Abstract
Mouse aortic smooth muscle cells (SMCs) were loaded for 72 h with cholesterol by using cholesterol:methyl-β-cyclodextrin complexes, leading to ≈2-fold and ≈10-fold increases in the contents of total cholesterol and cholesteryl ester, respectively. Foam-cell formation was demonstrated by accumulation of intracellular, Oil Red O-stained lipid droplets. Immunostaining showed decreased protein levels of smooth muscle α-actin and α-tropomyosin and increased levels of macrophage markers CD68 and Mac-2 antigen. Quantitative real-time RT-PCR revealed that after cholesterol loading, the expression of SMC-related genes α-actin, α-tropomyosin, myosin heavy chain, and calponin H1 decreased (to 11.5 ± 0.5%, 29.3 ± 1.4%, 23.8 ± 1.4%, and 3.8 ± 0.5% of unloaded cells, respectively; P < 0.05 for all), whereas expression of macrophage-related genes CD68, Mac-2, and ABCA1 mRNA increased (to 709 ± 84%, 330 ± 11%, and 207 ± 13% of unloaded cells, respectively; P < 0.05 for all), thereby demonstrating that the protein changes were regulated at the mRNA level. Furthermore, these changes were accompanied by a gain in macrophage-like function as assessed by phagocytotic activity. Expression of vascular cell adhesion molecule 1 and monocyte chemoattractant protein 1, known responders to inflammation, were not changed. In conclusion, cholesterol loading of SMC causes phenotypic changes regulated at the mRNA level that result in a transdifferentiation to a macrophage-like state. This finding suggests that not all foam cells in lesions may have a macrophage origin, despite what is indicated by immunostaining for macrophage-related markers. Furthermore, inflammatory changes in foam cells observed in vivo may not be simple consequences of cholesterol accumulation.
Keywords: foam cell, cyclodextrin, gene expression, cholesteryl, ester
The accumulation in the arterial wall of cholesterol and cholesteryl esters (CE) within macrophages, resulting in a “foamy” histopathological appearance, is a hallmark of atherosclerosis (1). The identification of foam cells as macrophages is typically confirmed histochemically by positive immunostaining with macrophage markers. When recruitment and entry of monocytes/macrophages were disrupted by targeted or natural gene mutations (2-6) in mouse models susceptible to atherosclerosis, however, although reduced, the persistence of foam cells that stained positively with macrophage-specific markers was significant. Although the presence of such cells could be due to redundancy of monocyte/macrophage recruitment or retention factors, it is also possible that some foam cells may have originated from other cell types, in particular, smooth muscle cells (SMCs).
In support of this notion, studies (e.g., ref. 7) have shown that some lesional foam cells have morphologic characteristics of SMCs, although these are typically found in advanced lesions. Because SMCs maintained in tissue culture can be induced to accumulate CE (8-10), we undertook an in vitro evaluation of the early phenotypic changes at the molecular level as the cholesterol and CE content of SMCs increased. Toward this goal, mouse SMCs were loaded with free cholesterol (FC) complexed to methyl-β-cyclodextrin, which are water-soluble, cyclic polysaccharides that can enhance the solubility of hydrophobic compounds, such as cholesterol (11). Within 48-72 h, as CE accumulated, the cells assumed the typical appearance of foam cells. Strikingly, at the protein and mRNA levels, this relatively rapid phenotypic change was associated with a decline and increase, respectively, in the expression of SMC- and macrophage-related genes. Furthermore, the changes in protein and gene expression were associated with the acquisition of macrophage-like functional properties, as assessed by phagocytotic activity. These results imply that the phenotype of SMCs in vivo may be similarly “plastic” and that cells of SMC origin that become loaded with cholesterol may constitute a significant subpopulation of neointimal macrophage marker-positive foam cells, even in early lesions.
Materials and Methods
Cell Culture. Mouse aortic SMCs were obtained from thoracic aortas of 8-week-old C57BL/6 mice (The Jackson Laboratory). Adventitia and endothelium were removed after digestion of the aortic segments with collagenase type II (Worthington; 175 units/ml). The media were further digested with a mixture containing collagenase type II (175 units/ml) and elastase (Sigma; 0.5 mg/ml), which yielded ≈100,000 cells per aorta. Cells were grown in DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin, and incubated at 37°C with 5% CO2/95% air. SMC lineage was confirmed by the presence of immunoreactivity for α-actin (Sigma) in >99% of the cells. All the following experiments involving SMCs were performed by using cells with a passage number ≤5. The mouse cell lines, Lewis lung carcinoma (LLC; originally from a C57BL/6 mouse) and NIH/3T3 fibroblasts, were kindly provided by A. Hidalgo (Mount Sinai School of Medicine, New York) and maintained in the same culture condition as SMCs.
Cell Metabolic Activity Assay. Cell metabolic activity, a standard index of cytotoxicity, was determined as described (12). Subconfluent (90-95% confluent) SMCs were treated for 72 h with 5, 10, and 20 μg/ml cholesterol:methyl-β-cyclodextrin complexes (Chol:MβCD; see below) in 0.2% BSA. CellTiter 96 Aqueous One Solution Reagent (Promega) was then added and incubated with the cells for 1 h. The reagent was converted by metabolically active cells into a colored formazan product measurable at 490 nm. Cytotoxicity is inversely related to the relative metabolic activity (i.e., formazan production).
