Abstract
The antennal imaginal disc was transplanted between pre-metamorphic male larvae of two different Lepidopteran moth species. Following adult eclosion, electrophysiological recordings were made from 33 central olfactory neurons in the antennal lobes of both Helicoverpa zea donor to Heliothis virescens recipient (Z-V) and reciprocal (V-Z) transplants. Under the influence of sensory neuron input derived from the transplanted antennal imaginal disc, most antennal lobe projection neurons (29/33) were classified as belonging to physiological categories encountered previously in donor species males. Furthermore, when stained, many of these neurons had dendritic arbors restricted to donor-induced glomerular locations predicted by their physiology. However, some neurons with unexpected physiological profiles were also identified (4/33), but only in V-Z transplants. These profiles help to explain why some V-Z bilateral transplants were able to respond to both pheromone blends in flight tunnel bioassays, an unforeseen result counter to the assumption that a donor antenna develops a normal donor antennal olfactory receptor neuron compliment. Stainings of several neurons in V-Z transplant males also revealed unusual morphological features including multiglomerular dendritic arbors and “incorrect” glomerular locations. These results indicate a developmental plasticity in the final dendritic arborization pattern of central olfactory neurons including an ability to colonize and integrate inputs across topographically novel donor glomeruli, different from those found in the normal recipient antennal lobe.
Indexing terms: olfaction, antennal lobe, glomerulus, Heliothis virescens, Helicoverpa zea, behavior, pheromone, imaginal disc
Introduction
Olfactory glomeruli form a spatial interface between the sensory epithelium and brain in many, divergent animal taxa (Hildebrand and Shepherd, 1997). A growing body of evidence suggests that glomeruli are not only distinct anatomical structures but also functional units in olfactory coding in that neurons housed within these structures are activated in a manner that allows an across-glomerular combinatorial pattern to represent a given odorant (Friedrich and Korsching, 1997; Joerges et al., 1997; Galizia et al., 2000; Lei et al., 2002). Because the numbers of glomeruli are finite such patterns facilitate the recognition of a much broader array of odorants than could be discriminated if single glomeruli were activated only by a single odorant. In some cases however, such as moth pheromone discrimination, single odorants are represented specifically by a single glomerulus in the sexually-dimorphic male macroglomerular complex (MGC). Often moth pheromone blends are mixtures of two or more odorants and accurate discrimination therefore also requires an across-glomerular or ‘across-label’ pattern of activity (Vickers et al., 1998; Christensen and White, 2000; Vickers and Christensen, 2003). The narrowly-tuned responses of moth pheromone-sensitive neurons represents one end of the olfactory spectrum where unambiguous detection and discrimination of the conspecific pheromone blend has a clear adaptive function, one subject to strong selective pressures.
Olfactory processing in Heliothis virescens and Helicoverpa zea
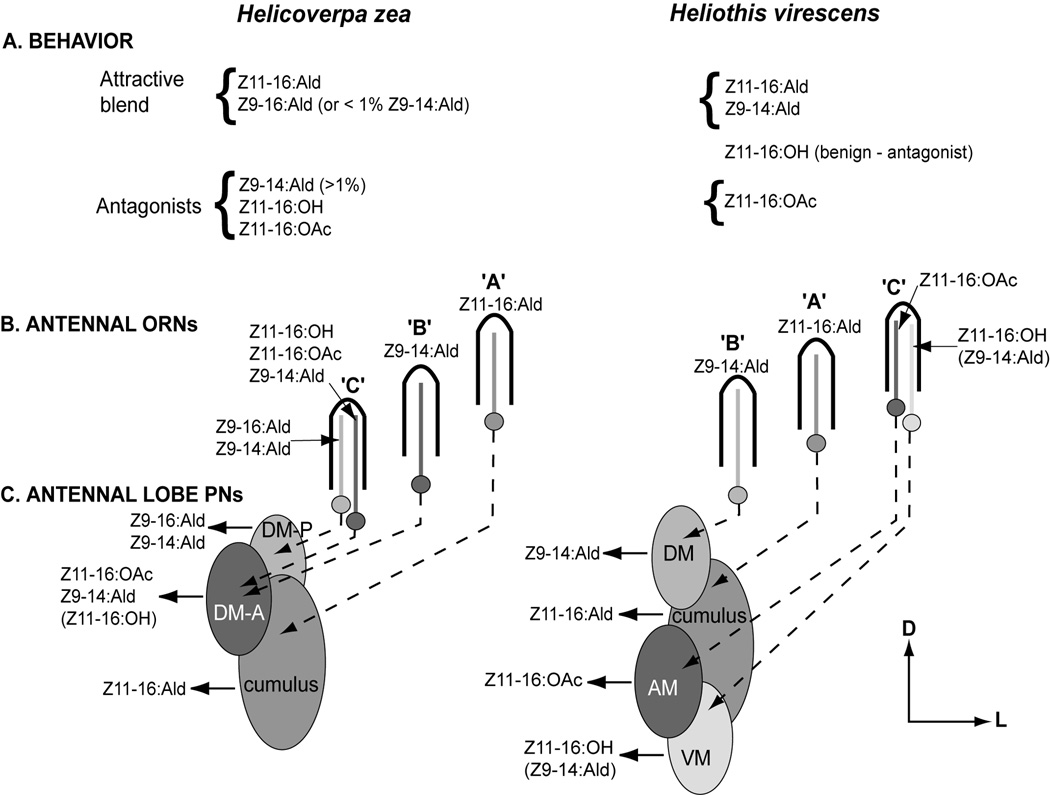
Several studies have examined pheromone-mediated behavior and peripheral/central processing of pheromonal information in two heliothine moths, the tobacco budworm, Heliothis virescens, and the corn earworm, Helicoverpa zea (Vetter and Baker, 1983, 1984; Almaas and Mustaparta, 1990, 1991; Almaas et al., 1991; Christensen et al., 1991; 1995; Hansson et al., 1995; Berg et al., 1995, 1998; Cossé et al., 1998; Vickers et al., 1991, 1998; Baker et al., 2004). Thus, the specificities of sensory and central neurons for pheromonal ligands and chemicals that act as interspecific antagonists of male upwind flight behavior are well understood. The salient features of the pheromone processing peripheral and central olfactory pathways have been reviewed recently (Vickers et al., 2003) and are summarized in Figure 1.
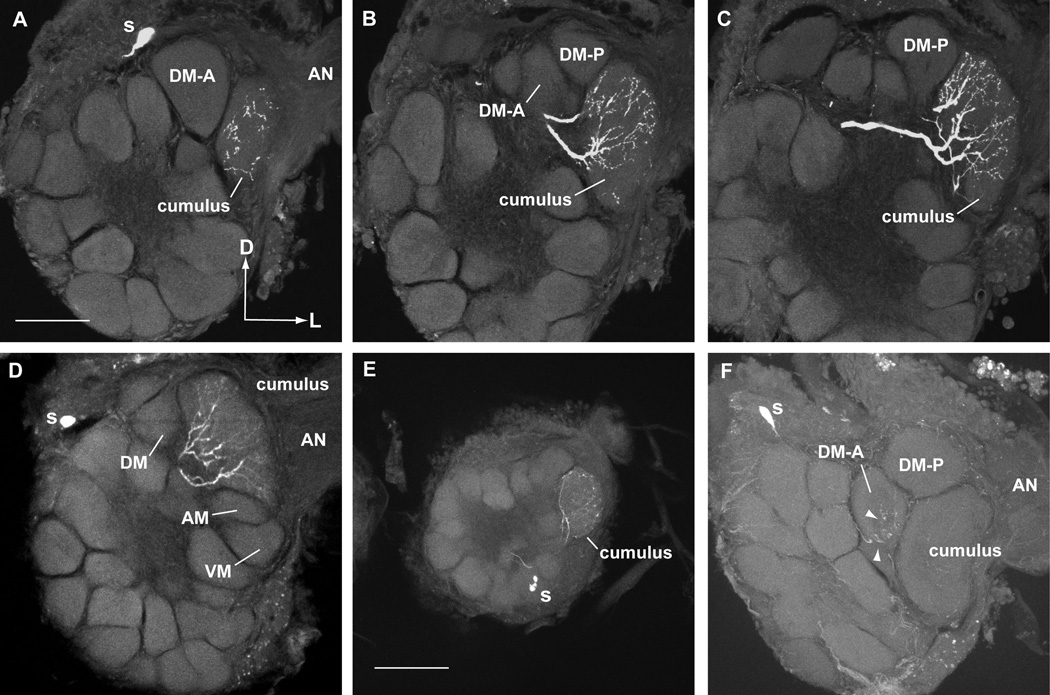
Figure 1.
Schematic of pheromone-mediated behavior and olfactory pathways of male Helicoverpa zea and Heliothis virescens modified from Vickers et al. (2003) to include central olfactory projections neurons (PNs). A. Attractive pheromone mixtures for the two species differ by a single secondary component, Z9-16:Ald for H. zea and Z9-14:Ald for H. virescens. Several additional compounds when singly added to an attractive blend antagonize upwind flight responses by males. B. Antennal olfactory receptor neurons (ORNs) are housed in sensilla along the antenna. Three different sensilla populations (A, B, and C types) have been characterized on the antennae of H. zea and H. virescens. These sensilla house ORNs with the odorant specificities indicated. The axonal projections of these different ORNs are not entirely understood but known and anticipated targets in the sexually-dimorphic male macroglomerular complex (MGC) are indicated. C. The MGCs of both species are dominated by a large glomerulus, the cumulus, with 2 (H. zea) or 3 (H. virescens) smaller, satellite glomeruli. These additional glomeruli were named according to their anatomical position relative to the cumulus (DM-A: dorso-medial anterior; DM-P: dorso-medial posterior; DM: dorso-medial; AM: antero-medial; VM: ventro-medial). Central PNs with dendritic arbors restricted to individual glomeruli (i.e. uniglomerular) have previously been shown to respond specifically to the odorants indicated (Christensen et al., 1991, 1995; Vickers et al., 1998) with the following exceptions: 1. PNs in the H. zea DM-A glomerulus are expected to respond to Z11-16:OH in addition to Z11-16:OAc and Z9-14:Ald; 2. PNs tuned to Z11-16:OH and Z9-14:Ald arborize in the VM glomerulus of H. virescens (Kleineidam et al., in prep.; Vickers, unpublished observations). The specificities of central PNs closely matched those of peripheral ORNs (H. zea: Cossé et al., 1998; H. virescens: Baker et al., 2004). D, dorsal; L, lateral.
Glomerular development
In holometabolous insects (those that undergo a complete metamorphosis) adult olfactory glomeruli develop during metamorphosis, replacing the larval olfactory noduli. The adult antennal structure is formed from an imaginal disc that underlies the larval antennal peg (Sanes and Hildebrand, 1976). Adult antennal sensory neurons send their axons into the larval antennal lobe. The construction of glomeruli results from interactions between ingrowing sensory axons, central interneurons and supporting tissues such as glial cells (Oland and Tolbert, 1996; Rössler et al., 1998; Tolbert et al., 2004). Just before the antennal nerve enters the antennal lobe (AL), the axons of olfactory receptor neurons (ORNs) fasiculate and then project to their glomerular targets (Rössler et al., 1999a). The spatial location of the target olfactory glomerulus appears to be consistent and, in those species for which maps have been constructed (typically species with a small number of glomeruli such as Drosophila, several moth species, honeybee, and zebrafish), the glomerular architecture is also consistent across individuals (Rospars, 1983; Flanagan and Mercer, 1989; Rospars and Hildebrand, 1992; Baier and Korsching, 1994; Laissue et al., 1999). The formation of such precise maps and the connections made within these structures between sensory and central pathways is therefore a pre-requisite for the unambiguous and accurate discrimination of olfactory signals. Recent evidence from Drosophila suggests that sensory and central projection neurons (PNs) are independently pre-specified to grow to certain areas of the AL during metamorphosis (Jefferis et al., 2001, 2004). However, the exact nature of the interactions between the different cell types of the olfactory system that give rise to the final anatomical structure remain unclear.
