Abstract
In rheumatoid arthritis, peripheral blood T cells have age-inappropriate telomeric erosion. We examined whether HLA-DRB1*04 alleles, the major susceptibility genes for this disease, confer risk for T cell senescence. In healthy individuals, HLA-DRB1*04 alleles were associated with excessive loss of telomeres in CD4+ T cells. Accelerated telomeric erosion occurred during the first two decades of life and was followed by reduced homeostatic T cell proliferation during adulthood. Premature telomeric loss also affected granulocytes, suggesting that the hematopoietic stem cell is the primary target. Telomeric repair mechanisms were intact in HLA-DRB1*04+ donors. We propose that HLA-DRB1*04 alleles or genes in linkage disequilibrium regulate stem cell replication and contribute to the accumulation of senescent and autoreactive T cells in rheumatoid arthritis.
In patients with rheumatoid arthritis (RA), the diversity of the T cell antigen receptor (TCR) repertoire is contracted (1). Naive and memory T cells have a premature shortening of telomeres that precedes physiologic age-dependent telomeric erosion by ≈25 yr (2). Cellular senescence of T cells results in a restricted clonal burst of naive T cells and a shift in the expression of regulatory molecules on memory T cells. Specifically, expression of the costimulatory molecule CD28 is lost, whereas HLA class I-recognizing receptors are gained (3-5).
Possible mechanisms of age-inappropriate telomeric erosion and accelerated cellular senescence include a primary defect in peripheral T cell turnover. Cytokines have been implicated in driving homeostatic T cell replication, and their disease-related production could enhance T cell proliferation and exhaust the replicative potential (6-8). Alternatively, given that the peripheral T cell pool is under homeostatic control, insufficient release of newly formed T cells could accelerate proliferation of peripheral T cells to fill the compartment (9, 10). This model has been supported by the observation that the number of T cells bearing TCR excision circles (TRECs) was reduced (2). TREC-expressing T cells are thought to reflect thymic production, which declines exponentially with age (11, 12). Individuals with RA already had markedly fewer TREC+ T cells in young adulthood. Also, progression of the disease did not affect the normal annual loss of TRECs and telomeres in peripheral T cells, suggesting that these abnormalities are not a consequence of RA but that they precede the onset of the disease (13). We have proposed that the primary defect is acquired before adulthood and is possibly a component of the genetic predisposition to RA.
The HLA haplotype is the major genetic risk factor for RA (14, 15). A collection of HLA-DRB1 alleles that share a sequence stretch (the shared epitope) confer disease susceptibility (16, 17). Among shared-epitope-positive HLA-DRB1 alleles, the strongest association is found with the HLA-DRB1*04 alleles HLADRB1*0401, HLA-DRB1*0404, HLA-DRB1*0405, and HLADRB1*0408. Also, the clinical phenotype of RA is different in patients with HLA-DRB1*04 and is characterized by more severe and extraarticular disease (18, 19). The classic paradigm suggests that the shared peptide sequence forms a pocket that selects and presents arthritogenic antigens and induces autoreactive T cell responses (20). This model is difficult to reconcile with the influence of HLA-DRB1*04 alleles on disease phenotype, raising the possibility of alternative biological functions of MHC class II molecules or a role for genes inherited in linkage disequilibrium. Recognition of self-MHC molecules by the TCR is important during thymic T cell development and in peripheral T cell homeostasis (21-23). T cells require self-MHC molecules for survival and homeostatic proliferation (9). Measurements of in vivo lifespans of lymphocytes in humans have emphasized that the need for T cell replacement is enormous (24). With age-dependent decline in thymic T cell production, replication of naive T cells becomes important in maintaining the T cell pool (25).
In this study, we investigated whether haplotypes encoding disease-associated HLA-DRB1*04 alleles affect T cell turnover and senescence. We report that age-inappropriate shortening of telomeres in CD4 T cells occurs independently of the rheumatoid disease process and is present in healthy adult HLA-DR4+ donors. Premature loss of telomeres also affects granulocytes, implying increased replicative stress on hematopoietic stem cells (HSCs). We propose that increased HSC turnover is genetically determined and contributes to the immunosenescent and autoimmune manifestations of RA.
Methods
Study Design and Population. The Mayo Clinic Institutional Review Board approved this study, and study participants gave informed consent. Healthy donors, who did not have a history of inflammatory or malignant disease, were enrolled. All donors were typed for their HLA-DRB1 alleles (Biotest Diagnostics, Denville, NJ), and all donors expressing a disease-associated HLA-DRB1*04 allele (HLA-DRB1*0401, HLA-DRB1*0404, HLA-DRB1*0405, or HLA-DRB1*0408) were included. For each HLA-DRB1*04+ donor, an age-matched HLA-DRB1*04- donor was selected. Patients with RA fulfilled the American College of Rheumatology criteria (26). Clinical data were obtained by retrospective chart review. Patients with a history of chemotherapy or radiotherapy were excluded.
Heparinized venous cord blood samples were collected from live-born infants. Semen was collected from healthy fertile donors.
Cell Purification. Peripheral blood mononuclear cells and granulocytes were separated by using Ficoll-Paque (Amersham Biosciences) or PrepaCyte (BioE, St. Paul, MN). Granulocytes were isolated from the red-cell pellet of Ficoll-Paque-separated cells by sedimentation with 3% dextran (Amersham Biosciences) in 0.9% NaCl. CD4+, CD4+CD45RO+, and CD4+CD45RO- T cells were separated with Ab-coated magnetic beads (Miltenyi Biotec, Auburn, CA).
