Abstract
There is currently no available experimental system wherein human cancer cells can be grown in the context of a mixed population of normal differentiated human cells for testing biological aspects of cancer cell growth (e.g., tumor cell invasion and angiogenesis) or response to anti-cancer therapies. When implanted into immunocompromised mice, human embryonic stem cells develop teratomas containing complex structures comprising differentiated cell types representing the major germ line-derived lineages. We sought to determine whether human cancer cells would grow within such teratomas and display properties associated with malignancy, such as invasiveness and recruitment of blood vessels. HEY ovarian cancer cells stably expressing an H2A-GFP fusion protein (HEY-GFP) injected into mature teratomas developed into tumors, which allowed tracking of tumor cell invasion and recruitment of human teratoma-derived blood vessels. This provides a straightforward and powerful approach to studying the biological properties of cancer cells within the microenvironment of normal differentiated human cells.
Keywords: cancer cell invasiveness, tumorigenesis, angiogenesis
Numerous models have been developed to study human tumorigenesis properties, such as proliferation, migration, invasion, neoangiogenesis, and metastasis, as well as for the study of anti-cancer treatments. Among others, these include in vitro systems such as focus formation in tumor cell culture explants and continuous cell lines grown on tissue culture plates, or, alternatively, anchorage-independent growth in soft agar. The major in vivo models involve the injection of tumor cells at various sites in immunocompromised mice. The foregoing experimental models are not particularly amenable to the investigation of interactions of tumor cells with the surrounding microenvironment of adjacent normal differentiated human cell tissues and structures. It has been shown that tumor progression is associated with extensive remodeling of adjacent tissues to provide a supportive environment for tumor growth, angiogenesis, invasion, and metastasis of cancer cells (1-4). Thus, proteases, heparanase, and other enzymes expressed by cancer cells or adjacent stromal cells contribute to these processes by activating and releasing cytokines and growth factors and degrading extracellular matrix components that support growth and invasion of cancer cells. Different malignant tumors present different “cancer-specific” patterns of gene expression necessary for nonneoplastic tissue remodeling that contribute to the crucial interplay between cancer cells and different types of surrounding nonneoplastic stromal cells. As a result of the recent increased appreciation of the important role for the tissue microenvironment, anti-cancer therapeutic strategies have been targeted to frustrate stromal response factors that support tumor growth. Among others, these have included protease inhibitors, inhibitors of heparanase, and, most strikingly, antiangiogenic molecules (5-8). Purely in vitro models such as focus formation and anchorage-independent growth are not particularly well suited for studying such mechanisms or testing the potential efficacy of therapies directed at the tumor microenvironment. Accordingly, most preclinical studies have been conducted using injection of tumor cells in immunocompromised mice. However, even these in vivo models depend upon a murine rather than human tissue microenvironment. Thus, it is the murine neoangiogenic response that has been the target in preclinical testing of antiangiogenic agents using existing experimental model systems. Other recent studies have focused on identifying and characterizing subpopulations within tumor masses that display specific tumorigenic properties, such as proliferation and invasiveness (9, 10). Once again, the conclusions are based on measurement of these properties within a surrounding murine, rather than human, tissue microenvironment.
Accordingly, we sought to develop a model system in which the specific properties of tumorigenesis related to the surrounding human cellular microenvironment could be studied. When implanted into immunocompromised mice, human embryonic stem (hES) cells developed teratomas containing complex structures, comprising differentiated cell types representing derivatives of all three major embryonic lineages (11-14). Therefore, we sought to determine whether human cancer cells would grow within such teratomas and display tumorigenic properties that specifically relate to the surrounding human cellular microenvironment, such as invasiveness and recruitment of blood vessels. In the current study we report the feasibility of this approach, using as a model system, ovarian cancer cells stably expressing an H2A-GFP fusion protein, which allow the monitoring of tumor cell growth and invasion within the human teratoma, as well as tracking of the angiogenic response originating in the human teratoma.