Imaginal disc transplants
The formation of olfactory glomeruli that results from interactions between the developing adult antenna from imaginal disc tissue and the AL central neurons allows for the use of transplantation techniques to challenge the developing adult brain with a set of in-growing ORNs that differ in terms of both the pre-specified sensory map and the odorant tuning properties of certain ORNs (Fig. 1). The first antennal imaginal disc transplants were performed between male and female Manduca sexta (Schneiderman et al., 1982, 1986). Some resultant gynandromorphic females exhibited upwind flight responses to a female pheromone blend. Subsequently, Rössler et al. (1999b) demonstrated that the female AL in transplant recipient females exhibited a male MGC. These studies demonstrated that formation of a specific glomerular array, such as the male MGC, was largely due to influences of the ingrowing ORN axons, but showed also that PNs in the female AL could arborize within the novel set of induced MGC glomeruli and process the pheromonal signal.
In more recent studies, involving inter-species imaginal disc transplantation between H. virescens and H. zea, a majority of individuals displayed an MGC arrangement that most closely resembled that of the donor, although other developmental end-points were possible, including a normal recipient MGC (Vickers et al., 2003). However, wind tunnel bioassays of these same individuals revealed that some males had unusual behavioral responses (Vickers et al., 2003). This was particularly conspicuous in H. virescens to H. zea transplants (V-Z transplants), where some transplant males flew upwind to the H. zea pheromone blend. This was not expected because the normal H. virescens antenna does not contain ORNs that respond to the H. zea secondary component, (Z)-9-hexadecenal (Z9-16:Ald) (see Figure 1). Electrophysiological recordings from antennal ORNs demonstrated that in some transplant males sensory cells had been induced to develop that responded to the H. zea component (Ochieng et al., 2003). These results added to the information obtained with M. sexta by showing that plasticity can occur both with respect to the development of novel sensory inputs as well as in the ability of AL PNs to make functional connections with a different MGC glomerular array.
However, any conclusions from these studies concerning plasticity of AL PN connectivity/functionality were inferred because they were based on the results of flight tunnel behavioral tests. The physiological tuning properties and morphological characteristics of central olfactory neurons in transplant recipient individuals have either been examined only in limited detail (gynandromorphic transplants between M. sexta males and females, Schneiderman et al., 1982) or not at all (inter-race transplants between E- and Z- race Ostrinia nubilalis, Linn et al., 1999; and interspecies transplants between H. virescens and H. zea, Vickers et al., 2003). In this study we report on the characteristics of central olfactory PNs in both V-Z and the reciprocal Z-V transplant individuals. We show that recipient PNs with dendritic arbors restricted to single donor-induced glomeruli assume the same physiological profiles as their spatial counterparts in normal individuals, but also that PNs can alter their arborization patterns to accommodate a different, and unexpected glomerular configuration.
Materials and Methods
Insects
Colonies of H. zea and H. virescens were maintained at the Geneva, NY laboratory on a pinto-bean diet as described by Jurenka et al. (1991).
Transplantation procedure
The transplantation procedure has been described in detail previously (Vickers et al., 2003). Transplants were conducted between H. virescens donor and H. zea recipient (referred to as V-Z transplants) and the reciprocal H. zea donor to H. virescens recipient (Z-V transplants). Both imaginal discs were harvested from a single individual donor larva prior to transplantation into the same individual recipient (host imaginal discs were excised and discarded prior to transplantation). Transplants were conducted in groups such that either V-Z or Z-V transplants were available for a period of time.
Chemicals
Behavior
The pheromone components (Z)-11-hexadecenal (Z11-16:Ald), (Z)-9- tetradecenal (Z9-14:Ald) and Z9-16:Ald were obtained from Bedoukian Research, Inc. (Danbury, CT). Purity of each individual stock solution was verified as >95% by gas chromatography (GC). Stock solutions were prepared in high performance liquid chromatography-grade hexane.
Neurophysiology
High purity odorant stock solutions (>95% purity as determined by GC) were obtained from Sigma-Aldrich (St. Louis, MO) and Bedoukian Research, Inc. (Danbury, CT). These included the following compounds: Z11-16:Ald, Z9-14:Ald, Z9-16:Ald, (Z)-11-hexadecenol (Z11-16:OH) and (Z)-11-hexadecenyl acetate (Z11-16:OAc). Serial dilutions of each compound were made in high purity hexane. Individual compounds were loaded onto 3.5×0.7 cm filter paper strips (Whatman No. 1) at a dosage of 10ng. Two pheromone blend mixtures were also tested: 10ng Z11-16:Ald + either 0.5ng Z9-14:Ald (H. virescens 2-mix) or 0.5ng Z9-16:Ald (H. zea 2-mix). The ratios of pheromone components in these blends have previously been shown to be behaviorally active (albeit at higher loading dosages). On a few occasions, variations of these two blends were used that included more compounds in addition to the binary mixture indicated (Heliothis subflexa 3-mix: Z11-16:Ald, Z9-16:Ald and Z11-16:OH in a ratio of 1/0.5/0.1; H. zea 4-mix: Z11-16:Ald, Z9-16:Ald, (Z)-7-hexadecenal (Z7-16:Ald) and hexadecenal (16:Ald) in a ratio of 1:0.05/0.01/0.05; H. virescens 6-mix: Z11-16:Ald, Z9-14:Ald, Z9-16:Ald, Z7-16:Ald, tetradecanal, and 16:Ald in a ratio of 1/0.1/0.01/0.01/0.05/0.5).
Flight tunnel
Flight tunnel assays were conducted as described previously (Vickers et al., 2003) in the wind tunnel facility in Geneva, NY. Briefly, transplant males were flown to two different pheromone blends: 300µg Z11-16:Ald admixed with either 5% Z9-14:Ald (H. virescens blend) or 2% Z9-16:Ald (H. zea blend) on rubber septa (A.H. Thomas Co.). Septa were cleaned in hexane before initial loading and use. The first lure tested on any given day was selected at random. Between five and ten normal H. virescens or H. zea males were also flown to each lure during each day of testing to verify activity of the odor source. Males were scored for taking flight (initiating flight without orientation to the pheromone plume), upwind anemotactic flight in the plume, and source contact (locating and landing on the rubber septum odor source). Males were placed into one of three categories depending upon their behavioral responses: 1. source contact only to H. virescens blend, 2. source contact only to H. zea blend, 3. source contact to both blends.
Differences between response categories were established with a χ2 2×2 test of independence (P<0.05). After behavioral tests, males were packaged individually in a 1oz clear plastic medicine cup with lid and shipped overnight to the University of Utah.
Neurophysiology
Animals were selected for neurophysiological evaluation based upon the presence of an antenna with a normal appearance on the right side of the head. No data relating to behavioral performance of individual male moths sent to Utah was provided until the experiment had concluded.
Neurophysiological procedures used for recording from the AL of heliothine moths have been described in detail previously (Christensen et al., 1991; 1995; Vickers et al., 1998). All single unit intracellular recordings and subsequent staining were made from the right AL. Borosilicate glass microelectrodes were filled at the tip with 4% Lucifer yellow CH (LY; Sigma-Aldrich, St. Louis, MO) in a 0.2M solution of LiCl and then backfilled with 2M LiCl. Microelectrode resistance varied between 150 and 400 MΩ. Once a stable penetration had been established, individual odorant and blend stimuli were directed at the ipsilateral antenna. Stimuli were delivered as a series of five 40 ms pulses delivered over 1.2 s. Following physiological characterization, negative direct current (up to 2 nA) was passed through the electrode in order to iontophorese LY into the neuron. Wholemount and sectioned material were examined on a Zeiss LSM 510 laser scanning confocal microscope (25mW Argon laser light source, excitation: 458/514 nm dichroic, emission: LP 475 nm). Brains were scanned with either a 20X objective (wholemount and some sectioned material) or 40X (sectioned material only). Serial optical images were collected at intervals of 1 or 2 µm. Details of the histologic procedures used to prepare brains for microscopic examination have been reported previously (Vickers et al., 1998). Intracellular activity was monitored continuously on oscilloscope and recorded on a VHS tape PCM recorder (A.R. Vetter, PA). Spike trains were digitized and analyzed with DataPac 2000 software (Run Technologies, Laguna Niguel, CA). Acquisition was initiated on the rising phase of the first stimulus pulse with 2 s pre- and 4 s post-trigger data captured. Each spike was assigned a time value based upon positive transition across a threshold level. The threshold level was set manually for each spike train taking into consideration various factors including action potential amplitude and background noise. From the spike-time stamp data, instantaneous frequencies were calculated and plotted as instantaneous spike frequency histograms (IFTs) to facilitate comparison of responses within and between neurons. In most cases, excitatory responses in the form of action potential bursts phase-locked to the delivery of each odor pulse were observed. Occasionally the resting membrane potential baseline was stable enough to observe excitatory post-synaptic potentials in the absence of spiking responses. In a few instances, spiking was reduced following stimulation but in all such cases the baseline was too unstable to permit observation of any underlying inhibitory post-synaptic potentials. The classification of neurons into different physiologic categories was therefore based upon the transient, stimulus-induced excitatory responses exhibited by each neuron.
Image Processing and Figure preparation
Each half-tone figure panel was composed from either single or consecutive (2–15 successive focal planes at 1- or 2-µm intervals) optical sections. Projection of sections was performed with Zeiss LSM Image Examiner software and composite images were exported as JPEG files. Image cropping and other adjustments to brightness and contrast were made only when necessary. These operations were performed in Adobe Photoshop. Final half-tone panels were assembled and labeled in Adobe Illustrator.
