Abstract
Nitric oxide (NO) exerts both antiatherogenic and proatherogenic effects, but the cellular and molecular mechanisms that contribute to modulation of atherosclerosis by NO are not understood completely. The cGMP-dependent protein kinase I (cGKI) is a potential mediator of NO signaling in vascular smooth muscle cells (SMCs). Postnatal ablation of cGKI selectively in the SMCs of mice reduced atherosclerotic lesion area, demonstrating that smooth muscle cGKI promotes atherogenesis. Cell-fate mapping indicated that cGKI is involved in the development of SMC-derived plaque cells. Activation of endogenous cGKI in primary aortic SMCs resulted in cells with increased levels of proliferation; increased levels of vascular cell adhesion molecule-1, peroxisome proliferator-activated receptor γ, and phosphatidylinositol 3-kinase/Akt signaling; and decreased plasminogen activator inhibitor 1 mRNA, which all are potentially proatherogenic properties. Taken together, these results highlight the pathophysiologic significance of vascular SMCs in atherogenesis and identify a key role for cGKI in the development of atherogenic SMCs in vitro and in vivo. We suggest that activation of smooth muscle cGKI contributes to the proatherogenic effect of NO and that inhibition of cGKI might be a therapeutic option for treating atherosclerosis in humans.
Atherosclerosis causes heart attack and stroke, the major causes of death in industrial nations. The pathophysiology of atherogenesis is complex. It is considered to be a chronic inflammatory condition that results from the interaction between modified lipoproteins and various cell types, including leukocytes, platelets, and cells of the vessel wall (1). The development of an atherosclerotic plaque involves, in addition to inflammation, the phenotypic modulation of vascular smooth muscle cells (SMCs) to proliferating and dedifferentiated cells. However, the mechanisms that contribute to the development of plaque SMCs and their pathophysiologic significance are not well understood (2).
The signaling molecule nitric oxide (NO) has critical roles in the pathogenesis of atherosclerosis (3). Analysis of transgenic mice that lack or overexpress NO synthases indicated that NO exerts both protective (4, 5) and atherogenic (6-9) effects. The double role of NO might explain why NO-generating drugs (e.g., glyceryl trinitrate) have not been reported to limit the progression of atherosclerosis in humans. The opposing actions of NO on atherogenesis might depend on the spatiotemporal profile of its production and are likely mediated by different cellular and molecular mechanisms. Signaling pathways in SMCs that contribute to NO modulation of atherogenesis have not been identified. In vascular SMCs, NO is thought to exert many of its effects by activation of soluble guanylyl cyclase, synthesis of the second messenger cGMP, and activation of cGMP-dependent protein kinase I (cGKI) (10). The analysis of the functional significance of cGKI in NO/cGMP signaling is complicated by the existence of multiple receptors for cGMP (11) and by the lack of highly specific agonists and inhibitors of cGKI (12, 13). Here, we have studied the consequences of postnatal SMC-specific inactivation of the cGKI gene by using a spatiotemporally controlled Cre/lox system for combined gene targeting and cell-fate mapping in mice. Our results show that smooth muscle cGKI modulates the properties of aortic SMCs in vitro and in vivo and promotes atherosclerosis.
Materials and Methods
Experimental Animals. The generation of mice carrying a conditional loxP-flanked cGKI allele (L2) or a recombined cGKI-null allele (L-) and the detection of the cGKI WT(+), L2, and L- alleles by PCR have been described (14). Mice carrying the SM-CreERT2 knock-in allele (Cre) were genotyped as described (15). Mice carrying the ROSA26 Cre reporter (R26R) (16) or ApoE-null (17) alleles were obtained from The Jackson Laboratory and genotyped according to published protocols (available at www.jax.org). Mice with modified cGKI alleles were crossed with SM-CreERT2 knock-in mice to generate “pro-mutant” mice (genotype cGKIL-/L2; SM-CreERT2(Cre/+)) and control mice (genotype cGKI+/L2; SM-CreERT2(Cre/+)). For analysis of atherosclerosis, all mice had an ApoE-/- genotype. To induce the Cre-mediated conversion of the cGKI L2 allele into the L- allele in SMCs, mice were injected i.p. with 1 mg of tamoxifen (Sigma) for 5 consecutive days (15). For experiments, litter-matched female control and pro-mutant mice on a mixed 129Sv/C57BL6 genetic background were used with the investigator unaware of the genotype of the mice. Experiments had been approved by the local government's committee on animal care and welfare in Munich.
5-Bromo-4-chloro-3-indolyl β-d-Galactoside (X-Gal) Staining and Immunohistochemistry. LacZ activity was detected by staining aortas with X-Gal as described (15). For immunohistochemistry, animals were deeply anesthetized and perfused with 10% phosphate-buffered formalin. Aortas were dissected and postfixed overnight in the same fixative, embedded in paraffin, and cut at 8-μm thickness. Sections were stained by using rabbit antisera to cGKI (18) or proliferating cell nuclear antigen (PCNA; Santa Cruz Biotechnology), goat anti-CD68 (Santa Cruz Biotechnology), and a mouse monoclonal antibody to α-smooth muscle actin (Sigma). For detection of primary antibodies, we used the avidin-biotin method with diaminobenzidine or Vector blue substrate as a chromogen (Vector Laboratories). For detection of α-smooth muscle actin, the MOM Basic immunodetection kit (Vector Laboratories) was used. Control sections were processed in the absence of primary antibodies. Stained sections were mounted in 80% glycerol containing 1 μg/ml Hoechst 33258 (Sigma) to visualize cell nuclei. To determine the percentage of cGKI- or cGKI/PCNA-positive SMCs, immunostained cells and total cells (Hoechst 33258-stained) were counted in sections from five mice (three sections per mouse).