Cholesterol Loading. Cholesterol was delivered to cells by using Chol:MβCD complex purchased from Sigma as “water-soluble cholesterol” (catalog no. C4951) containing ≈50 mg of cholesterol/g solid (molar ratio, 1:6 cholesterol/MβCD). All treatment concentrations involving Chol:MβCD were based on cholesterol weight.
Subconfluent SMCs were incubated with Chol:MβCD (10 μg/ml) in 0.2% BSA for 72 h. Subconfluent LLC cells and NIH/3T3 fibroblasts were incubated with a lower concentration of Chol:MβCD (5 μg/ml), because they were more susceptible to the toxicity of cholesterol loading. Cells incubated with 0.2% BSA for 72 h without Chol:MβCD treatment served as controls for all experiments. To determine whether the changes in gene expression were regulated by the cellular content of FC or EC, in some experiments cells were preincubated overnight with the acyl-CoA:cholesterol acyltransferase (ACAT) inhibitor (13, 14) F-1394 (1 μM), and then incubated with Chol:MβCD in the presence of F-1394. In some experiments, to test the effects of tumor necrosis factor α (TNF-α) on the stimulation of monocyte chemoattractant protein 1 (MCP-1) and vascular cell adhesion molecule 1 (VCAM-1) expression, control or cholesterol-loaded cells were treated with 30 ng/ml TNF-α (Roche Applied Science) for 2 h (Susan Idel, personal communication) after cholesterol loading.
Cellular Cholesterol and Protein Determinations. Cellular lipids were extracted by using a hexane/isopropyl alcohol (3:2) mixture, followed by cellular protein extraction with 0.2 N NaOH as described (15). Total cholesterol and FC were determined by using kits from Roche Applied Science. CE content was determined by subtracting FC from total cholesterol. Cellular protein content was determined based on a Lowry assay by using a Sigma kit.
Histo- and Immunohistochemistry. After treatment, mouse SMCs in Lab-Tek chamber slides (Nalge Nunc) were fixed in 4% paraformaldehyde in PBS and stained with Oil Red O (for neutral lipids), CD68 (macrophage marker), or smooth muscle α-actin, as described (16). In addition, immunostaining was also performed with monoclonal antibodies directed toward either the SMC marker, α-tropomyosin (Sigma; dilution, 1:500,000) or the macrophage marker, Mac-2 (Cedarlane, ON, Canada; dilution, 1:1,000).
For the purposes of this report, cells that, by means of light microscopy, had an obvious increase in droplets stained by Oil Red O were deemed foam cells.
Quantitative Real-Time RT-PCR (QRT-PCR). Total RNA was extracted by using a kit from Qiagen (Valencia, CA). The mRNA levels for specific genes were determined by QRT-PCR as described (17), using 10 ng of total RNA. The primer and probe sequences for 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase (GenBank accession no. M62766), Mac-2 (X16934), smooth muscle myosin heavy chain (NM_013607), calponin H1 (Z19542), and α-tropomyosin (NM_024427) are listed in Table 1, and those for CD68, ATP-binding cassette transporter 1 (ABCA1), α-actin, MCP-1, VCAM-1, and cyclophilin A (loading control for all marker genes), are the same as described (17, 18). QRT-PCR standard curves (for each mRNA species) were constructed by using serial dilutions of mouse total RNA isolated from liver (HMG-CoA reductase), elicited peritoneal macrophages (CD68, Mac-2, and ABCA1), lesion-enriched aortic arch (MCP-1 and VCAM-1), or normal aorta (α-actin, smooth muscle myosin heavy chain, α-tropomyosin, and calponin H1). All data were normalized to cyclophilin A content and expressed as fold change over the controls.
Table 1. Primer and probe sequences for QRT-PCR.
| Genes | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| HMG-CoA reductase | GGGAGCATAGGCGGCT | TGCGATGTAGATAGCAGTGACA | CAACGCCCACGCAGCAACA |
| Calponin H1 | AGTACTGCCTGAACCCGGAGT | GTTGTGCGGGTGGTGATTG | CCCAGAGCTGAGTGAGCCCACCC |
| Myosin heavy chain | CATGGACCCGCTAAATGACA | CAATGCGGTCCACATCCTTC | TCACTCCTCAATGCCTCCTCTGACAAGTTT |
| α-Tropomyosin | AGCTCGACAAAGAGAACGCC | ATCTTCCAGCTGCTTGCTCC | TGGATCGAGCTGAGCAAGCGGA |
| Mac-2 | AGGAGAGGGAATGATGTTGCC | GGTTTGCCACTCTCAAAGGG | TCCACTTTAACCCCCGCTTCAATGAGA |
All sequences are from 5′ to 3′. The probes are labeled at the 5′ and 3′ positions with 6-carboxyfluorescein reporter and 6-carboxytetramethylrhodamine quencher, respectively.