Results
Behavior
H. virescens donor to H. zea recipient (V-Z transplants)
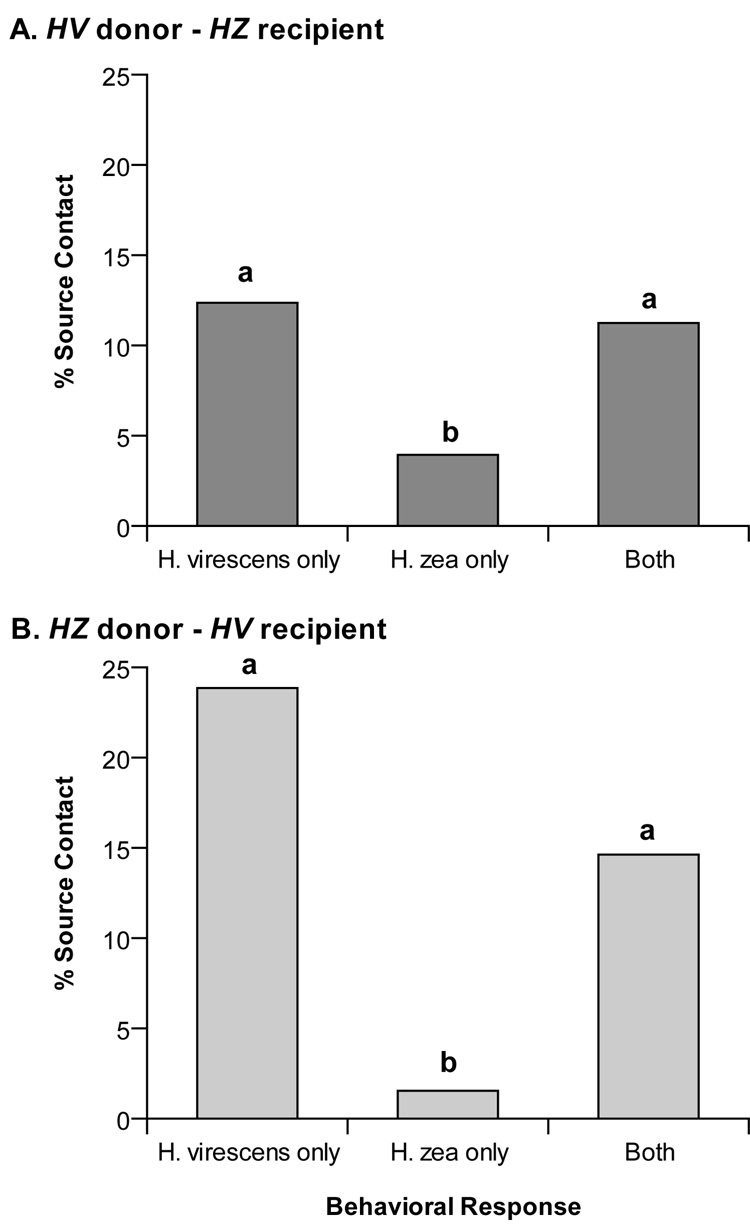
A total of 178 V-Z transplant males were assayed in the flight tunnel to both pheromone blends with 27.5% contacting the odor source (Fig. 2A). Males exhibited a preference for the H. virescens blend with 12.4% responding positively only to this blend, compared with 3.9% to the H. zea blend (χ2 = 8.838; p = 0.003). Additionally, 11.2% responded with upwind flight and source contact to both pheromone blends. This value was not significantly different than the proportion responding to the H. virescens blend alone (12.4%; χ2 = 0.108; p = 0.7424), but was greater than the proportion responding to the H. zea blend alone (3.9%; χ2 = 7.041; p = 0.008). Thus, the majority of males of this transplant type exhibited a donor-type behavioral preference.
Figure 2.
Behavioral responses of bilateral transplant males to H. virescens and H. zea pheromone blends. Individual transplant males were tested to both native pheromone blends (H. virescens and H. zea) presented in a randomly determined order and assigned to one of three categories based upon source contact responses. Categories with no letter in common were significantly different according to a χ2 2×2 test of independence (P<0.05). A. Behavioral responses of H. virescens donor – H. zea recipient (V-Z) transplant males (N = 178). The majority of responding males flew to the donor (H. virescens) blend. Interestingly, some V-Z transplant males were capable of responding to the H. zea blend containing Z9-16:Ald (‘H. zea only’ and ‘Both’). This behavioral response was counter to the assumption that a normal H. virescens antenna had developed in these males because neurophysiological responses to this odorant have not been reported in previous studies of ORNs on normal H. virescens male antennae. B. Behavioral responses of H. zea donor – H. virescens recipient (Z-V) transplant males (N = 130). In contrast to the V-Z transplant males, Z-V males did not prefer the donor blend but instead responded well to the recipient blend. In fact, the overall pattern of behavioral responses from Z-V transplant males was similar to that of V-Z males.
H. zea donor to H. virescens recipient (Z-V transplants)
Of the 130 Z-V transplant males assayed to both pheromone blends, 40% succeeded in contacting the odor source (Fig. 2B). In contrast to the V-Z transplant males, the majority of individuals exhibited a recipient-type behavioral preference. Although more Z-V transplant males exhibited positive behavioral responses, the distribution of males amongst the different categories was similar to the reciprocal V-Z group. A significantly greater proportion of Z-V males responded to the H. virescens blend compared with the H. zea blend (23.9% vs.1.5%; χ2 = 34.373; p < 0.0001). A lower, but not significant, proportion of Z-V transplant males responded to both blends compared with the H. virescens blend (14.6% vs. 23.9%; χ2 = 3.594; p = 0.058). However, the proportion of responders to both blends was significantly greater than the proportion to the H. zea blend alone (14.6% vs. 1.5%; χ2 = 17.114; p <0.0001).
Neurophysiology
Recordings were attempted in 54 V-Z and 53 Z-V individual bilateral transplant males. Activity in response to specific odorant stimuli or blends was noted in 23 V-Z and 20 Z-V males respectively. Of these 18 and 14 neurons in these respective transplant categories were physiologically characterized, digitized and analyzed further. The remaining recordings were not suitable for analysis either because the signal-to-noise ratio was not great enough or the complete set of odorant stimuli were not delivered due to deterioration in the quality of recording or loss of contact with the neuron.
V-Z transplants
In V-Z transplants 18 neurons were physiologically characterized in 15 individuals. In 9 individuals one or more PNs were stained with LY. Neurons were classified into 5 different physiological categories. Four of these categories have been characterized previously (Christensen et al., 1995; Vickers et al., 1998) while the fifth category was comprised of PNs with physiological profiles that have not previously been identified in H. virescens males.
1. Z11-16:Ald
Physiology
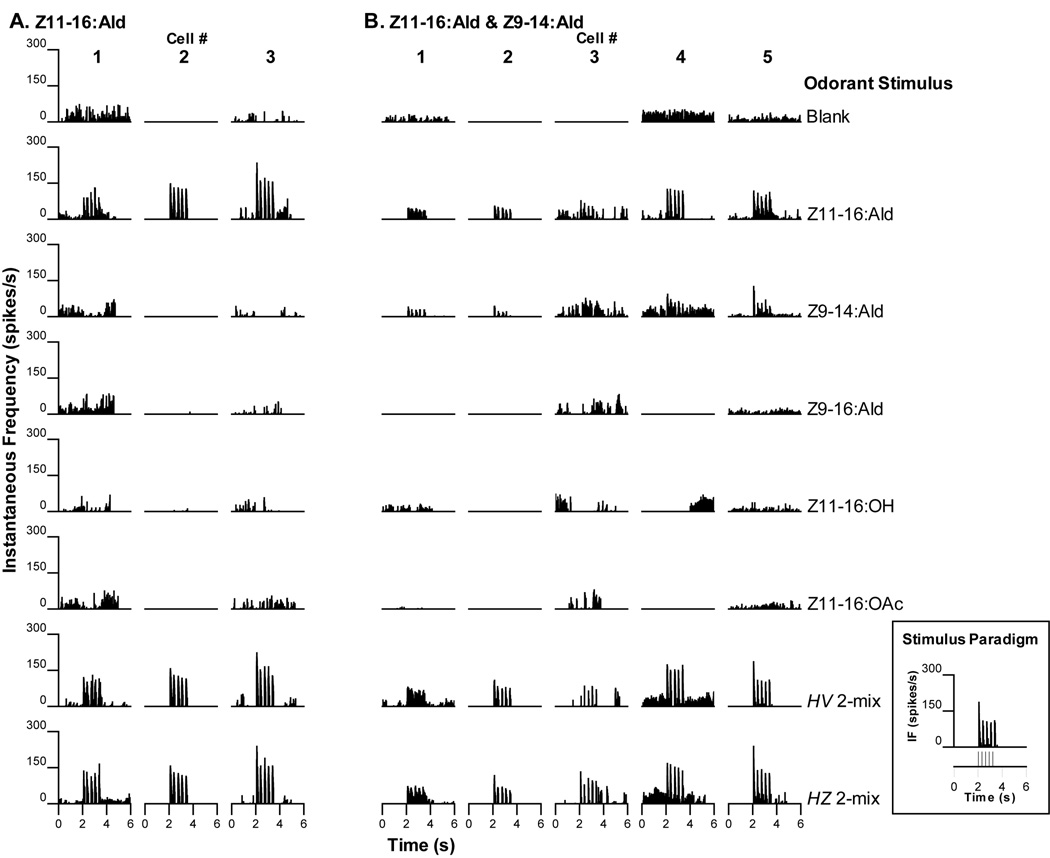
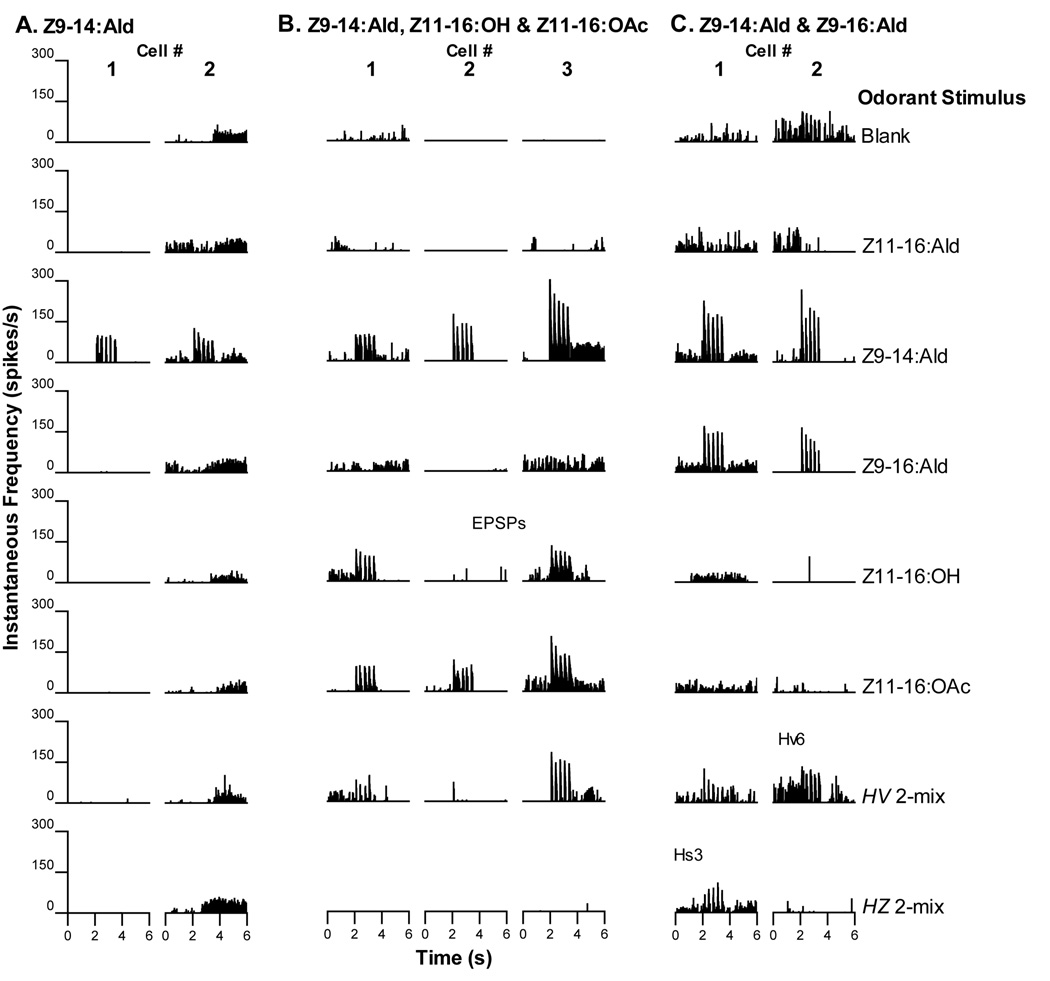
Three PNs responded with phasic bursts of action potentials to the delivery of each stimulus pulse of Z11-16:Ald (Fig. 3A). The responses of these PNs were qualitatively similar whether Z11-16:Ald was presented singly or as a part of a binary blend (Fig. 3A).