Flow Cytometry. Fresh peripheral blood mononuclear cells were surface-stained with peridinin chlorophyll protein- or phycoerythrin-conjugated mAb against CD28, CD45RO, CD4, or CD8 (Becton Dickinson). The cells were permeabilized with PBS containing 0.1% Triton X-100 (Sigma), stained with FITC-conjugated anti-Ki-67 mAb (Beckman Coulter), and fixed with 2% paraformaldehyde in PBS. For intracellular stains, 50,000 events were collected on a FACScan flow cytometer (Becton Dickinson).
HLA-DRB1*04 Typing. DNA was amplified by seminested PCR with HLA-DRB1*04-specific primers (5′-GCCGCTGCACTGTGAAGCTCTC-3′ and 5′-CCCCACAGCACGTTTCTTG-3′ or 5′-GTTTCTTGGAGCAGGTTAAAC-3′). PCR products were sequenced to determine HLA-DRB1*04 subtypes.
DNA Extraction. DNA from granulocytes and CD4+ T cells was extracted by using the QIAamp blood kit (Qiagen, Valenica, CA). Sperm (0.5 ml of semen) were lysed (10 mM Tris·HCl, pH 8.0/10 mM EDTA/100 mM NaCl/40 mM DTT/2% SDS/100 μg/ml proteinase K) at 55°C overnight. Each sample was extracted twice with phenol/chloroform/isoamyl alcohol (25:24:1, vol/vol) and then precipitated with ethanol.
Telomere Length Analysis. Telomeric restriction fragment (TRF) lengths were analyzed by Southern blotting as described (2). Results are given as the median TRF length of the sum equation of the telomere-signal distribution.
Telomerase Activity. Telomerase activity was assayed with the TRAPeze telomerase detection kit (Serologicals, Clarkston, GA).
Quantification of TRECs. Signal joint TREC concentrations were analyzed by competitive PCR as described (2, 12).
Cell Proliferation Assays. CD4+CD45RO- T cells were activated with immobilized anti-CD3 and anti-CD28 Ab, maintained in 20 units per ml IL-2, and restimulated every 14 days. Cells were counted at regular intervals, and the mean population doubling was calculated as described (2).
Statistical Analysis. Age-dependent variables (median TRF lengths, the number of TRECs per 106 CD4+ T cells, and the frequency of Ki-67+ cells) in HLA-DR4+ and HLA-DR4- individuals were compared by regression analysis using SAS statistical software, Version 8 (SAS Institute, Cary, NC). Parameters in age-matched groups were compared by using the Wilcoxon rank sum test (sigmastat software, SPSS, Chicago).
Results
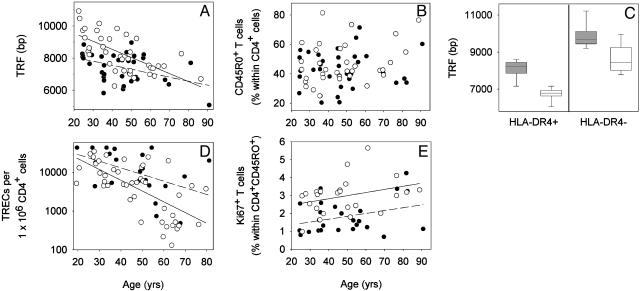
HLA-DR4 Affects Telomeric Length in CD4+ T Cells. We determined TRF lengths in CD4+ T cells of 37 HLA-DR4+ healthy individuals (ages 24-90 yr, mean age 44.8 yr) who expressed one of the RA-associated HLA-DRB1*04 alleles (HLA-DRB1*0401, HLA-DRB1*0404, HLA-DRB1*0405, or HLA-DRB1*0408) and from 37 HLA-DR4- age-matched healthy controls (ages 23-86 yr, mean age 44.8 yr). In HLA-DR4- individuals, TRF length in CD4+ T cells progressively declined with age by 50 bp/yr (r2 = 0.43, P < 0.001) (Fig. 1A). Telomeric sequences in HLADR4+CD4+ T cells were shorter (P = 0.002). By age 20 yr, HLA-DR4+CD4+ T cells lacked ≈1,500 bp in telomeric sequences compared with the HLA-DR4- cells. The subsequent age-dependent decline in telomeric lengths was less pronounced in HLA-DR4+ individuals (25 bp/yr), but this difference was not significant (P = 0.057). Accelerated telomeric loss in HLADR4+ individuals could reflect the expansion of memory T cells, which have shorter telomeres than naive T cells. The phenotypic distinction between naive and memory T cells can be complicated by the reversion of memory T cells to the naive CD45RO- phenotype. Because such phenotypic shifts are less frequent for CD45RO on CD4+ memory T cells, we focused on this population. As shown in Fig. 1B, the frequencies of CD45RO+ memory cells were indistinguishable in HLA-DR4- and HLADR4+ individuals. To obtain direct evidence for the involvement of naive and memory cells, we studied 25- to 40-yr-old donors, in whom the differences in TRF length are most evident. CD4+CD45RO- and CD4+CD45RO+ T cells from eight HLADR4+ (mean age 33.2 ± 5.6 yr) and eight age-matched HLADR4- (mean age 32.4 ± 5.3 yr) individuals were analyzed separately (Fig. 1C). As expected, memory T cells had shorter telomeres than naive T cells. However, telomeres in both lymphocyte subsets were significantly shorter in HLA-DR4+ than in HLA-DR4- individuals (P < 0.001). In fact, TRF lengths in HLA-DR4+ naive T cells were similar to those in HLA-DR4- memory T cells.
