Abstract
Mammary epithelial cells constitutively expressing Id-1 protein are unable to differentiate, acquire the ability to proliferate, and invade the extracellular matrix. In addition, Id-1 is aberrantly over-expressed in aggressive and metastatic breast cancer cells, as well as in human breast tumor biopsies from infiltrating carcinomas, suggesting Id-1 might be an important regulator of breast cancer progression. We show that human metastatic breast cancer cells become significantly less invasive in vitro and less metastatic in vivo when Id-1 is down-regulated by stable transduction with antisense Id-1. Expression of the matrix metalloproteinase MT1-MMP is decreased in proportion to the decrease in Id-1 protein levels, representing a potential mechanism for the reduction of invasiveness. Further, to more accurately recapitulate the biology of and potential therapeutic approaches to tumor metastasis, we targeted Id-1 expression systemically in tumor-bearing mice by using a nonviral approach. We demonstrate significant reduction of both Id-1 and MT1-MMP expressions as well as the metastatic spread of 4T1 breast cancer cells in syngeneic BALB/c mice. In conclusion, our studies have identified Id-1 as a critical regulator of breast cancer progression and suggest the feasibility of developing novel therapeutic approaches to target Id-1 expression to reduce breast cancer metastasis in humans.
The Id (inhibitor of DNA binding) genes were originally identified in murine myoblasts, where they prevented myogenic basic helix-loop-helix (bHLH) transcription factors from binding muscle-specific regulatory elements (1). These transcription factors are key regulators of tissue-specific gene expression in a number of mammalian and nonmammalian organisms, and constitutive expression of Id proteins has been shown to inhibit the differentiation of various tissues (2). bHLH proteins act as obligate dimers, dimerizing through HLH domains, and bind to DNA through the composite basic domains to activate the transcription of target genes containing E-boxes (CANNTG) in their promoters. Id proteins dimerize with bHLH proteins, but the Id-bHLH heterodimers fail to bind to DNA because Id proteins lack the basic domains necessary for DNA interaction.
Four members of the Id gene family have been described to date: Id-1, Id-2, Id-3, and Id-4. The different family members localize to different chromosomes and show marked differences in their pattern of expression and function (3, 4). Although the family members are similar in the HLH sequence, the regions outside the HLH domain are distinct for each member and may determine the tissue specificity of Id function, as well as the binding specificity for particular bHLH proteins.
We previously developed a line of murine mammary epithelial cells (MEC), SCp2 cells, which originated from a midpregnant mouse mammary gland (5, 6). A role for HLH Id proteins in the differentiation of SCp2 cells was suggested by our finding that Id-1 expression declined to undetectable levels when the cells were induced to differentiate in culture on treatment with extracellular matrix and lactogenic hormones (7). A similar decline was observed in lactating MEC in vivo (8). Conversely, when SCp2 cells were transfected with a constitutively expressed Id-1 gene, the transfected cells failed to differentiate, even in the presence of extracellular matrix and lactogenic hormones, and then proliferated and became invasive (9).
We also found that high Id-1 levels correlated with high invasiveness in a panel of human breast cancer cell lines and that infection of a noninvasive breast cancer cell line with Id-1 rendered it invasive (9-11). Further, using immunohistochemistry, we examined a limited number of human breast cancer biopsies for Id-1 expression (10). Almost all of the ductal carcinomas examined in situ were negative or weakly positive for Id-1 staining, whereas the majority of infiltrating grade III carcinomas of ductal origin were strongly Id-1 positive. These findings suggested that Id-1 might serve as a reliable marker for breast cancer progression, invasion, and metastasis. We determined that Id-1 regulates different aspects of breast cancer biology, namely the expression of genes controlling cell cycle progression and invasion, as well as proteins involved in cell-cell interaction (9, 12).