Phagocytosis of Latex Beads by SMCs. Mouse aortic SMCs (plated on a glass coverslip) preincubated with or without Chol:MβCD (10 μg/ml, 72 h) in 0.2% BSA were incubated with 1-μm Fluoresbrite YG Microsphere latex beads (Polysciences; 4.55 × 106 beads per ml) for 24 h. Coverslips with cells were then washed extensively in PBS, fixed with 2% paraformaldehyde in PBS, counterstained with 4′,6-diamidino-2-phenylindole (for nuclei) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (for membrane) according to the manufacturer's instructions (Molecular Probes), inverted, and mounted on a glass slide with VectorShield mounting medium (Vector Laboratories). Digital images were captured by a fluorescent microscope by using different excitation wavelengths for latex beads, 4′,6-diamidino-2-phenylindole, and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine. To quantitatively determine the uptake of latex beads by SMCs, the number of beads and nuclei were counted in five randomly selected fields in each experimental condition. Phagocytotic activity was expressed as the number of beads divided by the number of nuclei in the fields.
Statistics. Within an experiment, duplicate or triplicate wells were used for each condition or treatment. Data are expressed as average ± SEM. All experiments were repeated at least once. PRISM software (GraphPad, San Diego) was used to analyze differences between samples, either by the two-sample student t test or, for Fig. 7, by one-way ANOVA with the Bonferroni posttest for differences between selected pairs of samples. P values of <0.05 were considered significant.
Fig. 7.
The effects of FC on SMC and macrophage-related gene expression. Subconfluent mouse SMCs were pretreated overnight with or without an ACAT inhibitor F-1394 (1 μM), then treated with (Chol, Chol + ACAT inhibitor) or without (Control, ACAT inhibitor alone) Chol:MβCD (10 μg/ml) in 0.2% BSA (72 h) in the presence of F-1394. Total RNA was extracted and subjected to QRT-PCR analysis. All data are averages ± SEM from independent duplicates. *,α-actin was not detected. †, P < 0.01 vs. Chol.
Results
Cytotoxicity. Chol:MβCD delivers cholesterol rapidly and directly to the plasma membrane (11), and excessive FC in the plasma or ER membrane may lead to cytotoxicity and cell death (19, 20). To determine the maximum subtoxic dose, mouse aortic SMCs were treated with increasing concentrations of Chol:MβCD for 72 h. As shown in Fig. 1, at concentrations up to 10 μg/ml, Chol:MβCD had no or little effect on SMC metabolic activity and cellular protein content. Therefore, 10 μg/ml was used in the following cholesterol-loading experiments for SMCs.
Fig. 1.
Cytotoxicity of Chol:MβCD. Subconfluent mouse SMCs were treated with increasing concentrations of Chol:MβCD in 0.2% BSA for 72 h. Cells were then incubated for 1 h with CellTiter 96 Aqueous One Solution Reagent, convertible to a colored formazan product by metabolically active cells. (A) Relative metabolic activity was determined by spectrophotometric measurement (OD = 490 nm) of the accumulation of the formazan product in the medium. (B) Total cellular protein was determined by using a Sigma kit. See Materials and Methods for details. Data are averages ± SEM from triplicate wells and repeated at least once.
Chol:MβCD Treatment of SMC-Induced Cholesterol and CE Accumulation. After incubation with Chol:MβCD for 72 h, SMCs assumed the appearance of foam cells with Oil Red O-stained lipid droplets present throughout the cytosol of most cells (Fig. 2 A and Inset); in contrast, no lipid droplets were found in control cells incubated with BSA. Biochemical measurements were consistent with the staining results: compared with control SMCs, cholesterol loading led to ≈2-fold increase in total cholesterol levels (32.7 ± 1.5 vs. 56.1 ± 0.9 μg/mg cellular protein, P < 0.00005; Fig. 2B) and ≈10-fold increase in CE (1.3 ± 0.2 vs.12.6 ± 3.5 μg/mg cellular protein, P <0.02). That this degree of sterol accumulation was a significant metabolic perturbation was demonstrated by down-regulation of the mRNA of HMG-CoA reductase to ≈30% of the control level (Fig. 2C).
Fig. 2.
Cholesterol loading leads to smooth muscle foam-cell formation. Subconfluent mouse SMCs were treated with (Chol) or without (Control) Chol:MβCD (10 μg/ml) in 0.2% BSA (72 h). Cells were fixed in 10% neutral-buffered formaldehyde and stained with Oil Red O (magnification: A, ×100; Inset, ×400) or harvested to determine cellular cholesterol (B) or to extract RNA for QRT-PCR determination of HMG-CoA reductase mRNA levels (C). The numerical data are averages ± SEM from independent duplicates.
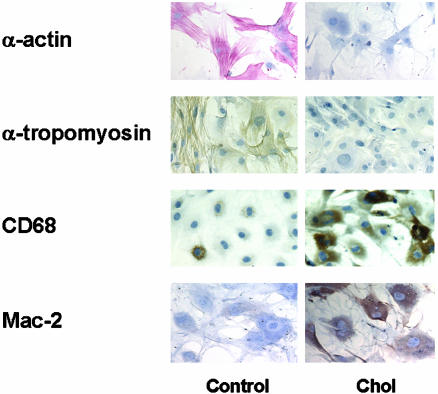
The Effects of Cholesterol Loading on the Expression of SMCs and Macrophage-Related Proteins. To characterize the phenotypic changes associated with SMC foam-cell formation at the end of the 72-h treatment period, cholesterol-loaded and control SMC were immunostained with antibodies specific for proteins commonly taken as characteristic for the smooth muscle or macrophage phenotype. As shown in Fig. 3, as expected, the control SMCs stained heavily with antibodies to α-actin and α-tropomyosin. Notably, after cholesterol loading, the staining for both diminished. In contrast, only a few control cells stained positively with antibodies recognizing the macrophage-related proteins (CD68 and Mac-2), but after cholesterol loading, the increase in the number of positively stained cells and in the intensity of staining was striking.
