Abstract
The precise function of cis elements in regulating V(D)J recombination is still controversial. Here, we determined the effect of inactivation of the TCRβ enhancer (Eβ) on cleavage and rearrangement of Dβ1, Dβ2, Jβ1, and Jβ2 gene segments in CD4-CD8- [double-negative (DN)] and CD4+CD8+ [double-positive (DP)] thymocytes. In Eβ-deficient mice, (i) Dβ1 rearrangements were more severely impaired than Dβ2 rearrangements; (ii) most of the Dβ and Jβ cleavages and rearrangements occurred in DP, rather than in DN, thymocytes; and (iii) most of the 3′ Dβ1 cleavages were coupled to 5′ Dβ2 cleavages instead of to Jβ cleavages, resulting in nonstandard Dβ1-Dβ2-Jβ2 joints. These findings suggest that the Eβ regulates TCRβ rearrangement by promoting accessibility of Dβ and Jβ gene segments in DN thymocytes and proper pairing between Dβ1 and Jβ gene segments for cleavage and joining in DP thymocytes.
The variable regions of Ig and T cell receptor (TCR) genes are assembled from V, D, and J gene segments via a site-specific DNA recombination process (1, 2). During recombination in developing lymphocytes, two rearranging gene segments are first juxtaposed in cis into a synapse (paired complex) (3-6). Within the synapse, the lymphocyte-specific proteins recombination-activating gene (RAG)1 and -2 cut DNA at the junction of recombination signal sequences (RSSs) and coding sequences (7), producing covalently sealed coding ends and blunt signal ends (SEs) (8, 9). The resulting double-strand breaks are then repaired by ubiquitously expressed proteins involved in nonhomologous end joining (1, 10). The sealed coding ends are rapidly and efficiently opened, modified, and joined to produce the continuous coding sequences. The SEs are joined directly to produce signal joints (SJs), which are usually deleted from the chromosomes.
V(D)J recombination is tightly regulated in the context of lymphocyte development, exhibiting lineage, developmental stage, and allele specificity. Although recombination at different TCR and Ig loci is mediated by the same recombinase complex and conserved RSSs, complete rearrangements of TCR genes are limited to T cells, whereas complete rearrangements of Ig genes are limited to B cells (11). Within the appropriate cell lineage, recombination is regulated temporally and in a stage-specific manner (12-14). For example, TCRβ rearrangement occurs in CD4-CD8- [double-negative (DN)] thymocytes before TCRα rearrangement, which occurs in CD4+CD8+ [double-positive (DP)] thymocytes. Moreover, in a given lymphocyte, only one of two alleles usually undergoes functional rearrangement, a process known as allelic exclusion (11).
Studies have shown that transcriptional regulatory cis elements, such as promoters and enhancers, play a critical role in targeting specific gene segments for recombination (12, 14, 15). At the TCRβ locus, deletion of the PDβ1 promoter immediately upstream of the Dβ1 gene segment severely impairs Dβ1 rearrangement (16, 17). Deletion of the TCRβ enhancer (Eβ) severely impairs both Dβ1 and Dβ2 rearrangement (18, 19). To date, most evidence suggests that promoters and enhancers target gene segments for recombination by promoting their access to RAG-mediated cleavage (12, 14, 15). Thus, in the absence of the PDβ1 promoter, the Dβ1 region in DN thymocytes was hypoacetylated and hypermethylated (20). Similarly, in the absence of the Eβ (Eβ-/-), the entire Dβ-Jβ region became hypoacetylated, hypermethylated, and inaccessible to nuclease cleavage (21). In Eβ-/- mice, however, it was also reported that the levels of Dβ and Jβ joints were much more severely reduced than the levels of Dβ and Jβ SEs (22). Based on these observations, the Eβ was proposed to play a significant role in postcleavage steps of recombination.
Several factors have complicated the elucidation of the precise effects of Eβ inactivation on TCRβ rearrangement. One factor is the complexity of the genomic organization of the TCRβ locus and the consequent complexity of TCRβ rearrangement. The murine TCRβ locus consists of many Vβ gene segments and two clusters of Dβ and Jβ gene segments (Fig. 5A, which is published as supporting information on the PNAS web site). Each Jβ cluster includes six functional Jβ gene segments and one pseudogene segment. Because of the arrangement of gene segments in the locus, Dβ1 can recombine with every Jβ segment in both Jβ1 and Jβ2 clusters, whereas Dβ2 recombines only with Jβ2 gene segments. Another factor is the complexity of TCRβ rearrangements during T cell development. Although TCRβ rearrangement is thought to occur in DN thymocytes (23), studies have shown that it can also occur in DP thymocytes (16). The precise effect of Eβ inactivation on Dβ1, Dβ2, Jβ1, and Jβ2 rearrangements in both DN and DP thymocytes has not been examined comprehensively.
We have studied the effect of Eβ inactivation on TCRβ rearrangement by assessing cleavages and rearrangements of Dβ1, Dβ2, Jβ1, and Jβ2 gene segments in both DN and DP thymocytes. Complementing these analyses, we have also measured the levels of Dβ1 and Dβ2 gene segments that remain in germ-line configuration in DN and DP thymocytes. Our findings suggest that Eβ regulates TCRβ rearrangement by promoting access of Dβ and Jβ gene segments in DN thymocytes and proper pairing between Dβ1 and Jβ2 gene segments for cleavage and rearrangement in DP thymocytes.
