Abstract
The increase in life expectancy is accompanied by the growing burden of chronic diseases. Hearing loss is perhaps the most prevalent of all chronic diseases. In addition to age-related hearing loss, a substantial number of cases of audiological impairment are either congenital in nature or acquired during childhood. The permanence of hearing loss is mainly due to the inability of the cochlear sensory epithelium to replace lost mechanoreceptor cells, or hair cells. Generation of hair cells from a renewable source of progenitors that can be transplanted into damaged inner ears is a principal requirement for potential cell replacement therapy in this organ. Here, we present an experimental protocol that enables us to routinely create inner ear progenitors from murine embryonic stem cells in vitro. These progenitors express a comprehensive set of marker genes that define the developing inner ear, in particular the organ's developing sensory patches. We further demonstrate that cells that express markers characteristic of hair cells differentiate from embryonic stem cell-derived progenitors. Finally, we show that these progenitors integrate into the developing inner ear at sites of epithelial injury and that integrated cells start expressing hair cell markers and display hair bundles when situated in cochlear or vestibular sensory epithelia in vivo.
Slowing or reversing hearing loss is a major challenge for modern medicine. Mechanical wear and tear, pharmaceutical assaults, and age-related hair cell loss cause a progressive diminishment of hearing throughout life. Underlying the irreversibility of hearing loss in mammals is the incapacity of inner ear sensory epithelia to replace lost mechanoreceptor cells, or hair cells. Hair cell replacement, either by stimulation of regeneration or by transplantation of progenitor cells capable of differentiating into hair cells, remains the ultimate goal in the development of treatment applications to reconstruct damaged inner ears. As an initial step in this direction, we sought to explore whether a stepwise approach of differentiating embryonic stem (ES) cells could lead to inner ear progenitor cells and eventually to hair cells.
Generation of specific cell types from ES cells can be directed by applying cues that are involved in normal development. Such guided differentiation of cells derived from embryoid bodies has led, for example, to the generation of dopaminergic and motor neurons, among other cell types (1-4).
The inductive signals that lead to the generation of the ear anlage, the otic vesicle, and finally to the differentiation of the inner ear cell types are gradually being unraveled. Several growth factors have been implicated to play roles during inner ear development based on their mitogenic function, their survival-promoting activity, or their ability to induce certain cellular phenotypes. These molecules include epidermal growth factor (EGF) (5), insulin-like growth factor 1 (IGF-1) (5-7), and members of the fibroblast growth factor family (8-10). The observation that the early otic placode possesses extensive autonomy and does not require additional external signals to form all major inner ear cell types (11, 12) led us to investigate whether it is possible to enrich for a population of inner ear-like progenitor cells at a stage that is equivalent to the otic placode from differentiating ES cells. We hypothesized that growth factors that have been shown to mitogenically or trophically promote inner ear progenitors, such as EGF, IGF-1, and basic fibroblast growth factor (bFGF), could be used to selectively enrich the cell population for inner ear progenitor cells. After withdrawal of mitogenic supplements, these progenitors should be able to differentiate without external factors (1, 4, 11, 12) into various inner ear cell types, including the ear's featured sensory receptors, hair cells.
Materials and Methods
ES Cell Culture, Embryoid Body Formation, Progenitor Cells, and in Vitro Differentiation. Three ES cell lines were used in this study: R1, YC5/EYFP, and ROSA26. All three lines behaved similarly. ES cells were maintained on mitotically inactivated primary mouse embryonic fibroblasts or on gelatin-coated culture plates in the presence of 1,000 units·ml-1 leukemia inhibitory factor (Chemicon) in ES medium consisting of knockout DMEM supplemented with 15% FCS, 100 mM MEM nonessential amino acids, 0.55 mM 2-mercaptoethanol, l-glutamine, and antibiotics (all from Invitrogen/GIBCO/BRL). Embryoid bodies formed in 30-μl drops, each containing 300 ES cells, hanging from the undersurface of a lid of a 10-cm tissue culture dish (13). Plating embryoid body-derived cells onto adhesive tissue culture surface initiated the step for progenitor cell enrichment. After an initial 16 h of culture in ES cell medium to promote adhesion, cells were incubated for 10 days in serum-free medium with N2 supplement, EGF at 20 ng·ml-1, and IGF-1 at 50 ng·ml-1. bFGF at 10 ng·ml-1 was used to further expand the cells for 8 days. We use the term “enriched progenitor cells” for this cell population throughout this article. All growth factors and supplements were obtained from R & D Systems or Invitrogen/GIBCO/BRL. After selection, we removed the growth factors to initiate differentiation in serum-free medium with N2 supplement. Cells were analyzed by RT-PCR or immunocytochemistry after 10-14 days of differentiation. We use the term “differentiated cells” for this cell population throughout this article.
