fig. 2]. This suggests that the more frequently a subadult female is exposed to a mature male of a given phenotype, the less likely she is to copulate with a male of a different phenotype as an adult, suggesting a type of frequency-dependent mate choice (27, 28).
Fig. 2.
A comparison of the average number of times a female was exposed to males of a given phenotype between females that copulated (filled bars) and those that did not copulate (open bars). Shared letters indicate no significant difference. Females that did not copulate with unfamiliar male phenotypes were exposed significantly more times than females that copulated with unfamiliar males, suggesting that increased exposure to one male phenotype decreases the probability of copulation with an unfamiliar male phenotype [F(1,40) = 4.78, P = 0.035].
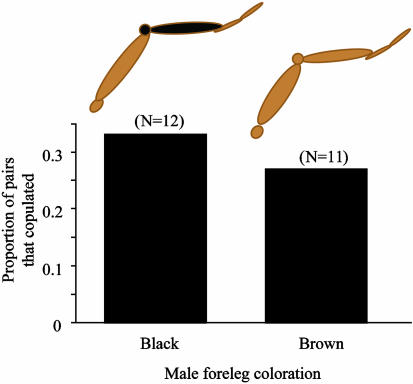
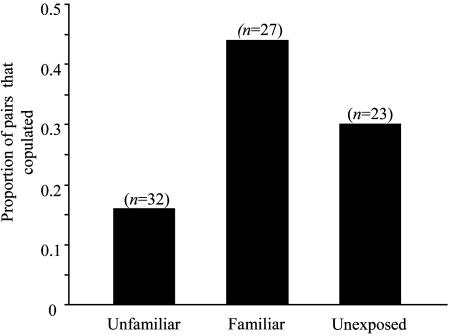
Unlike the case with the exposed females, mate choice of unexposed adult females was not influenced by male phenotype (n = 23, χ2 = 0.1, P = 0.75; Fig. 3), suggesting again that there is no innate bias or preference for any one particular phenotype. The proportion of females that mated, however, did depend on whether the females were unexposed, exposed to familiar males, or exposed to unfamiliar males (χ2 = 6.04, P = 0.049; Fig. 4). The highest proportion of matings occurred with exposed females in the familiar-phenotype treatment, suggesting that prior experience increases a female's likelihood to mate with a familiar male and decreases a female's likelihood to mate with an unfamiliar male. When results from all exposed females were combined, there was no evidence for an effect of early exposure on the likelihood to mate (χ2 = 0.07, P = 0.79) thus, exposure itself does not seem to influence a female's willingness to mate.
Fig. 3.
The proportion of unexposed females that mated with males of a given phenotype. Male phenotype had no effect on female mate choice for unexposed females (χ2 = 0.1, P = 0.75).
Fig. 4.
The effect of exposure on probability of mating. When all three exposure categories are compared, the proportion of pairs mating depends on the exposure treatment (χ2 = 6.04, P = 0.049). However, when all exposed females are grouped together, there is no difference between the proportion of exposed versus unexposed females that mate (χ2 = 0.07, P = 0.79).
Discussion
These results clearly demonstrate that a female wolf spider's experience during her penultimate life stage can affect her mate choice as an adult. Subadult females that were exposed to courtship advances from mature, phenotypically manipulated males not only mated more frequently with males of a familiar phenotype, but they also were more likely to cannibalize males of an unfamiliar phenotype. Thus, the socially influenced origin of female mating preference described here could impose incredibly strong selection pressures on male maturation times, male mating strategies, and ultimately secondary sexual ornamentation, because females that gain their mating preferences through subadult experience are choosing mates versus meals. In contrast to exposed females, the adult mate choice of unexposed females was not influenced by male phenotype, demonstrating again that there is no innate bias or preference for any one particular phenotype.