Taking these findings together, we have shown that Id-1 protein may serve as a key regulator in breast cancer progression. However, to our knowledge, no study has been conducted to determine whether reduction of Id-1 expression in cancer cells would significantly affect their malignant phenotype in vivo. We hypothesized that a reduction in Id-1 expression might reduce not only the invasiveness of breast cancer cells in vitro but also their ability to metastasize in vivo. Here we show that the use of a plasmid-based antisense technology to target Id-1 expression is potentially a highly effective therapeutic strategy against breast cancer progression.
Methods
pLXSN-Id1 Antisense Retroviral Vector and Virus Production. The full-length human Id-1 cDNA (13) was cloned in an antisense orientation into the pLXSN expression vector. This pLXSN-Id1 antisense vector was transfected into the TSA54 packaging cell line (Cell Genesis, Foster City, CA) by using calcium phosphate. Twenty-four hours after transfection, culture medium was harvested twice at 4-h intervals and frozen at -80°C.
Cell Culture and Retroviral Infection. Human breast cell lines MCF10A, T47D, MCF-7, ZR75-1, MDA-MB436, and MDAMB231 were purchased from the American Type Culture Collection. The human breast cell line 184 was obtained from P. Yaswen and M. R. Stampfer (Lawrence Berkeley National Laboratory, Berkeley, CA), and the murine breast cancer cell line 4T1 was obtained from S. Ostrand-Rosenberg (University of Maryland, Baltimore). All breast cancer cell lines were grown in RPMI medium 1640 containing 10% FBS and insulin (5 μg/ml, Sigma). Approximately 8 reverse transcriptase units of either pLXSN or pLXSN-Id1 antisense retrovirus was mixed with 5 ml of medium containing 4 μg/ml Polybrene and added to the cells in 100-mm dishes. Cell expressing the retroviral genes were selected in neomycin (800 μg/ml) and pooled.
Northern, Western, and Immunofluorescence Analyses. Total cellular RNA was isolated and purified as described (14). Blots were hybridized with 32P-labeled probes prepared by random oligonucleotide priming. The Id-1 probe was as described (9). Proteins were separated by SDS/PAGE using 10% acrylamide gels and blotted onto nitrocellulose membranes. Blots were probed with anti-Id-1 (10), anti-membrane-type matrix metalloproteinase 1 (MT1-MMP), or anti-actin (Chemicon) antibodies. Membranes were incubated with horseradish peroxidase-labeled secondary antibodies, and binding was detected by using ECL (Amersham Biosciences). For the immunofluorescence experiments, 2 × 105 cells (MDA-MB436 or MDA-MB231) infected either with pLXSN control or with pLXSN-Id1 antisense vector were plated on coverslips. The coverslips were fixed in 1% paraformaldehyde and incubated with 1 μg/ml anti-Id-1 antibody (same batch of antibody used for the immunohistochemistry experiments), followed by incubation with a FITC-conjugated secondary antibody. The coverslips were stained with 4′,6-diamidino-2-phenylindole to visualize the nuclei.
Boyden Chamber Invasion Assays. Invasion assays were performed as described (9, 10). Filters were coated with 10-12 μl of ice-cold Matrigel (10 mg/ml protein; Collaborative Research). Cells (50,000 per well for the MDA-MB231 cells and 100,000 per well for the MDA-MB436 cells) were added to the upper chamber in 200 μl of serum-free medium [containing 0, 0.3, 3, or 30 μM mitomycin C (Sigma)]. After a 20-h incubation, cells on the lower side of the filter were fixed and counted by using light microscopy. Cells were assayed in triplicate or quadruplicate.
Analysis of Lung Metastasis in nude Mice. To produce experimental lung metastasis, groups of 10 5- to 6-wk-old female athymic BALB/c nude mice (Simenson Laboratories, Gilroy, CA) were injected with MDA-MB231 infected with pLXSN control vector, MDA-MB231 infected with Id-1 in an antisense orientation, or parental MDA-MB231 cells. The cells were trypsinized and resuspended in culture media at a density of 2 × 106 per 200 μl and injected into the lateral tail vein of each mouse. All mice were killed 7 wk after injection. The lungs were dissected out, infused with 15% India ink intratracheally, and fixed in Fekete's solution. Visible lung metastases were counted by using a dissecting microscope.