Analysis of Atherosclerosis. Pro-mutant and control mice on an ApoE-deficient genetic background (genotype cGKIL-/L2; SMCreERT2(Cre/+); ApoE-/- and cGKI+/L2; SM-CreERT2(Cre/+); ApoE-/-, respectively) were fed an atherogenic diet (20% fat, 1.5% cholesterol by weight; Altromin, Lage, Germany) beginning at 5 weeks of age. All animals were injected with tamoxifen at 4 and 8 weeks of age. After 8 and 16 weeks on the atherogenic diet, plasma lipid profiles (Medical Service Laboratorium, Munich) and atherosclerotic lesions in the aorta were analyzed. Animals were deeply anesthetized and perfused with 10% phosphate-buffered formalin. Aortas were isolated, postfixed in the same fixative overnight, and stained with oil red O (Sigma) as described (19). Two different methods were used for quantitative estimation of lesion area. In one method, aortas were mounted between two glass slides and photographed from each side. In the other method, aortas were cut open longitudinally, pinned to Sylgard 184 (Dow-Corning), and photographed. A 5-mm scale bar was used for calibration. Images were scanned, and the oil red O-positive area in the aortic arch was determined by using uthscsa image tool, version 2.00 (University of Texas Health Science Center, San Antonio, TX). The mean lesion areas obtained by using each method were not significantly different. Blood pressure and heart rate were measured by using the tail-cuff method on conscious restrained mice.
Analysis of Primary Aortic SMCs. Aortic SMCs were isolated from WT mice and cGKI-/- mice (14) and grown in DMEM supplemented with 10% FCS as described (15). Cell numbers were determined from photomicrographs of 12-well plates by using uthscsa image tool, version 2.00. For measuring [3H]thymidine incorporation, cells were plated in 96-well plates. After 48-h incubation in the absence of serum, cells were treated with platelet-derived growth factor (PDGF)-BB and drugs for 20 h and then for 4 h with the addition of [3H]thymidine (1 μCi/ml; 1 Ci = 37 GBq). Western blot analysis was performed by using antibodies to cGKI (18), vascular cell adhesion molecule 1 (VCAM-1), peroxisome proliferator-activated receptor γ (PPAR-γ; Santa Cruz Biotechnology), p44/42 mitogen-activated protein kinase (MAPK), Akt, phospho-Akt (phosphorylated at either Thr-308 or Ser-473), and phospho-forkhead transcription factor (p-FKHR; Ser-256; Cell Signaling Technology, Beverly, MA). The level of plasminogen activator inhibitor 1 (PAI-1) mRNA was estimated by semiquantitative RT-PCR using the hypoxanthine phosphoribosyltransferase mRNA as an internal standard (20). A 525-bp fragment of the PAI-1 cDNA was amplified by using primers OK15 (5′-AGCAACAAGTTCAACTACACTGAG-3′) and OK16 (5′-AAGGCTCCATCACTTGCCCCA-3′). For quantification of signals, Western blots or images of DNA gels were scanned and analyzed by using AIDA software, version 2.11 (Raytest, Straubenhardt, Germany).
Statistics. Data are presented as mean ± SEM. Statistical analyses were performed by t test or ANOVA, followed by Newman-Keuls post hoc test. Significance was determined at P < 0.05.
Results and Discussion
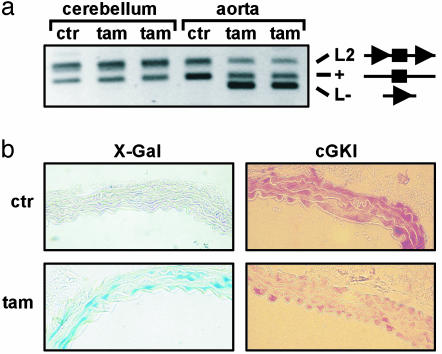
Temporally Controlled Ablation of cGKI in SMCs. Mice with a global cGKI deficiency show multiple phenotypes, and most of them die at an age of 3-6 weeks (18), precluding the analysis of atherosclerotic lesion development. To circumvent these limitations and study the specific function of cGKI in SMCs, we generated SMC-specific cGKI knockout (ko) mice by ligand-activated Cre/loxP-mediated recombination (20, 21). In mice carrying a conditional cGKI allele (L2) with two loxP sites flanking the critical exon 10 of the cGKI gene, Cre-mediated recombination of the loxP sites results in the excision of exon 10 and thus in a cGKI-null allele (L-) (14). Conversion of the cGKI L2 into the L- allele takes place only in cells expressing active Cre recombinase. We generated mice carrying the cGKI L2 allele as well as the SM-CreERT2 knock-in allele (15), which expresses the tamoxifen-dependent CreERT2 recombinase under the control of the endogenous SMC-specific SM22 gene promoter. Treatment of these pro-mutant mice with tamoxifen activates the CreERT2 recombinase and results in ablation of cGKI selectively in SMCs. By PCR analysis of genomic DNA isolated from various tissues, the recombined cGKI L- allele was undetectable in vehicle-treated mice, whereas it was detected in the aortas of tamoxifen-treated mice (Fig. 1a). Recombination was undetectable in the cerebellum and other non-smooth muscle tissues of tamoxifen-treated mice (Fig. 1a and data not shown). LacZ staining of aortic sections from mice carrying the R26R allele (16) showed tamoxifen-inducible recombination in aortic SMCs (Fig. 1b Left). Importantly, immunohistochemistry demonstrated that the cGKI protein was ablated in ≈55% of aortic SMCs within 3 weeks after tamoxifen treatment (Fig. 1b Right).