Materials and Methods
Targeting Vector and Mice. The targeting vector used for electroporation into J1 embryonic stem (ES) cells consisted of a floxed phosphoglycerate kinase (PGK) promoter-driven neomycin resistance gene (neo) flanked upstream by a 3.1-kb BamHI-HpaI fragment and downstream by a 4.3-kb NcoI-StuI fragment (Fig. 5A). We introduced eight copies of either Gal4- or LexA-binding sequences upstream of neo. A PGK promoter-driven thymidine kinase gene (tk) was inserted upstream of the 3.1-kb fragment. ES clones with proper deletion of Eβ were identified by Southern blotting and injected into C57BL/6 blastocysts to generate chimeric mice, which were bred with the deleter strain to remove the floxed neo (24). A single loxP site and Gal4 (or LexA) DNA sequences were left in place of Eβ in the final mutant chromosomal configuration. Heterozygous mutant mice were bred with each other to generate homozygous mutant mice and were analyzed in a mixed 129 × C57BL/6 background at 6-8 weeks of age. Different mouse strains were all maintained under specific pathogen-free conditions. Thymocyte DNA of Eβ-/- mice was kindly provided by P. Ferrier (Centre d'Immunologie de Marseille-Luminy, Marseille, France).
Assays for Germ-Line Dβ1 and Dβ2, Coding Joints (CJs), SEs, and SJs. The semiquantitative nested PCR assays for JAK3, Dβ1-Jβ1, Dβ1-Jβ2, and Dβ2-Jβ2 CJs and SJs, and the ligation-mediated (LM)-PCR assays for 3′ Dβ1 and 5′ Jβ1 SEs were performed as described (9, 16, 22). Dβ1-Jβ1, Dβ2-Jβ2, and Cβ1 probes were as described (16). JAK3 oligonucleotide probe for hybridization was 5′-GAGAGACACCTTAAGTACATC-3′. PCR products were cloned into pCR2.1-TOPO (Invitrogen) for sequencing. LM-PCR assays for 3′ Dβ2, 5′ Jβ2, 5′ Dβ1, and 5′ Dβ2 SEs, Dβ1-Dβ2 CJs, and germ-line Dβ1 and Dβ2 are described in Supporting Methods, which is published as supporting information on the PNAS web site.
Results
Eβ Inactivation Preferentially Impairs Dβ1 Rearrangement in DN Thymocytes. Deletion of the Eβ enhancer (Eβ-/-), by removing a 560-bp HpaI-NcoI fragment, has been shown to impair TCRβ rearrangement (18, 19). In our study, the same 560-bp Eβ was replaced with a concatamer of eight copies of either Gal4- or LexA-binding sequences to inactivate the enhancer and to afford future opportunities to target heterologous proteins to the locus (Fig. 5A; see Materials and Methods). Based on thymocyte numbers, surface phenotype, and levels of Dβ to Jβ rearrangements (Fig. 5 B and C and data not shown), both replacement mutations had the same effects on TCRβ rearrangement and T cell development as the deletion mutation. Therefore, the results from only one mutation, the Gal4 replacement (referred to as EβR/R), are presented below.
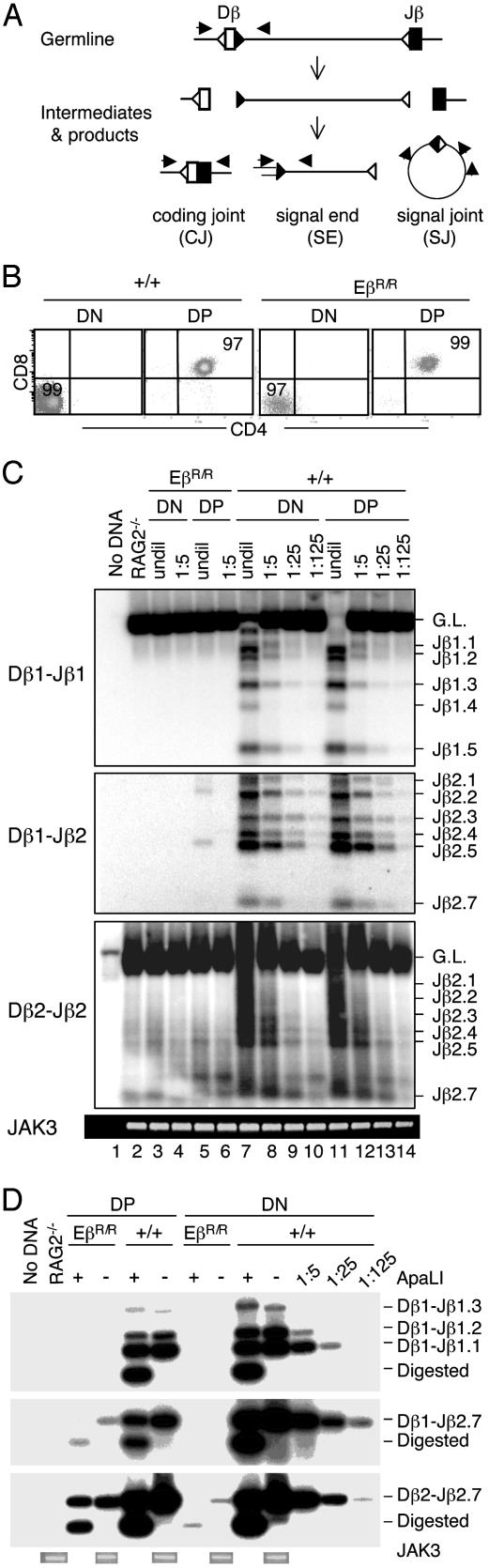
To determine the effect of Eβ inactivation on TCRβ rearrangement, we isolated DN and DP thymocytes of wild-type and EβR/R mice by cell sorting and measured the levels of Dβ1-Jβ1, Dβ1-Jβ2, and Dβ2-Jβ2 CJ by semiquantitative PCR assays (Fig. 1 A). The purity of sorted DN and DP populations was >97% (Fig. 1B). In wild-type DN and DP thymocytes, diverse PCR products, i.e., Dβ1 to different Jβ1 or Jβ2 rearrangements, and Dβ2 to different Jβ2 rearrangements, were detected (Fig. 1C, lanes 7-14). In contrast, no Dβ1-Jβ1 CJ were detected in either DN or DP thymocytes of EβR/R mice (Fig. 1C Top). Dβ1-Jβ2 CJ were not detectable in DN thymocytes of EβR/R mice, and their levels in DP thymocytes of EβR/R mice were only ≈1% of those in wild-type DN or DP thymocytes (Fig. 1C Middle). Although Dβ2-Jβ2 CJ were detected in both DN and DP thymocytes of EβR/R mice, the levels were reduced ≈25-fold as compared with those in wild-type DN and DP thymocytes (Fig. 1C Bottom). The differences in the levels of Dβ1-Jβ1, Dβ1-Jβ2, and Dβ2-Jβ2 CJ in DN and DP thymocytes suggest that various rearrangements are affected differently by the Eβ inactivation in a developmental stage-specific manner.