Plasmid Construction and Purification for the Nonviral Systemic Gene Delivery Experiments. Vector control plasmid p4694 was constructed by removing the SalI-digested luciferase gene from p4458 that contained the Epstein-Barr nuclear antigen-1-coding sequence and Epstein-Barr virus family of repeats sequences (15). The remaining vector fragment was blunt-ended with the Klenow fragment of DNA polymerase, and new multiple cloning sites were ligated into PstI and NotI sites. Luciferase control plasmid p4726 was constructed by ligating the 1.6-kb NotI DNA fragment of p4379 (15) to NotI-digested p4694. The mouse antisense Id-1-targeting plasmid was constructed by ligating full-length mouse Id-1 cDNA (≈1.2 kb) in an antisense orientation into SalI- and NotI-digested and blunt-ended p4694. The CC3-containing plasmid was constructed as described (16).
In Vivo Gene Delivery and Analysis of Antimetastatic Activities.Murine 4T1 breast cancer cells were freshly thawed and grown in 5% FBS in MEM for 48 h. On day zero, groups of 10 mice were injected with 50,000 4T1 cells in 200 μl of culture medium into the tail vein of each syngeneic 8-wk-old female BALB/c (Simenson Laboratories) mouse. On day 3 after tumor cell injection, each mouse was injected with 25 μg of plasmid DNA complexed with the cationic liposome N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride at 1 μg of DNA per 24 nmol of liposome prepared as described (16). Mice were killed 21-25 days after tumor injection. The lungs were dissected out, weighed, and infused transtracheally as described (17). The potential statistical significance of differences of total lung tumor numbers between various groups was analyzed by using the unpaired two-tailed Student's t test.
Immunohistochemistry. Formalin-fixed paraffin-embedded tumor tissue sections (obtained from California Pacific Medical Center, San Francisco) were used to determine Id-1 protein expression in 21 cases of infiltrating grade I ductal carcinoma, 15 cases of infiltrating grade II ductal carcinoma, and 22 cases of infiltrating grade III ductal carcinoma. Paraffin sections were prepared as described (10). The slides were incubated with 1 μg/ml anti-Id-1 antibody overnight at 4°C. Control slides were incubated with rabbit Ig or with Id-1 blocking peptide. To determine Id-1 expression, we semiquantitatively assessed the percentage of positive tumor cells (score 2-4 points) and the staining intensity (score 1-3 points). Points for percentage of positive cells and staining intensity (levels of expression) were added, and specimens were attributed to the three groups according to their overall scores (negative if <2 points; weak if 2-4 points; strongly positive if 5-7 points). To analyze the expression levels of Id-1 and MT1-MMP after systemic transfection by cationic liposome-DNA complex (CLDC)-based delivery, mice were injected as described above and killed 2 days later. The lungs were infused with 5% formalin in PBS. Slides were incubated with 1 μg/ml anti-Id-1 or anti-MT1-MMP antibody (Chemicon) overnight at 4°C.