Fig. 1.
The influence of HLA-DR4 on CD4+ T cell homeostasis. (A) TRF lengths were analyzed in CD4+ T cells of 37 HLA-DR4+ (•) and 37 age-matched HLA-DR4- (○) donors. Results are shown as a function of age. The regression line for the HLA-DR4+ individuals (dashed line) was significantly shifted toward shorter telomeric length compared with HLA-DR4- individuals (solid line) (P = 0.002), and the slope of the age-dependent decline after age 20 yr was slightly reduced (P = 0.056). (B) The frequencies of CD4+CD45RO+ memory T cells were not significantly different between HLA-DR4+ and HLA-DR4- individuals (P = 0.63), indicating that telomere shortening was not a reflection of expanded memory T cells. (C) CD4+CD45RO- naive (shaded bars) and CD4+CD45RO+ memory (unshaded bars) T cells were analyzed for TRF length. HLA-DR4+ (mean age 33.1 yr) and HLA-DR4- (mean age 32.4 yr) individuals differed significantly for naive (P < 0.001) and memory (P < 0.001) cells. Results are expressed as box plots displaying the medians as horizontal lines, 25th and 75th percentiles as boxes, and 10th and 90th percentiles as whiskers. (D) The frequency of TRECs in peripheral CD4+ T cells was determined in HLA-DR4+ (•) and HLA-DR4- (○) individuals by competitive PCR. Regression lines indicate an annual decline in the frequency of TREC+ cells of 3.6% in HLA-DR4- (solid line) and 2.7% in HLA-DR4+ (dashed line) individuals. (E) The frequency of peripheral blood mononuclear cells in the cell cycle from HLA-DR4+ (•) and HLA-DR4- (○) individuals was analyzed by flow cytometry. Regression lines are shown for HLA-DR4+ (dashed line) and HLA-DR4- (solid line) individuals.
CD4+ T Cell Turnover in HLA-DR4+ Individuals Is Reduced. Accelerated telomeric erosion could result from increased replication of mature peripheral CD4+ T cells to compensate either for increased attrition by apoptosis or for reduced production of new T cells. The frequency of TREC+ T cells is a direct measure of peripheral naive T cell homeostasis as long as the naive to memory T cell ratio is conserved (Fig. 1B). Peripheral TREC concentrations depend on thymic output and are affected also by the proliferative rate of naive T cells (12, 27). Quantification of TRECs demonstrated the expected age-dependent decline of recent thymic emigrants in HLA-DR4- donors (Fig. 1D); TREC concentrations decreased by 3.6% per yr, accumulating to a 98% reduction by age 80 yr. The HLA-DR4 haplotype was associated with a slightly lower annual decline of 2.7%, leading to an enrichment of TREC+CD4 T cells in HLA-DR4+ donors. These data suggested a reduced rather than increased dilution of thymic emigrants in the peripheral T cell pool of HLA-DR4+ individuals.
The turnover of memory cells is higher than that of naive cells and can be reliably estimated by in vivo labeling or from the frequency of T cells expressing Ki-67 (24). We measured the frequency of Ki-67+CD4+CD45RO+ T cells in relation to the HLA-DRB1 genotype. The number of Ki-67+CD4+ T cells varied from 0.98% to 5.65% in HLA-DR4- donors (Fig. 1E). Frequencies of cycling CD4+ T cells increased with age and were almost doubled by age 80 yr. In HLA-DR4+ individuals, the frequencies of Ki-67+CD4+ T cells showed a trend to be lower over the entire age range (0.69-4.2% of CD4+ T cells).
These data show that age-appropriate telomeric erosion was not correlated with accelerated T cell division of either naive or memory T cells at the time when the individuals were studied. Increased T cell turnover at an early age cannot be excluded as the mechanism of telomere loss; however, it should have caused a dilution of TREC+ cells.
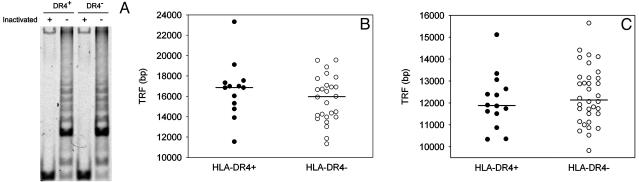
Telomeric Repair Is Intact in HLA-DR4+ Individuals. Premature shortening of telomeres in HLA-DR4+ T cells could reflect an anomaly in telomeric repair. We measured telomerase activity in T cells from HLA-DR4+ and HLA-DR4- individuals in CD45RO-CD4+ T cells before and after stimulation with anti-CD3 and anti-CD28 mAbs. Unstimulated T cells had no significant telomerase activity (data not shown). After stimulation, telomerase activity was equally induced in all donors regardless of their HLA-DRB1 genotype (Fig. 2A). Furthermore, telomeric erosion was not accelerated in HLA-DR4+ T cells that were driven to proliferate for eight generations by repeated stimulation in vitro (data not shown). However, induction of telomerase activity in T cells after in vitro stimulation may insufficiently mimic the in vivo situation in which telomerase activity is highly compartmentalized. Induction of telomerase in HLA-DR4+ T cells in a particular microenvironment could be impaired, leading to telomere shortening.