Fig. 1.
Tamoxifen-induced ablation of cGKI in aortic SMCs. Four-week-old mice were injected with either vehicle (ctr) or tamoxifen (tam) for 5 consecutive days and then analyzed at an age of 8 weeks. (a) PCR analysis of Cre-mediated recombination in tissues from cGKI+/L2; SM-CreERT2(Cre/+) mice. PCR products amplified from the cGKI L2, WT (+), and L- alleles are indicated. In the corresponding diagrams, exon 10 of the cGKI (▪) and loxP (▸) sites are indicated. (b) Detection of β-galactosidase activity by X-Gal (Left) and immunohistochemical staining of cGKI (Right) on aortic sections from R26R+/-; SM-CreERT2(Cre/+) mice and from cGKIL-/L2; SM-CreERT2(Cre/+) mice, respectively. (Original magnification of photomicrographs was ×400.)
Postnatal SMC-Specific Ablation of cGKI Attenuates Atherosclerosis. To study the role of cGKI in atherogenesis, we induced the SMC-specific cGKI ko in ApoE-/- mice that develop complex atherosclerotic lesions similar to those in humans (17). Tamoxifen-treated SMC-specific cGKI ko mice (cGKIsmko mice; genotype cGKIL-/L2; SM-CreERT2(Cre/+); ApoE-/-) and tamoxifen-treated control mice (genotype cGKI+/L2; SM-CreERT2(Cre/+); ApoE-/-) were fed an atherogenic diet for 8 or 16 weeks. Plasma lipid levels, body weight, heart-to-body weight and kidney-to-body weight ratios, blood pressure, and heart rate were not significantly different between cGKIsmko and control mice after 8 (data not shown) and 16 (Table 1) weeks on the atherogenic diet. Thus, ablation of cGKI in SMCs did not alter basal physiological parameters that could affect atherosclerotic lesion development (1, 4).
Table 1. Physiological parameters of mice after 16 weeks on an atherogenic diet.
| Parameter | Control | cGKIsmko |
|---|---|---|
| (n = 7) | (n = 5) | |
| Total cholesterol, mg/dl | 459 ± 41 | 540 ± 61 |
| Triglyceride, mg/dl | 84 ± 12 | 88 ± 4 |
| HDL, mg/dl | 92 ± 10 | 91 ± 10 |
| LDL, mg/dl | 350 ± 34 | 432 ± 56 |
| (n = 15) | (n = 11) | |
| Body weight, g | 25.1 ± 0.5 | 24.9 ± 0.8 |
| Heart-to-body weight, mg/g | 4.0 ± 0.1 | 4.0 ± 0.2 |
| Kidney-to-body weight, mg/g | 5.4 ± 0.2 | 5.6 ± 0.2 |
| (n = 9) | (n = 6) | |
| Mean blood pressure, mmHg | 81 ± 2 | 86 ± 3 |
| Heart rate, beats per minute | 633 ± 17 | 662 ± 16 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
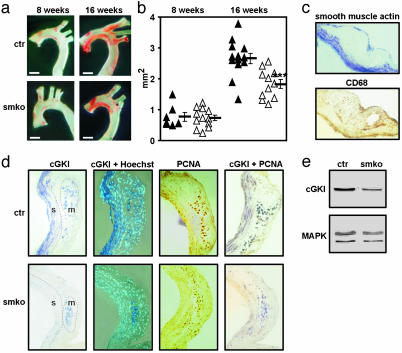
After 8 weeks on the atherogenic diet, the mean atherosclerotic lesion area in the aortic arch was similar in cGKIsmko and control mice. However, after 16 weeks on the atherogenic diet, atherosclerosis was significantly attenuated in cGKIsmko mice compared with control mice (Fig. 2 a and b). The mean lesion areas in the aortic arch of cGKIsmko and control mice were 1.81 ± 0.14 mm2 and 2.63 ± 0.16 mm2, respectively (P < 0.001). The mean lesion areas in the brachiocephalic artery, left carotid artery, and left subclavian artery of cGKIsmko and control mice were 0.77 ± 0.09 mm2 and 1.28 ± 0.09 mm2, respectively (P < 0.01). Thus, mosaic inactivation of the cGKI gene in ≈55% of the aortic SMCs (see Fig. 1) reduced the atherosclerotic lesion area by ≈40%. Immunohistochemistry (Fig. 2d Left) and Western blot analysis (Fig. 2e) confirmed the reduction of cGKI-positive SMCs in atherosclerotic lesions of cGKIsmko mice. Expression of cGKI was detected in 24 ± 3% and 55 ± 3% of the SMCs in lesions from cGKIsmko and control mice, respectively. In contrast, cGKI expression in plaque macrophages was not reduced in cGKIsmko mice (Fig. 2d Left). Interestingly, the proliferation marker PCNA was expressed in ≈75% of the nuclei of cGKI-positive SMCs independent of the genotype of the mice (Fig. 2d Right). These results indicated that smooth muscle cGKI promotes the development of atherosclerotic lesions, perhaps by the modulation of the SMC phenotype in plaques.