Materials and Methods
Cell Culture. The human undifferentiated embryonic stem cell clone H9.1 (15) was kindly provided by J. Itskovitz-Eldor (Technion and Rambam Medical Center, Haifa, Israel), and cells were grown on a mitomycin C-treated mouse embryonic fibroblast feeder layer as described (14). The HEY cell line that was initiated from a disaggregated xenograft ovarian tumor (16, 17) was grown in RPMI medium 1640 supplemented with 10% FCS and 1% l-glutamine (Biological Industries, Kibbutz Beit Haemek, Israel).
Reporter Plasmid and Stable Transfection. The cDNA coding region for the GFP fused downstream to the histone H2A (18) (kindly provided by M. Brandeis, Hebrew University, Jerusalem), was inserted into AgeI and NotI restriction sites of the pEGFP-N1 expression vector (Clontech). Stable transfection of HEY cells was carried out using FuGENE 6 reagent (Roche) and 300 μg/ml G418 (Life Technologies) for selection of stable clones.
Teratoma Formation. Undifferentiated hES cells were harvested using 1 mg/ml collagenase type IV (Life Technologies) and injected into the hind limb of severe combined immunodeficient (SCID)/beige mice (≈5 × 106 cells per injection). Teratomas were palpable after 4 weeks. Sixty-one days after initial injection of H9.1 cells, 106 HEY-GFP cells were injected into the teratoma and permitted to grow for an additional 21 days. Control noninjected teratomas, HEY-GFP-injected teratomas, and tumor nodules derived from direct injection of HEY-GFP were all harvested at 82 days.
Histological Analysis. Teratomas were harvested, fixed for 48 h in 10% neutral buffered formalin, transferred into 70% ethanol, and processed using a routine wax-embedding procedure for histologic examination. Six-micrometer paraffin sections were mounted on Super FrostPlus microscope slides (Menzel-Glaser, Braunschweig, Germany) and stained with hematoxylin/eosin.
Immunohistochemistry. Slides were deparaffinized using xylene and rehydrated through a series of gradients of alcohol to water. Antigens were retrieved using microwave exposure at 90°C for 8 min in a citrate buffer (pH 6.1). Endogenous peroxidase enzyme activity was blocked by using 3% hydrogen peroxidase in methanol for 30 min at room temperature. Slides were washed in distilled water and in PBS, pH 7.4, and then were blocked using 10% nonimmune goat serum for 1 h (GFP) and 24 h (CD31 and CD34) at 4°C. Slides were incubated for 24 h at 4°C with the following primary antibodies: rabbit polyclonal anti-GFP (1:2,000; Molecular Probes), mouse monoclonal anti-human CD34 (1:50; DAKO), rabbit polyclonal anti-mouse CD31 (1:2,000; kindly provided by J. Madri, Yale University, New Haven, CT), followed by incubation with goat anti-rabbit or anti-mouse biotinylated secondary antibody. Preimmune rabbit or mouse sera were used as negative controls. Detection was accomplished using a Histostain-SP (3-amino-9-ethylcarbazole) kit (Zymed). Counterstaining was carried out using hematoxylin.
Results
Establishment of a Human Tissue Microenvironment Surrounding Tumor Tissue. Eighty-two days after the i.m. injection of undifferentiated hES cells (H-9.1 clone) into the hind limb musculature of SCID/beige mice, typical nodules appeared and increased in size progressively, as has been described (15). Stained sections of such nodules reveal them to be teratomas containing numerous and varied complex differentiated structures (data not shown), as has been reported (11). Injection of 106 HEY-GFP cells at day 61 into such teratomas in SCID/beige mice (Fig. 1) yielded a different gross morphologic appearance compared with the direct hind limb i.m. injection of an equal aliquot of HEY-GFP cells. In the case of direct i.m. injection of tumor cells, the well described appearance of small nodules at each injection site was observed, in which there was poor demarcation of the nodule from the surrounding murine muscle tissue and a markedly hemorrhagic surface. In contrast, after injection of an equal aliquot of cells at day 61 into the teratoma, the teratoma surface appeared well circumscribed, with relatively few blood vessels and absence of hemorrhage. HEY-GFP cells were injected into the teratomas, and staining of sections with the Gomori technique for reticulin fibers (19) was used to detect the histochemical appearance of the mixed teratoma structures obtained 21 days after injection of HEY-GFP cells into these teratomas. Histologic appearance at lower-power magnification revealed these mixed structures to be comprised of regions with the typical appearance of teratomas derived from hES cells with a variety of mature differentiated structures (Fig. 2A); regions of tumor cells with the appearance of a homogeneous mass of cells with the characteristic morphology of adenocarcinoma (Fig. 2B) and also exhibiting high proliferative capacity as exhibited by the number of cells in mitosis and by proliferating cell nuclear antigen staining (data not shown); as well as boundary regions in which tumor cells appeared adjacent to differentiated teratoma structures such as the neurovascular bundle shown in Fig. 2C.