RT-PCR. Total RNA was extracted from cultured cells by using RNeasy kits (Qiagen, Valencia, CA). For reverse transcription with Superscript II reverse transcriptase (Invitrogen), we used 2 μg total RNA, treated with RNase-free DNase (Roche Molecular Biochemicals). PCR primer pairs were selected to discriminate between cDNA and genomic DNA by using individual primers specific for different exons, when possible. The identity of the PCR products was confirmed by sequencing. Control PCRs from mock reverse transcription reactions lacking enzyme did not produce products. Cycling parameters for different products were optimized to generate products at the linear portion of the product accumulation curve. Underlying this optimization was a series of pilot experiments to determine the sample (ES cells, embryoid bodies, progenitors, or differentiated cells) that produced the highest amount of amplification product. Specific cycling parameters were a denaturation step at 94°C for 1 min followed by x cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 45 s. The number of cycles (x) was 22 for Otx2, 25 for GAPDH, 30 for myosin VIIA, espin, Brn3.1, and α9 acetylcholine receptor, and 32 for all other primer pairs. The optimized conditions were held constant for each sample to assure valid comparison of the results. The data presented are representative of at least four identical independent experiments. We used the following primer pairs (protein, forward, reverse, and cDNA product length): nestin, GCCGAGCTGGAGCGCGAGTTAGAG, GCAAGGGGGAAGAGAAGGATGTCG, 694 bp; Pax2, CCAAAGTGGTGGACAAGATTGCC, GGATAGGAAGGACGCTCAAAGAC, 544 bp; BMP7, TGGGCTTCTGAGGAGGGCTGGTTG, TGGCGTGGTTGGTGGCGTTCAT, 484 bp; Jagged-1, CAGAATGACGCCTCCTGTCG, TGCAGCTGTCA ATCACT TCG, 361 bp; Otx2, CCATGACCTATACTCAGGCT TCAGG, GA AGCTCCATATCCCTGGGTGGAAAG, 211 bp; Math1, AGATCTACATCA ACGCTCTGTC, ACTGGCCTCATCAGAGTCACTG, 449 bp; myosin VIIA, CTCCCTCTACATCGCTCTGTTCG, AAGCACCTGCTCCTGCTCGTCCACG, 628 bp; espin, CAGCCTGAGTCACCGCAGCCTC, TGACCTGTCGCTGCCAGGGCGCG, 475 bp; α9 acetylcholine receptor, GAAGAACGTCATCTCCTACGGCTG, CAGCTCTCACCCACATCGTAGAC, 441 bp; p27Kip1, CTGGAGCGGATGGACGCCAGAC, CGTCTGCTCCACAGTGCCAGC, 525 bp; GA PDH, A ACGGGA AGCCCATCACC, CAGCCT TGGCAGCACCAG, 442 bp.
Chimeras. EGF/IGF-1-selectively enriched and bFGF-expanded ROSA26-derived progenitors were plated into bacterial Petri dishes to allow the formation of small cell aggregates. Aggregates were injected with beveled micro glass pipettes into the right otic vesicles of stage-16 to -17 chicken embryos. The left otic vesicles did not receive cell grafts and served as controls for β-galactosidase (β-gal) staining experiments. Specimens were fixed for 2-6 h in 4% paraformaldehyde in PBS at pH 7.3, cryoprotected for 24 h in 30% sucrose in PBS at pH 7.3, and embedded in optimal cutting temperature compound (O.C.T. Tissue-Tek, Sakura Finetek, Torrance, CA); 12- to 14-μm frozen sections were cut with a microtome (CM3050, Leica, Nussloch, Germany), collected on microslides (Utrastick Gold Seal, Portsmouth, NH), and stored frozen at -80°C. Otic vesicle sections were obtained by cutting the hindbrain regions of 3.5-day embryos in cross section, which was also the plane of sectioning for obtaining longitudinal cochlear duct sections of specimens at their sixth day of embryonic development. Cross-sections of vestibular sensory epithelia of 14-day-old embryos were cut from dissected inner ears. Immunolabeling was initiated by rehydrating and blocking the sections for 1 h with 0.1% Triton X-100 in PBS supplemented with 1% BSA and 5% goat serum (PBT1) and by following the protocol described below.