Fig. 2.
Telomere shortening in HLA-DR4+ individuals is acquired, but telomeric repair is intact. (A) CD4+CD45RO- T cells were stimulated with immobilized anti-CD3 and anti-CD28 Abs, and the cells were assayed after 4 days. A representative result of seven experiments before and after heat inactivation of telomerase activity is shown. (B) TRF lengths were analyzed in sperm from 13 HLA-DR4+ (•) and 26 age-matched HLA-DR4- (○) donors. (C) TRF lengths were determined in CD4+ T cells that were obtained from 14 HLA-DR4+ (•) and 34 HLA-DR4- (○) newborns (mean gestational ages 37.0 and 38.0 weeks, respectively). TRF lengths were indistinguishable between the two groups (P = 0.53). Median telomeric lengths are indicated by horizontal lines.
Telomeric repair is particularly important in sperm, a highly proliferative cell population. Instead of losing telomeres, sperm show age-dependent extension of telomeric ends (28). We collected semen from 39 healthy men and measured TRF lengths. The median lengths of 16,851 bp and 15,960 bp in HLA-DR4+ and HLA-DR4- donors, respectively, were indistinguishable (P = 0.30). Compared with lymphocytes, telomeres were elongated in both groups, compatible with hypercompensatory telomeric repair (Fig. 2B). These data provide further evidence that HLA-DR4+ individuals do not have a generalized defect in inducing telomerase activity. In addition, telomere shortening in HLA-DR4+ individuals was tissue-specific, suggesting that our findings did not reflect subtelomeric polymorphisms.
Premature Loss of Telomeres in HLA-DR4+ T Cells Is Acquired During Childhood. To determine whether HLA-DR4+ individuals were born with T cells possessing shortened telomeres, we determined TRF lengths in 14 HLA-DR4+ and 34 HLA-DR4- newborns (Fig. 2C). The TRF lengths of CD4+ T cells were indistinguishable between the two groups (P = 0.53). As reported (29), telomeric ends were markedly longer in cord blood T cells than in adult T cells, and the loss of telomeres in the first 20 yr of life exceeded that of the subsequent 60 yr. It is during these first 20 yr that telomeric erosion is accelerated in HLA-DR4+ individuals.
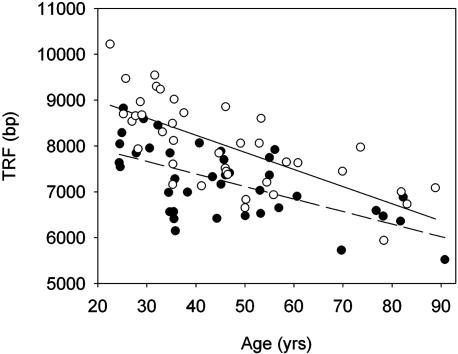
HLA-DR4+ Granulocytes Have Premature Telomeric Erosion. The major determinant of T cell homeostasis during childhood and early adolescence is the production rate of the thymus. The frequencies of TREC+CD4+ T cells in HLA-DR4+ donors demonstrated intact thymic function. We, therefore, examined whether prethymic cells were affected by accelerated telomeric erosion. Granulocytes have a survival time of only a few days and their TRF lengths closely reflect the proliferative history of bone marrow stem cells (30). Telomeric length in granulocytes significantly correlated with age (P < 0.001; Fig. 3). The slope of the curves reflecting the yearly decline was not different between HLA-DR4+ and HLA-DR4- individuals (27 and 37 bp/yr, respectively, P = 0.31). HLA-DR4+ granulocytes had significantly shorter telomeres throughout the entire age range (P = 0.004). In support of a mechanism affecting all hematopoietic lineages, telomeres of CD8+ T cells derived from HLA-DR4+ individuals were also shortened (J.J.G. and C.M.W., unpublished data).
Fig. 3.
Premature telomeric erosion in granulocytes of HLA-DR4+ donors. TRF lengths were measured in granulocytes from 39 HLA-DR4+ (•) and 39 HLA-DR4- (○) donors. The age dependency of telomeric loss in both cohorts is indicated by regression lines (dashed line, HLA-DR4+; solid line, HLA-DR4-). Granulocytes from HLA-DR4+ donors had significantly shorter telomeres (P = 0.004). The annual loss during adult life did not differ significantly (P = 0.24).
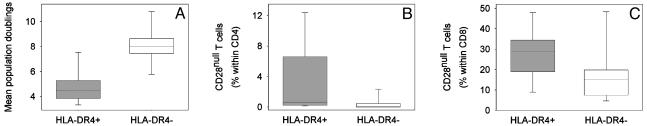
Telomere Shortening in HLA-DR4+ T Cells Correlates with Functional Markers of Cellular Senescence. Results described in Fig. 2 showed that adult HLA-DR4+ individuals did not have increased T cell turnover. On the contrary, turnover of naive and memory T cells tended to be decreased, which is indicative of cellular senescence. To examine whether accelerated telomeric erosion limited the ability of T cells to divide, the in vitro replicative potential of naive T cells from HLA-DR4+ and HLA-DR4- age-matched individuals was compared. To control for the influence of age, only healthy individuals 28-38 yr of age were included. The mean age of the HLA-DR4+ individuals was 31.6 ± 6.3 yr compared with 32.3 ± 7.0 yr for the HLA-DR4- donors. Results are shown as the mean population doublings after three in vitro polyclonal stimulations over a culture period of 4 weeks (Fig. 4A), at which time the growth rates reached a plateau. Growth rates and maximal clonal expansion were significantly reduced for HLA-DR4+ naive T cells (P = 0.007).