Fig. 2.
SMC-specific ablation of cGKI attenuates atherosclerosis. (a) Photomicrographs of oil red O-stained atherosclerotic lesions in the aortic arch of control (ctr, Upper) and cGKIsmko (smko, Lower) mice after 8 (Left)or16(Right) weeks on the atherogenic diet. (Bars indicate 1 mm.) (b) Lesion areas in the aortic arch of ctr (▴) and smko (▵) mice after 8 or 16 weeks on the atherogenic diet. Horizontal bars indicate the corresponding mean lesion area for each group of animals (***, P < 0.001 vs. control). Lesion areas were obtained from oil red O-stained aortas mounted between two glass slides. (c) Immunostaining of an atherosclerotic plaque for α-smooth muscle actin (blue) to detect SMCs (Upper) and for CD68 (brown) to detect macrophages (Lower). Sections were from a control mouse after 16 weeks on the atherogenic diet. (d) Immunohistochemical analysis of atherosclerotic plaques in ctr (Upper) and smko (Lower) mice after 16 weeks on the atherogenic diet. Serial sections were stained for cGKI (blue), PCNA (brown), or cGKI and PCNA (purple). Cell nuclei were visualized with the fluorescent dye Hoechst 33258. SMCs (s) and macrophages (m) were identified by staining with antibodies to α-smooth muscle actin and CD68, respectively (data not shown). The corresponding regions are indicated in Left. (Original magnification of photomicrographs was ×200.) (e) Western blot analysis of cGKI expression in extracts from atherosclerotic aortic arches of ctr and smko mice after 16 weeks on the atherogenic diet. Loading of gels was controlled by staining for MAPK.
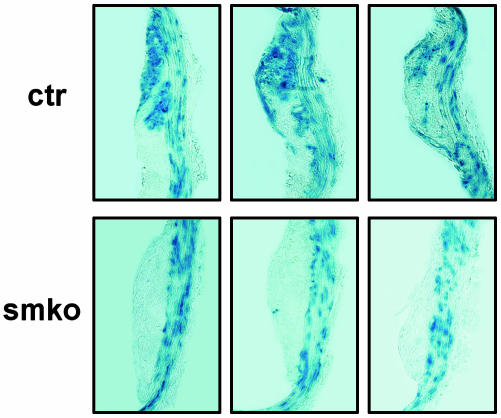
cGKI Contributes to the Development of SMC-Derived Plaque Cells. To determine the effect of cGKI deficiency on the properties of plaque SMCs, the fate of SMCs during the development of atherosclerotic lesions was followed in control and cGKIsmko mice. Arterial SMCs were genetically marked by tamoxifen-induced recombination of the R26R allele and subsequent activation of LacZ expression in control mice (genotype cGKI+/L2; SM-CreERT2 (Cre/+); ApoE-/-; R26R+/-) and in cGKIsmko mice (genotype cGKIL-/L2; SM-CreERT2(Cre/+); ApoE-/-; R26R+/-). After 16 weeks on the atherogenic diet, plaques were stained with X-Gal for LacZ activity. Thus, blue-stained cells in the lesions of control and cGKIsmko mice should have been derived from WT and cGKI-deficient SMCs, respectively. In control mice, blue-stained cells were detected both in the media and inside the atherosclerotic plaques (Fig. 3). In contrast, blue-stained cells were almost exclusively detected in the media but not inside the lesions of cGKIsmko mice (Fig. 3). The ratio of lesional cells to medial SMCs was reduced by ≈32% in cGKIsmko mice compared with control mice (0.63 ± 0.11 vs. 0.92 ± 0.10). These results indicated that cGKI is critically involved in the development of SMC-derived plaque cells during atherogenesis. Thus, the reduced oil red O-positive area in cGKIsmko mice (Fig. 2 a and b) can be attributed, at least in part, to a reduced number of SMC-derived foam cells. If cGKI deficiency results in a cell-autonomous defect in SMCs and a selective loss of SMC-derived plaque cells, then the relative content of plaque macrophages should be increased in cGKIsmko mice. However, the ratio of plaque macrophages to total lesional cells was not significantly different between cGKIsmko and control mice (0.57 ± 0.03 vs. 0.49 ± 0.05). Taking these results together, it appears that loss of cGKI in SMCs not only results in decreased development of SMC-derived plaque cells but also affects paracrine mechanisms that lead to reduced recruitment and/or proliferation of plaque macrophages in cGKIsmko mice.
Fig. 3.
cGKI is involved in the development of SMC-derived plaque cells. Serial sections of atherosclerotic plaques in control (ctr, Upper) and cGKIsmko (smko, Lower) mice were stained with X-Gal. All mice carried the R26R allele and were injected with tamoxifen at 4 weeks of age. The atherogenic diet was started at 5 weeks of age, followed by a second tamoxifen injection at 8 weeks of age. After 16 weeks on the atherogenic diet, the fate of the SMCs that had been genetically labeled by tamoxifen-induced recombination of the R26R allele and subsequent activation of LacZ expression was analyzed by X-Gal staining. Blue-stained cells represent SMC-derived cells. (Original magnification of photomicrographs was ×200.)