Immunocytochemistry and Immunohistochemistry. Cells were fixed for 15 min with 4% paraformaldehyde in PBS. Cellular membranes were permeabilized for 15 min with PBT1. Fixed and permeabilized cells or rehydrated sections were then incubated for 2 h in antiserum diluted in PBT1 1:200 for monoclonal antibody to β-gal (Promega), 1:5,000 for polyclonal antibody to espin (ref. 14, gift from A. J. Hudspeth, The Rockefeller University, New York), 1:3,000 for polyclonal antibody to myosin VIIA (gift from A. El-Amraoui and C. Petit, Pasteur Institute, Paris), 1:1,000 for monoclonal antibody to nestin (Developmental Studies Hybridoma Bank, Iowa City, IA), 1:2,000 for polyclonal antibody to parvalbumin 3 (15), 1:100 for monoclonal antibody to Math1 (Developmental Studies Hybridoma Bank), and 1:100 for polyclonal antibody to Brn3.1 (Covance, Princeton). Samples were washed three times for 15 min each with PBS. FITC-, tetramethylrhodamine B isothiocyanate-, and Cy-5-conjugated anti-rabbit and anti-mouse secondary antibodies (Jackson ImmunoResearch) were used to detect primary antibodies. F-actin was labeled with tetramethylrhodamine B isothiocyanate-conjugated phalloidin (Sigma), and cell nuclei were stained by exposure to 4,6-diamidino-2-phenylindole with VectaShield (Vector Laboratories). Staining was visualized with epifluorescence microscopy (Axioskop 2 Mot Axiocam, Zeiss) or confocal microscopy (TCS, Leica). The fraction of immunopositive cells of the total number of cells was established in double-blind fashion by counting ≈300 cells in 10 randomly selected microscopic fields per experiment, of which at least three were conducted for each determination.
Results
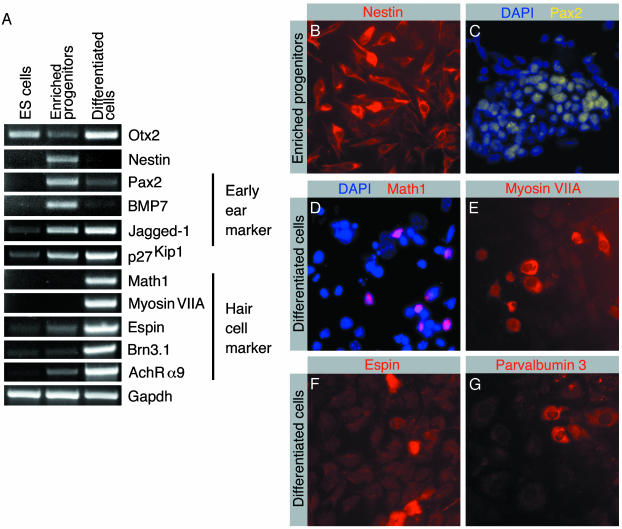
Differentiation of ES cells can be induced in vitro by allowing them to develop aggregates, called embryoid bodies (13). We initiated enrichment for potential inner ear progenitor cells by culturing embryoid body-derived cells for 10 days in the presence of EGF and IGF-1. The resulting cell population was further expanded for an additional 8-day period with bFGF. Following this treatment regimen, we observed that 96 ± 2.45% (mean ± SD; n = 3) of the cells express the intermediate filament protein nestin, a marker of neural progenitor cells (16) (Fig. 1 A and B). RT-PCR analysis of the enriched progenitor cells for the presence of marker genes characteristic for the developing inner ear revealed expression of the otic placode and otic vesicle markers Pax2 (17), BMP7 (18), and Jagged-1 (19, 20) (Fig. 1 A and C). Otx2, a murine homologue of the Drosophila melanogaster Orthodenticle gene, is an ES cell marker (1), which is also expressed in the otic vesicle and more abundantly expressed during subsequent inner ear morphogenesis (21). This expression profile is also reflected in our in vitro differentiation system (Fig. 1 A). The expression of early developmental and differentiated cell markers in embryoid bodies (data not shown) was similar to the expression pattern that we observed in ES cells, which is an observation also made when generating dopaminergic neurons from ES cells (1).