Fig. 4.
Premature senescence of HLA-DR4+ naive and memory T cells. (A) CD4+CD45RO- T cells were purified from age-matched HLA-DR4+ (31.6 ± 6.3 yr, gray) and HLA-DR4- (32.3 ± 7.0 yr, white) donors and stimulated every 2 weeks. Mean population doublings are shown for 30 days, when stimulation-induced proliferation reached a plateau. Box plots represent the results from seven experiments. The replicative potential was significantly reduced in HLA-DR4+ naive T cells (P = 0.007). (B and C) The frequency of CD28null T cells, as a marker of in vivo immunosenescence, in HLA-DR4+ (gray) and age-matched HLA-DR4- (white) individuals was determined by flow cytometry. The frequencies of CD28nullCD4+ (B)(P < 0.001) and CD28nullCD8+ (C)(P = 0.005) T cells were significantly increased in HLA-DR4+ donors.
An indirect marker of replicative senescence in memory T cells is the loss of CD28 (31). We determined the frequencies of CD28nullCD4+ and CD28nullCD8+ T cells from 28 HLA-DR4+ and 28 age-matched HLA-DR4- individuals who were 30-60 yr of age (Fig. 4B). As described, CD8+ T cells contained a higher proportion of CD28null T cells than did CD4+ T cells (32). HLA-DR4+ individuals had significantly increased frequencies of both CD28nullCD4+ (P < 0.001) and CD28nullCD8+ (P = 0.005) T cells.
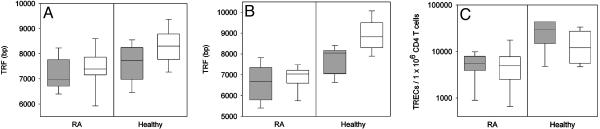
HLA-DR4-Associated Telomeric Erosion and RA. The HLA-DR4 haplotype is the major genetic risk factor for RA, raising the possibility that increased replicative stress of HSCs and premature aging of lymphocytes are relevant to disease. We compared telomeric repeats in granulocytes from HLA-DR4+ and HLA-DR4- healthy donors and patients with RA, focusing on the donors who were 20-40 yr of age. The median TRF length for granulocytes was 8,304 bp in the HLA-DR4- donors, compared with 7,737 bp in the HLA-DR4+ cohort (P = 0.004; Fig. 5A). The patients with RA shared with healthy HLA-DR4+ individuals the loss of telomeres in granulocytes, implying increased replicative history of HSCs (median TRF length 7,217 bp; P < 0.001, compared with HLA-DR4- donors, and P = 0.48, compared with HLA-DR4+ donors). In contrast with healthy donors, telomeric erosion in patients with RA was detected regardless of the HLA-DR haplotype.
Fig. 5.
Premature telomeric erosion in lymphocytes and granulocytes and decreased frequencies of TREC+ T cells in patients with RA. Telomere lengths were compared in granulocytes (A) and CD4+ T cells (B) from HLA-DR4- (white) and HLA-DR4+ (gray) healthy individuals and patients with RA, all 20-40 yr of age. Patients with RA shared with HLA-DR4+ individuals accelerated telomeric erosion in granulocytes and CD4 T cells. (C) Patients with RA had a significant reduction in TREC frequencies (P = 0.002).
Likewise, healthy HLA-DR4+ donors and patients with RA shared age-inappropriate erosion of telomeres in CD4+ T cells (Fig. 5B). With a median TRF length of 8,821 bp, HLA-DR4- individuals had preserved significantly more telomeric repeats than HLA-DR4+ individuals (median TRF length 8,039 bp; P < 0.001). Telomeric loss was even more pronounced in patients with RA (median TRF length 6,841 bp; P < 0.001, compared with HLA-DR4- donors, and P = 0.002, compared with HLADR4+ donors).
Although healthy HLA-DR4+ individuals and patients with RA had shortening of telomeres in both granulocytes and lymphocytes, they were distinguishable by the number of TREC+ T cells (Fig. 5C). Median TREC frequencies tended to be increased in HLADR4+ donors compared with HLA-DR4- individuals (30,090 per 106 CD4+ T cells and 12,030 per 106 CD4+ T cells, respectively; P = 0.08). In contrast, TREC levels were reduced in patients with RA irrespectively of their HLA-DRB1 genotype (P = 0.002, compared with healthy HLA-DR4+ individuals, and P < 0.001, compared with healthy HLA-DR4- donors).