Activation of cGKI in Primary Aortic SMCs Stimulates Phenotypic Modulation and Phosphatidylinositol 3-Kinase (PI3K)/Akt Signaling and Suppresses PAI-1 Expression. The in vivo analysis of atherosclerosis and fate-mapping experiments strongly suggested that activation of cGKI in vascular SMCs promotes the phenotypic modulation of medial SMCs to proliferating and dedifferentiated cells that are involved in lesion development. The role of cGKI in SMC growth and dedifferentiation was analyzed further by using primary aortic SMCs from both WT and conventional cGKI ko mice (14, 18). When cultivated in vitro, SMCs convert from a contractile and differentiated state to a synthetic and dedifferentiated state. This phenotypic modulation is associated with the activation of cell proliferation and a characteristic morphological change to elongated cells with a “hill-and-valley” growth pattern (22). Similar changes might occur in vivo during the formation of atherosclerotic lesions (2).
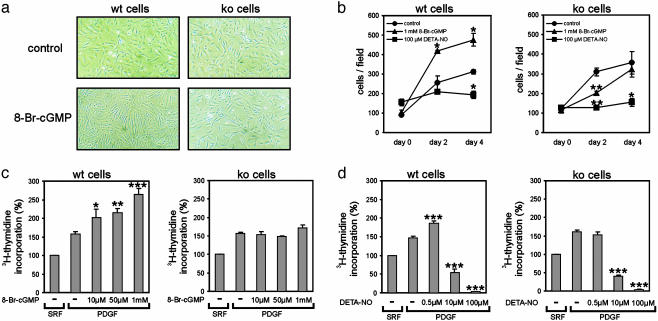
The membrane-permeable cGMP analogue 8-Br-cGMP (1 mM), which activates endogenous cGKI in primary aortic SMCs (23), stimulated the growth and morphological change of WT SMCs and slightly inhibited the growth of cGKI-deficient cells without an effect on their morphology (Fig. 4 a and b). In contrast to cGMP, the NO-generating compound diethylenetriamine NONOate {DETA-NO; (z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate} (Qbiogene-Alexis, Grünberg, Germany) (100 μM) inhibited the proliferation of both WT and ko cells (Fig. 4b). Likewise, DNA synthesis induced by PDGF was dose-dependently enhanced by 8-Br-cGMP in WT but not in ko cells (Fig. 4c). Interestingly, PDGF-stimulated DNA synthesis was increased also by a low concentration of DETA-NO (0.5 μM) in WT but not in ko cells, whereas it was strongly inhibited by higher concentrations of DETA-NO (10 μM and 100 μM) in both WT and ko cells (Fig. 4d). Together, these results indicated that (i) activation of cGKI promotes the growth of primary aortic SMCs, (ii) low concentrations of NO can exert a proliferative effect by activation of cGKI, and (iii) higher concentrations of NO inhibit SMC growth by a cGKI-independent mechanism. These results are in line with previous studies that showed that NO (24-26) as well as cGMP (24) can stimulate SMC proliferation and that the anti-proliferative effect of NO is not mediated by cGKI (27). Thus, it is tempting to speculate that activation of cGKI is involved in a variety of vasculoproliferative processes under pathological conditions. This view is supported by the recent finding that ischemia-induced angiogenesis is impaired in cGKI-deficient mice (28). Others reported that cGMP and cGKI inhibit SMC proliferation in vitro (for reviews, see refs. 29 and 30). Some of the previous studies used subcultured (repeatedly passaged) SMCs and/or pharmacological cGKI inhibitors of uncertain efficiency (13). In contrast, we used primary cells and a genetic rather than pharmacological approach to analyze the functional relevance of cGKI. Subculture of SMCs might result in down-regulation of cGKI expression (31) and/or alterations in other signaling components and proliferative responses. In this respect, it is interesting that 8-Br-cGMP slightly inhibited the growth of cGKI-deficient SMCs (Fig. 4b). This cGKI-independent effect of 8-Br-cGMP might be mediated by cross-activation of cAMP-dependent protein kinase (32) and could explain the reported growth-inhibitory action of this drug in subcultured SMCs that do not contain cGKI or express the enzyme at a low level.
Fig. 4.
Activation of cGKI promotes aortic SMC growth. Primary aortic SMCs were isolated from WT (wt) or conventional cGKI ko mice. (a) Photomicrographs of cells grown in serum-containing medium in the absence (control) or presence (8-Br-cGMP) of 1 mM 8-Br-cGMP for 96 h. (Original magnification was ×100.) (b) Cells were grown in serum-containing medium in the absence (control; •) and presence of 1 mM 8-Br-cGMP (▴) or 100 μM DETA-NO (▪). Cells were counted shortly before (day 0), 48 h after (day 2), and 96 h after (day 4) the addition of drugs (*, P < 0.05; **, P < 0.01 vs. control). (c and d) DNA synthesis in WT (wt) and ko cells grown in serum-free medium (SRF) and then stimulated with 10 ng/ml PDGF-BB (PDGF) in the absence (-) or presence of the indicated concentrations of 8-Br-cGMP (c) or DETA-NO (d) for 24 h. [3H]Thmidine was added for the last 4 h (*, P < 0.05; **, P < 0.01; ***, P < 0.001, vs. PDGF alone).