Fig. 1.
Analysis of expression of markers by ES cells, in vitro selectively enriched progenitor cells, and cells after in vitro differentiation. (A) RT-PCR-based analysis of expression of marker transcripts. Enriched progenitor cells were analyzed after expansion with bFGF. Differentiated cells were analyzed after 14 days of in vitro differentiation. Expression analysis of the ubiquitously expressed GAPDH is shown for reference. (B) Virtually all selected progenitors express nestin. (C) The majority of cells display Pax2 immunoreactivity. Nuclei are visualized with blue 4,6-diamidino-2-phenylindole staining. (D) After 14 days of in vitro differentiation, nuclear immunoreactivity for the hair cell marker Math1 was detectable. (E) A subset of differentiated cells express the hair cell marker myosin VIIA. (F) The hair-bundle marker espin is detectable in differentiated cells. (G) The hair cell marker parvalbumin 3 is detectable in differentiated cells.
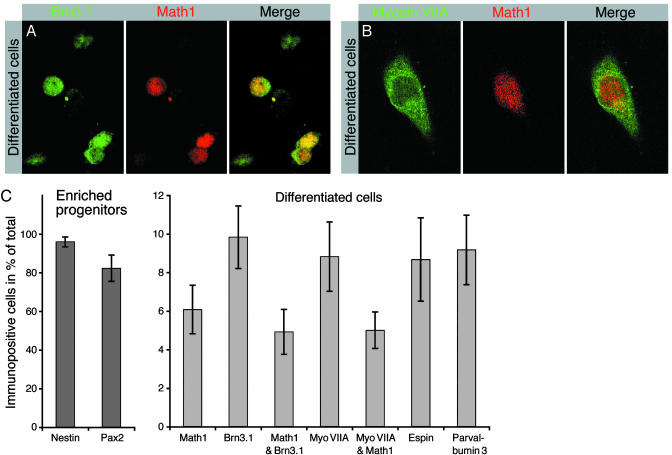
We next sought to determine the capacity of enriched inner ear progenitors to differentiate into mature cells types. In vitro differentiation of ES cell-derived progenitors can be initiated by removal of mitogens (1, 4, 22-24). We induced differentiation of enriched progenitors by withdrawing growth factors and continued culturing in defined medium. After 10-14 days of differentiation, we analyzed the cultures for expression of marker genes by RT-PCR and immunocytochemistry. We found that nestin expression was down-regulated, indicative of cell differentiation (Fig. 1 A). In concordance with cell differentiation, we detected reduced expression of the early otic vesicle marker genes Pax2 (17) and BMP7 (18) (Fig. 1 A). Presence of differentiating inner ear cell types was indicated by detection of mRNA encoding markers for the maturing sensory epithelia [Math1 (25), Brn3.1 (26), and Jagged-1 (19, 20)], hair cell markers [myosin VIIA (27), espin (14, 28), parvalbumin 3 (15), and α9 acetylcholine receptor (29)], and p27Kip1, a marker expressed in developing sensory epithelia and supporting cells (30) (Fig. 1 A and D-G). Consistent with the roles of Math1 and Brn3.1 for maturation and survival of newly generated hair cells (25, 26), we found that after in vitro differentiation 81 ± 14% (mean ± SD; n = 6) of Math1-expressing cells coexpressed Brn3.1 (Fig. 2 A and C). In addition, we found that 83 ± 9% (mean ± SD; n = 6) of Math1-expressing differentiated cells coexpressed myosin VIIA (Fig. 2 B and C).
Fig. 2.
Coexpression of hair cell markers by differentiated cells. (A) Expression of the transcription factors Brn3.1 and Math1 in nuclei of differentiated cells. The majority of Math1-expressing cells coexpress Brn3.1. (B) A large fraction of Math1-expressing cells coexpress the hair cell marker protein myosin VIIA. (C) Quantification of the number of cells expressing otic vesicle and hair cell markers at the progenitor cell stage (dark gray bars) and after in vitro differentiation (light gray bars). The individual bars visualize the fraction of immunopositive cells of the total number of cells. Shown are mean values and standard deviations determined in three to seven independent experiments for each data set.