Fig. 1.
Schematic representation of the experimental protocol.
Fig. 2.
Photomicrographs of teratoma, tumor, and boundary region using reticulin staining with the Gomori technique. (A) Typical appearance of teratoma-derived structure in SCID/beige mice, with derivatives of three embryonic germ layers. se, stratified epithelium of ectodermal origin; m, smooth muscle of mesodermal origin; ce, columnar epithelium with goblet cells of endodermal origin. (B) Homogeneous mass of HEY ovarian carcinoma tumor cells (tu). (C) Boundary region of tumor cells (tu) adjacent to a differentiated teratoma structure consisting of a neurovascular bundle with a venule (v), an arteriole (a), and a nerve (n). (Bars: 200 μm.)
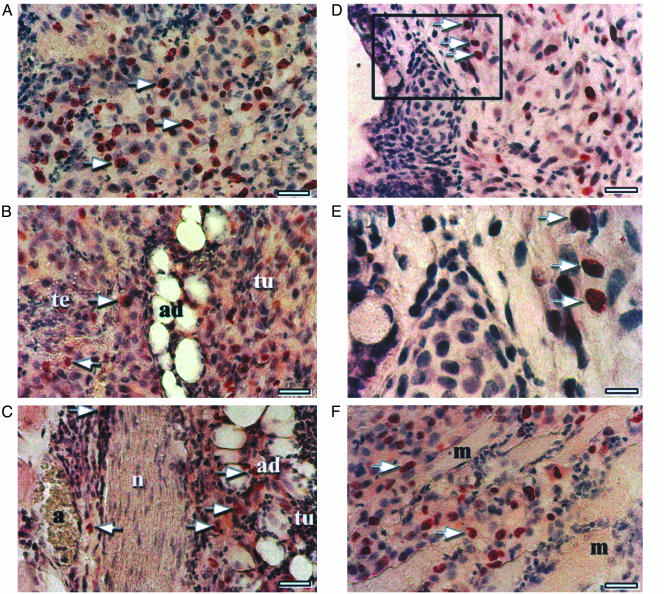
Identification of Tumor Cell Invasiveness into the Human Tissue Microenvironment. Because invasiveness is a hallmark characteristic of tumorigenesis, and because of the appearance of tumor cells adjacent to hES cell-derived teratoma structures, we sought to track the possible migration and infiltration of tumor cells into normal differentiated tissue. For this purpose, because the HEY-GFP cells are stably transfected to constitutively express GFP, it was possible to track HEY-GFP cell infiltration using immunohistochemistry for GFP. Fig. 3A shows nuclear GFP-positive immunohistochemical staining in a field of tumor cells. Although there is variable intensity of staining, positive staining is easily detectable in tumor cell nuclei. Fig. 3 B-E shows GFP-positive cells that have invaded and interspersed among teratoma-derived differentiated cells and structures such as adipocytes and migrated to the other side of a neural structure (Fig. 3 B and C) or surrounding connective tissue (Fig. 3 D and E). In the case of nodules generated by direct i.m. injection of HEY-GFP ovarian cancer cells in SCID/beige mice, there is expected invasion of GFP-positive cells into the surrounding murine muscle tissue (Fig. 3F).
Fig. 3.
Infiltration of HEY-GFP cells into human teratoma-derived tissue. (A) Arrows indicate HEY-GFP-positive nuclear immunostaining of HEY-GFP cells in a field of tumor cells. (Bar: 50 μm.) (B and C) Arrows show migration of tumor (tu)-derived GFP-positive cells into the teratoma (te)-derived adipocytes (ad) and crossing adjacent nerve tissue (n). (Bar: 50 μm.) (D) Arrows show invasion of HEY-GFP-positive cells into the surrounding connective tissue. (Bar: 50 μm.) (E) Enlargement of Inset in D. (Bar: 20 μm.) (F) Invasion of tumor-derived GFP-positive cells into surrounding mouse muscle cells after direct i.m. injection. (Bar: 50 μm.)