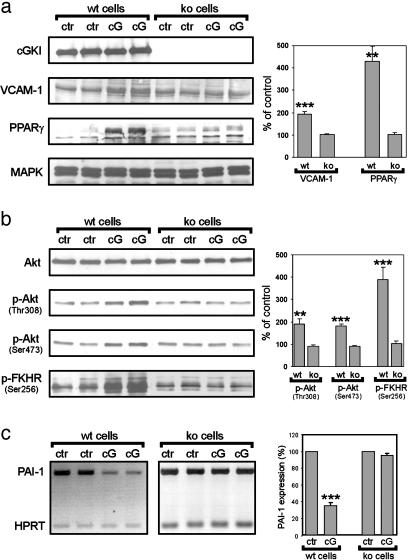
Western blot analysis (Fig. 5a) showed that activation of cGKI was associated with increased expression of proteins that have been implicated as markers for dedifferentiated SMCs, VCAM-1 (33, 34) and PPAR-γ (35, 36). Furthermore, cGKI signaling stimulated the PI3K/Akt pathway, as revealed by increased levels of its components phospho-Akt and p-FKHR (Fig. 5b). These results are consistent with the concept that NO/cGMP activates the PI3K/Akt pathway (37-40), which promotes PPAR-γ expression (41) and SMC proliferation (42, 43). Although alternative pathways cannot be excluded, these previous results and our present results strongly suggest that an interaction between cGKI and PI3K/Akt signaling promotes the phenotypic modulation of vascular SMCs. It is important to note that the cGKI-mediated increase in phospho-Akt and p-FKHR was detected in SMCs treated with 8-Br-cGMP for 6 days (Fig. 5b) but not in cells treated for 10-60 min (data not shown). Thus, increased Akt signaling is not a rapid direct effect of cGKI activation but might reflect the change in cell phenotype that develops over time.
Fig. 5.
Activation of cGKI modulates gene expression and PI3K/Akt signaling in aortic SMCs. Primary aortic SMCs were isolated from WT (wt) mice or conventional cGKI ko mice. (a and b) Cells were grown in serum-containing medium for 6 days in the absence (control, ctr) or presence of 1 mM 8-Br-cGMP (cG) and were then analyzed by Western blot analysis. (a) Detection of cGKI and the dedifferentiation-marker proteins VCAM-1 and PPAR-γ. Staining for MAPK indicated equal loading of gels. (b) Detection of Akt, phospho-Akt (p-Akt, phosphorylated at either Thr-308 or Ser-473), and p-FKHR (Ser-256). Histograms on the right show the quantitative analysis of Western blot signals from cGMP-treated cells that were normalized to signals under control conditions (**, P < 0.01; ***, P < 0.001, vs. ko). Data were obtained from three independent cell preparations, each of which was analyzed in duplicate (n = 6). (c) Cells grown in serum-free medium for 48 h were incubated in the absence (ctr) or presence (cG) of 1 mM 8-Br-cGMP for 12 h. RNA was isolated and analyzed by semiquantitative RT-PCR. PCR products corresponding to the PAI-1 mRNA and to the hypoxanthine phosphoribosyltransferase (HPRT) mRNA used as an internal control (coamplified in the same reaction) are indicated. The histogram on the right shows the quantitative analysis of the level of PAI-1 mRNA (***, P < 0.001 vs. ko). Data were obtained from four independent cell preparations, each of which was analyzed in duplicate (n = 8).
The analysis of plaque composition in cGKIsmko and control mice (see above) suggested that cGKI may also regulate factors that are secreted by SMCs and affect matrix remodeling as well as the recruitment and proliferation of other plaque cells, including macrophages. It has been shown that NO and cGMP suppress the expression of PAI-1 (44), which is secreted by SMCs and limits plaque growth and abnormal matrix remodeling (45). Indeed, semiquantitative RT-PCR (Fig. 5c) and Northern blot analysis (data not shown) showed that activation of cGKI suppresses the level of PAI-1 mRNA in primary aortic SMCs.
This study supports the notion that spatiotemporally controlled genetic engineering in mice is a powerful tool to study the consequences of a defined somatic gene mutation during postnatal life. Using this technique, we showed that postnatal ablation of cGKI selectively in murine SMCs attenuates the development of SMC-derived plaque cells and atherosclerotic lesions, demonstrating a proatherogenic role for smooth muscle cGKI. Activation of endogenous cGKI in primary aortic SMCs led to cells with increased levels of proliferation; increased levels of VCAM-1, PPAR-γ, and PI3K/Akt signaling; and decreased levels of PAI-1 mRNA, which all are potentially proatherogenic properties. These results suggest that cGMP/cGKI signaling promotes the development of SMCs with increased atherogenic potential in vitro and in vivo. However, the cGKI substrate protein(s) and the molecular mechanism(s) that are involved in cGKI-mediated phenotypic modulation are presently unknown. Our finding that smooth muscle cGKI promotes atherosclerosis and the recent demonstration that cGKI stimulates platelet activation (46) raise concerns that, in addition to their short-term beneficial effects, cGMP-elevating drugs may have undesired long-term effects that could perhaps even exacerbate atherosclerosis and its complications. Indeed, it has been reported that long-term nitrate therapy in chronic coronary artery disease is associated with a significantly increased mortality risk (47). Taken together with these reports, the present study proposes that cGMP/cGKI signaling contributes to the proatherogenic effect of NO and that inhibition of cGKI might be a novel therapeutic option to treat atherosclerosis in humans.