Discussion
Here, we provide evidence that the HLA-DR4 haplotype imposes premature replicative senescence on both the myeloid and lymphoid hematopoietic lineages. Although the functional consequences for the myeloid lineage are not understood, replicative stress of lymphoid precursors is an important mechanism leading to immunosenescence. The functional integrity of the adaptive immune system depends greatly on its ability to respond to antigenic stimuli with a clonal burst of antigen-specific lymphocytes, and this ability is compromised in senescent T cells. The proliferative capacity of T cells is also critical in maintaining T cell numbers and diversity within the peripheral T cell compartment. Thymic output of newly generated T cells declines markedly with age, and maintenance of the adult lymphocyte pool relies on the self-replication of mature T cells (9, 12, 33).
We report that the survival and replication of hematopoietic precursor cells is influenced by polymorphisms of the HLA-DR4 haplotype. Genetic control of HSC turnover has been suggested by a high concordance of telomeric lengths in monozygotic twins (34). Recent linkage studies have mapped the gene that is associated with increased frequencies of HSCs in AKR/J mice to the MHC region (35). Chromosome 6p21.31, where the human MHC is encoded, contains several candidate genes with a possible role in HSC proliferation. Alternatively, HLA-DR polymorphisms may directly affect the survival and replication of the common precursor of granulocytes and lymphocytes. By recognizing HLA-D antigen complexes, T cells could critically regulate stem cell homeostasis. The only known functions of HLA-DR are its selective binding of peptides and stimulation of the TCR. Engagement of the TCR with self-MHC/peptide ligands has been established as a prerequisite for T cell survival (36). The HLA-DR4 haplotype is complex, as evidenced by the multitude of HLA-DRB1*04 alleles. We did not find a preferential association of telomere shortening with the frequent alleles HLA-DRB1*0401 or HLA-DRB1*0404, and our sample size was too small to assess associations with the less frequent alleles. Linkage studies will be needed to define the contributions of HLA-DRB1 polymorphisms and genes in linkage disequilibrium to the accelerated telomeric erosion in hematopoietic cell lineages.
A model of HLA-DR-dependent T cell stimulation regulating the survival and turnover of precursor cells of the myeloid and lymphoid lineages is not without precedent. In allogeneic bone marrow transplantation, T cells are critical in HSC survival and engraftment and are considered essential to secure success of the transplant (37). The nature of the facilitating T cells and their mode of action are insufficiently understood. HLA-DR4 may be a stronger facilitator of T cell activation in the bone marrow environment, possibly affecting the production of stromal cell-derived cytokines and growth factors and modulating the persistence and replenishment of HSCs.
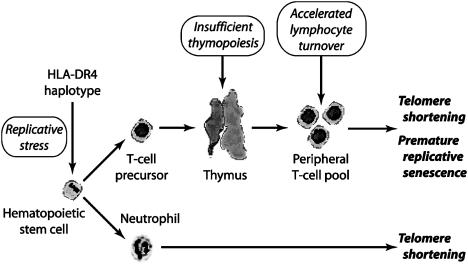
The influence of HLA-DR4 on telomere shortening coincided with the time period of maximal replicative stress for HSCs. Telomeric loss of granulocytes and lymphocytes is highest within the first year of life (29) because of the demand for the establishment of lymphoid organs and the rapid increase in the size of the T cell compartment. In newborns, we did not detect an influence of the HLA-DR4 haplotype on telomere shortening, whereas, in 20-yr-old donors, the difference between HLADR4+ and HLA-DR4- subjects was maximal. After this age, annual telomeric loss was not accelerated by the HLA-DR4 haplotype. Critical events, either endogenous or exogenous, appear to have occurred in childhood and early adolescence. The nature of such events is currently purely speculative. HLA-DR4+ individuals could be particularly susceptible to certain infections that impose excessive proliferative stress on the hematopoietic system and thereby set the stage for developing RA later in life. The correlation between the HLA-DR4 haplotype and T cell senescence explains some of the immunologic abnormalities in RA (Fig. 6). Patients with RA shared with healthy HLA-DR4+ donors the accelerated telomeric loss in granulocytes and T cells. Thus, premature senescence of myeloid and lymphoid precursor cells is not a consequence of disease; rather, it precedes the onset of disease. In contrast to healthy HLA-DR4+ donors, who were able to preserve TREC+ T cells likely through a decline in homeostatic T cell proliferation, donors with RA had a reduction in TREC-bearing cells. One possible explanation includes excessive proliferative turnover of T cells in RA, leading to dilution of TREC+ T cells that are released from the thymus. We have not detected an increase in Ki-67+ T cells in the blood of patients with RA (data not shown), but these patients could experience transient episodes of accelerated T cell replication that exhaust homeostatic control. Alternatively, patients with RA may have a second defect that restricts the functionality of the thymus. A combination of both abnormalities could act synergistically to accelerate replicative senescence of T cells.
Fig. 6.
Model of the mechanisms leading to telomere shortening in RA.