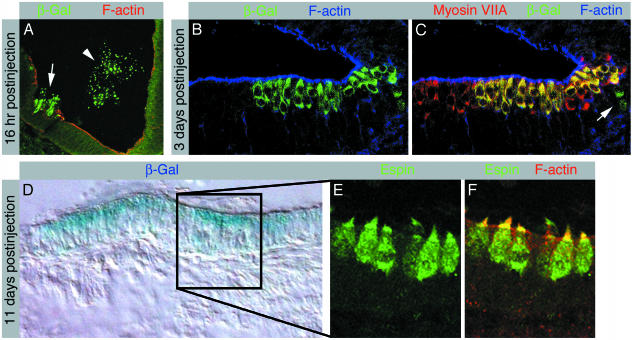
The ultimate goal of a potential therapeutic application for deafness or vestibular dysfunction with ES cell-derived progenitor cells is to generate hair cells in vivo. We therefore assessed the potential to generate hair cells in the living animal by grafting the selectively enriched inner ear progenitors, before differentiation, derived from ROSA26 ES cells into the otic vesicle of chicken embryos. ROSA26 ES cells carry the lacZ gene encoding bacterial β-gal that is ubiquitously expressed in ROSA26-6 mice (31). Shortly after the injection of progenitor cells, we found grafted cells integrated into the developing otic vesicle epithelium (Fig. 3A). Integration occurred preferentially in areas of the inner ear epithelium that were injured during the micro-surgical procedure. Several days postinjection, we found cells positive for the β-gal marker located in the developing cochlear hair cell layers (Fig. 3B). Murine cells that were situated luminally in the sensory epithelia expressed the hair cell marker myosin VIIA; this protein is also present in the adjacent chicken hair cells that did not express β-gal (Fig. 3C). Progenitor derivatives that integrated elsewhere did not up-regulate hair cell markers (Fig. 3C). We did not encounter β-gal-positive cells in control sections that were made from the uninjected ears and processed in parallel. Murine hair cells continued to mature, and we found that β-gal-positive cells, observed in chimeric inner ears at day 14 of embryonic development (Fig. 3D), expressed the hair cell and hair-bundle marker espin and displayed prominent cytomorphological specializations highly reminiscent of F-actin-rich hair bundles (Fig. 3 E and F).
Fig. 3.
Grafted inner ear progenitor cells integrate into the developing inner ear and differentiate into hair cells. (A) Cross section of the developing chicken inner ear at embryonic day 3.5, 16 h postinjection of ROSA26-derived progenitor cells. β-gal-expressing murine cells invade the epithelium that surrounds the lumen of the otic vesicle (arrow). The arrowhead labels β-gal-positive murine cells that remain in the lumen of the otic vesicle. F-actin labeling reveals disruptions of the epithelium, caused by injury during the grafting procedure. (B-C) Longitudinal section of a developing cochlear sensory epithelium at embryonic day 6, 3 days postinjection, immunolabeled with antibodies for β-gal (B) and myosin VIIA (C). F-actin labeling is shown for orientation. (B) β-gal-positive cells occur in the developing sensory patch. (C) Myosin VIIA-positive early hair cells can be detected in the cochlea at embryonic day 6. Merging the confocal images reveals a patch of myosin VIIA-positive hair cells that express β-gal. β-gal-positive cells observed in the developing supporting cell layer, located below the apical cell layer of early hair cells, do not express myosin VIIA (arrow). (D) At day 14 of embryonic development, 11 days postinjection, we found patches of β-gal-positive cells in inner ear sections. The patch of β-gal-positive cells in a utricular section, here revealed with X-gal histochemistry, is surrounded by cells that did not display blue X-gal staining, which served as negative control for the staining method. (E) Enlargement of an area with β-gal-positive cells exposes espin-positive cells with cylindrical hair cell morphology that display espin-positive hair bundles. (F) Espin-positive hair bundles are rich in F-actin, here apparent as yellow staining in the merged confocal images.