Acknowledgments
We thank Pascal Weinmeister for technical help and Carsten Wotjak for advice in statistical evaluation. This work was supported by grants from the Deutsche Forschungsgemeinschaft, VolkswagenStiftung, and Fonds der Chemischen Industrie.
Abbreviations: cGKI, cGMP-dependent protein kinase I; DETA-NO, diethylenetriamine NONOate; ko, knockout; MAPK, mitogen-activated protein kinase; p-FKHR, phospho-forkhead transcription factor; PAI-1, plasminogen activator inhibitor 1; PCNA, proliferating cell nuclear antigen; PDGF, platelet-derived growth factor; PI3K, phosphatidylinositol 3-kinase; PPAR-γ, peroxisome proliferator-activated receptor γ; SMC, smooth muscle cell; VCAM-1, vascular cell adhesion molecule 1; X-Gal, 5-bromo-4-chloro-3-indolylβ-d-galactoside.
References
- 1.Ross, R. (1999) N. Engl. J. Med. 340, 115-126. [DOI] [PubMed] [Google Scholar]
- 2.Dzau, V. J., Braun-Dullaeus, R. C. & Sedding, D. G. (2002) Nat. Med. 8, 1249-1256. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones, D. M. & Bloch, K. D. (1996) Annu. Rev. Med. 47, 365-375. [DOI] [PubMed] [Google Scholar]
- 4.Knowles, J. W., Reddick, R. L., Jennette, J. C., Shesely, E. G., Smithies, O. & Maeda, N. (2000) J. Clin. Invest. 105, 451-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhlencordt, P. J., Gyurko, R., Han, F., Scherrer-Crosbie, M., Aretz, T. H., Hajjar, R., Picard, M. H. & Huang, P. L. (2001) Circulation 104, 448-454. [DOI] [PubMed] [Google Scholar]
- 6.Shi, W., Wang, X., Shih, D. M., Laubach, V. E., Navab, M. & Lusis, A. J. (2002) Circulation 105, 2078-2082. [DOI] [PubMed] [Google Scholar]
- 7.Ozaki, M., Kawashima, S., Yamashita, T., Hirase, T., Namiki, M., Inoue, N., Hirata, K., Yasui, H., Sakurai, H., Yoshida, Y., et al. (2002) J. Clin. Invest. 110, 331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlencordt, P. J., Chen, J., Han, F., Astern, J. & Huang, P. L. (2001) Circulation 103, 3099-3104. [DOI] [PubMed] [Google Scholar]
- 9.Detmers, P. A., Hernandez, M., Mudgett, J., Hassing, H., Burton, C., Mundt, S., Chun, S., Fletcher, D., Card, D. J., Lisnock, J., et al. (2000) J. Immunol. 165, 3430-3435. [DOI] [PubMed] [Google Scholar]
- 10.Pfeifer, A., Ruth, P., Dostmann, W., Sausbier, M., Klatt, P. & Hofmann, F. (1999) Rev. Physiol. Biochem. Pharmacol. 135, 105-149. [DOI] [PubMed] [Google Scholar]
- 11.Beavo, J. A. & Brunton, L. L. (2002) Nat. Rev. Mol. Cell Biol. 3, 710-718. [DOI] [PubMed] [Google Scholar]
- 12.Schwede, F., Maronde, E., Genieser, H. & Jastorff, B. (2000) Pharmacol. Ther. 87, 199-226. [DOI] [PubMed] [Google Scholar]
- 13.Burkhardt, M., Glazova, M., Gambaryan, S., Vollkommer, T., Butt, E., Bader, B., Heermeier, K., Lincoln, T. M., Walter, U. & Palmetshofer, A. (2000) J. Biol. Chem. 275, 33536-33541. [DOI] [PubMed] [Google Scholar]
- 14.Wegener, J. W., Nawrath, H., Wolfsgruber, W., Kuhbandner, S., Werner, C., Hofmann, F. & Feil, R. (2002) Circ. Res. 90, 18-20. [DOI] [PubMed] [Google Scholar]
- 15.Kuhbandner, S., Brummer, S., Metzger, D., Chambon, P., Hofmann, F. & Feil, R. (2000) Genesis 28, 15-22. [DOI] [PubMed] [Google Scholar]
- 16.Soriano, P. (1999) Nat. Genet. 21, 70-71. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, S. H., Reddick, R. L., Piedrahita, J. A. & Maeda, N. (1992) Science 258, 468-471. [DOI] [PubMed] [Google Scholar]
- 18.Pfeifer, A., Klatt, P., Massberg, S., Ny, L., Sausbier, M., Hirneiss, C., Wang, G. X., Korth, M., Aszodi, A., Andersson, K. E., et al. (1998) EMBO J. 17, 3045-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guevara, N. V., Kim, H. S., Antonova, E. I. & Chan, L. (1999) Nat. Med. 5, 335-339. [DOI] [PubMed] [Google Scholar]
- 20.Feil, R., Brocard, J., Mascrez, B., LeMeur, M., Metzger, D. & Chambon, P. (1996) Proc. Natl. Acad. Sci. USA 93, 10887-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzger, D. & Feil, R. (1999) Curr. Opin. Biotechnol. 10, 470-476. [DOI] [PubMed] [Google Scholar]
- 22.Chamley-Campbell, J., Campbell, G. R. & Ross, R. (1979) Physiol. Rev. 59, 1-61. [DOI] [PubMed] [Google Scholar]