Results
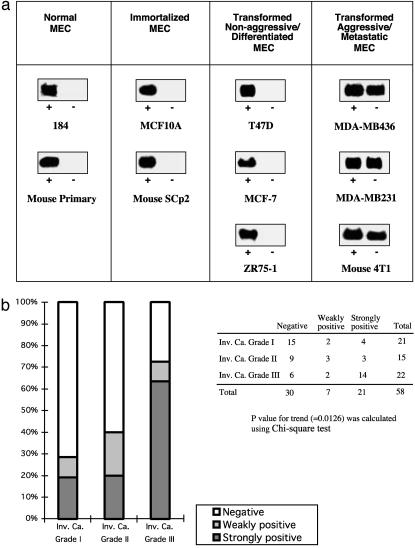
We characterized Id-1 expression in a panel of nonaggressive cell lines, including normal MEC (human 184 and mouse primary cells), immortalized MEC (human MCF10A and mouse SCp2), as well as the transformed nonaggressive/differentiated MEC [T47D, MCF-7, and ZR75-1, which do not metastasize when injected into nude mice (18)]. In all of these cell lines, Id-1 mRNA was highly expressed in the presence of serum/growth factors but down-regulated on removal of growth factor (Fig. 1a). Conversely, Id-1 mRNA was constitutively expressed only in aggressive and invasive breast cancer cells MDA-MB436 and MDA-MB231 of human origin, as well as in metastatic cells 4T1 of murine origin, in either the presence or absence of serum/growth factors.
Fig. 1.
Id-1 as a marker of breast cancer cell progression. (a) Id-1 mRNA was constitutively expressed in aggressive and metastatic breast cancer cells (MDA-MB436, MDA-MB231, and 4T1) in the presence (+) or absence (-) of serum/growth factors. In normal MEC (human 184 and mouse primary cells), immortalized MEC (human MCF10A and mouse SCp2), and transformed nonaggressive/differentiated MEC (T47D, MCF-7, and ZR75-1), Id-1 mRNA was highly expressed in the presence (+) of serum/growth factors but was down-regulated on serum starvation (-). (b) Id-1 expression in human breast cancer biopsies. A total of 58 infiltrating carcinomas were analyzed by immunohistochemistry using an antiserum directed against Id-1. The majority of the grade I invasive carcinomas (Inv. Ca.) showed negative or weakly positive signals for Id-1 (white and light gray, respectively), whereas the majority of the grade III invasive carcinomas showed strongly positive Id-1 immunoreactivity (dark gray).
We further examined Id-1 expression in a new and expanded panel of human breast cancer biopsies. A total of 58 cases of infiltrating carcinomas were analyzed for Id-1 expression by using a specific anti-Id-1 antibody. Multiple parallel assays, including Western blotting, immunofluorescence (Fig. 2a), and in situ hybridization (data not shown), were performed to verify the specificity and accuracy of this antibody. As described (10, 19), the majority of the Id-1-positive cells showed prominent cytoplasmic staining. Only ≈20% of the grade I invasive carcinoma biopsies showed strong Id-1 staining, whereas >60% of the grade III invasive carcinoma investigated showed strong Id-1 expression (Fig. 1b). These data further support our hypothesis that Id-1 is not only a significant marker for human breast tumor progression but also a potent target for anti-breast cancer therapy because Id-1 is overexpressed in a high proportion of aggressive breast carcinomas. We therefore investigated whether targeting Id-1 gene expression in breast tumor cells would reduce the invasive phenotype of metastatic cells in vitro and/or in vivo.
Fig. 2.
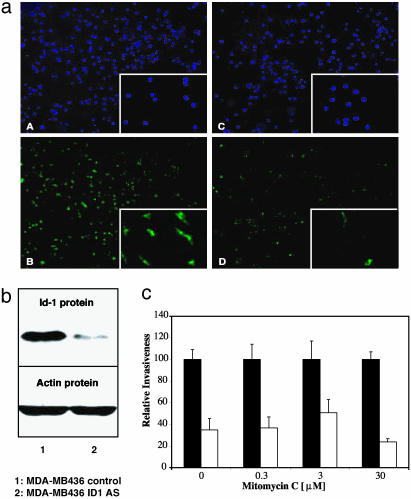
Targeting Id-1 expression in human MDA-MB436 breast cancer cells reduced their invasiveness in vitro.(a) Id-1 protein expression detected by immunofluorescence. (A and B) Nuclei staining (4′,6-diamidino-2-phenylindole; DAPI) and Id-1 staining in MDA-MB436 infected with pLXSN control, respectively. (C and D) Nuclei staining (DAPI; C) and Id-1 staining (D) in MDA-MB436 infected with pLXSN-Id1 antisense. The Insets show, at a higher magnification, the results corresponding to MDA-MB231 cells. (b) Western blot analysis of Id-1 protein expression in MDA-MB436 breast cancer cells. Lane 1, control cells infected with the empty vector pLXSN; lane 2, cells infected with pLXSN-Id1 antisense construct (Id1 AS). Actin was used as an internal control. (c) Boyden chamber invasion assay comparing the invasive ability of the infected MDA-MB436 cell populations in the absence or presence of mitomycin C. Shown are MDA-MB436 pLXSN control (▪) and MDA-MB436 pLXSN-Id1 AS (□) in the absence or presence of 0.3, 3, or 30 μM mitomycin C. Data are presented as relative invasiveness, where the respective controls are set as 100%. The cell populations infected with Id-1 antisense construct had significantly lower invasive potential than the control populations (P < 0.05).