Fig. 1.
Eβ inactivation preferentially impairs Dβ1 rearrangement. (A) Schematic diagrams of germ-line Dβ and Jβ gene segments and their rearrangement intermediates and products. Dβ and Jβ gene segments are shown as open and filled boxes, respectively. RSSs are shown as either open or filled triangles. Arrows indicate directions of PCR primers used for assaying germline Dβ gene segment, CJ, SEs, and SJ. Double lines indicate oligonucleotide linker for LM-PCR assay. (B) Flow cytometry reanalysis of purified DN and DP thymocytes from wild-type and EβR/R mice. CD4 and CD8 staining profiles are shown for live cells. Numbers indicate the percentages of cells in the gated areas. (C) PCR assays for Dβ1-Jβ1, Dβ1-Jβ2, and Dβ2-Jβ2 CJ in DN and DP thymocytes. Various joints in purified DN and DP thymocytes of wild-type (+/+) and EβR/R mice were measured by a semiquantitative PCR. DNA was either undiluted (undil) or serially diluted every 5-fold into RAG2-/- kidney DNA and then amplified. JAK3 was amplified to verify DNA quality and relative amount. PCR products were separated on agarose gel and hybridized with specific Dβ probes. Rearrangements to different Jβ are labeled. G.L., germ-line. Representative data from one of the four experiments are shown. (D) PCR assays for Dβ-Jβ SJ in DN and DP thymocytes. The levels of Dβ1-Jβ1, Dβ1-Jβ2.7, and Dβ2-Jβ2.7 SJ in DN and DP thymocyte DNA were assayed by PCR followed by hybridization with Dβ-specific oligonucelotide probes. Half of the PCR products were digested with ApaL1 before electrophoresis. Representative data from one of the four experiments are shown.
To confirm these observations, we measured the levels of SJ in DN and DP thymocytes (Fig. 1 A). As expected, abundant Dβ1-Jβ1, Dβ1-Jβ2.7, and Dβ2-Jβ2.7 SJ were detected in both DN and DP thymocytes of wild-type mice (Fig. 1D). Consistent with an absence of Dβ1 rearrangements, no Dβ1-Jβ1 and Dβ1-Jβ2.7 SJ were detected in DN thymocytes of EβR/R mice, and the level of Dβ1-Jβ2.7 SJ was reduced ≈125-fold in DP thymocytes of EβR/R mice. Dβ2-Jβ2.7 SJ were more abundant in both DN and DP thymocytes of EβR/R mice, but the levels were still at least 25-fold lower than those in wild-type DN or DP thymocytes. Nevertheless, SJ products from both wild-type and EβR/R mice were digested equally by ApaL1, which is created by precise ligation of two SEs. These results show that SJ levels completely mirror CJ levels in both DN and DP thymocytes of EβR/R mice, providing further support for the differential effects of the Eβ inactivation on Dβ1 and Dβ2 rearrangements in DN and DP thymocytes.
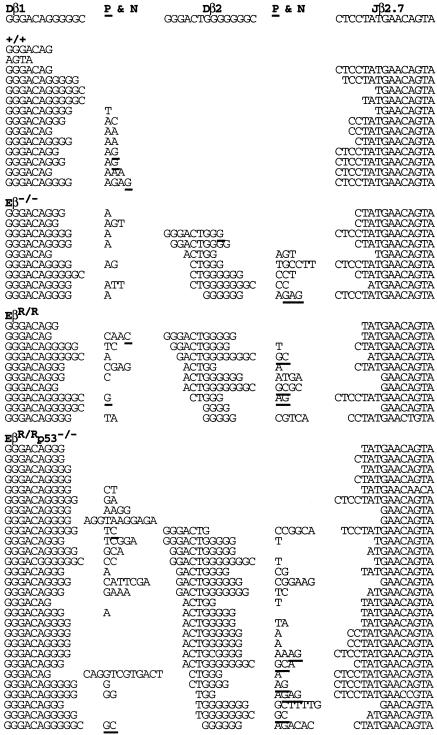
Most “Dβ1-Jβ2” Joints in EβR/R Mice Are Actually Dβ1-Dβ2-Jβ2 Rearrangements. To investigate the effect of Eβ inactivation on the quality of Dβ-Jβ joints, we sought to clone and sequence the rare Dβ1-Jβ1, Dβ1-Jβ2, and Dβ2-Jβ2 CJ found in EβR/R as well as Eβ-/- mice. Despite repeated efforts, no Dβ1-Jβ1 CJ could be cloned from thymic DNA of both EβR/R and Eβ-/- mice, further supporting a critical role of the Eβ in Dβ1-Jβ1 CJ formation. The low levels of Dβ2-Jβ2 CJ detected in EβR/R mice were indistinguishable from those of wild-type mice with regard to the frequencies and average lengths of nucleotide deletions or additions (Fig. 6B, which is published as supporting information on the PNAS web site) (25, 26). In contrast, many Dβ1-Jβ2 CJ had unusually long nucleotide “additions” in both EβR/R and Eβ-/- mice and also in EβR/R mice on a p53-deficient background (EβR/Rp53-/-) (Figs. 2 and 6A). A closer examination revealed that most of these additional nucleotides shared partial identity to the Dβ2 gene segment. If a stretch of nucleotides that share four or more identical nucleotides with the Dβ2 is taken as evidence that the rearrangements involve the Dβ2 gene segment, then 50 of the 63 supposedly Dβ1-Jβ2 CJ were actually Dβ1-Dβ2-Jβ2 rearrangements (79%). Only 13 of the 63 CJ appeared to be bona fide Dβ1-Jβ2 rearrangements (21%). Both the normal Dβ1-Jβ2 and the nonstandard Dβ1-Dβ2-Jβ2 CJ in Eβ-mutant mice had nucleotide deletions and N-region nucleotide additions with similar frequencies and average lengths, indicating normal joint formation. Based on the 12/23 rule (27), Dβ1-Dβ2 rearrangements are permissible, but their formation has not been reported in any complete TCRβ joint. The generation of the nonstandard Dβ1-Dβ2-Jβ2 CJ further demonstrates a preferential effect of the Eβ inactivation on the normal Dβ1 rearrangements.