Figure 3.
Central PNs responsive to Z11-16:Ald were often encountered in H. virescens donor – H. zea recipient (V-Z) transplant males. A. Three PNs responded only to stimulation with Z11-16:Ald with no observable responses to other odorant stimuli. Blends containing Z11-16:Ald elicited similar responses as the Z11-16:Ald alone. B. As well as exhibiting responses to Z11-16:Ald, five PNs also responded weakly to Z9-14:Ald. Neurons with response profiles similar to those illustrated in A. and B. have been encountered before in normal H. virescens males (Christensen et al., 1995; Vickers et al., 1998). Inset: stimulus paradigm – 5 ×40ms stimulus pulses delivered over 1.2s with first pulse occurring at the 2s mark.
Morphology
In one preparation two PNs were stained (Fig. 5A, physiology shown in Fig. 3A, cell #2). Both neurons had somata in the medial cell body cluster and dendritic arborizations restricted only to the cumulus. In this particular case, the arrangement of MGC glomeruli resembled that of the recipient, H. zea, as opposed to the imaginal disc donor, H. virescens. Such PNs have been stained previously and shown to arborize in the cumulus of H. virescens and H. zea (Christensen et al., 1995; Vickers et al., 1998).
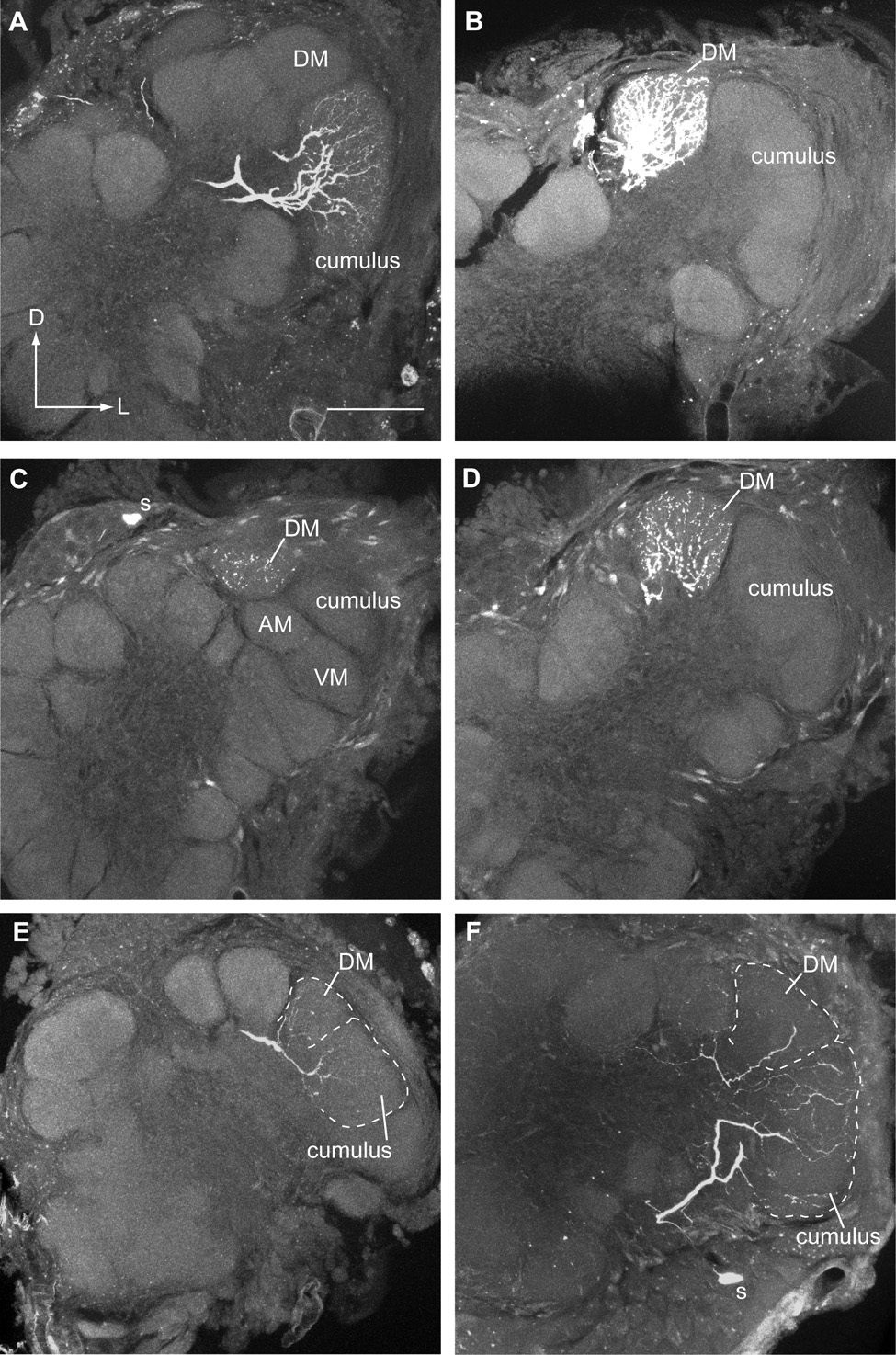
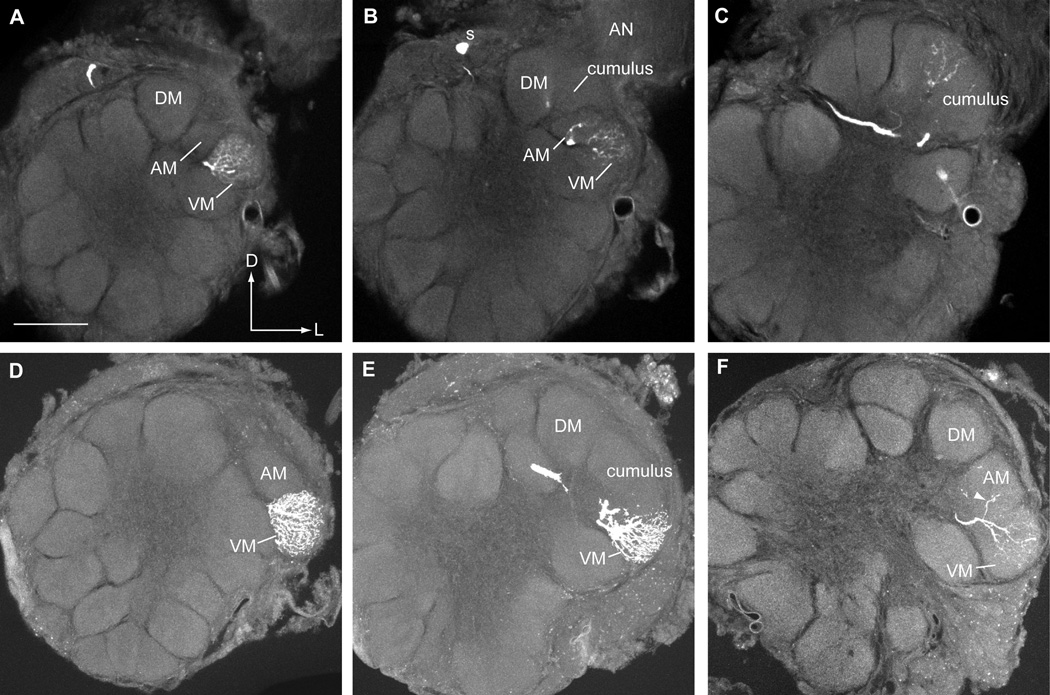
Figure 5.
Confocal photomicrographs of olfactory PNs stained with Lucifer Yellow in five different V-Z transplant males. Based upon their physiological responses, these PNs were expected to arborize within specific glomeruli of an induced H. virescens MGC. A. A PN responsive to Z11-16:Ald with a dense dendritic arbor restricted to the cumulus. As expected this cell responded strongly to stimulation with Z11-16:Ald (Fig. 3A, cell #2). This PN had a cell body in the medial cluster (not visible in this confocal iamge) and projected an axon through the IACT. The overall structure of the MGC was similar to that of a normal recipient H. zea . B. A uniglomerular PN with a dendritic arbor restricted to the DM glomerulus in an induced H. virescens MGC. This PN responded strongly to stimulation with Z9-14:Ald (Fig. 4A, cell #s 2 and 4 were recorded from this preparation). C–D. A second PN also responded exclusively to Z9-14:Ald (Fig. 4A, cell #1) and arborized extensively in the DM glomerular neuropil. C. All four glomeruli associated with a normal H. virescens MGC can be resolved in this section. D. In a confocal image taken from the subsequent histological section only the cumulus and DM glomerulus were visible, AM and VM having receded. The dendritic arbor clearly remained restricted to the DM glomerulus. E–F. Two PNs excited by both Z11-16:Ald and Z9-14:Ald exhibited differing morphologies. The morphological appearance of the MGC in both individuals appeared to be similar to that of the antennal disc donor, H. virescens. Dashed lines indicate the approximate borders of the named glomeruli. E. A medial cell body (not visible in this section) PN (Fig. 3B, cell #4) had dendritic arbors in both the cumulus and DM. F. A PN with a cell body (s) in the lateral cluster (Fig. 3B, cell #5) arborized extensively in the cumulus and had a secondary arbor in DM. This PN also had weak branches in other glomeruli outside the MGC. Although lateral cell body PNs have been documented previously in normal H. virescens males, relatively little is known about the physiological responses of this group of PNs. Scale bar = 50µm, same for all images. D, dorsal; L, lateral.
2. Z11-16:Ald and Z9-14:Ald
Physiology
Five PNs responded to stimulation with bursts of action potentials to presentation of either Z11-16:Ald or Z9-14:Ald (Fig. 3B). Generally, Z11-16:Ald pulses elicited a higher rate of spiking than Z9-14:Ald alone. Each odor pulse resulted in a discrete volley of action potentials that were most readily observed when either Z11-16:Ald, H. virescens 2-mix or H. zea 2-mix was the stimulus. Indeed, these neurons appeared to be more strongly excited by the presence of these latter blend stimuli in terms of actual peak frequencies achieved. This type of physiology has been reported previously in normal H. virescens males (Christensen et al., 1995). In addition, temporal resolution of the pulsatile stimulus delivery appeared to be enhanced in response to either binary blend. Because the responses to Z9-14:Ald alone were weaker, the ability of each PN to follow the temporal pattern of odor delivery was not as evident.