Fig. 3.
Immunostaining of SMCs for SMC and macrophage-related proteins. Subconfluent mouse SMCs were treated with (Chol) or without (Control) Chol:MβCD (10 μg/ml) in 0.2% BSA (72 h). Cells were stained with antibodies for α-actin (red stain, ×200), α-tropomyosin (brown stain, ×200), CD68 (brown stain, ×200), and Mac-2 (brown stain, ×200).
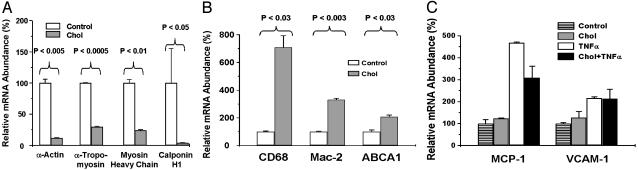
The Effects of Cholesterol Loading on the Abundance of SMCs and Macrophage-Related mRNA Species. To further pursue the phenotypic changes associated with cholesterol loading, we determined the abundance of mRNA species for several SMCs (21) and macrophage-related proteins, including those examined by immunohistochemistry. As shown in Fig. 4A, consistent with the immunostaining (Fig. 3), SMC foam cells had dramatically decreased mRNA levels of α-actin (11.5 ± 0.5% of control, P < 0.005), α-tropomyosin (29.3 ± 1.4%, P < 0.0005), smooth muscle myosin heavy chain (23.8 ± 1.4%, P < 0.01), and calponin H1 (3.8 ± 0.5%, P < 0.05).
Fig. 4.
QRT-PCR determination of SMC and macrophage-related gene expression in SMCs. Subconfluent mouse SMCs were treated with (Chol) or without (Control) Chol:MβCD (10 μg/ml) in 0.2% BSA (72 h). In C, control or cholesterol-loaded cells were treated with 30 ng/ml TNF-α for 2 h after cholesterol loading. Total RNA was extracted and subjected to QRT-PCR analysis of smooth muscle marker genes (A), macrophage marker genes (B), and macrophage-related inflammation genes (C). All data are averages ± SEM from independent duplicates.
In the BSA-treated control cells, the CD68 mRNA level was low (≈5% of the level in lesional macrophages; J.X.R. and E.A.F., unpublished observations) and similar to that in cells maintained in 10% FBS/DMEM (data not shown), consistent with the immunostaining data. On cholesterol loading, the cells had increased levels of CD68 mRNA (to 709 ± 84% of control, P < 0.03). Similarly, the expression of another macrophage marker, Mac-2, increased to 330 ± 11% of controls (P < 0.003) (Fig. 4B). These changes in mRNA abundance, therefore, are consistent with the immunostaining findings, which showed obvious decreases and increases, respectively, in SMCs and macrophage-related protein signals.
The mRNA for ABCA1, a key regulator of cholesterol efflux from peripheral cells to high-density lipoprotein (22) and associated predominantly with macrophages in vivo (23), increased to 207 ± 13% (P < 0.03) in cholesterol-loaded SMCs (Fig. 4B). Because macrophage foam-cell formation is thought to initiate or promote an inflammatory state, we also assayed the mRNA levels of two atherosclerosis-related genes, MCP-1 and VCAM-1, known to be strong responders to inflammatory stimuli and to be expressed in both SMCs and macrophages. Surprisingly, no differences were found in expression of MCP-1 and VCAM-1 between the controls and cholesterol-loaded cells, despite these genes responding to TNF-α treatment in both cell groups (Fig. 4C).
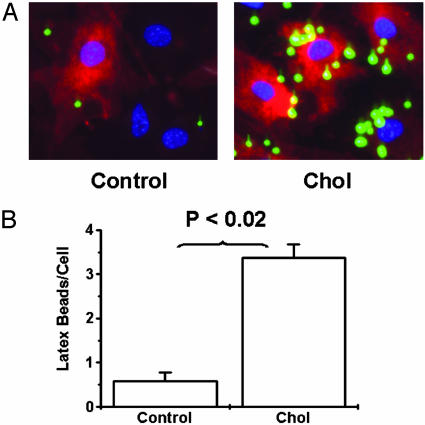
The Effects of Cholesterol Loading on Phagocytotic Activity of SMCs.To investigate whether cholesterol-loaded SMCs acquire functional aspects of macrophages, SMCs with or without cholesterol loading were incubated with 1-μm latex beads as part of the protocol commonly used to study phagocytotic activity of leukocytes (24). As shown in Fig. 5, control SMCs had very low phagocytotic activity (0.6 ± 0.2 beads per cell). After cholesterol loading, however, accumulation of latex beads increased (3.4 ± 0.3 beads per cell, P < 0.02 vs. control). This finding indicated that, in addition to the increases in macrophage-related gene and protein expression in cholesterol-loaded SMCs, evidence of the acquisition of functional properties of macrophages also existed.
Fig. 5.