Fig. 2.
Most of the rare Dβ1-Jβ2 joints in Eβ-mutant mice are actually Dβ1-Dβ2-Jβ2 rearrangements. PCR assays for Dβ-Jβ rearrangements in thymocytes of wild-type (+/+), Eβ-/-, EβR/R, and EβR/Rp53-/- mice were carried out as in Fig. 1C. Rearrangement products were cloned and sequenced. Sequences of Dβ1-Jβ2.7 joints are shown, and the rest is shown in Fig. 6. Germ-line sequences are shown for comparison. N-nucleotides are indicated, and possible P-nucleotides are underlined.
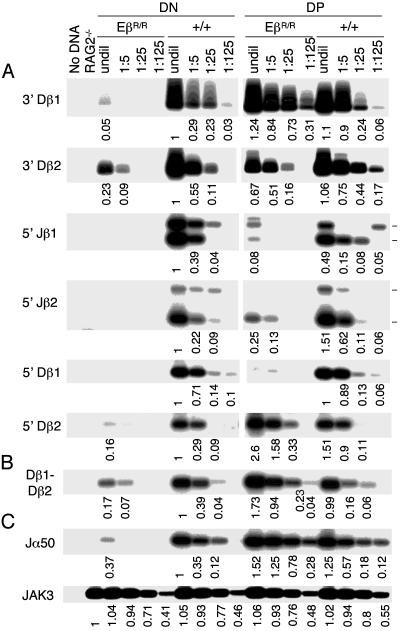
EβR/R Mutation Impairs Dβ and Jβ Cleavages in DN but Not in DP Thymocytes. To examine the molecular basis for the reduced Dβ-Jβ rearrangements in EβR/R mice, we measured the levels of SEs derived from RAG-mediated cleavage at the 3′ end of Dβ and 5′ end of Jβ in DN and DP thymocytes by LM-PCR (Fig. 1 A). In wild-type DN thymocytes, SEs of 3′ Dβ1, 3′ Dβ2, 5′ Jβ1, and 5′ Jβ2 were readily detected (Fig. 3A Left). By comparison, in DN thymocytes of EβR/R mice, the levels of 3′ Dβ1 and 3′ Dβ2 SEs were reduced ≈100- and 15-fold, respectively, and no Jβ SEs were detected. Because there are seven Jβs, the level of cleavage at each Jβ might have been below the detection limit. These results suggest that in the absence of Eβ, the Dβ and Jβ gene segments are inaccessible to RAG-mediated cleavages in DN thymocytes.
Fig. 3.
Eβ inactivation preferentially impairs Dβ and Jβ cleavages in DN thymocytes. (A) LM-PCR assays for Dβ and Jβ SEs. DNA from DN and DP thymocytes of wild-type and EβR/R mice was diluted and used to assay for SEs derived from cleavages at 3′ Dβ1, 3′ Dβ2, 5′ Jβ1(Jβ1.1, Jβ1.2, Jβ1.3, and Jβ1.4), 5′ Jβ2(Jβ2.1, Jβ2.2, Jβ2.3, Jβ2.4, and Jβ2.5), 5′ Dβ1, and 5′ Dβ2 by LM-PCR. PCR products were separated by agarose gels and hybridized with specific Dβ or Jβ probes. For Jβ1 and Jβ2 SEs, only the most abundant Jβ1.1 and Jβ1.2 or Jβ2.1 and Jβ2.2 are shown (marked). The numbers indicate the band intensities normalized to JAK3 (C), with the level of SEs in wild-type DN thymocytes as 1. Representative data from one of the four experiments are shown. (B) PCR assays for Dβ1-Dβ2 CJ. The levels of Dβ1-Dβ2 CJ in DN and DP thymocytes of wild-type and EβR/R mice were assayed by PCR followed by hybridization with a Dβ1 oligonucleotide probe. Representative data from one of the four experiments are shown. (C) Controls for PCR assays. Efficiencies of the LM-PCR were controlled by assaying for Jα50 SEs. The relative amounts of DNA in different samples were estimated by PCR assays for JAK3. The linear range values (25-fold dilution) were used for normalization. Representative data from one of the four experiments are shown.
In wild-type mice, the levels of 3′ Dβ1 SEs were similar between DN and DP thymocytes, whereas the levels of 3′ Dβ2 SEs were ≈2-fold higher in DP than in DN thymocytes (Fig. 3A Right). In contrast, in EβR/R mice, the levels of 3′ Dβ1 and 3′ Dβ2 SEs were, respectively, 125- and 10-fold higher in DP than in DN thymocytes. Sequence analysis revealed that the PCR products were derived from precise cleavage of Dβ1 or Dβ2 gene segment at the junction of RSS and coding sequences (data not shown). Compared with those in wild-type DN thymocytes, the levels of 3′ Dβ1 SEs were ≈3-fold higher in DP thymocytes of EβR/R mice, whereas the levels of 3′ Dβ2 SEs were about the same. Compared with those in wild-type DP thymocytes, the levels of 3′ Dβ1 SEs were about the same, whereas the levels of 3′ Dβ2 SEs were ≈5-fold lower in DP thymocytes of EβR/R mice. Thus, in DP thymocytes of EβR/R mice, both Dβ1 and Dβ2 gene segments are accessible to RAG-mediated cleavage.