Morphology
Neurons were stained in three separate preparations (Fig. 5E, F; Fig. 6A–C). All three PNs had dendritic arbors in two or more glomeruli and in each case the MGC arrangement most closely resembled that of the imaginal disc donor, H. virescens. Two PNs had primary arbors in both the cumulus and DM (Fig. 5E, F; physiology shown in Fig. 3B, cell #s 4 and 5, respectively). The location of the neuronal cell bodies in these two preparations was, however, different (one medial and the other lateral). The lateral cell body neuron had additional weak arbors in other glomeruli outside the MGC (Fig. 5F). Little is known about PNs with somata in the lateral cell body cluster but they have been reported previously in H. virescens males (Vickers et al., 1998). The remaining preparation revealed a medial cell body neuron with a denser arbor in VM than either the cumulus or AM (Fig. 6A–C; physiology shown in Fig. 3B, cell #3). Christensen et al. (1995) reported a large group of PNs with this physiological profile in normal H. virescens males but none were successfully stained.
Figure 6.
Several olfactory PNs exhibited a normal physiological response profile but had dendritic arbors in an unexpected glomerular location or arborized within two or more glomeruli in V-Z transplant males. A–C. A series of confocal images at different depths through the antennal lobe revealed a single PN with a medial cell body (s) and dendritic arbors in VM, AM and the cumulus. The MGC clearly resembled that of the antennal imaginal disc donor, H. virescens. The most apparent arbor was in VM with weaker arbors in AM and the cumulus. This PN responded to Z11-16:Ald and Z9-14:Ald (Fig. 3B, cell #3). D–E. Confocal images from two consecutive histological sections revealed a PN with a uniglomerular arbor restricted to the VM glomerulus. The anatomy of the antennal lobe indicated that a donor MGC (H. virescens) had formed. This PN had a medial cell body (not visible in these images) and responded to stimulation with Z11-16:OAc (Fig. 4B, cell #1). Such PNs have been encountered in normal H. virescens males but arborized only in AM (Vickers et al., 1998). F. Another medial cell body PN sensitive to Z11-16:OAc (Fig. 4B, cell #2) was shown to have an unusual multiglomerular dendritic arbor. This PN arborized strongly in VM and had a weaker secondary arbor in AM (indicated by arrowhead). Again the arrangement of MGC glomeruli most closely matched that of the antennal disc donor, H. virescens. Scale bar = 50µm, same for all images. D, dorsal; L, lateral.
3. Z9-14:Ald
Physiology
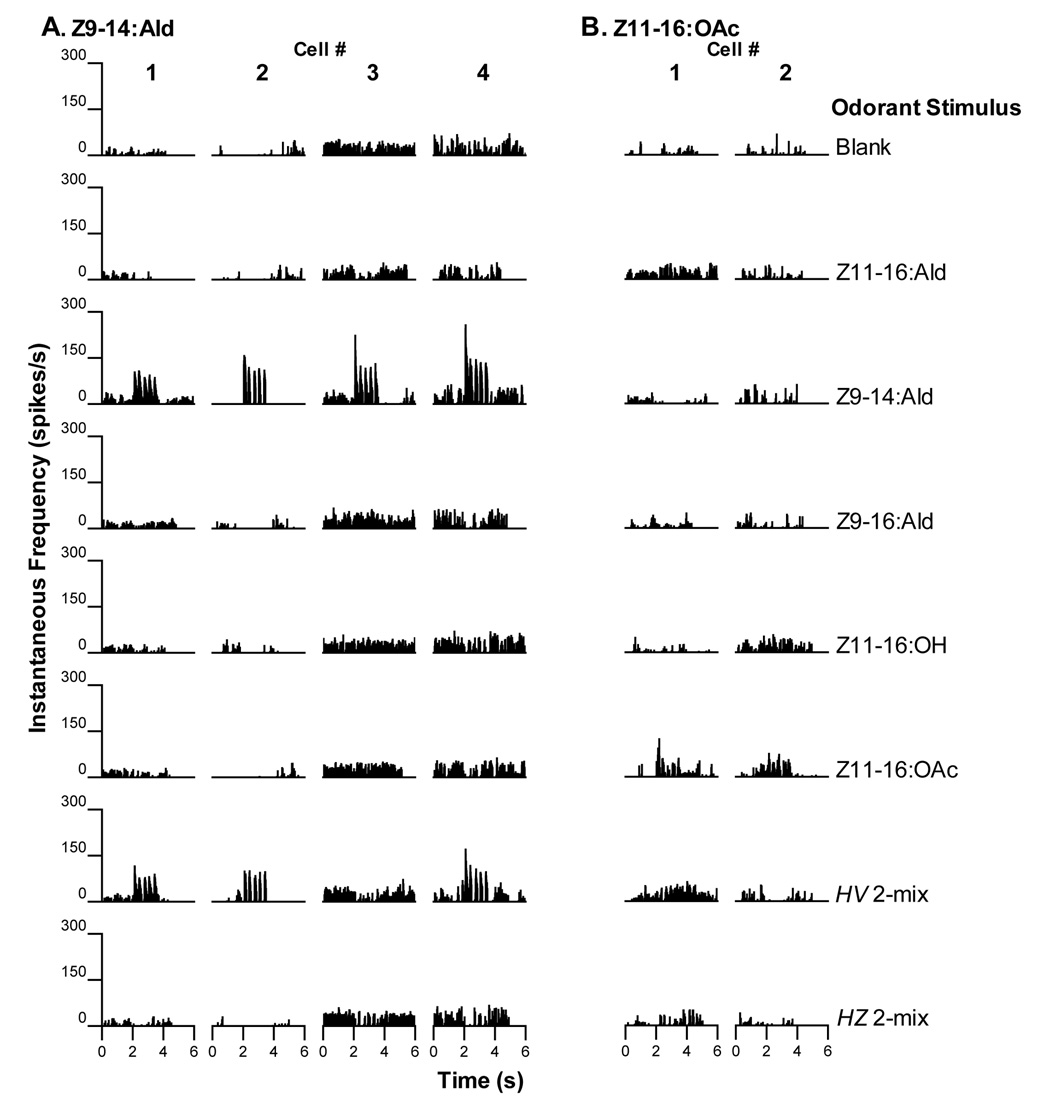
A third physiological category contained 4 PNs that responded only to stimulation with Z9-14:Ald (Fig. 4A). Lower instantaneous rates of spiking were achieved in response to pulses of the H. virescens 2-mix that contained a smaller amount of Z9-14:Ald (0.5ng loading dosage compared to 10ng Z9-14:Ald alone). Two PNs were recorded from the same individual (Fig. 4A, cell #s 2 and 4). Neurons with a similar physiological response profile were identified previously in normal H. virescens males (Christensen et al., 1995; Vickers et al., 1998).
Figure 4.
Two additional neuronal types have been encountered before in the antennal lobes of normal H. virescens males and were also encountered in V-Z transplant males A. Four PNs responded with excitatory bursts of action potentials to stimulation with Z9-14:Ald only. These PNs were not excited by other single odorant stimuli but responded to the H. virescens 2-mix blend that contained a small amount of Z9-14:Ald with the exception of cell #3. B. Two PNs responded weakly to stimulation with Z11-16:OAc. Other stimuli did not elicit excitatory responses.
Morphology
Projection neurons were stained in two individuals. In both cases, a single PN with a soma located in the medial cell body cluster and a dendritic arbor restricted to DM were evident (Fig. 5B–D; physiology shown in Fig. 4A, cell #s 1, 2 and 4). In both cases, the MGC structure resembled that of the imaginal disc donor, H. virescens. Previous studies revealed that Z9-14:Ald sensitive PNs with somata located in the medial cluster also had dendritic arbors restricted to DM (Vickers et al. 1998).
4. Z11-16:OAc
Physiology
Two neurons exhibited relatively weak excitatory responses when stimulated with pulses of Z11-16:OAc (Fig. 4B). Neither excitatory nor inhibitory responses were evoked by other stimuli. Vickers et al. (1998) described PNs with similar physiological response properties.
Morphology
In both cases a single PN with a medial cluster soma was stained with LY (Fig. 6D–F; physiology shown in Fig. 4B, cell #s 1 and 2) and the MGC arrangement appeared to be that of the donor, H. virescens. In one case, a strong dendritic arbor was restricted to the VM glomerulus whereas the other PN, as well as having a well-defined arbor in VM, appeared to also have a secondary dendritic process in the AM glomerulus. In their previous study Vickers et al. (1998) reported that medial cluster cell body PNs tuned to Z11-16:OAc arborized only in the AM glomerulus.
5. Unusual Physiology
Whereas the 4 physiologically defined PN groups described above have been identified beforehand in the ALs of normal H. virescens males, a number of PNs did not fit into any previously described category.
Physiology
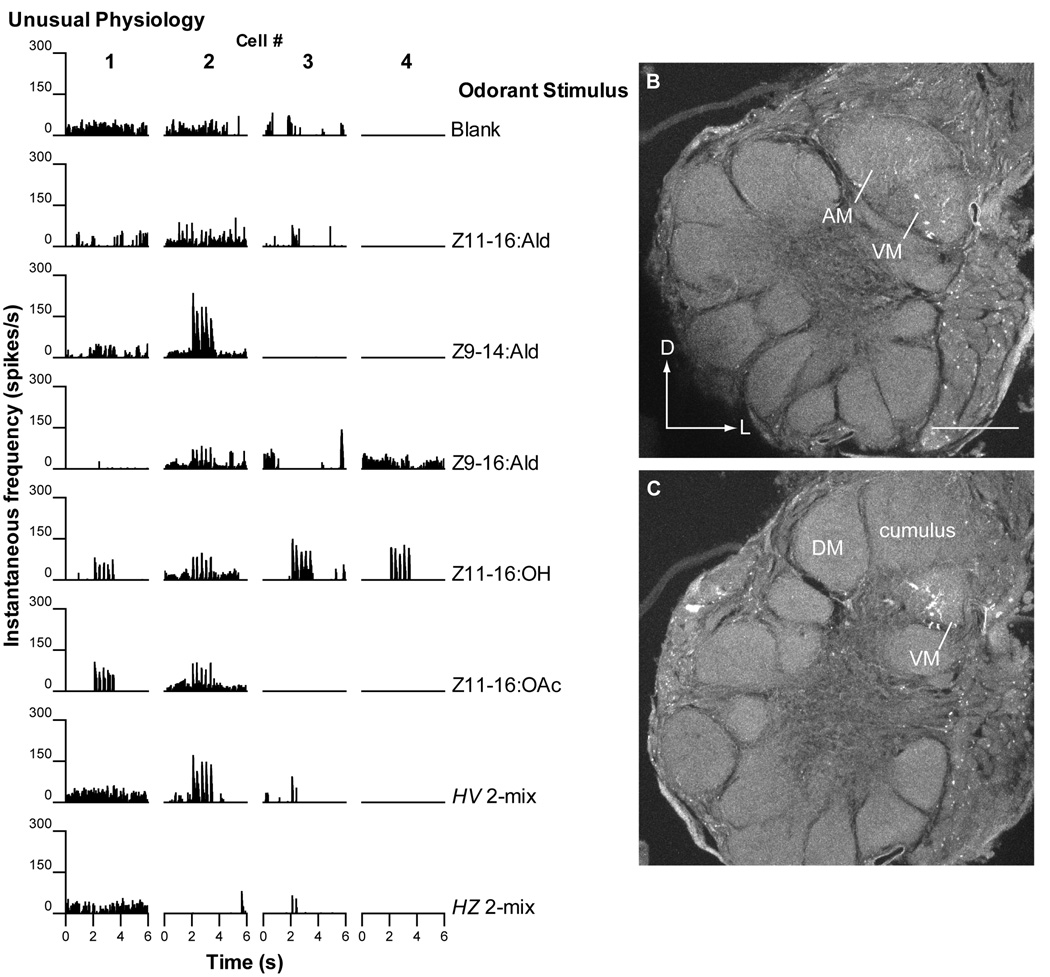
The four PNs placed into this category all exhibited different physiological profiles (Fig. 7A). One PN responded to Z9-14:Ald, Z11-16:OH and Z11-16:OAc (Fig. 7A, cell #1). Importantly, a second PN, in addition to responding to these three odorants also responded to Z9-16:Ald (Fig. 7A, cell #2). Perhaps significantly this individual moth also responded to the H. zea pheromone blend with upwind flight and location of the odor source. Two further PNs responded most strongly to Z11-16:OH (Fig. 7A, cell #s 3 and 4). One of these PNs also responded weakly to Z11-16:Ald.