Cholesterol loading increases the phagocytotic activity of SMCs. (A) Subconfluent mouse SMCs were treated with (Chol) or without (Control) Chol:MβCD (10 μg/ml) in 0.2% BSA (72 h), followed by incubation with 1-μm latex beads (green) for 20 h. Cells were then washed extensively, fixed, counterstained with 4′,6-diamidino-2-phenylindole (blue, for nuclei) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (red, for cell membrane), and subjected to fluorescent microscopy. (Magnification, ×200.) (B) The number of cells and latex beads were also counted to obtain numerical data for phagocytotic activity. All data are averages ± SEM from independent duplicates.
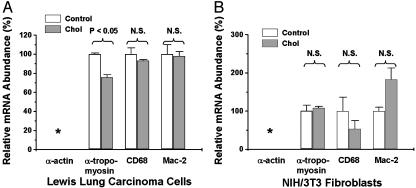
The Effects of Cholesterol Loading on LLC Cells and NIH/3T3 Fibroblasts. To determine whether gaining macrophage-like features on cholesterol loading was a general phenomenon independent of cell type, we studied LLC cells, originally established from the lung of a C57BL/6 mouse in which the murine primary LLC had been implanted (25), and NIH/3T3 mouse fibroblasts. As shown in Fig. 6, α-tropomyosin, CD68, and Mac-2 mRNAs were detected in LLC cells and NIH/3T3 fibroblasts, but α-actin was not detectable in either cell line. For LLC cells, a slight decrease occurred in α-tropomyosin (to 75.8 ± 2.7% of control, P < 0.05) after the cells were loaded with cholesterol (confirmed by cholesterol measurement; data not shown). The abundance of CD68 and Mac-2 mRNAs, however, were not changed and were similar to the levels in SMC control cells (data not shown). For the NIH/3T3 fibroblasts, no significant difference was apparent in α-tropomyosin, CD68, or Mac-2 mRNAs between the control and cells loaded with cholesterol (confirmed by cholesterol measurement; data not shown). Thus, the large increase in macrophage-related gene expression observed in cholesterol-loaded SMCs does not appear to be a nonspecific or general cellular response.
Fig. 6.
The effects of cholesterol loading on SMC and macrophage-related gene expression in LLC cells (A) and NIH/3T3 fibroblasts (B). Subconfluent murine LLC cells or NIH/3T3 fibroblasts were treated with (Chol) or without (Control) Chol:MβCD (5 μg/ml) in 0.2% BSA (72 h). Total RNA was extracted and subjected to QRT-PCR analysis. All data are averages ± SEM from independent duplicates. *, α-actin was not detected.
The Effects of Increased Cellular FC on the Abundance of SMCs and Macrophage-Related mRNA Species. About 50% of the FC delivered to SMCs by Chol:MβCD were converted to CE (Fig. 2). In a recent collaborative study (20), we found differential effects of FC and CE on gene expression related to the ER stress response and apoptosis. To determine whether FC and CE also have differential effects on the changes in SMC gene expression associated with cholesterol loading, we treated the cells with Chol:MβCD (10 μg/ml) in the presence of an ACAT inhibitor, Fujirebio compound F-1394 [which blocks cholesterol esterification (13)]. At the highest subtoxic concentration of F-1394 (1 μM), SMCs had a 50% reduction in CE content with no decrease in total cellular cholesterol content compared with cells treated with Chol:MβCD alone. As shown in Fig. 7, the preferential increase in FC content either tended to further decrease or significantly increase, respectively, SMC or macrophage-related gene expression associated with cholesterol loading, although the degree of the enhancement was variable depending on the gene (i.e., greater for α-actin or CD68 than for α-tropomyosin or Mac-2, respectively).
Discussion
Much attention has been focused on the macrophage foam cell as central to atheroma formation (see ref. 19 for a recent review). In general, it is assumed that foam cells originate primarily from the circulating monocytes that are recruited into the vessel wall. Whereas lipid-laden, SMC-derived, foam cells have been demonstrated in vitro (8, 10) and in atherosclerotic plaques (e.g., ref. 7), these cells are usually assumed to occur relatively late in lesion development. In addition, they retain sufficient phenotypic features to be identifiable by light (26) or electron microscopy (7) as SMCs in origin.
Although SMCs have previously been induced to accumulate cholesterol in vitro (8-10), for either in vitro or in vivo SMC foam cells, information is scant about phenotypic changes at the protein or RNA level. The results of the present study show that, with cholesterol loading in vitro, SMCs rapidly assume a foam-cell appearance and lose the expression of commonly accepted markers of the SMC phenotype. Concurrently, increases occur in the expression of macrophage-related genes and proteins and the acquisition of macrophage function (as assessed by phagocytotic activity). Notably, the gain of macrophage characteristics was not a general consequence of cholesterol loading, as demonstrated by the results for the murine LLC cell line and NIH/3T3 fibroblasts. Our findings, therefore, lead to the provocative possibility that, in addition to the previously recognized foam cells with SMC appearance, a subpopulation exists of foam cells traditionally classified as macrophages, which are actually arterial SMCs that have been induced by cholesterol accumulation to significantly alter their phenotype.