3′ Dβ1 and 5′ Dβ2 Cleavages Are Coupled in DP Thymocytes of EβR/R Mice. As in DN thymocytes, the presence of 3′ Dβ SEs in DP thymocytes of wild-type mice was associated with the presence of a proportional 5′ Jβ SEs (Fig. 3A Right). Although the levels of Jβ1 SEs were slightly lower in DP than in DN thymocytes, the levels of Jβ2 SEs were slightly higher in DP than in DN thymocytes. In contrast, whereas the overall levels of 3′ Dβ1 and 3′ Dβ2 SEs in DP thymocytes of EβR/R mice were similar to those in DN or DP thymocytes of wild-type mice, the levels of Jβ1 and Jβ2 SEs in DP thymocytes of EβR/R mice were ≈25- and 5-fold lower, respectively. Thus, most of 3′ Dβ1 cleavages in DP thymocytes of EβR/R mice are not associated with corresponding Jβ cleavages.
Because most of the Dβ1 rearrangements in DP thymocytes of EβR/R mice involve Dβ1-Dβ2-Jβ2 joint formation, 3′ Dβ1 cleavage might be coupled to 5′ Dβ2 cleavage. To test this possibility, we measured the levels of SEs derived from cleavages at 5′ Dβ1 and 5′ Dβ2 in DN and DP thymocytes. Abundant 5′ Dβ1 and 5′ Dβ2 SEs were detected in both DN and DP thymocytes of wild-type mice, whereas very few 5′ Dβ SEs were detected in DN thymocytes of EβR/R mice (Fig. 3A). Although almost no 5′ Dβ1 SEs were detected in DP thymocytes of EβR/R mice, abundant 5′ Dβ2 SEs were detected. Sequence analysis of the PCR products confirmed that they were derived from cleavage at the junction of RSS and 5′ Dβ2 coding sequence (data not shown). Consistently, the levels of Dβ1-Dβ2 rearrangements were ≈5-fold higher in DP thymocytes of EβR/R mice than in DP or DN thymocytes of wild-type mice (Fig. 3B). These findings suggest that most of 3′ Dβ1 and 5′ Dβ2 cleavages are coupled and involved in the nonstandard Dβ1-Dβ2 rearrangements.
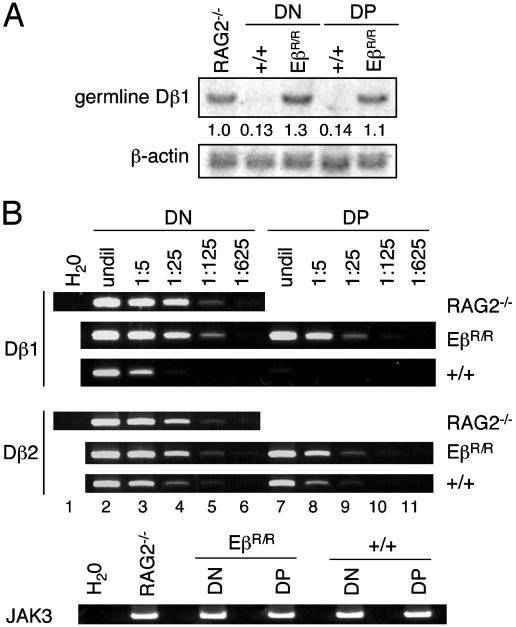
Dβ Cleavages Occur in a Small Fraction of DP Thymocytes of EβR/R Mice. Because SEs tend to accumulate in nondividing DP thymocytes (7, 9, 28), the steady-state levels of SEs measured by LM-PCR might overestimate the actual cleavages at a specific locus. To estimate the extent of Dβ cleavage in DP thymocytes of EβR/R mice, we measured the levels of germ-line Dβ1 by Southern blotting. The levels of germ-line Dβ1 in DN and DP thymocytes of EβR/R mice were as high as in RAG2-/- kidney DNA, whereas the levels were reduced ≈10-fold in DN and DP thymocytes of wild-type mice (Fig. 4A). Thus, most of the Dβ1 gene segment remains in germ-line configuration in DN and DP thymocytes of EβR/R mice but has undergone rearrangement in DN and DP thymocytes of wild-type mice.
Fig. 4.
Most of Dβ1 and Dβ2 gene segments are in germ-line configuration in EβR/R thymocytes. (A) Southern blotting assays for germ-line Dβ1 levels. Ten micrograms of DNA from DN and DP thymocytes of wild-type and EβR/R mice were digested with HindIII followed by hybridization with a Dβ1-Jβ1 intronic probe and then a β-actin probe. The numbers indicate the relative levels of germ-line Dβ1 after normalization to the β-actin levels. (B) The levels of germ-line Dβ1 and Dβ2 in DN and DP thymocyte DNA were assayed by PCR and compared with those in kidney DNA of RAG2-/- mice. DNA samples were either undiluted (undil) or diluted every 5-fold before PCR assay. JAK3 amplification was as in Fig. 1. The PCR assays were performed twice with the same results. One set of data is shown.