Figure 7.
Some olfactory PNs in V-Z transplant males exhibited unusual physiology and morphology. A. Four olfactory PNs in V-Z transplant males displayed responses to one or more odorants that placed them in a category outside those described previously in normal H. virescens. One PN (cell #1) responded to Z9-14:Ald, Z11-16:OH and Z11-16:OAc. In addition to the 3 odorants that stimulated cell 1, a second PN (cell #2) in a different individual also responded to Z9-16:Ald. The response to Z9-16:Ald is important because many of these transplant males responded behaviorally to the H. zea pheromone blend suggesting the existence of a pathway for detection of Z9-16:Ald (which has not been shown to drive olfactory PNs in previous studies of H. virescens males). Two additional PNs responded to Z11-16:OH (cell #s 3 and 4) with one responding weakly to Z11-16:Ald (cell #3). These latter 3 PNs (cell #s 2–4) exhibited physiological response profiles that were also dissimilar to those previously reported for normal H. zea males. B–C. In one preparation a single PN was stained. B. Confocal images from one section revealed only sparse dendritic arbors in the AM glomerulus and a slightly denser arbor in the VM glomerulus. C. In the subsequent histological section, AM has receded, but evidence of the dendritic arbor in VM remained. This arrangement of glomeruli indicated the development of an H. virescens donor antennal imaginal disc. Interestingly, the excitatory responses to Z9-14:Ald, Z11-16:OH and Z11-16:OAc (Fig. 7A, cell #1) indicated that this PN might be integrating normal H. virescens ORN inputs to both the AM and VM glomeruli (see Fig. 1). PNs with such a profile would be expected in the DM-A glomerulus of a normal H. zea male leading to the suggestion that in the absence of a DM-A structure the PNs that typically arborize there seek other structures - in this case, the induced and topographically novel AM and VM glomeruli. Scale bar = 50µm, same for all images. D, dorsal; L, lateral.
Morphology
One preparation in this category had a single stained PN (Fig. 7B, C). This PN had a soma in the medial cell body cluster and a multiglomerular dendritic arbor with weaker branches in the AM glomerulus and more robust staining in the VM glomerulus of an H. virescens MGC. Two PNs were characterized in this individual (Fig. 7A, cell #s 1 and 4). Based upon the length of the recording and period of current injection, the PN that responded to Z9-14:Ald, Z11-16:OH and Z11-16:OAc was most likely stained (Fig. 7A, cell #1).
Z-V transplants
In Z-V transplants, 15 PNs recorded from 14 individuals were physiologically characterized. Based upon their physiology these PNs were classified into four categories, all of which have been identified in normal H. zea males previously (Christensen et al., 1991; Vickers et al., 1998).
1. Z11-16:Ald
Physiology
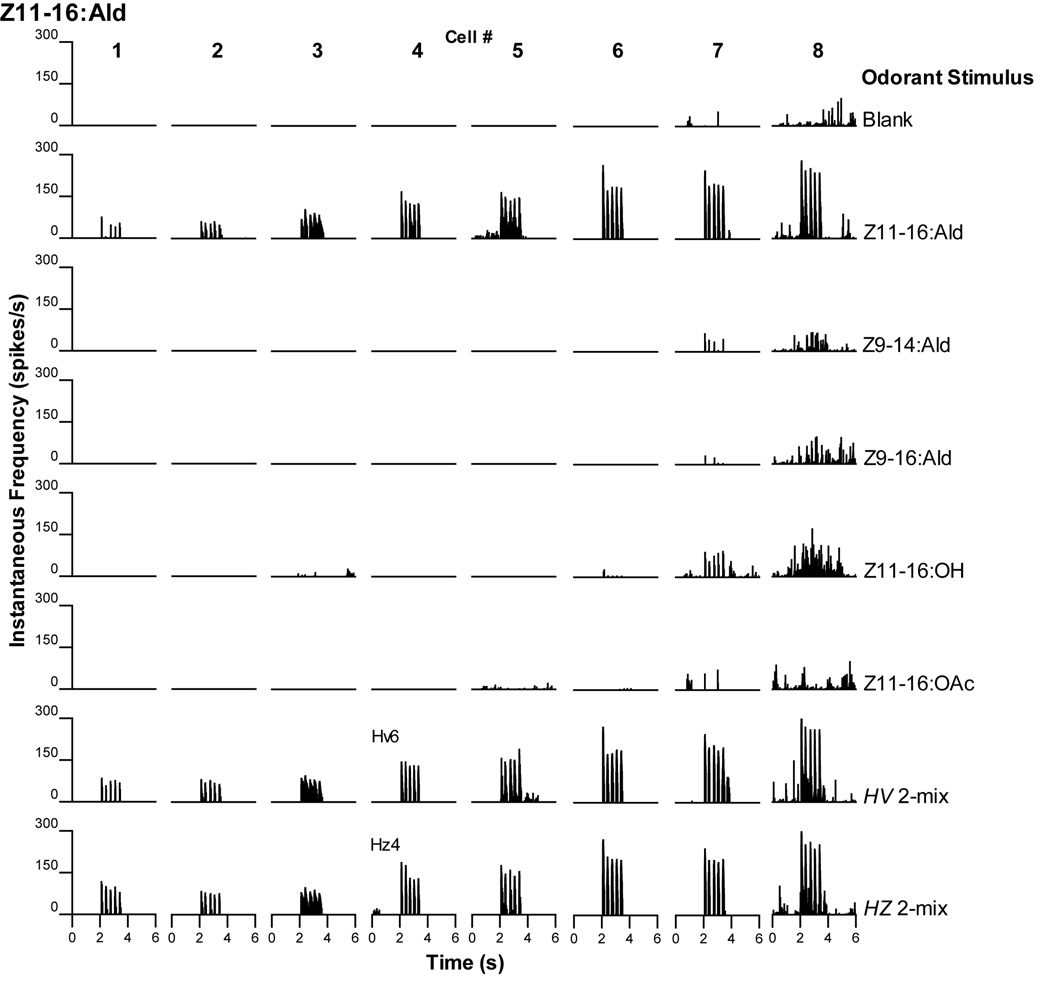
Eight PNs responded with excitatory bursts of action potentials to presentation with Z11-16:Ald (Fig. 8). The level of excitation (as determined by the peak instantaneous spike frequencies in each volley of action potentials) varied amongst these neurons. Six PNs were exclusively stimulated by Z11-16:Ald and there appeared to be little difference between responses to Z11-16:Ald presented alone or as part of a mixture (Fig. 8, cell #s 1–6). The remaining two PNs also exhibited positive responses to additional compounds (Fig. 8, cell #s 7 and 8). Both neurons clearly responded to Z11-16:OH but much more weakly than to Z11-16:Ald. Responses elicited by other compounds were even weaker and often only barely above background.
Figure 8.
The most commonly encountered olfactory PNs in Z-V transplant males responded to Z11-16:Ald. All PNs responded to presentation of Z11-16:Ald alone with little observable difference to mixtures containing the same dosage of this odorant. Two PNs responded to stimulation with other odorants (cell #s 7 and 8) but these responses were much less robust than those to Z11-16:Ald. Hv6, H. virescens 6-mix used as stimulus; Hz4, H. zea 4-mix used as stimulus (see Materials and Methods for details).
Morphology
Neurons were stained in 4 preparations. In three instances dendritic arbors were restricted to the cumulus (Fig. 10). Two of these preparations had neurons with medial cell bodies (one appeared to have an H. zea MGC configuration whereas the other appeared to be H. virescens) (Fig. 10A–D; physiology shown in Fig. 8, cell #s 4 and 6). In the third individual the neuronal cell body was located in the lateral cluster and the MGC had an H. zea arrangement (Fig. 10E; physiology shown in Fig. 8, cell #8). In a fourth preparation at least two neurons were stained with LY, one local interneuron (LN) and one PN (images not shown). Although the MGC was that of H. virescens, it appeared that the PN dendritic arbors were likely restricted to the cumulus but precise distinction between LN and PN arbors was not possible in this instance.
Figure 10.
Confocal photomicrographs of olfactory PNs stained with Lucifer Yellow from four Z-V transplant males had dendritic arbors restricted to a single glomerulus. In three of the preparations shown here the organization of MGC glomeruli was clearly that of the antennal imaginal disc donor, H. zea (A–C, E, F). A–C. Confocal images of 3 different histological sections through the same preparation revealed a single PN with a cell body (s) located in the medial cluster and an extensive dendritic arbor restricted to the cumulus. This PN responded to Z11-16:Ald (Fig. 8, cell #4). D. A Z11-16:Ald PN (Fig. 8, cell #6) with a medial cell body (s) arborized in the cumulus. In this preparation the MGC resembled that of a normal recipient (H. virescens) rather than the antennal imaginal disc donor (H. zea). E. PNs with somata in the lateral cell body cluster (s) are less common in normal H. zea and H. virescens males. In this confocal image taken with a 20X objective from the wholemount, at least two neurons were stained with overlapping dendritic arbors apparently restricted to the cumulus. These PNs projected directly to the lateral horn via the outer antenno-cerebral tract. Physiological recordings from this preparation revealed strong responses to stimulation with Z11-16:Ald but also excitatory responses to other odorants (Fig. 8, cell #8). F. A weakly stained PN with a medial cell body (s) and arbors (indicated by arrowheads) restricted to the DM-A glomerulus. This PN responded to stimulation with Z9-14:Ald, Z11-16:OH and Z11-16:OAc (Fig. 9B, cell #3). Scale bars = 50µm (same for A–D, F) and 100µm (E). AN, antennal nerve; D, dorsal; L, lateral.
2. Z9-14:Ald
Physiology
Two PNs were excited only by presentation of Z9-14:Ald to the ipsilateral antenna (Fig. 9A). One PN appeared to be inhibited by stimulation with Z11-16:Ald (Fig. 9A, cell #2). Neither PN was excited by the H. virescens 2-mix even though that mixture contained a small amount of Z9-14:Ald. In one case, this lack of response to the mixture might have been due to inhibitory input from Z11-16:Ald pathways that suppressed any Z9-14:Ald-mediated excitatory activity. Neurons with a similar physiological profile were reported previously in normal H. zea males (Christensen et al. 1991; Vickers et al. 1998).
Figure 9.