Alteration of the differentiated, contractile phenotype of SMCs is known to play an integral role in the formation of atherosclerotic lesions (27). All four smooth muscle markers examined in the present study are related to SMCs contraction; α-actin and myosin heavy chain are essential components of smooth muscle contractile machinery (21). Calponin H1 is an actin-associated smooth muscle-specific protein and a potential modulator of contraction (28). α-Tropomyosin is considered to play an important part in the regulation of contraction process (29). Down-regulation of these genes has also been observed at early stages of atherogenesis, associated with SMC proliferation and migration into the neointima. As noted earlier, we studied SMCs with a passage number ≤5, which avoids their assumption of a proliferative phenotype (30), thereby making it unlikely that the gene expression down-regulation we observed was a consequence of increased proliferation. Because the cells studied had low passage numbers, and proliferation was further minimized during the experiments (as reflected by the constancy of cellular metabolic activity and protein content; Fig. 1) by incubation in serum-free medium, it is also unlikely that the down-regulation of SMC-related genes or the up-regulation of macrophage-related genes was a result of cultures being overtaken by a rapidly dividing subpopulation of aberrant SMCs that had macrophage-like properties before cholesterol loading.
The down-regulation in SMC foam cells (Figs. 3 and 4A), therefore, suggests that cholesterol may lead to the alteration of SMCs to a state associated with features that are proatherogenic (27). Consistent with this notion is the report that enzymatically degraded, nonoxidized LDL induced human vascular SMC activation and foam-cell transformation, with the authors interpreting the data as evidence for a de-differentiation process (10). In light of the present results for macrophage-related proteins (see below), rather than representing a de-differentiating process, cholesterol loading may be more appropriately considered as part of a transdifferentiation program.
The conversion of SMCs to macrophage-like cells may also speaks to two other observations in the mouse atherosclerosis literature. The first is the finding of significant amounts of macrophage marker-positive foam cells in atherosclerotic lesions in mice deficient or impaired in genes responsible for monocyte recruitment (2, 3), leukocyte adhesion (4, 5), and macrophage proliferation and differentiation (6). Although undoubtedly this finding reflects, in part, biological redundancy in monocyte- and macrophage-related factors, it is certainly plausible that some of the persistent “macrophages” were once SMCs. The second observation is from our previous study (16), in which the acute and sustained elevation of HDL levels in apoE-/- mice (naturally low in plasma HDL) remodeled advanced plaques, so that a marked increase occurred in the thickness of the fibrous cap (as assessed by α-actin staining). If the changes we have described were reversible on cholesterol efflux, perhaps the increased HDL restored the SMC phenotype to cells that had originally migrated to the subendothelium from the medial layer and assumed macrophage-like features after cholesterol loading.
Surprisingly, cholesterol-loaded SMCs, despite taking on characteristics of macrophages (usually considered to be an inflammatory cell), did not appear to be significantly activated, at least by the criteria of the induction of MCP-1 or VCAM-1 (Fig. 4C), proatherogenic molecules whose genes are highly regulated by NF-κB. One possible explanation for this finding is that cholesterol loading somehow made the cells generally unresponsive to inflammatory stimuli. This possibility is unlikely, however, based on the comparable increases in control and loaded SMCs in MCP-1 and VCAM-1 mRNA after TNF-α treatment. Another possible explanation for these results is that cholesterol accumulation per se is not an inflammatory stimulus or that it is, but that induction of inflammatory molecules occurs at a later time. Support for the former possibility comes from the above-cited study (10), in which activation of SMCs was accomplished by delivery of cholesterol in the form of modified LDL [which contains the inflammatory factors (31, 32)], and from studies by Lawn and colleagues (33), which have shown that loading of macrophage cell lines with oxidized LDL leads to increased expression of inflammatory genes and greater responsiveness of these genes to lipopolysaccharide stimulation (34).
By using cyclodextrins to deliver cholesterol, we have been able to separate the effects of cholesterol itself from other components of modified lipoproteins, which, as the reports above suggest, taken with the present results, may exert effects independent of, or perhaps synergistic with, cholesterol accumulation. Cyclodextrins are effective in delivering cholesterol to a wide array of cell types, such as fibroblasts, macrophages, and hepatoma cells (e.g., see reports of Rothblat and colleagues, such as ref. 11). Notably, in the present study, SMC foam-cell formation was achieved without causing cytotoxicity by using a relatively low concentration of cyclodextrin-cholesterol complexes [≈<1:20 as in previous studies (11)]. Again, the lack of inflammatory activation in our studies, compared with those using modified and oxidized LDL, may also reflect this low level of cytotoxicity, given that oxidized LDL is known to have adverse effects on cell viability.
What are the mechanisms by which cholesterol loading leads to the conversion of SMCs to macrophage-like cells? Although the “candidate factor” approach we have taken cannot detect all the induced changes, we know that at least some are at the mRNA level, making transcriptional regulation a likely key component of the response to cholesterol loading. In addition, the potentiation of the effects when cholesterol esterification was inhibited indicates a FC-sensitive pathway. Given that, in a collaborative study, we have recently reported a preferential effect of FC on gene expression related to the ER stress pathway in macrophages (20), it is possible that those results and the present findings are related.
Two transcription factors strongly regulated by cellular cholesterol content are sterol-responsive element-binding proteins (SREBP) and liver X receptors (LXR). The former is an unlikely candidate for the regulator of SMC transdifferentiation based on our results for HMG-CoA reductase (Fig. 2C), which suggest that the SREBP pathway was down-regulated under our experimental conditions. LXR, on the other hand, is implicated by virtue of its natural ligands, including oxysterols derived from the oxidation of FC, which occurs in most, if not all, cells that accumulate cholesterol.