Identification of Blood Vessel Origin Adjacent to and Within the Tumor. Besides invasion into surrounding normal differentiated tissue, tumor cells elicit a number of stromal responses that modulate tumor growth. Among these, tumor-induced neoangiogenesis has been extensively studied (20). Growth of tumor-derived nodules in immunocompromised mouse models has been used extensively to demonstrate tumor neoangiogenesis, which is thought to make an indispensable contribution to sustained tumor growth (20). Blood vessels of murine origin have been shown to grow within the tumor nodules, and antiangiogenic agents have been shown to disrupt this effect and induce tumor regression in such experimental models. We sought to determine whether tumors growing within hES-derived teratomas would elicit the growth of teratoma-derived blood vessels of human origin adjacent to and within the tumor. To distinguish mouse-derived from human-derived blood vessels, we used CD34 and CD31 human- and mouse-specific surface endothelial marker antibodies, respectively.
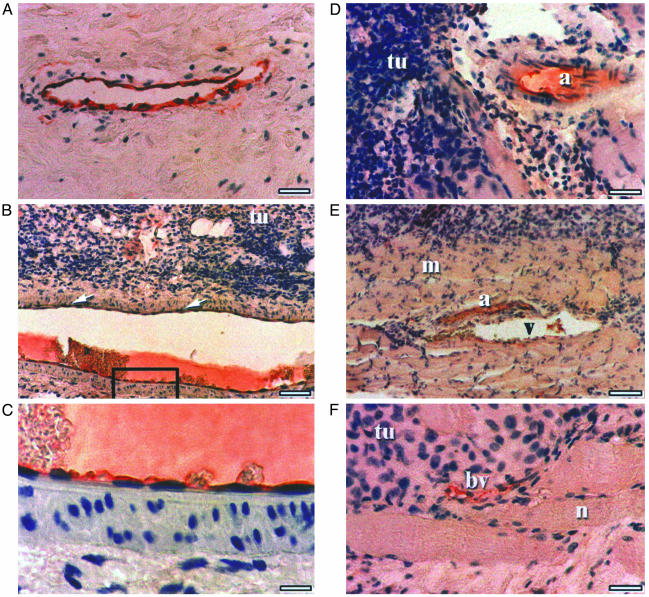
Fig. 4A shows a positive control of immunostaining with human-specific CD34 antibody of blood vessels in a specimen of human breast carcinoma. Fig. 4 B and C are photomicrographs showing low- and higher-power magnifications of CD34-positive immunostaining of an arteriole adjacent to a mass of HEY tumor cells growing within a human teratoma. Specificity of staining of the endothelial cell layer is evident in the higher-power magnification. Fig. 4 D-F shows a variety of additional human CD34-positive-staining blood vessels of various sizes and configuration (arteriole, venule, capillary, and immature small blood vessel characteristic of tumor-induced angiogenesis) adjacent to and within tumor cells. Hepatic sinusoid endothelium immunostained by mouse-specific CD31 antibody is shown in normal mouse liver tissue (Fig. 5A) and in a HEY-derived tumor nodule after direct i.m. injection in SCID/beige mice (Fig. 5B). In contrast, mouse-specific CD31 immunostaining is not evident in HEY ovarian cancer-derived cells growing within an hES teratoma, despite the presence of blood vessels adjacent to and within the tumor mass (Fig. 5C).
Fig. 4.
Detection of tumor neoangiogenesis using human-specific CD34 antibodies. (A) Positive control. A specimen of human breast carcinoma stained with anti-CD34 human-specific antibody is shown. (Bar: 50 μm.) (B) Photomicrographs showing low-power magnifications of CD34-positive immunostaining of an arteriole adjacent to a mass of HEY tumor cells growing within a human teratoma. (Bar: 100 μM.) (C) Higher-power magnification of the inset from B demonstrating specificity of staining of the endothelial cell layer. (Bar: 20 μM.) (D-F) Additional human CD34 positively staining blood vessels adjacent to and within tumor cells. a, arteriole; v, venule; bv, blood vessel; tu, tumor. (Bars: 50 μm in D and F and 100 μm in E.)