The extent to which prior experience affects current behavior is often overlooked in behavioral studies, which tend to focus instead on the equivalent of snapshots of an animal's life (i.e., individual foraging bouts or individual mate-choice experiences). Nonetheless, several studies on insects have demonstrated that experience during the adult stage affects subsequent adult foraging or oviposition behavior (reviewed in ref. 29). However, fewer studies exist that address how, or if, immature or subadult experience affects adult behavior, and those that do exist remain somewhat controversial (29). Within the arachnids, immature experience has been shown to affect subsequent hunting behavior, learning, and central nervous system development in another wolf spider (30). Until this study, however, to my knowledge no information has been available on invertebrates in general regarding the potential connection between immature or subadult experience and adult mate choice. In fact, Alexander et al. (31) lay out several reasons why they believe that social learning regarding mate choice does not exist in insects, and presumably arachnids, in contrast to vertebrates. They argue that instead of relying on social influences, insects are likely restricted to the use of thresholds for mate choice (31). An exception to their ideas, however, recently emerged in a study by Wagner et al. (32), in which the mating preference of female crickets was shown to be influenced by prior adult experience, suggesting that social interactions indeed may influence mating preferences in insects. Adult female crickets were separated into two groups: one group experienced three low chirp-rate songs (known to be unattractive), whereas the second group experienced a low chirp-rate song followed by a high chirp-rate song (known to be attractive) followed by another low chirp-rate song. After exposure to a high chirp-rate song, females in the second group significantly decreased their response to the last low chirp-rate song as opposed to females that only experienced low chirp-rate songs (32). These results suggest that exposure to more variable song types or to more attractive songs make females even less attracted to unattractive songs. Although Wagner et al. were able to show that acoustic experience in the variable field cricket can influence female response to song (as measured by time spent in association with a speaker), unlike the study described here, it is not clear how or if their scenario in nature could influence the evolution of male cricket song rate. Furthermore, similar to the many studies of socially influenced mating preferences in vertebrates, the cricket study described above shows the influence of adult experience on subsequent adult behavior within a very short time window.
In contrast to prior experiments whether in vertebrates or invertebrates, this wolf spider study distinguishes itself from previous examples of socially influenced mating preferences such as sexual imprinting or mate-choice copying based on the life stage during which the social influence acts. For example, although mate-choice copying involves prior adult experience, sexual imprinting generally involves “early” social interactions, often during a critical period (33). The phenomenon described in this study, however, involves the penultimate, or subadult, life stage: the equivalent of “teenagers.” Unlike processes of sexual imprinting, the influence of subadult experience on adult mate choice described here cannot involve parental phenotypes, because (i) spiders do not exhibit parental care and (ii) the subadult females used in this study were already ≈10 months old. Because of the 1-year life span of S. uetzi, all parents are generally dead within 1-2 months of their offspring hatching (i.e., 8 months before the female exposure treatments). Also, unlike scenarios of prior adult experience, my results imply that subadult female wolf spiders are not only able to learn male phenotypes but that they retain this memory through their final maturation molt and for another 11-20 days beyond maturation and ultimately incorporate this memory into adult mate-choice decisions. The effect of prior subadult female exposure then does not manifest itself for almost 3 weeks, and one molt, after the experience itself. This study suggests that the complexity of factors involved in mate-choice decisions of arthropods, at least wolf spiders, may not be much less than those involved in vertebrate decision making.
The evolutionary implications of subadult experience influencing adult mate choice are potentially very different from those involving what we traditionally think of as “imprinting” or mate-choice copying. Unfortunately, it is currently difficult to predict the trajectory of male secondary sexual trait evolution via a process of female choice where females attain mating preferences based on subadult experience. Exploring the evolutionary outcomes of selection from such a process is a future direction for which modeling may prove to be very useful. Initially, intuition suggests that male traits will simply go to fixation. For example, the more common phenotype will presumably mate more frequently, because it will also be the familiar phenotype, and given that the male phenotype is heritable, it will ultimately spread throughout the population. However, it is not as simple as it first seems, because the phenotype frequency that ultimately will influence adult mate choice in this scenario will occur relatively early in the season, while females are still subadults. It is the frequency of male phenotypes during the female penultimate life stage that will influence adult female mating preferences. Currently, we do not know how or if the natural frequency of phenotypes in the population varies throughout a season. A common phenotype early in the season may not be a common phenotype later in the season and vice versa. For example, different phenotypes may experience differing levels of selection based on factors such as variable predation risk, and this could influence the frequency of different phenotypes through time. Furthermore, it is unlikely that all females in a population will gain exposure to a mature male during their subadult stage, and, as such, the effect that I describe in this study is realistically likely to affect only a subset of females. Unexposed females are presumably using other means by which to choose mates. Regardless, the acquisition of mating preferences through subadult experience is likely to affect the maintenance of alternative male phenotypes in the population and may even select for variable male strategies such as actively seeking interactions with subadult females. More studies clearly are needed, both empirical and theoretical, to explore the possible evolutionary effects of this socially influenced origin of female mating preference.