Additional olfactory PNs in Z-V transplant males exhibited physiological response properties that were similar to those described previously in studies of normal H. zea males indicating that the donor antennal imaginal disc not only controls the fate of the glomerular architecture but also the physiological profiles of central PNs. A. Two PNs were excited only when presented with Z9-14:Ald. B. Three PNs exhibited excitatory responses to 3 odorants: Z9-14:Ald, Z11-16:OH and Z11-16:OAc. Cell #1 was approximately equally sensitive to all 3 odorants. Cell #2 exhibited stronger responses to Z9-14:Ald compared to Z11-16:OAc. Although stimulation with Z11-16:OH did not elicit action potentials, strong excitatory post-synaptic potentials (EPSPs) were observed. Cell #3 also responded most vigorously to Z9-14:Ald with weaker responses to Z11-16:OAc and Z11-16:OH. This type of PN would be expected to arborize in the DM-A glomerulus of a normal H. zea MGC. C. Two PNs responded to stimulation with both Z9-14:Ald and Z9-16:Ald. Responses to Z9-14:Ald were slightly more vigorous compared to Z9-16:Ald. Blends containing either of these odorants elicited weak responses likely because the dosages of these odorants were much lower in each of the blends. Neurons with this type of physiology were encountered in H. zea males and arborized in the DM-P glomerulus (Fig. 1). Hv6, H. virescens 6-mix used as stimulus; Hs3, H. subflexa 3-mix used as stimulus (see Materials and Methods for details).
Morphology
Both preparations had one or more stained neurons (images not shown). In one AL a strongly stained LN (cell body in the lateral cluster) and a PN with a dendritic arbor restricted to a single glomerulus were stained. It was not possible to determine if this glomerular location was within the confines of the MGC in this specimen. In the second preparation, dendritic arbors of two PNs were restricted to two posteriorly located glomeruli neither of which belonged to the MGC.
3. Z11-16:OH/Z11-16:OAc/Z9-14:Ald
Physiology
A third group of PNs also exhibited strong responses to stimulation with Z9-14:Ald. However, unlike the previous category, these PNs also were excited by presentation of Z11-16:OH and Z11-16:OAc (Fig. 9B). One PN failed to produce any action potentials when stimulated with Z11-16:OH but clear excitatory post-synaptic potentials were observed (Fig. 9B, cell #2). A group of PNs were previously identified in normal H. zea that likely correspond to the PNs reported here. While these PNs responded to both Z11-16:OAc and Z9-14:Ald, Z11-16:OH was not used as a test odorant in that study (Vickers et al., 1998).
Morphology
One individual had a stained PN identified as belonging to this category. The single PN had a medial cell body and a dendritic arbor restricted to the DM-A glomerulus of a donor H. zea MGC (Fig. 10F; physiology shown in Fig. 9B, cell #3). PNs responsive to Z9-14:Ald and Z11-16:OAc were shown to arborize in the H. zea DM-A glomerulus by Vickers et al. (1998).
4. Z9-16:Ald/Z9-14:Ald
Physiology
Two PNs were classified as responding to both Z9-14:Ald and Z9-16:Ald (Fig. 9C). In each case, responses were stronger to Z9-14:Ald than Z9-16:Ald. Both PNs responded to the small amount of Z9-14:Ald present in the H. virescens 2-mix. However, only one PN responded to presentation of a mixture containing Z9-16:Ald (Fig. 9C, cell #1 - likely because this particular PN was tested to a mixture containing a higher dosage of Z9-16:Ald than that contained in the H. zea 2-mix to which most cells were tested in these experiments).
Morphology
Neither PN recorded from in this category was stained. A previous study revealed that such PNs had dendritic arbors restricted to the DM-P glomerulus of the normal H. zea MGC (Vickers et al., 1998).
Discussion
Pre-metamorphic transplantation of antennal imaginal discs between two different species of heliothine moth combined with behavioral, neuroanatomical and neurophysiological examination of olfactory PNs in individual post-metamorphic males has illuminated a variety of issues relating to plasticity in the development and functional organization of olfactory systems with respect to the discrimination of odor mixtures.
Plasticity in PN-Glomerular Associations
The results of the present study (see also Vickers et al. 2003), clearly support the hypothesis that peripheral sensory afferents dictate the final structural arrangement of glomeruli in the central olfactory neuropil (Tolbert et al., 2004). Results of recent studies with Drosophila have indicated that PNs also are pre-specified to grow to certain topographic locations of the antennal lobe even before the arrival of sensory afferents (Jeffris et al. 2004). However, our transplant results show that central PNs derived from recipient tissue arborized within topographically distinct donor-induced glomeruli, which differed spatially from those expected in the normal recipient, and established functional synaptic contacts. These results imply that there must be some plasticity in the final patterns of PN dendritic arborization. For example, in V-Z transplants, PNs derived from the recipient H. zea tissue invaded the induced and therefore novel AM and VM glomeruli (Fig. 6, Fig 7), PNs that presumably would have arborized in the DM-A glomerulus of the normal recipient H. zea MGC (see Fig. 1). The response profiles of PNs typically present in these structures (Z11-16:OAc in AM and Z11-16:OH/Z9-14:Ald in VM) match the expected physiological response profiles of PNs in the DM-A glomerulus of normal H. zea males (Z11-16:OAc/Z9-14:Ald/Z11-16:OH). We speculate that targeting cues produced by the H. virescens C-type ORNs growing into the H. zea brain are recognized by PNs that normally invade DM-A. Furthermore, unusual responses associated with PNs arborizing within AM and VM suggests that the spatial location of a particular glomerulus (imposed by the in-growing sensory axons) might be under separate control from the ultimate physiological tuning properties of the sensory neurons that terminate in a particular structure.
Plasticity in Discrimination of Agonist/Antagonist Mixtures
Our transplant studies also show that changes occurred in the discrimination of agonist/antagonist blends indicating that, whereas the MGC configuration of glomeruli was largely dictated by donor sensory afferents, the functional interpretation of those inputs depended on the specific PNs that arborized in the novel donor glomerular array. In H. zea, for example, the satellite DM-P glomerulus conveyed information about the presence of Z9-16:Ald, the agonist minor component in the H. zea pheromone, while PNs in the DM-A glomerulus responded to the antagonistic odorants Z9-14:Ald and Z11-16:OAc (and likely Z11-16:OH as well) (Vickers et al., 1998). Given these pathways, the upwind flight response of V-Z transplants to the H. virescens blend was unexpected because the ratio of Z9-14:Ald used was antagonistic to H. zea males (Vickers et al., 1991). Our recordings indicated that Z9-14:Ald-specific PNs existed in the V-Z transplant AL (Fig. 4A), as they do in H. zea males. When successfully stained, these PNs arborized within the DM glomerulus, a structure induced by the sensory afferents of the H. virescens donor imaginal disc. Our behavioral results indicated that these Z9-14:Ald-specific PNs participated in an agonistic pathway and they may be the same neurons that would normally arborize within the corresponding recipient H. zea agonist glomerulus, DM-P (Fig. 1). In addition, we noted several PNs that responded to both Z11-16:Ald and Z9-14:Ald when presented singly (Fig. 3B). Neurons with a similar specificity were reported previously in the ALs of H. virescens and H. zea (Christensen et al. 1991, 1995). In H. zea males, these PNs were multiglomerular, arborizing in all 3 MGC glomeruli (Christensen et al., 1991). Interestingly, in V-Z transplant males such PNs were also multiglomerular (Fig. 5E, F; Fig. 6A–C) but their arborization patterns were not consistent, suggesting that Z9-14:Ald information might be available in MGC locations other than DM (PNs associated with the VM glomerulus in H. virescens males responded to Z11-16:OH and Z9-14:Ald). It seems likely that these multiglomerular PNs played some role in the behavioral preference that V-Z transplant males exhibited for the H. virescens pheromone blend.
For the agonist pathways described above to result in upwind flight behavior in V-Z transplants, the H. zea brain would also need to ignore other Z9-14:Ald inputs that might result in an antagonistic response. Specifically this involves pathways in the H. virescens VM glomerulus (Fig. 1). We observed variability in both the dendritic aborization patterns and physiological response profiles of PNs invading the induced AM and VM glomeruli in V-Z transplant males. Two PNs responded to Z11-16:OAc (Fig. 4B), one arborizing in VM and the other in both AM and VM (Fig. 6D–F) (expected target AM only – see Fig. 1). A third PN responded to Z11-16:OH and Z9-14:Ald, in addition to Z11-16:OAc, and also arborized in AM and VM (Fig. 7). In H. zea males, PNs responding to all three compounds would be expected in the DM-A glomerulus, and signify the presence of behavioral antagonists. Thus PNs arborizing in AM and VM ranged in physiological profile from H. virescens types to H. zea types and if these neurons are normally associated with the antagonistic DM-A glomerulus in H. zea males the variability observed in transplant PN responses also implies that V-Z transplant males might exhibit variable behavioral responses to the inclusion, or exclusion, of antagonistic odorants into an agonist blend.
The preference of the reciprocal Z-V transplant males for the blend containing Z9-14:Ald was similarly unexpected because Z9-14:Ald input normally associated with other MGC regions did not mediate antagonistic behavioral responses. For example, in normal H. zea, Z9-14:Ald activated PNs were associated with the DM-A glomerulus, neurons that were also excited by Z11-16:OAc (and likely Z11-16:OH). All three of these compounds were antagonistic to H. zea male flight behavior (Vickers et al., 1991; Fadamiro et al., 1999; Quero and Baker, 1999). However, even though such PNs were found here in Z-V transplant males (including one PN in a male that responded to the H. virescens blend) with arbors restricted to DM-A (Fig. 10F), males were still able to respond to the H. virescens blend suggesting that the H. virescens brain interpreted the Z9-14:Ald inputs through the induced H. zea MGC array in an agonistic manner. Perhaps PNs normally associated with the benign VM location in normal H. virescens males were recruited to this novel location. If so these males should also fail to be antagonized by Z11-16:OAc, which is normally antagonistic to H. virescens males.
The fact that many individual Z-V transplant males responded well to both H. virescens and H. zea blends indicated that they were unable to effectively discriminate between Z9-14:Ald and Z9-16:Ald. This might be due to the existence of PNs activated by both odorants (Fig. 9C). However, if this central pathway mediated attraction to both blends then one would predict that equal numbers of males should respond only either to the H. virescens or H. zea blends, which they did not (Fig. 2). The lack of antagonism by Z9-14:Ald (indicated by the preference of males for the H. virescens blend) provided a clear indication that other central pathways must play a role in mediating agonistic behavioral responses in Z-V transplant males.
The lack of expected olfactory discrimination as measured by behavioral responses of both transplant types despite the existence of appropriate olfactory pathways has several possible explanations. These include a failure to activate antagonistic pathways because certain inputs have a lowered synaptic strength in the AL. Such effects might also be compounded by the altered spatial arrangement of the MGC that disrupts interactions mediated by inhibitory local interneurons, changing the across-label activity patterns (Christensen and White, 2000) between glomeruli thereby allowing males to respond positively to blends that would otherwise be discriminated against.