Discussion
The principal requirement for developing applications for the replacement of degenerated inner ear hair cells is a renewable source of progenitor cells that are capable of differentiating into hair cells. Our stepwise guidance protocol enriches for such a population of nestin-positive progenitor cells that express markers that are also present in the otic placode and otic vesicle. Inner ear progenitor cells, defined by the expression of nestin and multiple otic placode markers (Fig. 1 A-C), can be selectively enriched by 10-day treatment of embryoid body-derived cell populations with EGF/IGF-1 and by continued treatment with EGF/IGF-1 in combination with bFGF. This combination of growth factors seems to promote inner ear cell fate in our cultures, which is in accord with previous observations that implicated these factors in increasing the number of hair cells in ear development or promoting limited hair cell regeneration (6-10).
The differentiation of ES cell-derived progenitors is accompanied by a decrease in the fraction of the cells that express the progenitor marker nestin and the early otic marker Pax2; neither protein was detectable by immunocytochemical methods in differentiated cell populations (data not shown). Before differentiation, the majority of the selected progenitors expressed nestin and Pax2 (Fig. 2C). This decrease in expression was also apparent in our RT-PCR analysis (Fig. 1 A). Differentiation is complemented by an increase of cells expressing markers for maturing and adult sensory epithelia, including Math1, Brn3.1, myosin VIIA, espin, and parvalbumin 3 (Fig. 2C). Although the markers assessed are not individually specific for the developing ear, the expression of a combination of early and intermediate markers (Pax2, BMP7, Jagged-1, p27Kip1, Math1, and Brn3.1), followed by the onset of markers for differentiated hair cells (myosin VIIA, espin, parvalbumin 3, AchRα9), and accompanied by coexpression of Math1 with Brn3.1 and of Math1 with myosin VIIA, are fully consistent with a progressive differentiation of hair cell-like cells from ES cells, via progenitor cells.
The expression dynamics of key markers of developing inner ear sensory epithelia, when compared among ES cells, enriched progenitors, and differentiated cells (Fig. 1 A) recapitulate the in vivo situation where Math1 and Brn3.1 are important for maturing hair cells (25, 26) and the expression of Jagged-1 (19, 20) and p27Kip1 (30) is maintained in supporting cells. The formation of the sensory primordium in mouse inner ear does not require Math1 expression, but hair cell progenitors that have exited the cell cycle require Math1 for maturation (32); Math1-null mice fail to generate hair cells (25). Brn3.1-null mice initially generate hair cells, but the cells rapidly disappear during late gestation and early postnatal life (26). Although Math1, Brn3.1, and myosin VIIA are individually expressed in other cell types of the body, the three markers seem to be expressed simultaneously only in hair cells (25-27, 32-36).
Hair cell-like cells generated in our cultures are derived from Pax-2-expressing and nestin-positive progenitor cells because virtually all selectively enriched progenitor cells express these two markers (Fig. 2C). In the developing otic vesicle, Pax-2 expression can be detected in the majority of cells that form the presumptive inner ear sensory epithelia (37). We hypothesize that the hair cell marker-positive cells that differentiate in our cultures are differentiating in similar fashion as in vivo, where not all cells of the developing sensory patches differentiate into hair cells.
We used chicken-mouse chimeras to demonstrate that ES cell-derived inner ear progenitor cells can integrate into the developing inner ear sensory patches and that these progenitor cells can differentiate into hair cells in vivo. Invasion of the epithelium lining the inner lumen of the otic vesicle by transplanted murine progenitors happened preferentially when the epithelium was slightly injured. Creating an epithelial wound, in particular, damage of the apical epithelial surface, seemed to promote progenitor cell integration (Fig. 3A). Murine cells that were situated luminally in developing inner ear sensory patches expressed hair cell marker proteins with a similar time course as adjacent chicken hair cells. Murine cells that occurred outside the developing hair cell patches did not express hair cell makers. From these results, we conclude that the grafted inner ear progenitor cells adopt a hair cell phenotype in vivo when positioned in a suitable environment. Consequently, our results demonstrate that murine progenitor cell derivatives are able to respond to local cues controlling avian hair cell differentiation. We cannot exclude that cell fusion between injected ES cellderived progenitors and chicken cells accounts for the acquisition of hair cell markers in our grafting experiments. Nevertheless, we argue that fusion is a rather rare event leading to chimeric cells types, which would not explain the high efficacy of our injection experiments (In 30 of 50 cases, we found β-galpositive cells in the inner ear of the host) and the high reliance of specific up-regulation of hair cell markers of correctly positioned cells.