We also measured the levels of germ-line Dβ1 and Dβ2 by PCR. In RAG2-/- mice, germ-line Dβ1 was detected faintly by PCR after 125-fold dilution of kidney DNA (Fig. 4B, lane 5). In DP thymocytes of wild-type mice, germ-line Dβ1 was not detected in undiluted DNA (lane 7), indicating that less than one in 125 of Dβ1 remains in germ-line configuration. In DN thymocytes of wild-type mice, germ-line Dβ1 was detected after 5-fold but not 25-fold dilution (lane 3). Based on the intensities of PCR and Southern blot products, ≈10% of Dβ1 remains in germ-line configuration in DN thymocytes. In contrast, the levels of germ-line Dβ1 in DN thymocytes of EβR/R mice were as high as in RAG2-/- mice (lanes 2-6), consistent with Southern blotting analysis. In DP thymocytes of EβR/R mice, Dβ1 product was detected after 25- but not 125-fold dilution of DNA sample, suggesting <10% of Dβ1 are cleaved. By an analogous comparison, most of Dβ2 remained in germ-line configuration in DN and DP thymocytes of EβR/R mice, whereas significant fractions of Dβ2 gene segment had undergone rearrangements in DN and DP thymocytes of wild-type mice. Together, these results show that in EβR/R mice, most Dβ1 and Dβ2 remain in germ-line configuration in DN thymocytes and a small fraction (<10%) of Dβ1 and Dβ2 are cleaved in DP thymocytes.
Discussion
We report here a comprehensive analysis of the effect of Eβ inactivation on cleavage and rearrangement of Dβ1, Dβ2, Jβ1, and Jβ2 in both DN and DP thymocytes. Consistent with previous reports (18, 19, 22, 29, 30), we found that Eβ inactivation results in a severe reduction of Dβ to Jβ rearrangement, a diminished Dβ cleavage in DN thymocytes, and a block of αβ T cell development. Our detailed analyses also reveal additional effects of Eβ inactivation on TCRβ rearrangement. We found that in EβR/R mice, (i) Dβ1 rearrangements are more severely impaired than Dβ2 rearrangements; (ii) most of the Dβ and Jβ cleavages and rearrangements occur in DP, rather than in DN, thymocytes; and (iii) most of the 3′ Dβ1 cleavages are coupled to 5′ Dβ2 cleavages, instead of to Jβ cleavages, resulting in the nonstandard Dβ1-Dβ2-Jβ2 joints. However, we did not find any significant evidence for a role of Eβ in postcleavage steps of recombination. These findings help elucidate the precise roles of Eβ in regulating TCRβ rearrangement during different stages of T cell development.
Eβ Regulates Accessibility of Dβ and Jβ Gene Segments in DN Thymocytes. In DN thymocytes of EβR/R mice, almost all Dβ1 and Dβ2 gene segments were in germ-line configuration (Fig. 4). The levels of Dβ1 and Dβ2 SEs were reduced ≈100- and 15-fold, respectively, as compared with those in DN thymocytes of wild-type mice (Fig. 3). These findings suggest that in the absence of Eβ, both Dβ1 and Dβ2 gene segments in DN thymocytes are inaccessible to RAG-mediated cleavage. The significantly higher level of Dβ2 than Dβ1 cleavages observed in DN thymocytes of EβR/R mice is consistent with a higher level of Dβ2 than Dβ1 rearrangements (Fig. 1). It is also consistent with previous findings showing that in DN thymocytes of Eβ-/- mice, the Dβ1-Jβ1 region was more hypermethylated, more hypoacetylated, and less accessible to restriction enzyme digestion than the Dβ2-Jβ2 region (21). Recombination accessibility has often been correlated with increased transcription, hypomethylation, histone acetylation, and nuclease sensitivity (12-14). Thus, in DN thymocytes, the major mechanism by which Eβ regulates TCRβ rearrangement is to promote access of Dβ and Jβ gene segments, especially Dβ1 and Jβ1, to RAG cleavage.
In a previous study (22), abundant Dβ and Jβ SEs were detected in DN thymocytes of Eβ-/- mice. What might account for the apparently different results between our study and the previous ones? In our study, DN and DP thymocytes were purified directly from either wild-type or EβR/R mice, whereas in the previous study, DN thymocytes were obtained by breeding the Eβ-/- mutation onto a CD3ε-/- or TCRδ-/- background. One possibility is that the forced arrest of thymocyte development at the DN stage by either the CD3ε-/- or TCRδ-/- mutation in Eβ-/- mice might have prolonged the survival of DN thymocytes, leading to Dβ and Jβ cleavages that would otherwise have occurred in DP thymocytes.
Eβ Confers Accessibility to an Extended Dβ1-Jβ1 Region in DP Thymocytes. Based on the significant levels of 3′ Dβ1 and 3′ Dβ2 SEs and diminished germ-line Dβ1 and Dβ2 gene segments (Figs. 3 and 4), both Dβ1 and Dβ2 gene segments become more accessible to RAG-mediated cleavage in DP thymocytes of EβR/R mice. These findings suggest that Eβ is not essential for Dβ accessibility in DP thymocytes. In DP thymocytes of EβR/R mice, Jβ1 and Jβ2 SEs also became detectable, although the levels were significantly lower than in wild-type DN or DP thymocytes. In particular, only a low level of Jβ1 SEs was detected (Fig. 3), and no Dβ1-Jβ1 joints were ever detected (Fig. 1). Besides the Eβ, access of both the Dβ1 and Jβ1 gene segments also depends on the presence of the PDβ1 promoter (16, 17, 20). In the absence of the Eβ, the PDβ1 might confer some degree of access to the proximal Dβ1 gene segment but not the more distal Jβ1 gene segments in DP thymocytes. Extending the accessible region to Jβ1 gene segments in DP thymocytes evidently still requires the presence of a functional Eβ.