Fig. 5.
Photomicrographs of mouse-specific CD31 immunostaining. (A) Positive control of normal mouse hepatic sinusoidal endothelium stained with anti-CD31 mouse-specific antibody. cv, central hepatic vein. (Bar: 50 μm.) (B) Mouse-specific anti-CD31 immunostaining of blood vessels in a tumor nodule after direct i.m. injection. (Bar: 50 μm.) (C) Absence of signal in mouse-specific anti-CD31 antibody-stained section containing a neurovascular structure. (Bar: 50 μm.)
Thus, human ovarian cancer cells elicit a neoangiogenic response, which is of murine origin in the case of tumors growing directly within the surrounding murine tissue, and which is of human origin in the case of growth within normal differentiated human tissue of embryonic stem cell origin. Other blood vessels within the teratoma tissue itself were stained with either CD31 or CD34, reflecting neoangiogenesis of murine origin at early stages of teratoma generation in the mouse and subsequent differentiation of hES cells into blood vessels of human cellular origin, and did not differ in appearance between control and tumor-injected teratomas.
Discussion
It has long been appreciated that the stroma surrounding tumor is frequently modified in terms of cellular composition and extracellular matrix during the course of tumor growth (21, 22). Furthermore, the “tumor microenvironment” has been shown to greatly influence tumorigenicity properties both at the site of the primary tumor and at metastatic sites (2). Thus, for example, it has been demonstrated that human prostatic carcinoma-associated fibroblasts can promote carcinogenesis in human prostate epithelial cells that have been initiated but are not yet tumorigenic (23). Conversely, in the case of ovarian cancer cell lines, it was shown that tumor growth induced by injection of these cell lines in nude mice could be reduced by coinjection of normal bovine or human ovarian stromal cells, but not by coinjection of other stromal cell types (24). Interestingly, however, the normal ovarian stromal cells did not appear to survive long-term incubation at the tumor site, which was instead supported by the recruitment of host murine stromal cells. Normal keratinocytes have also been shown to suppress early stages of neoplastic progression in skin epithelia (25).
In the current study we have developed a straightforward experimental model for growth of human tumor cells within the surrounding microenvironment of normal human differentiated cells. This was enabled by the availability of hES cells, which develop into teratomas when grown in immunocompromised mice. Numerous previous models have been developed for human tumorigenesis to study properties of tumor cells such as proliferation, migration, invasion, angiogenesis, and metastasis among others, as well as for the study of anti-cancer treatments (26). Such models range from purely in vitro systems to growth in vivo after s.c., i.m., or i.p. injection in immunocompromised mice. However, these experimental models of human tumor cell growth do not permit the study of properties of tumor cells related to their growth within the microenvironment of adjacent normal differentiated human cell tissues. The latter situation has added potential experimental and clinical importance in elucidating properties that modulate tissue invasion, reactive sclerosis, angiogenesis, and responses to certain anti-cancer regimens. Thus, for example, antiangiogenic agents have been tested using experimental models in which tumor cells have been grown in immunocompromised mice, where they elicit an angiogenic response involving the growth within the tumor of blood vessels of murine host origin (20, 27, 28). The subsequent step of research testing in humans in the clinical setting omits an important step in which the effects of such agents are examined in tumors growing in the microenvironment of human tissue, where the neoangiogenesis response is comprised of human blood vessels. The current model provides just such a platform, with the key observation of blood vessels of human origin. Conversely, candidate anti-cancer agents, particularly those directed at stromal responses, may be frequently discarded before clinical testing, when they fail to inhibit cancer cell growth or induce regression using in vitro models or models of tumor nodule growth directly in immunocompromised mice. Anti-cancer and immunotargeting therapeutic agents that depend on a microenvironment of surrounding normal human differentiated tissues would not be adequately evaluated in such experimental systems, whereas the currently described model provides a potential experimental platform for studying such agents. Numerous additional refinements and applications can be considered for the model system described. Among others, these include quantitation of tumor proliferation after in vivo pulse labeling with BrdUrd, comparative quantitation of neoangiogenic responses (29), and comparison of properties related to local microenvironment among different human tumor cell types, including tumor cells harvested from clinical samples. The current approach could also be combined with recently described experimental methods using avian retroviral vectors to introduce transcriptional regulators into mammalian cells (30).