Our report provides a protocol to generate inner ear progenitors in large numbers from a proliferative supply, which should make it possible to develop and study in vivo transplantation of hair cell progenitors into damaged mammalian inner ears. These studies complement the recent discovery of adult inner ear stem cells (38) and could be the foundation of assessing the ultimate therapeutic potential of ES cell-derived inner ear progenitors in cell replacement therapy to functionally restore hearing in deaf patients.
Acknowledgments
We thank Drs. M. Applebury, J. T. Corwin, J. Cyr, P. G. Gillespie, W. F. Sewell, and the members of our group for valuable discussions and for suggestions on the manuscript. H.L. is supported in part by National Nature Science Foundation of China Grant 30271397. G.R. is a Research Fellow of the Thomas Wickham Jones Foundation. This work was supported by National Institutes of Health Grant DC006167 (to S.H.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ES, embryonic stem; EGF, epidermal growth factor; IGF-1, insulin-like growth factor 1; bFGF, basic fibroblast growth factor; β-gal, β-galactosidase.
References
- 1.Lee, S. H., Lumelsky, N., Studer, L., Auerbach, J. M. & McKay, R. D. (2000) Nat. Biotechnol. 18, 675-679. [DOI] [PubMed] [Google Scholar]
- 2.Lumelsky, N., Blondel, O., Laeng, P., Velasco, I., Ravin, R. & McKay, R. (2001) Science 292, 1389-1394. [DOI] [PubMed] [Google Scholar]
- 3.Doetschman, T. C., Eistetter, H., Katz, M., Schmidt, W. & Kemler, R. (1985) J. Embryol. Exp. Morphol. 87, 27-45. [PubMed] [Google Scholar]
- 4.Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. (2002) Cell 110, 385-397. [DOI] [PubMed] [Google Scholar]
- 5.Pickles, J. O. & van Heumen, W. R. (1997) Dev. Neurosci. 19, 476-487. [DOI] [PubMed] [Google Scholar]
- 6.Leon, Y., Vazquez, E., Sanz, C., Vega, J. A., Mato, J. M., Giraldez, F., Represa, J. & Varela-Nieto, I. (1995) Endocrinology 136, 3494-3503. [DOI] [PubMed] [Google Scholar]
- 7.Leon, Y., Sanz, C., Frago, L. M., Camarero, G., Canon, S., Varela-Nieto, I. & Giraldez, F. (1999) Horm. Metab. Res. 31, 126-132. [DOI] [PubMed] [Google Scholar]
- 8.Ladher, R. K., Anakwe, K. U., Gurney, A. L., Schoenwolf, G. C. & Francis-West, P. H. (2000) Science 290, 1965-1967. [DOI] [PubMed] [Google Scholar]
- 9.Represa, J., Leon, Y., Miner, C. & Giraldez, F. (1991) Nature 353, 561-563. [DOI] [PubMed] [Google Scholar]
- 10.Zheng, J. L., Helbig, C. & Gao, W. Q. (1997) J. Neurosci. 17, 216-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson, G. J., Howard, M. & Lewis, J. (1990) Dev. Biol. 137, 243-257. [DOI] [PubMed] [Google Scholar]
- 12.Corwin, J. T. & Cotanche, D. A. (1989) J. Comp. Neurol. 288, 529-537. [DOI] [PubMed] [Google Scholar]
- 13.Spector, D. L., Goldman, R. D. & Leinwand, L. A. (1998) in Cells: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), Vol. 1, pp. 8.1. [Google Scholar]
- 14.Li, H., Liu, H., Balt, S., Mann, S., Corrales, E. C. & Heller, S. (2003) J. Comp. Neurol., in press. [DOI] [PubMed]
- 15.Heller, S., Bell, A., Denis, C. S., Choe, Y. & Hudspeth, A. J. (2002) J. Assoc. Res. Otolaryngol. 3, 488-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lendahl, U., Zimmerman, L. B. & McKay, R. D. (1990) Cell 60, 585-595. [DOI] [PubMed] [Google Scholar]
- 17.Groves, A. K. & Bronner-Fraser, M. (2000) Development (Cambridge, U.K.) 127, 3489-3499. [DOI] [PubMed] [Google Scholar]