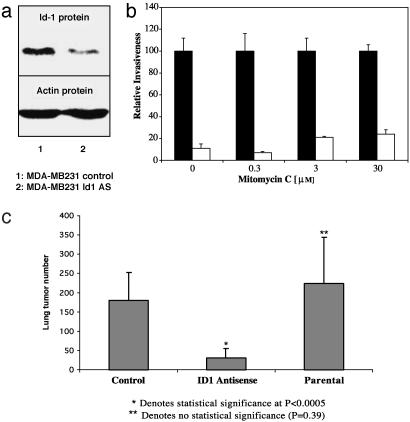
Targeting Id-1 Reduces Human Breast Cancer Cell Invasion in Vitro and in Vivo. The full-length human Id-1 cDNA was expressed in an antisense orientation in the human metastatic breast cancer cell lines MDA-MB436 and MDA-MB231. Using immunofluorescence as well as Western blotting, we determined that levels of Id-1 protein were substantially reduced in MDA-MB436 (Fig. 2 a and b) and in MDA-MB231 (Figs. 2a and 3a) cells infected with pLXSN-Id1 antisense construct compared with pLXSN-control-infected cells. We then assessed the ability of the different populations of stably infected cells expressing various levels of Id-1 to migrate and invade a reconstituted basement membrane in a Boyden chamber (20). The invasive activity of each cell population directly correlated with the level of its Id-1 protein expression (Figs. 2c and 3b). Specifically, the populations of MDA-MB436 and MDA-MB231 that expressed high levels of Id-1 (pLXSN-control) were significantly more invasive than the Id-1 antisense-infected cells that expressed low levels of Id-1 (pLXSN-Id1 antisense cells). To study the effect of Id-1 targeting on invasion/migration only, and not on proliferation, we performed the invasion assays in the presence of three different biologically active concentrations of mitomycin C (0.3, 3, and 30 μM) (Figs. 2c and 3b). The same pattern of reduction in invasiveness was documented, suggesting that the effect of Id-1 targeting on invasion/migration is independent of its effect on cell proliferation.
Fig. 3.
Targeting Id-1 expression in human MDA-MB231 breast cancer cells reduced their invasiveness in vitro and their metastatic spread in vivo. (a) Id-1 protein expression in MDA-MB231 breast cancer cells. Lane 1, control cells infected with the empty vector pLXSN; lane 2, cells infected with pLXSN-Id1 antisense construct (Id1 AS). Actin was used as an internal control. (b) Boyden chamber invasion assay comparing the invasive ability of the infected MDAMB231 cell populations in the absence or presence of mitomycin C. Shown are MDA-MB231 pLXSN control (▪) and MDA-MB231 pLXSN-Id1 antisense (AS) (□) in the absence or presence of 0.3, 3, or 30 μM mitomycin C. Data are presented as relative invasiveness, where the respective controls are set as 100%. The cell populations infected with Id-1 antisense construct had significantly lower invasive potential than the control populations (P < 0.001). (c) Targeting Id-1 expression in MDA-MB231 cells reduced not only their invasiveness in vitro but also their metastatic spread in vivo. The graph shows total lung tumor numbers in nude mice that were injected systemically with MDA-MB231 infected with pLXSN vector (Control), MDA-MB231 infected with pLXSN-Id1 antisense construct (ID1 Antisense), or parental MDA-MB231 (Parental) cells. Compared with control: *, P < 0.0005; **, P = 0.39 (not significant).