In the current study, we have focused on ovarian cancer cell lines, which may be particularly amenable to study by the current model system. It has been shown that critical mediators of ovarian cancer aggressiveness, such as IL-8, may not be adequately reflected in a murine host system (31). Furthermore, ovarian cancer ascites fluid may serve as a source of cells whose tumorigenesis can be studied in the currently described model. In this regard, the effect of the potent ovarian cancer growth factor lysophosphatidic acid (LPA), which is known to stimulate expression of IL-8 (32), can be more readily analyzed by administration of inhibitors or by genetic modulation of the expression of the enzymatic and regulatory pathways involved in the elaboration of LPA and IL-8 of tumor or stromal origin. Extension of this model to other cell types in future studies will be of interest. Indeed, comparative quantitation of the human neoangiogenic response and application to a variety of different tumor types represent the next most important steps in extending this model.
We have also tested a number of other methods of obtaining growth of tumor cells in conjunction with hES cells. Of interest, mixing of various tumor cell lines with undifferentiated hES cells in a culture dish, resulted in marked inhibition of hES differentiation. While this precluded the development of a wholly in vitro experimental model using this approach, this finding will be of future interest in terms of exploring the mechanisms whereby some kinds of tumor cells inhibit hES cell differentiation.
In conclusion, we demonstrate a structure consisting of human tumor cells growing within human teratomas derived from hES cells. The growth, invasion, and human blood vessel neoangiogenic response observed in these structures indicate that they may serve as an important experimental model for investigating and manipulating the local microenvironment in tumor cell growth within the context of differentiated human cell structures and tissues, and thereby contribute to the progress of cancer research.
Acknowledgments
We thank Michal Amit, Israel Vlodavsky, Ofer Ben-Yitzchak, and Yoram Reiter for helpful input, Sara Selig for critical reading of the manuscript, and Galit Paor and Ruth Tal for excellent technical assistance. This research was supported by grants from the Annie Chutick Endowment and the Rappaport Institute.
Abbreviations: hES, human embryonic stem; SCID, severe combined immunodeficient.
References
- 1.Hanahan, D. & Weinberg, R. A. (2000) Cell 100, 57-70. [DOI] [PubMed] [Google Scholar]
- 2.Bissell, M. J. & Radisky, D. (2001) Nat. Rev. Cancer 1, 46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fidler, I. J. (2002) Semin. Cancer Biol. 12, 89-96. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, A. F., Groom, A. C. & MacDonald, I. C. (2002) Nat. Rev. Cancer 2, 563-572. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen, M., Lund, L. R., Romer, J., Almholt, K. & Dano, K. (1998) Curr. Opin. Cell Biol. 10, 667-771. [DOI] [PubMed] [Google Scholar]
- 6.Wylie, S., MacDonald, I. C., Varghese, H. J., Schmidt, E. E., Morris, V. L., Groom, A. C. & Chambers, A. F. (1999) Clin. Exp. Metastasis 17, 111-117. [DOI] [PubMed] [Google Scholar]