MHC-linked T cell senescence has implications for understanding comorbidities in RA and the limitations of immunosuppression. Equally important is the integration of this biological mechanism into concepts of how MHC class II haplotypes confer disease risk. The most widely accepted model is that RA-associated alleles, through the shared epitope, preferentially bind disease-inducing antigens and elicit pathogenic immune responses (16, 20). Although this model is appealing, data supporting antigen-specific responses as a primary cause of RA are lacking. Epidemiological studies of RA favor a regulatory function of the relevant HLA gene(s). Disease-associated alleles expressing the shared epitope are not equal in their impact on the disease. Patients with disease-associated HLA-DRB1*04 alleles have far more severe RA than patients with other shared epitope alleles. The disease follows a particularly aggressive course in hosts with two HLA-DRB1*04 alleles (19). Frequently, these patients develop extraarticular manifestations (18). Our study, therefore, focused on disease-associated HLA-DRB1*04 alleles. Whether the observed accelerated telomeric erosion is a common feature of shared epitope alleles or whether it explains the unique role of RA-associated HLA-DRB1*04 alleles remains to be studied. Preliminary analysis of telomeres in HLA-DR4- individuals did not show a striking association with other shared epitope haplotypes, such as HLA-DR1.
How could premature replicative senescence of myeloid and lymphoid precursors affect pathomechanisms of RA? Perturbations in T cell homeostasis could contribute to autoimmunity through several different pathways (38). Replicative senescence in regulatory T cells could cause deficiency of anti-inflammatory and anti-autoimmune processes (39). Dominance of peripheral instead of central selection mechanisms in shaping the TCR repertoire could favor the expansion of autoreactive clonotypes (13). Finally, escape of senescent T cells from the regulatory confines of the immune system may be the dominant driving force behind RA. Senescent T cells are not inert, and they can be potent effector cells that are difficult to control. Although deficient in the expression of CD28, they typically secrete large amounts of IFN-γ, have cytolytic capabilities, use MHC class I-recognizing receptors to costimulate TCR triggering, and have several defects in apoptotic pathways (40-43).
Acknowledgments
We thank Cynthia Crowson for statistical analysis, Alan Thornhill, M.D. (Department of Reproductive Endocrinology), and Jane M. Jaquith (Division of Rheumatology) for recruiting patients, James W. Fulbright for assistance in preparing this manuscript, and Linda H. Arneson for secretarial support. This work was funded by National Institutes of Health Grants R01 AR42527, R01 AR41974, R01 AI44142, and R01 AG15043, and by the Mayo Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HSC, hematopoietic stem cell; RA, rheumatoid arthritis; TCR, T cell antigen receptor; TREC, TCR excision circle; TRF, telomeric restriction fragment.
References
- 1.Wagner, U. G., Koetz, K., Weyand, C. M. & Goronzy, J. J. (1998) Proc. Natl. Acad. Sci. USA 95, 14447-14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koetz, K., Bryl, E., Spickschen, K., O'Fallon, W. M., Goronzy, J. J. & Weyand, C. M. (2000) Proc. Natl. Acad. Sci. USA 97, 9203-9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young, N. T., Uhrberg, M., Phillips, J. H., Lanier, L. L. & Parham, P. (2001) J. Immunol. 166, 3933-3941. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt, D., Goronzy, J. J. & Weyand, C. M. (1996) J. Clin. Invest. 97, 2027-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namekawa, T., Snyder, M. R., Yen, J. H., Goehring, B. E., Leibson, P. J., Weyand, C. M. & Goronzy, J. J. (2000) J. Immunol. 165, 1138-1145. [DOI] [PubMed] [Google Scholar]
- 6.Ku, C. C., Murakami, M., Sakamoto, A., Kappler, J. & Marrack, P. (2000) Science 288, 675-678. [DOI] [PubMed] [Google Scholar]
- 7.Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. (1998) Immunity 8, 591-599. [DOI] [PubMed] [Google Scholar]
- 8.Napolitano, L. A., Grant, R. M., Deeks, S. G., Schmidt, D., De Rosa, S. C., Herzenberg, L. A., Herndier, B. G., Andersson, J. & McCune, J. M. (2001) Nat. Med. 7, 73-79. [DOI] [PubMed] [Google Scholar]
- 9.Freitas, A. A. & Rocha, B. (2000) Annu. Rev. Immunol. 18, 83-111. [DOI] [PubMed] [Google Scholar]
- 10.Goldrath, A. W. & Bevan, M. J. (1999) Nature 402, 255-262. [DOI] [PubMed] [Google Scholar]
- 11.Kong, F. K., Chen, C. L., Six, A., Hockett, R. D. & Cooper, M. D. (1999) Proc. Natl. Acad. Sci. USA 96, 1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Douek, D. C., McFarland, R. D., Keiser, P. H., Gage, E. A., Massey, J. M., Haynes, B. F., Polis, M. A., Haase, A. T., Feinberg, M. B., Sullivan, J. L., et al. (1998) Nature 396, 690-695. [DOI] [PubMed] [Google Scholar]
- 13.Goronzy, J. J. & Weyand, C. M. (2001) Trends Immunol. 22, 251-255. [DOI] [PubMed] [Google Scholar]
- 14.Jawaheer, D., Seldin, M. F., Amos, C. I., Chen, W. V., Shigeta, R., Monteiro, J., Kern, M., Criswell, L. A., Albani, S., Nelson, J. L., et al. (2001) Am. J. Hum. Genet. 68, 927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornelis, F., Faure, S., Martinez, M., Prud'homme, J. F., Fritz, P., Dib, C., Alves, H., Barrera, P., de Vries, N., Balsa, A., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 10746-10750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nepom, G. T. (1998) Adv. Immunol. 68, 315-332. [DOI] [PubMed] [Google Scholar]
- 17.Winchester, R. (1994) Adv. Immunol. 56, 389-466. [DOI] [PubMed] [Google Scholar]
- 18.Weyand, C. M., Xie, C. & Goronzy, J. J. (1992) J. Clin. Invest. 89, 2033-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weyand, C. M., McCarthy, T. G. & Goronzy, J. J. (1995) J. Clin. Invest. 95, 2120-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinigaglia, F. & Nagy, Z. A. (2001) in Rheumatoid Arthritis, eds. Goronzy, J. J. & Weyand, C. M. (Karger, Basel), pp. 36-50.