- 23.Feil, R., Gappa, N., Rutz, M., Schlossmann, J., Rose, C. R., Konnerth, A., Brummer, S., Kuhbandner, S. & Hofmann, F. (2002) Circ. Res. 90, 1080-1086. [DOI] [PubMed] [Google Scholar]
- 24.Hassid, A., Arabshahi, H., Bourcier, T., Dhaunsi, G. S. & Matthews, C. (1994) Am. J. Physiol. 267, H1040-H1048. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan, T. R., Li, D., Laubach, V. E., Shesely, E. G., Zhou, N. & Johns, R. A. (2000) Am. J. Physiol. 279, L641-L650. [DOI] [PubMed] [Google Scholar]
- 26.Thomae, K. R., Nakayama, D. K., Billiar, T. R., Simmons, R. L., Pitt, B. R. & Davies, P. (1995) J. Surg. Res. 59, 337-343. [DOI] [PubMed] [Google Scholar]
- 27.Ignarro, L. J., Buga, G. M., Wei, L. H., Bauer, P. M., Wu, G. & del Soldato, P. (2001) Proc. Natl. Acad. Sci. USA 98, 4202-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamahara, K., Itoh, H., Chun, T. H., Ogawa, Y., Yamashita, J., Sawada, N., Fukunaga, Y., Sone, M., Yurugi-Kobayashi, T., Miyashita, K., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 3404-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lincoln, T. M., Dey, N. & Sellak, H. (2001) J. Appl. Physiol. 91, 1421-1430. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar, R. & Webb, R. C. (1998) J. Vasc. Res. 35, 135-142. [DOI] [PubMed] [Google Scholar]
- 31.Cornwell, T. L., Soff, G. A., Traynor, A. E. & Lincoln, T. M. (1994) J. Vasc. Res. 31, 330-337. [DOI] [PubMed] [Google Scholar]
- 32.Cornwell, T. L., Arnold, E., Boerth, N. J. & Lincoln, T. M. (1994) Am. J. Physiol. 267, C1405-C1413. [DOI] [PubMed] [Google Scholar]
- 33.Cuff, C. A., Kothapalli, D., Azonobi, I., Chun, S., Zhang, Y., Belkin, R., Yeh, C., Secreto, A., Assoian, R. K., Rader, D. J. & Pure, E. (2001) J. Clin. Invest. 108, 1031-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun, M., Pietsch, P., Schror, K., Baumann, G. & Felix, S. B. (1999) Cardiovasc. Res. 41, 395-401. [DOI] [PubMed] [Google Scholar]
- 35.Bishop-Bailey, D., Hla, T. & Warner, T. D. (2002) Circ. Res. 91, 210-217. [DOI] [PubMed] [Google Scholar]
- 36.Law, R. E., Goetze, S., Xi, X. P., Jackson, S., Kawano, Y., Demer, L., Fishbein, M. C., Meehan, W. P. & Hsueh, W. A. (2000) Circulation 101, 1311-1318. [DOI] [PubMed] [Google Scholar]
- 37.Begum, N., Sandu, O. A., Ito, M., Lohmann, S. M. & Smolenski, A. (2002) J. Biol. Chem. 277, 6214-6222. [DOI] [PubMed] [Google Scholar]
- 38.Ciani, E., Virgili, M. & Contestabile, A. (2002) J. Neurochem. 81, 218-228. [DOI] [PubMed] [Google Scholar]
- 39.Falcone, S., Mauro, L., de Rose, G., Paolucci, C., Sciorati, C., Ando, S. & Clementi, E. (2002) Biochem. J. 366, 165-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kook, H., Itoh, H., Choi, B. S., Sawada, N., Doi, K., Hwang, T. J., Kim, K. K., Arai, H., Baik, Y. H. & Nakao, K. (2003) Am. J. Physiol. 284, H1388-H1397. [DOI] [PubMed] [Google Scholar]
- 41.Fu, M., Zhu, X., Wang, Q., Zhang, J., Song, Q., Zheng, H., Ogawa, W., Du, J. & Chen, Y. E. (2001) Circ. Res. 89, 1058-1064. [DOI] [PubMed] [Google Scholar]
- 42.Duan, C., Bauchat, J. R. & Hsieh, T. (2000) Circ. Res. 86, 15-23. [DOI] [PubMed] [Google Scholar]
- 43.Jung, F., Haendeler, J., Goebel, C., Zeiher, A. M. & Dimmeler, S. (2000) Cardiovasc. Res. 48, 148-157. [DOI] [PubMed] [Google Scholar]
- 44.Bouchie, J. L., Hansen, H. & Feener, E. P. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 1771-1779. [DOI] [PubMed] [Google Scholar]
- 45.Luttun, A., Lupu, F., Storkebaum, E., Hoylaerts, M. F., Moons, L., Crawley, J., Bono, F., Poole, A. R., Tipping, P., Herbert, J. M., et al. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 499-505. [DOI] [PubMed] [Google Scholar]
- 46.Li, Z., Xi, X., Gu, M., Feil, R., Ye, R. D., Eigenthaler, M., Hofmann, F. & Du, X. (2003) Cell 112, 77-86. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura, Y., Moss, A. J., Brown, M. W., Kinoshita, M. & Kawai, C. (1999) Am. Heart J. 138, 577-585. [DOI] [PubMed] [Google Scholar]