- 7.Goldshmidt, O., Zcharia, E., Abramovitch, R., Metzger, S., Aingorn, H., Friedmann, Y., Schirrmacher, V., Mitrani, E. & Vlodavsky, I. (2002) Proc. Natl. Acad. Sci. USA 99, 10031-10036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang, J., Frischer, J. S., Serur, A., Kadenhe, A., Yokoi, A., McCrudden, K. W., New, T., O'Toole, K., Zabski, S., Rudge, J. S., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 7785-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dick, J. E. (2003) Proc. Natl. Acad. Sci. USA 100, 3547-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. (2003) Proc. Natl. Acad. Sci. USA 100, 3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S. & Jones, J. M. (1998) Science 282, 1145-1147. [DOI] [PubMed] [Google Scholar]
- 12.Pera, M. F., Reubinoff, B. & Trounson, A. (2000) J. Cell Sci. 113, 5-10. [DOI] [PubMed] [Google Scholar]
- 13.Assady, S., Maor, G., Amit, M., Itskovitz-Eldor, J., Skorecki, L. K. & Tzukerman, M. (2001) Diabetes 50, 1691-1697. [DOI] [PubMed] [Google Scholar]
- 14.Tzukerman, M., Shachaf, C., Ravel, Y., Braunstein, I., Cohen-Barak, O., Yalon-Hacohen, M. & Skorecki, K. L. (2000) Mol. Biol. Cell 11, 4381-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amit, M., Carpenter, M. K., Inokuma, M. S., Chiu, C. P., Harris, C. P., Waknitz, M. A., Itskovitz-Eldor, J. & Thomson, J. A. (2000) Dev. Biol. 227, 271-278. [DOI] [PubMed] [Google Scholar]
- 16.Buick, R. N., Pullano, R. & Trent, J. M. (1985) Cancer Res. 45, 3668-3676. [PubMed] [Google Scholar]
- 17.Braunstein, I., Cohen-Barak, O., Shachaf, C., Ravel, Y., Yalon-Hacohen, M., Mills, G. B., Tzukerman, M. & Skorecki, K. L. (2001) Cancer Res. 61, 5529-5536. [PubMed] [Google Scholar]
- 18.Zur, A. & Brandeis, M. (2002) EMBO J. 21, 4500-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomori, G. (1937) Am. J. Pathol. 13, 993. [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman, J. (2002) Semin. Oncol. 6, Suppl. 16, 15-18. [DOI] [PubMed] [Google Scholar]
- 21.Cunha, G. R. & Matrisian, L. M. (2002) Differentiation 70, 469-472. [DOI] [PubMed] [Google Scholar]
- 22.Tlsty, T. D. (2001) Semin. Cancer Biol. 11, 97-104. [DOI] [PubMed] [Google Scholar]
- 23.Olumi, A. F., Grossfeld, G. D., Hayward, S. W., Carroll, P. R., Tlsty, T. D. & Cunha, G. R. (1999) Cancer Res. 59, 5002-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parrott, J. A., Nilsson, E., Mosher, R., Magrane, G., Albertson, D., Pinkel, D., Gray, J. W. & Skinner, M. K. (2001) Mol. Cell. Endocrinol. 175, 29-39. [DOI] [PubMed] [Google Scholar]
- 25.Javaherian, A., Vaccariello, M., Fusenig, N. E. & Garlick, J. A. (1998) Cancer Res. 58, 2200-2208. [PubMed] [Google Scholar]
- 26.Fidler, I. J. (2002) Differentiation 70, 498-505. [DOI] [PubMed] [Google Scholar]
- 27.Pawliuk, R., Bachelot, T., Zurkiya, O., Eriksson, A., Cao, Y. & Leboulch, P. (2002) Mol. Ther. 5, 345-351. [DOI] [PubMed] [Google Scholar]
- 28.Eisterer, W., Jiang, X., Bachelot, T., Pawluk, R., Abramovich, C., Leboulch, P., Hogge, D. & Eaves, C. (2002) Mol. Ther. 5, 352-359. [DOI] [PubMed] [Google Scholar]
- 29.Egami, K., Murohara, T., Shimada, T., Sasaki, K., Shintani, S., Sugaya, T., Ishii, M., Akagi, T., Ikeda, H., Matsuishi, T. & Imaizumi, T. (2003) J. Clin. Invest. 112, 67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pao, W., Klimstra, D. S., Fisher, G. H. & Varmus, H. E. (2003) Proc. Natl. Acad. Sci. USA 100, 8764-8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneda, J., Kuniyasu, H., Crispens, M. A., Price, J. E., Bucana, C. D. & Fidler, I. J. (1998) J. Natl. Cancer Inst. 90, 447-454. [DOI] [PubMed] [Google Scholar]
- 32.Fang, X., Schummer, M., Mao, M., Yu, S., Tabassam, F. H., Swaby, R., Hasegawa, Y., Tanyi, J. L., LaPushin, R., Eder, A., et al. (2002) Biochim. Biophys. Acta 1582, 257-264. [DOI] [PubMed] [Google Scholar]