- 21.Ernst, B., Lee, D. S., Chang, J. M., Sprent, J. & Surh, C. D. (1999) Immunity 11, 173-181. [DOI] [PubMed] [Google Scholar]
- 22.Goldrath, A. W. & Bevan, M. J. (1999) Immunity 11, 183-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viret, C., Wong, F. S. & Janeway, C. A., Jr. (1999) Immunity 10, 559-568. [DOI] [PubMed] [Google Scholar]
- 24.Hellerstein, M., Hanley, M. B., Cesar, D., Siler, S., Papageorgopoulos, C., Wieder, E., Schmidt, D., Hoh, R., Neese, R., Macallan, D., et al. (1999) Nat. Med. 5, 83-89. [DOI] [PubMed] [Google Scholar]
- 25.Haynes, B. F., Markert, M. L., Sempowski, G. D., Patel, D. D. & Hale, L. P. (2000) Annu. Rev. Immunol. 18, 529-560. [DOI] [PubMed] [Google Scholar]
- 26.Arnett, F. C., Edworthy, S. M., Bloch, D. A., McShane, D. J., Fries, J. F., Cooper, N. S., Healey, L. A., Kaplan, S. R., Liang, M. H. & Luthra, H. S. (1988) Arthritis Rheum. 31, 315-324. [DOI] [PubMed] [Google Scholar]
- 27.Hazenberg, M. D., Otto, S. A., Cohen Stuart, J. W., Verschuren, M. C., Borleffs, J. C., Boucher, C. A., Coutinho, R. A., Lange, J. M., de Wit, T. F., Tsegaye, A., et al. (2000) Nat. Med. 6, 1036-1042. [DOI] [PubMed] [Google Scholar]
- 28.Allsopp, R. C., Vaziri, H., Patterson, C., Goldstein, S., Younglai, E. V., Futcher, A. B., Greider, C. W. & Harley, C. B. (1992) Proc. Natl. Acad. Sci. USA 89, 10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rufer, N., Brummendorf, T. H., Kolvraa, S., Bischoff, C., Christensen, K., Wadsworth, L., Schulzer, M. & Lansdorp, P. M. (1999) J. Exp. Med. 190, 157-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson, J. D., Gale, R. E., Wynn, R. F., Dougal, M., Linch, D. C., Testa, N. G. & Chopra, R. (2000) Br. J. Haematol. 109, 272-279. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo, A. N., Weyand, C. M. & Goronzy, J. J. (2001) J. Biol. Chem. 276, 2565-2570. [DOI] [PubMed] [Google Scholar]
- 32.Posnett, D. N., Sinha, R., Kabak, S. & Russo, C. (1994) J. Exp. Med. 179, 609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackall, C. L., Hakim, F. T. & Gress, R. E. (1997) Semin. Immunol. 9, 339-346. [DOI] [PubMed] [Google Scholar]
- 34.Slagboom, P. E., Droog, S. & Boomsma, D. I. (1994) Am. J. Hum. Genet. 55, 876-882. [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison, S. J., Qian, D., Jerabek, L., Thiel, B. A., Park, I. K., Ford, P. S., Kiel, M. J., Schork, N. J., Weissman, I. L. & Clarke, M. F. (2002) J. Immunol. 168, 635-642. [DOI] [PubMed] [Google Scholar]
- 36.Tanchot, C., Rosado, M. M., Agenes, F., Freitas, A. A. & Rocha, B. (1997) Semin. Immunol. 9, 331-337. [DOI] [PubMed] [Google Scholar]
- 37.Marmont, A. M., Horowitz, M. M., Gale, R. P., Sobocinski, K., Ash, R. C., van Bekkum, D. W., Champlin, R. E., Dicke, K. A., Goldman, J. M. & Good, R. A. (1991) Blood 78, 2120-2130. [PubMed] [Google Scholar]
- 38.Theofilopoulos, A. N., Dummer, W. & Kono, D. H. (2001) J. Clin. Invest. 108, 335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi, S. (2000) Cell 101, 455-458. [DOI] [PubMed] [Google Scholar]
- 40.Vallejo, A. N., Schirmer, M., Weyand, C. M. & Goronzy, J. J. (2000) J. Immunol. 165, 6301-6307. [DOI] [PubMed] [Google Scholar]
- 41.Warrington, K. J., Takemura, S., Goronzy, J. J. & Weyand, C. M. (2001) Arthritis Rheum. 44, 13-20. [DOI] [PubMed] [Google Scholar]
- 42.Yen, J. H., Moore, B. E., Nakajima, T., Scholl, D., Schaid, D. J., Weyand, C. M. & Goronzy, J. J. (2001) J. Exp. Med. 193, 1159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markovic-Plese, S., Cortese, I., Wandinger, K. P., McFarland, H. F. & Martin, R. (2001) J. Clin. Invest. 108, 1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]