Peroxisome proliferator-activated receptor γ (PPARγ) is another candidate factor. Previous studies have indicated that PPARγ regulates gain of macrophage-like phenotype in primary smooth muscle culture (35) and that the uptake of oxidized LDL by macrophages (36) or of cholesterol (provided by albumin-cholesterol complexes) in endothelial cells (37) leads to PPARγ activation. At this time, however, we can only speculate on whether these or other factors are involved in the phenotypic changes we have described. Future experiments with more candidate factors and, ultimately, DNA microarrays will be needed to identify the transcriptional pathways altered by cholesterol loading that are responsible for the assumption by SMCs of a macrophage-like state.
In summary, we have characterized the phenotypic changes associated with mouse aortic SMC foam-cell formation at the protein and molecular level. These cells became macrophage-like, but without activation of genes known to be induced in the inflammatory state. The results suggest that lesional foam cells previously assumed to have a macrophage origin, based on immunostaining, might have originated in the SMC population. The further elucidation of the pathways in SMCs altered by cholesterol accumulation will not only illuminate an interesting area of developmental biology, but they will also likely increase our fundamental knowledge of plaque formation and remodeling.
Acknowledgments
We thank Dr. Robin P. Choudhury (Department of Cardiovascular Medicine, University of Oxford Medical School, Oxford) for helpful discussions when this study was initiated; Dr. Jun Kusunoki (Tsukuba Research Institute, Banyu Pharmaceutical Co., Ltd., Tokyo), Drs. Gwendalyn J. Randolph and Veronique Angeli (Institute for Gene Therapy and Molecular Medicine, Mount Sinai School of Medicine), and Dr. Susanne Idel (The Rockefeller University, New York) for assistance in cholesterol loading, phagocytotic activity, and TNF-α-treatment experiments; Dr. Andrés Hidalgo (Divisions of Hematology and Clinical Immunology, Department of Medicine, Mount Sinai School of Medicine) for kindly providing us the LLC cells and NIH/3T3 cells; and Mr. Otis Defreitas (Cardiovascular Institute, Mount Sinai School of Medicine) for technical help on immunohistochemical procedures. Fluorescent microscopy was performed at the Mount Sinai School of Medicine-Microscopy Shared Research Facility, supported, in part, with funding from National Institutes of Health-National Cancer Institute shared resources Grant 1 R24 CA095823-01. This work was supported by National Institutes of Health Grant HL61814 (to E.A.F.).
Abbreviations: CE, cholesteryl esters; Chol:MβCD, cholesterol:methyl-β-cyclodextrin; FC, free cholesterol; LLC, Lewis lung carcinoma; QRT-PCR, quantitative real-time RT-PCR; SMC, smooth muscle cell; ACAT, acyl-CoA:cholesterol acyltransferase; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemoattractant protein 1; VCAM-1, vascular cell adhesion molecule 1.
References
- 1.Stary, H. C., Chandler, A. B., Glagov, S., Guyton, J. R., Insull, W., Jr., Rosenfeld, M. E., Schaffer, S. A., Schwartz, C. J., Wagner, W. D. & Wissler, R. W. (1994) Circulation 89, 2462-2478. [DOI] [PubMed] [Google Scholar]
- 2.Boring, L., Gosling, J., Cleary, M. & Charo, I. F. (1998) Nature 394, 894-897. [DOI] [PubMed] [Google Scholar]
- 3.Gu, L., Okada, Y., Clinton, S. K., Gerard, C., Sukhova, G. K., Libby, P. & Rollins, B. J. (1998) Mol. Cell 2, 275-281. [DOI] [PubMed] [Google Scholar]
- 4.Dong, Z. M., Chapman, S. M., Brown, A. A., Frenette, P. S., Hynes, R. O. & Wagner, D. D. (1998) J. Clin. Invest. 102, 145-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cybulsky, M. I., Iiyama, K., Li, H., Zhu, S., Chen, M., Iiyama, M., Davis, V., Gutierrez-Ramos, J. C., Connelly, P. W. & Milstone, D. S. (2001) J. Clin. Invest. 107, 1255-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith, J. D., Trogan, E., Ginsberg, M., Grigaux, C., Tian, J. & Miyata, M. (1995) Proc. Natl. Acad. Sci. USA 92, 8264-8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faggiotto, A., Ross, R. & Harker, L. (1984) Arteriosclerosis 4, 323-340. [DOI] [PubMed] [Google Scholar]