Eβ Promotes Proper Pairing of Dβ1 and Jβ2 Gene Segments for Cleavage and Rearrangement. Compared with Jβ1, Jβ2 gene segments in DP thymocytes of EβR/R mice were more accessible (Fig. 3) and were involved in Dβ2-Jβ2 rearrangements (Figs. 1 and 2). However, only 20% of Dβ1 rearrangements observed in DP thymocytes of EβR/R mice were the normal Dβ1-Jβ2 joints (Figs. 2 and 6). These findings suggest that most of 3′ Dβ1 cleavages are not coupled with Jβ2 cleavages in the absence of Eβ, despite the lack of competition from Jβ1 gene segments. Instead, ≈80% of Dβ1 rearrangements were the nonstandard Dβ1-Dβ2-Jβ2 joints (Fig. 2) and the levels of Dβ1-Dβ2 joints in DP thymocytes were ≈5-fold higher in EβR/R than in wild-type mice (Fig. 3B). These results suggest that most 3′ Dβ1 cleavages are coupled with 5′ Dβ2 cleavages. In support of this interpretation, high levels of both 3′ Dβ1 and 5′ Dβ2 SEs were detected in DP thymocytes of EβR/R mice (Fig. 3). These findings suggest that Eβ is required for proper pairing between Dβ1 and Jβ2 gene segments for subsequent cleavage and rearrangement. In the absence of Eβ,Dβ1 appears to pair mostly with Dβ2 for cleavage and rearrangement in DP thymocytes, either because the Dβ2 gene segment is more accessible than the Jβ2 or because the Dβ2 is closer to the Dβ1 than the Jβ2 gene segments.
Does Eβ Play a Role in Postcleavage Steps of V(D)J Recombination? Based on significantly higher levels of Dβ and Jβ SEs than Dβ-Jβ CJ in both total and DN thymocytes of Eβ-/- mice, it was previously suggested that the Eβ plays a significant role in postcleavage steps of recombination (22). Although we also found significant levels of Dβ1 and Dβ2 SEs but little Dβ1 and Dβ2 CJ in DP thymocytes of EβR/R mice (Figs. 1 and 3), most Dβ1 and Dβ2 gene segments were in germ-line configuration (Fig. 4), indicating that Dβ1 and Dβ2 are cleaved only in a small fraction of DP thymocytes of EβR/R mice. The apparently high levels of Dβ1 and Dβ2 SEs observed in DP thymocytes of EβR/R mice as compared with those in wild-type DN thymocytes are probably because SEs tend to accumulate in nondividing DP thymocytes (7, 9, 28). Furthermore, because most 3′ Dβ1 cleavages were coupled with 5′ Dβ2 cleavages, the levels of Dβ1 to Jβ rearrangements would be expected to be reduced. Based on these considerations, it is questionable whether Eβ plays any significant role in postcleavage steps of recombination. Nevertheless, because of difficulties in qualifying the precise levels of SEs and CJ and in directly comparing the levels of SEs and CJ in DN and DP thymocytes, further studies are required to resolve this issue.
The Observed Effects Likely Reflect the Normal Function of Eβ in TCRβ Rearrangement. As with Eβ-/- mice (29), thymocytes in EβR/R mice consist of DN and DP cells, with almost no SP cells (Fig. 5C). Studies have shown that the development of DP thymocytes in Eβ-/- mice requires the presence of an intact TCRδ gene (29). Most likely, functional rearrangement and expression of the TCRδ (and probably TCRγ) lead to differentiation of thymocytes from DN to DP stage in Eβ-/- mice. The question that arises is whether the observed effect of Eβ inactivation on Dβ and Jβ rearrangement in DP thymocytes of EβR/R mice is an artifact resulting from the anomalous DP thymocyte differentiation or reflects a genuine role of Eβ in normal TCRβ rearrangement. The existing evidence supports the latter possibility.
During normal T cell development, TCRβ, -γ, and -δ rearrangements occur simultaneously in DN thymocytes (31, 32). Although the precise mechanism by which a developing thymocyte chooses to become an αβ or γδ T cell is unknown, expression of preTCR, consisting of TCRβ,pTα, and CD3 proteins, appears to be critical for αβ T cell commitment (33, 34). However, αβ lineage-committed thymocytes can be rescued by γδ TCR in the absence of TCRβ chain (35). Thus, DP thymocytes generated in EβR/R or Eβ-/- mice might have committed to αβ T cell lineage. In support of this interpretation, DP thymocytes of Eβ-/- mice rearrange and express TCRα (29), which normally occurs only in αβ but not in γδ T cells. Furthermore, we found significant levels of Dβ1, Dβ2, Jβ1, and Jβ2 SEs in DP thymocytes of wild-type mice (Fig. 3). Because SEs are ligated to form SJ before cell cycle progression (7, 9, 28), as occurs during normal DN to DP thymocyte development (36), Dβ and Jβ SEs detected in wild-type DP thymocytes are likely generated de novo in DP thymocytes. In support of this interpretation, residual germ-line Dβ1 gene segments observed in DN thymocytes were all gone in DP thymocytes (Fig. 4). The levels of germ-line Dβ2 gene segments were further reduced during DN to DP transition. Together, these observations strongly suggest that the observed effect of Eβ inactivation on Dβ and Jβ rearrangement in DP thymocytes of EβR/R mice reflects the normal function of the Eβ in TCRβ rearrangement.
Supplementary Material
Acknowledgments
We thank Dr. P. Ferrier for providing thymocyte DNA of Eβ-/- mice; Drs. Herman N. Eisen, Phillip Sharp, Tania Baker, and Mark Schlissel for review of the manuscript; and members of the Chen laboratory for helpful discussions. This work was supported in part by National Institutes of Health Grant AI 40416 (to J.C.), a Cancer Center Core Grant (to Richard Hynes), the Ministry of Health and Welfare, Republic of Korea (Grant 02-PJ1-PG3-20908-0002 to C.J.R.), and a Postdoctoral Fellowship from the American Cancer Society (to B.B.H.).
Abbreviations: TCR, T cell receptor; RAG, recombination-activating gene; RSS, recombination signal sequence; SE, signal end; SJ, signal joint; CJ, coding joint; DN, double negative; DP, double positive; LM-PCR, ligation-mediated PCR; Eβ, TCRβ enhancer.