Plasticity in Odorant Inputs
A third point from our transplant studies relating to plasticity in the interactions between peripheral and central processing pathways is that in addition to AL PNs being able to arborize in novel glomerular locations, they also can process information from a novel sensory input. Sensory ORNs responding to Z9-16:Ald, for example, have not been previously identified on the antennae of normal H. virescens males, despite extensive surveys (Almaas and Mustaparta, 1990, 1991; Baker et al., 2004). However, the ability of V-Z transplant males to respond to the H. zea blend (for which Z9-16:Ald is essential minor pheromone component) suggested that such inputs did occur. A previous electrophysiological study of antennal ORNs provided evidence for this induced H. zea-type sensory input on the transplant H. virescens antenna (Ochieng et al., 2003). In the present study a single PN in a V-Z transplant responded to Z9-16:Ald, as well as three other odorants (Fig. 7A). This unique type of physiological profile occurred in a male that responded behaviorally to the H. zea blend indicating that this pathway might well have activated agonistic circuits in the brain. While more recordings and stains are needed to determine the glomerular associations of such PNs, it seems reasonable to speculate from the multi-odorant sensitivity of these neurons that they will be associated with the AM and/or VM glomeruli. It is in these two latter glomeruli that PNs sensitive to Z11-16:OAc and Z11-16:OH/Z9-14:Ald respectively have been identified in H. virescens males (Vickers et al., 1998; Kleineidam et al., in prep; Vickers, personal observations). Thus, integration across these two glomeruli, in addition to the appearance of Z9-16:Ald sensitivity, could account for the physiological responses observed here.
Acknowledgements
We thank Karrie Catropia and Callie Musto (Cornell University) for rearing the heliothine moths and Karrie for conducting many of the flight tunnel tests. Kim Iceman and David Kelly (both supported by the Biology Undergraduate Research Program at the University of Utah) assisted with dissection, histology and confocal imaging of transplant males. A University of Utah Research Foundation seed grant (to NJV) provided initial support for some of the studies reported here.
Grant Sponsor: National Institutes of Health; Grant number: 1 R55 DC04443-01 (CEL)
References
- Almaas TJ, Mustaparta H. Pheromone reception in tobacco budworm moth, Heliothis virescens. J Chem Ecol. 1990;16:1331–1347. doi: 10.1007/BF01021030. [DOI] [PubMed] [Google Scholar]
- Almaas TJ, Mustaparta H. Heliothis virescens: response characteristics of receptor neurons in sensilla trichodea type 1 and 2. J Chem Ecol. 1991;17:953–972. doi: 10.1007/BF01395602. [DOI] [PubMed] [Google Scholar]
- Almaas TJ, Christensen TA, Mustaparta H. Chemical communication in heliothine moths: Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol A. 1991;169:249–258. [Google Scholar]
- Baier H, Korsching SI. Olfactory glomeruli in the zebrafish olfactory system form an invariant pattern and are identifiable across animals. J Neurosci. 1994;14:219–230. doi: 10.1523/JNEUROSCI.14-01-00219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TC, Ochieng’ SA, Cossé AA, Lee SG, Todd JL, Quero C, Vickers NJ. A comparison of responsces from olfactory receptor neurons of Heliothis subflexa and Heliothis virescens to components of their sex pheromone. J Comp Physiol A. 2004;190:155–165. doi: 10.1007/s00359-003-0483-2. [DOI] [PubMed] [Google Scholar]
- Berg BG, Tumlinson JH, Mustaparta H. Chemical communication in heliothine moths: Receptor neuron responses to pheromone compounds and formate analogues in the male tobacco budworm moth Heliothis virescens. J Comp Physiol A. 1995;177:527–534. [Google Scholar]
- Berg BG, Almaas TJ, Bjaalie JG, Mustaparta H. The macroglomerular complex of the antennal lobe in the tobacco budworm moth Heliothis virescens: specified subdivision in four compartments according to information about biologically significant compounds. J Comp Physiol A. 1998;183:669–682. [Google Scholar]
- Christensen TA, White J. Representation of olfactory information in the brain. In: Finger TE, Silver WL, Restrepo D, editors. The neurobiology of taste and smell. vol 2. New York: Wiley-Liss; 2000. pp. 201–232. [Google Scholar]
- Christensen TA, Mustaparta H, Hildebrand JG. Chemical communication in heliothine moths: Central processing of intra- and interspecific olfactory messages in the male corn earworm moth Helicoverpa zea. J Comp Physiol A. 1991;169:59–274. [Google Scholar]
- Christensen TA, Mustaparta H, Hildebrand JG. Chemical communication in heliothine moths: Parallel pathways for information processing in the macroglomerular complex of the male tobacco budworm moth Heliothis virescens. J Comp Physiol A. 1995;177:545–557. [Google Scholar]
- Cossé AA, Todd JL, Baker TC. Neurons discovered in male Helicoverpa zea antennae that correlate with pheromone-mediated attraction and interspecific antagonism. J Comp Physiol A. 1998;182:585–594. [Google Scholar]
- Fadamiro HY, Cossé AA, Baker TC. Fine-scale resolution of finely spaced pheromone and antagonist filaments by flying male Helicoverpa zea. J Comp Physiol A. 1999;185:131–141. [Google Scholar]
- Flanagan D, Mercer AR. An atlas and 3-D reconstruction of the antennal lobes in the worker honeybee, Apis mellifera L. (Hymenoptera: Apidae) Int J Morphol Embryol. 1989;18:145–159. [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Mustaparta H. Calcium responses to pheromone and plant odours in the antennal lobe of the male and female moth Heliothis virescens. J Comp Physiol A. 2000;186:1049–1063. doi: 10.1007/s003590000156. [DOI] [PubMed] [Google Scholar]
- Hansson BS, Almaas TJ, Anton S. Chemical communication in heliothine moths: Antennal lobe projection patterns of pheromone-detecting olfactory receptor neurons in the male Heliothis virescens (Lepidoptera: Noctuidae) J Comp Physiol A. 1995;177:535–543. [Google Scholar]
- Hildebrand JG, Shepherd GM. Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci. 1997;20:595–631. doi: 10.1146/annurev.neuro.20.1.595. [DOI] [PubMed] [Google Scholar]
- Jefferis GSXE, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Jefferis GSXE, Vyas RM, Berdnik D, Ramaekers A, Stocker RF, Tanaka NK, Ito K, Luo L. Developmental orign of wiring specificity in the olfactory system of Drosophila. Development. 2004;131:117–130. doi: 10.1242/dev.00896. [DOI] [PubMed] [Google Scholar]
- Joerges J, Kuttner A, Galizia C, Menzel R. Representations of odoours and odour mixtures visualized in the honeybee brain. Nature. 1997;387:285–288. [Google Scholar]
- Jurenka RA, Jacquin E, Roelofs WL. Control of the pheromone biosynthetic pathway in Helicoverpa zea by the pheromone biosynthesis activating neuropeptide. Arch Insect Biochem Physiol. 1991;17:81–91. [Google Scholar]
- Laissue PP, Reiter C, Hiesinger RR, Halter S, Fischbach KF, Stocker RF. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J Comp Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci. 2002;5:557–565. doi: 10.1038/nn0602-859. [DOI] [PubMed] [Google Scholar]
- Linn CE, Jr, Poole K, Zhang A, Roelofs W. Pheromone-blend discrimination by European corn borer moths with inter-race and inter-sex antennal transplants. J Comp Physiol A. 1999;184:273–278. [Google Scholar]
- Ochieng SA, Poole K, Linn CE, Jr, Vickers NJ, Roelofs WL, Baker TC. Unusual pheromone receptor neuron responses in heliothine moth antennae derived from inter-species imaginal disc transplantation. J Comp Physiol A. 2003;189:19–28. doi: 10.1007/s00359-002-0371-1. [DOI] [PubMed] [Google Scholar]
- Oland LA, Tolbert LP. Multiple factors shape development of olfactory glomeruli: Insights from an insect model system. J Neurobiol. 1996;30:92–109. doi: 10.1002/(SICI)1097-4695(199605)30:1<92::AID-NEU9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Quero C, Baker TC. Antagonistic effects of (Z)-11-Hexadecen-1-ol on the pheromone-mediated flight of Helicoverpa zea (Boddie) (Lepidoptera:Noctuidae) J Insect Behav. 1999;12:701–710. [Google Scholar]
- Rospars JP. Invariance and sex-specific variations of the glomerular organization in the antennal lobes of a moth, Mamestra brassicae and a butterfly, Pieris brassicae. J Comp Neurol. 1983;220:80–96. doi: 10.1002/cne.902200108. [DOI] [PubMed] [Google Scholar]
- Rospars JP, Hildebrand JG. Anatomical identification of glomeruli in the antennal lobes of the male sphinx moth, Manduca sexta. Cell Tissue Res. 1992;270:205–227. doi: 10.1007/BF00328007. [DOI] [PubMed] [Google Scholar]
- Rössler W, Tolbert LP, Hildebrand JG. Early formation of sexually dimorphic glomeruli in the developing olfactory lobe of the brain of the moth Manduca sexta. J Comp Neurol. 1998;396:415–428. [PubMed] [Google Scholar]
- Rössler W, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J Neurosci. 1999a;19:9865–9877. doi: 10.1523/JNEUROSCI.19-22-09865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler W, Randolph PW, Tolbert LP, Hildebrand JG. Axons of olfactory receptor cells of transsexually grafted antennae induce development of sexually dimorphic glomeruli in Manduca sexta. J Neurobiol. 1999b;38:521–541. doi: 10.1002/(sici)1097-4695(199903)38:4<521::aid-neu8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Hildebrand JG. Structure and development of antennae in a moth Manduca sexta. Dev Biol. 1976;51:282–299. [PubMed] [Google Scholar]
- Schneiderman AM, Matsumoto SG, Hildebrand JG. Trans-sexually grafted antennae influence development of sexually dimorphic neurons in moth brain. Nature. 1982;298:844–846. [Google Scholar]
- Schneiderman AM, Hildebrand JG, Brennan MM, Tumlinson JH. Trans-sexually grafted antennae alter pheromone-directed behaviour in a moth. Nature. 1986;323:801–803. doi: 10.1038/323801a0. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Oland LA, Tucker ES, Gibson NJ, Higgins MR, Lipscomb BW. Bidirectional influences between neurons and glial cells in the developing olfactory system. Prog Neurobiol. 2004;73:73–105. doi: 10.1016/j.pneurobio.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Vetter RS, Baker TC. Behavioral responses of male Heliothis virescens in a sustained-flight tunnel to combinations of seven compounds identified from female sex pheromone glands. J Chem Ecol. 1983;9:747–759. doi: 10.1007/BF00988780. [DOI] [PubMed] [Google Scholar]
- Vetter RS, Baker TC. Behavioral responses of male Heliothis zea moths in a sustained-flight tunnel to combinations of 4 compounds identified from female sex pheromone gland. J Chem Ecol. 1984;10:193–202. doi: 10.1007/BF00987848. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA. Functional divergence of spatially conserved olfactory glomeruli in two related moth species. Chem Senses. 2003;28:325–338. doi: 10.1093/chemse/28.4.325. [DOI] [PubMed] [Google Scholar]
- Vickers NJ, Christensen TA, Mustaparta H, Baker TC. Chemical communication in heliothine moths III. Flight behavior of male Helicoverpa zea and Heliothis virescens in response to varying ratios of intra- and interspecific sex pheromone components. J Comp Phys A. 1991;169:275–280. [Google Scholar]
- Vickers NJ, Christensen TA, Hildebrand JG. Combinatorial odor discrimination in the brain: attractive and antagonist odor blends are represented in distinct combinations of uniquely identifiable glomeruli. J Comp Neurol. 1998;400:35–56. [PubMed] [Google Scholar]
- Vickers NJ, Poole K, Linn CE., Jr Consequences of interspecies antennal imaginal disc transplantation on organization of olfactory glomeruli and pheromone blend discrimination. J Comp Neurol. 2003;466:377–388. doi: 10.1002/cne.10890. [DOI] [PubMed] [Google Scholar]