- 18.Oh, S. H., Johnson, R. & Wu, D. K. (1996) J. Neurosci. 16, 6463-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison, A., Hodgetts, C., Gossler, A., Hrabe de Angelis, M. & Lewis, J. (1999) Mech. Dev. 84, 169-172. [DOI] [PubMed] [Google Scholar]
- 20.Lanford, P. J., Lan, Y., Jiang, R., Lindsell, C., Weinmaster, G., Gridley, T. & Kelley, M. W. (1999) Nat. Genet. 21, 289-292. [DOI] [PubMed] [Google Scholar]
- 21.Morsli, H., Tuorto, F., Choo, D., Postiglione, M. P., Simeone, A. & Wu, D. K. (1999) Development (Cambridge, U.K.) 126, 2335-2343. [DOI] [PubMed] [Google Scholar]
- 22.Rietze, R. L., Valcanis, H., Brooker, G. F., Thomas, T., Voss, A. K. & Bartlett, P. F. (2001) Nature 412, 736-739. [DOI] [PubMed] [Google Scholar]
- 23.Tropepe, V., Coles, B. L., Chiasson, B. J., Horsford, D. J., Elia, A. J., McInnes, R. R. & van der Kooy, D. (2000) Science 287, 2032-2036. [DOI] [PubMed] [Google Scholar]
- 24.Gritti, A., Parati, E. A., Cova, L., Frolichsthal, P., Galli, R., Wanke, E., Faravelli, L., Morassutti, D. J., Roisen, F., Nickel, D. D. & Vescovi, A. L. (1996) J. Neurosci. 16, 1091-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bermingham, N. A., Hassan, B. A., Price, S. D., Vollrath, M. A., Ben-Arie, N., Eatock, R. A., Bellen, H. J., Lysakowski, A. & Zoghbi, H. Y. (1999) Science 284, 1837-1841. [DOI] [PubMed] [Google Scholar]
- 26.Xiang, M., Gan, L., Li, D., Chen, Z. Y., Zhou, L., O'Malley, B. W., Jr., Klein, W. & Nathans, J. (1997) Proc. Natl. Acad. Sci. USA 94, 9445-9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahly, I., El-Amraoui, A., Abitbol, M., Petit, C. & Dufier, J. L. (1997) Anat. Embryol. 196, 159-170. [DOI] [PubMed] [Google Scholar]
- 28.Zheng, L., Sekerkova, G., Vranich, K., Tilney, L. G., Mugnaini, E. & Bartles, J. R. (2000) Cell 102, 377-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuo, J., Treadaway, J., Buckner, T. W. & Fritzsch, B. (1999) Proc. Natl. Acad. Sci. USA 96, 14100-14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen, P. & Segil, N. (1999) Development (Cambridge, U.K.) 126, 1581-1590. [DOI] [PubMed] [Google Scholar]
- 31.Zambrowicz, B. P., Imamoto, A., Fiering, S., Herzenberg, L. A., Kerr, W. G. & Soriano, P. (1997) Proc. Natl. Acad. Sci. USA 94, 3789-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, P., Johnson, J. E., Zoghbi, H. Y. & Segil, N. (2002) Development (Cambridge, U.K.) 129, 2495-2505. [DOI] [PubMed] [Google Scholar]
- 33.Yang, Q., Bermingham, N. A., Finegold, M. J. & Zoghbi, H. Y. (2001) Science 294, 2155-2158. [DOI] [PubMed] [Google Scholar]
- 34.Xiang, M., Zhou, L., Macke, J. P., Yoshioka, T., Hendry, S. H., Eddy, R. L., Shows, T. B. & Nathans, J. (1995) J. Neurosci. 15, 4762-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Arie, N., McCall, A. E., Berkman, S., Eichele, G., Bellen, H. J. & Zoghbi, H. Y. (1996) Hum. Mol. Genet. 5, 1207-1216. [DOI] [PubMed] [Google Scholar]
- 36.Lumpkin, E. A., Collisson, T., Parab, P., Omer-Abdalla, A., Haeberle, H., Chen, P., Doetzlhofer, A., White, P., Groves, A., Segil, N. & Johnson, J. E. (2003) Gene Expr. Patterns 3, 659-662.12972002 [Google Scholar]
- 37.Lang, H., Bever, M. M. & Fekete, D. M. (2000) J. Comp. Neurol. 417, 205-220. [DOI] [PubMed] [Google Scholar]
- 38.Li, H., Liu, H. & Heller, S. (2003) Nat. Med. 9, 1293-1299. [DOI] [PubMed] [Google Scholar]