- 8.Wolfbauer, G., Glick, J. M., Minor, L. K. & Rothblat, G. H. (1986) Proc. Natl. Acad. Sci. USA 83, 7760-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pomerantz, K. B. & Hajjar, D. P. (1989) J. Lipid Res. 30, 1219-1231. [PubMed] [Google Scholar]
- 10.Klouche, M., Rose-John, S., Schmiedt, W. & Bhakdi, S. (2000) Circulation 101, 1799-1805. [DOI] [PubMed] [Google Scholar]
- 11.Christian, A. E., Haynes, M. P., Phillips, M. C. & Rothblat, G. H. (1997) J. Lipid Res. 38, 2264-2272. [PubMed] [Google Scholar]
- 12.Rong, J. X., Berman, J. W., Taubman, M. B. & Fisher, E. A. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 1617-1623. [DOI] [PubMed] [Google Scholar]
- 13.Kusunoki, J., Aragane, K., Yamaura, T. & Ohnishi, H. (1995) Jpn. J. Pharmacol. 67, 195-203. [DOI] [PubMed] [Google Scholar]
- 14.Kusunoki, J., Hansoty, D., Aragane, K., Fallon, J., Badimon, J. & Fisher, E. (2001) Circulation 103, 2604-2609. [DOI] [PubMed] [Google Scholar]
- 15.Brown, M. S., Ho, Y. K. & Goldstein, J. L. (1980) J. Biol. Chem. 255, 9344-9352. [PubMed] [Google Scholar]
- 16.Rong, J. X., Li, J., Reis, E. D., Choudhury, R. P., Dansky, H. M., Elmalem, V. I., Fallon, J. T., Breslow, J. L. & Fisher, E. A. (2001) Circulation 104, 2447-2452. [DOI] [PubMed] [Google Scholar]
- 17.Trogan, E., Choudhury, R. P., Dansky, H. M., Rong, J. X., Breslow, J. L. & Fisher, E. A. (2002) Proc. Natl. Acad. Sci. USA 99, 2234-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng, P., Horwitz, A., Waelde, C. A. & Smith, J. D. (2001) Biochim. Biophys. Acta 1534, 121-128. [DOI] [PubMed] [Google Scholar]
- 19.Tabas, I. (2002) J. Clin. Invest. 110, 583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng, B., Yao, P. M., Li, Y., Devlin, C. M., Zhang, D., Harding, H. P., Sweeney, M., Rong, J. X., Kuriakose, G., Fisher, E. A., et al. (2003) Nat. Cell Biol. 5, 781-792. [DOI] [PubMed] [Google Scholar]
- 21.Owens, G. K. (1995) Physiol. Rev. 75, 487-517. [DOI] [PubMed] [Google Scholar]
- 22.Young, S. G. & Fielding, C. J. (1999) Nat. Genet. 22, 316-318. [DOI] [PubMed] [Google Scholar]
- 23.Joseph, S. B., McKilligin, E., Pei, L., Watson, M. A., Collins, A. R., Laffitte, B. A., Chen, M., Noh, G., Goodman, J., Hagger, G. N., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 7604-7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vetvicka, V. & Fornusek, L. (1987) Biomaterials 8, 341-345. [DOI] [PubMed] [Google Scholar]
- 25.Bertram, J. S. & Janik, P. (1980) Cancer Lett. 11, 63-73. [DOI] [PubMed] [Google Scholar]
- 26.Ikeda, T., Shirasawa, T., Esaki, Y., Yoshiki, S. & Hirokawa, K. (1993) J. Clin. Invest. 92, 2814-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross, R. (1993) Nature 362, 801-809. [DOI] [PubMed] [Google Scholar]
- 28.Miano, J. M. & Olson, E. N. (1996) J. Biol. Chem. 271, 7095-7103. [DOI] [PubMed] [Google Scholar]
- 29.Perry, S. V. (2001) J. Muscle Res. Cell Motil. 22, 5-49. [DOI] [PubMed] [Google Scholar]
- 30.Christen, T., Bochaton-Piallat, M. L., Neuville, P., Rensen, S., Redard, M., van Eys, G. & Gabbiani, G. (1999) Circ. Res. 85, 99-107. [DOI] [PubMed] [Google Scholar]
- 31.Watson, A. D., Subbanagounder, G., Welsbie, D. S., Faull, K. F., Navab, M., Jung, M. E., Fogelman, A. M. & Berliner, J. A. (1999) J. Biol. Chem. 274, 24787-24798. [DOI] [PubMed] [Google Scholar]
- 32.Watson, A. D., Leitinger, N., Navab, M., Faull, K. F., Horkko, S., Witztum, J. L., Palinski, W., Schwenke, D., Salomon, R. G., Sha, W., et al. (1997) J. Biol. Chem. 272, 13597-13607. [DOI] [PubMed] [Google Scholar]
- 33.Shiffman, D., Mikita, T., Tai, J. T., Wade, D. P., Porter, J. G., Seilhamer, J. J., Somogyi, R., Liang, S. & Lawn, R. M. (2000) J. Biol. Chem. 275, 37324-37332. [DOI] [PubMed] [Google Scholar]
- 34.Mikita, T., Porter, G., Lawn, R. M. & Shiffman, D. (2001) J. Biol. Chem. 276, 45729-45739. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, K., Hirano, K., Nozaki, S., Takamoto, A., Nishida, M., Nakagawa-Toyama, Y., Janabi, M. Y., Ohya, T., Yamashita, S. & Matsuzawa, Y. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1027-1032. [DOI] [PubMed] [Google Scholar]
- 36.Tontonoz, P., Nagy, L., Alvarez, J. G., Thomazy, V. A. & Evans, R. M. (1998) Cell 93, 241-252. [DOI] [PubMed] [Google Scholar]
- 37.Meerarani, P., Smart, E. J., Toborek, M., Boissonneault, G. A. & Hennig, B. (2003) Metabolism 52, 493-500. [DOI] [PubMed] [Google Scholar]