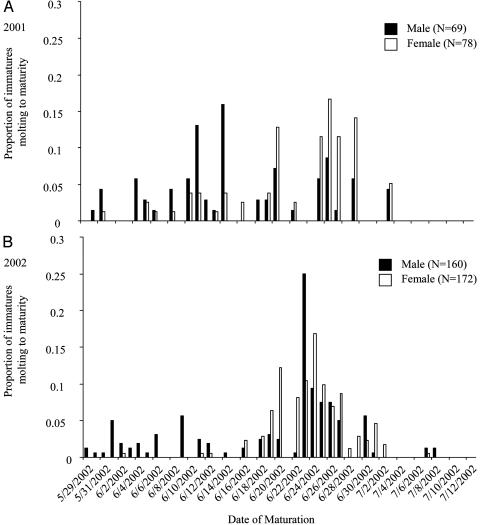
Under natural conditions, the question remains, however, as to how likely it is for a penultimate female S. uetzi to encounter a mature, courting conspecific male. Based on three lines of auxiliary evidence, I propose that, in the field, many subadult females are very likely to experience courtship advances from adult males and that this experience indeed can influence their adult mate choice. First, males tend to mature before females (34). Although early maturation of males is common among spiders in general, 2 years worth of data on maturation times provide support that S. uetzi males tend to mature earlier than females and thus are present in the environment during the female subadult stage (Fig. 5). Second, mature males initiate courtship after contact with mature female draglines/silk even in the absence of the female. After initiation of courtship, a male will direct his advances toward any spider present, even a subadult male (unpublished data), making it possible for subadult females to be the recipients of these advances. And third, although there may be relatively few mature S. uetzi females present early in the season to deposit draglines and associated pheromones, because this species is among the last to mature, there are many mature heterospecific females present depositing draglines (i.e., Schizocosa stridulans), and it is known that male wolf spiders will court the draglines of heterospecifics as well as conspecifics (25, 26). These three factors together create an environment in which there are mature males, subadult females, and mature female draglines throughout, providing ample opportunities for a subadult female to experience courtship advances from a mature male. This scenario also raises an intriguing problem of subadult females experiencing courtship advances from mature male heterospecifics, a topic that may be explored in future studies.
Fig. 5.
A comparison of maturation times for males and females from two field seasons, 2001 (A) and 2002 (B). All maturation times represent immatures that were collected from the field and molted to maturity in the laboratory. During collections, mature individuals were not collected, and thus this is a very conservative estimate of maturation times, because many males were already mature in the field.
In conclusion, this study demonstrates a basis for acquired mating preferences in a wolf spider and suggests that experiences during prereproductive life stages may exert a strong influence on adult reproductive behavior and ultimately on the evolution of mate traits. The results of this study have broad implications ranging from new hypotheses regarding variation in male courtship, mating and maturation strategies, influences of prior experience on male trait evolution, and the potential importance of learning and memory in arthropod mate choice. Based on the surprising findings of this study, current studies of mate choice may need to expand their scope and potentially incorporate or control for experiences during early life stages.
Acknowledgments
I thank G. Stratton and P. Miller for help with spider collecting as well as for providing food and lodging during collection times. D. Nee and K. Cuasay helped with exposure trials. B. Daley, D. Dean, N. Vandersal, D. Elias, A. Spence, K. Reeve, D. Papaj, T. Blackledge, J. Storz, and the behavior lunch bunch at Cornell University were all incredibly helpful in discussing results. D. Dean, S. Lynn, R. Hoy, K. Reeve, G. Patricelli, J. Storz, and the Papaj Laboratory also provided useful comments on an earlier draft of this article.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Andersson, M. (1994) Sexual Selection (Princeton Univ. Press, Princeton).