References
- 1.Gellert, M. (2002) Annu. Rev. Biochem. 71, 101-132. [DOI] [PubMed] [Google Scholar]
- 2.Fugmann, S. D., Lee, A. I., Shockett, P. E., Villey, I. J. & Schatz, D. G. (2000) Annu. Rev. Immunol. 18, 495-527. [DOI] [PubMed] [Google Scholar]
- 3.Eastman, Q. M., Leu, T. M. J. & Schatz, D. G. (1996) Nature 380, 85-88. [DOI] [PubMed] [Google Scholar]
- 4.van Gent, D. C., Hiom, K., Paull, T. T. & Gellert, M. (1997) EMBO J. 16, 2665-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steen, S. B., Gomelsky, L., Speidel, S. L. & Roth, D. B. (1997) EMBO J. 16, 2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillman, R. E., Wooley, A. L., Hughes, M. M., Wehrly, T. D., Swat, W. & Sleckman, B. P. (2002) J. Exp. Med. 195, 309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McBlane, J. F., van Gent, D. C., Ramsden, D. A., Romeo, C., Cuomo, C. A., Gellert, M. & Oettinger, M. A. (1995) Cell 83, 387-395. [DOI] [PubMed] [Google Scholar]
- 8.Roth, D. B., Menetski, J. P., Nakajima, P. B., Bosma, M. J. & Gellert, M. (1992) Cell 70, 983-991. [DOI] [PubMed] [Google Scholar]
- 9.Schlissel, M., Constantinescu, A., Morrow, T., Baxter, M. & Peng, A. (1993) Genes Dev. 7, 2520-2532. [DOI] [PubMed] [Google Scholar]
- 10.Grawunder, U. & Harfst, E. (2001) Curr. Opin. Immunol. 13, 186-194. [DOI] [PubMed] [Google Scholar]
- 11.Alt, F., Blackwell, T. K. & Yancopoulos, G. (1987) Science 238, 1079-1087. [DOI] [PubMed] [Google Scholar]
- 12.Sleckman, B. P., Gorman, J. R. & Alt, F. W. (1996) Annu. Rev. Immunol. 14, 459-481. [DOI] [PubMed] [Google Scholar]
- 13.Schlissel, M. S. & Stanhope-Baker, P. (1997) Semin. Immunol. 9, 161-170. [DOI] [PubMed] [Google Scholar]
- 14.Hempel, W. M., Leduc, I., Mathieu, N., Tripathi, R. K. & Ferrier, P. (1998) Adv. Immunol. 69, 309-352. [DOI] [PubMed] [Google Scholar]
- 15.Krangel, M. S. (2001) J. Exp. Med. 193, F27-F30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitehurst, C., Chattopadhyay, S. & Chen, J. (1999) Immunity 10, 313-322. [DOI] [PubMed] [Google Scholar]
- 17.Sikes, M. L., Suarez, C. C. & Oltz, E. M. (1999) Mol. Cell. Biol. 19, 2773-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouvier, G., Watrin, F., Naspetti, M., Verthuy, C., Naquet, P. & Ferrier, P. (1996) Proc. Natl. Acad. Sci. USA 93, 7877-7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bories, J.-C., Demengeot, J., Davidson, L. & Alt, F. W. (1996) Proc. Natl. Acad. Sci. USA 93, 7871-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitehurst, C. E., Schlissel, M. S. & Chen, J. (2000) Immunity 13, 703-714. [DOI] [PubMed] [Google Scholar]
- 21.Mathieu, N., Hempel, W. M., Spicuglia, S., Verthuy, C. & Ferrier, P. (2000) J. Exp. Med. 192, 625-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hempel, W. M., Stanhope-Baker, P., Mathieu, N., Huang, F., Schlissel, M. S. & Ferrier, P. (1998) Genes Dev. 12, 2305-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godfrey, D. I., Kennedy, J., Mombaerts, P., Tonegawa, S. & Zlotnik, A. (1994) J. Immunol. 152, 4783-4792. [PubMed] [Google Scholar]
- 24.Schwenk, F., Baron, U. & Rajewsky, K. (1995) Nucleic Acids Res. 23, 5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feeney, A. J. (1991) J. Exp. Med. 174, 115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogue, M., Candâeias, S., Benoist, C. & Mathis, D. (1991) EMBO J. 10, 3647-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonegawa, S. (1983) Nature 302, 575-581. [DOI] [PubMed] [Google Scholar]
- 28.Livak, F. & Schatz, D. G. (1996) Mol. Cell. Biol. 16, 609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leduc, I., Hempel, W. M., Mathieu, N., Verthuy, C., Bouvier, G., Watrin, F. & Ferrier, P. (2000) J. Immunol. 165, 1364-1373. [DOI] [PubMed] [Google Scholar]
- 30.Mathieu, N., Spicuglai, S., Gorbatch, S., Cabaud, O., Fernex, C., Verthuy, C., Hempel, W. M., Huber, A.-O. & Ferrier, P. (2003) J. Biol. Chem. 278, 18101-18109. [DOI] [PubMed] [Google Scholar]
- 31.Capone, M., Hockett, R. D., Jr. & Zlotnik, A. (1998) Proc. Natl. Acad. Sci. USA 95, 12522-12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak, F., Tourigny, M., Schatz, D. G. & Petrie, H. T. (1999) J. Immunol. 162, 2575-2580. [PubMed] [Google Scholar]
- 33.Groettrup, M., Ungewiss, K., Azogui, O., Palacios, R., Owen, M. J., Hayday, A. C. & von Boehmer, H. (1993) Cell 75, 283-294. [DOI] [PubMed] [Google Scholar]
- 34.Aifantis, I., Azogui, O., Feinberg, J., Saint-Ruf, C., Buer, J. & von Boehmer, H. (1998) Immunity 9, 649-655. [DOI] [PubMed] [Google Scholar]
- 35.Livak, F., Wilson, A., MacDonald, H. R. & Schatz, D. G. (1997) Eur. J. Immunol. 27, 2948-2958. [DOI] [PubMed] [Google Scholar]
- 36.Levelt, C. N. & Eichmann, K. (1995) Immunity 3, 667-672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.