- 2.Owens, I. P. F., Rowe, C. & Thomas, A. L. R. (1999) Trends Ecol. Evol. 14, 131-132. [DOI] [PubMed] [Google Scholar]
- 3.Dombrovsky, Y. & Perrin, N. (1994) Am. Nat. 144, 355-361. [Google Scholar]
- 4.Brown, L. (1981) Anim. Behav. 29, 375-382. [Google Scholar]
- 5.Bakker, T. C. M. & Milinski, M. (1991) Behav. Ecol. Sociobiol. 29, 205-210. [Google Scholar]
- 6.Milinski, M. & Bakker, T. C. M. (1992) Proc. R. Soc. London Ser. B 250, 229-233. [Google Scholar]
- 7.Collins, S. A. (1995) Anim. Behav. 49, 479-486. [Google Scholar]
- 8.Gabor, C. R. & Halliday, T. R. (1997) Behav. Ecol. 8, 162-166. [Google Scholar]
- 9.Kavaliers, M., Colwell, D. D., Braun, W. J. & Choleris, E. (2003) Anim. Behav. 65, 59-68. [Google Scholar]
- 10.White, D. J. & Galef, B. G. (2000) Anim. Behav. 59, 975-979. [DOI] [PubMed] [Google Scholar]
- 11.Laland, K. N. (1994) Theor. Popul. Biol. 45, 1-15. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima, A. & Aoki, K. (2002) Theor. Popul. Biol. 61, 73-81. [DOI] [PubMed] [Google Scholar]
- 13.Slagsvold, T., Hansen, B. T., Johannessen, L. E. & Lifjeld, J. T. (2002) Proc. R. Soc. London Ser. B 269, 1449-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendrick, K. M., Hinton, M. R., Atkins, K., Haupt, M. A. & Skinner, J. D. (1998) Nature 395, 229-230. [DOI] [PubMed] [Google Scholar]
- 15.Penn, D. & Potts, W. (1998) Proc. R. Soc. London Ser. B 265, 1299-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateson, P. (1978) Nature 273, 659-660. [DOI] [PubMed] [Google Scholar]
- 17.Bateson, P. (1985) Mate Choice (Cambridge Univ. Press, Cambridge, U.K.).
- 18.Laland, K. N. (1994) Evolution (Lawrence, Kans.) 48, 477-489. [DOI] [PubMed] [Google Scholar]
- 19.Aoki, K., Feldman, M. W. & Kerr, B. (2001) Evolution (Lawrence, Kans.) 55, 25-32. [DOI] [PubMed] [Google Scholar]
- 20.Hebets, E. A. & Uetz, G. W. (1999) Anim. Behav. 57, 865-872. [DOI] [PubMed] [Google Scholar]
- 21.Hebets, E. A. & Uetz, G. W. (2000) Behav. Ecol. Sociobiol. 47, 280-286. [Google Scholar]
- 22.Stratton, G. E. (1983) Anim. Behav. 31, 164-172. [Google Scholar]
- 23.McClintock, W. J. & Uetz, G. W. (1996) Anim. Behav. 52, 167-181. [Google Scholar]
- 24.Stratton, G. E. (1997) J. Arachnol. 25, 84-92. [Google Scholar]
- 25.Stratton, G. E. & Uetz, G. W. (1981) Science 214, 575-577. [DOI] [PubMed] [Google Scholar]
- 26.Uetz, G. W. & Denterlein, G. (1979) J. Arachnol. 7, 121-128. [Google Scholar]
- 27.Ehrman, L. & Parsons, P. A. (1981) Behavior Genetics and Evolution (McGraw-Hill, New York).
- 28.Ehrman, L. & Spiess, E. B. (1969) Am. Nat. 103, 675-680. [Google Scholar]
- 29.Barron, A. B. (2001) J. Insect Behav. 14, 725-737. [Google Scholar]
- 30.Punzo, F. & Ludwig, L. (2002) Anim. Cogn. 5, 63-70. [DOI] [PubMed] [Google Scholar]
- 31.Alexander, R. D., Marshall, D. C. & Cooley, J. R. (1997) in Mating Systems in Insects and Arachnids, eds. Choe, J. C. & Crespi, B. J. (Cambridge Univ. Press, Cambridge, U.K.), pp. 4-32.
- 32.Wagner, W. E., Smeds, M. R. & Wiegmann, D. D. (2001) Ethology 107, 769-776. [Google Scholar]
- 33.Alcock, J. (2001) Seventh Behavior (Sinauer, Sunderland, MA).
- 34.Foelix, R. (1996) Biology of Spiders (Oxford Univ. Press, New York).