We also performed experiments to determine the effects of Id-1 targeting on the proliferation/survival rate of these cell populations. We determined that the effects of the Id-1 antisense construct on MDA-MB231 cell proliferation were modest but significant (P < 0.003) (≈10-15% decrease of the labeling index of pLXSN-Id1 antisense cells compared with pLXSN-control) when cells were cultured in serum-free medium. There was no statistical difference in the apoptotic rate among these populations with various Id-1 levels (data not shown). On the basis of these results obtained by using cultured cells, we then tested whether stable reduction of Id-1 expression in MDA-MB231 tumor cells could reduce their metastatic spread. We have chosen the MDA-MB231 cell line as a model for the in vivo experiment for the reasons that (i) this aggressive cell line is among the few that metastasize in nude mice (18), and (ii) the effect of Id-1 targeting on cancer cells alone can be tested only on cell lines that express constitutively high levels of Id-1 protein independent of the nature of the microenvironment surrounding the tumor cells (i.e., serum, tissue, or extracellular matrix). nude mice injected with MDA-MB231 cells expressing antisense Id-1 exhibited significantly fewer lung tumors (P < 0.0005) than mice injected with the MDA-MB231 control or the parental MDAMB231 (Fig. 3c). These results further support the hypothesis that the invasive/growth and metastatic phenotypes in human breast cancer cells are directly related to the levels of expression of the Id-1 gene.
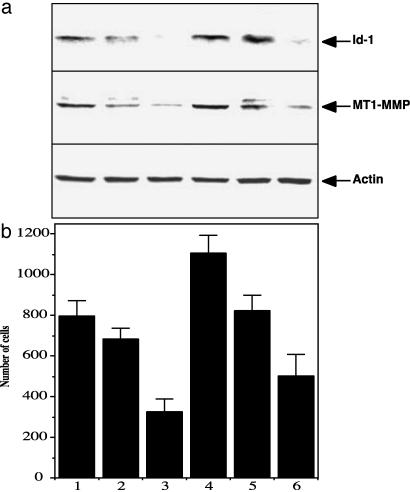
Targeting Id-1 Decreases the Expression of the Matrix Metalloproteinase MT1-MMP. Using the techniques of differential display and subtractive hybridization, we previously isolated a number of genes regulated by Id-1 in murine MEC. Among these were genes controlling cell cycle progression as well as cell-cell interaction (12). We also repeatedly pulled out one gene encoding the matrix metalloproteinase MT1-MMP, which was strongly up-regulated in cells transfected with Id-1 (unpublished data). Several groups have reported the role of this extracellular matrix-remodeling enzyme in increasing tumor invasion (21). Therefore, we investigated whether MT1-MMP was down-regulated in MDA-MB231 cells infected with an antisense Id-1 construct. We used six different subpopulations isolated from the pool MDA-MB231 antisense Id-1 cells that expressed different levels of Id-1 protein. We found that decreased levels of expression of MT1-MMP was highly correlated with decreased Id-1 protein expression and decreased invasion (Fig. 4).
Fig. 4.
Targeting Id-1 expression in MDA-MB231 cells down-regulated MT1-MMP expression that was correlated with the invasive ability of the cells. (a) Id-1 as well as MT1-MMP expression in six different subpopulations isolated from the pool population of MDA-MB231 breast cancer cells infected with pLXSN-Id1 antisense construct (as described for Fig. 3a). Actin was used as an internal control. (b) Boyden chamber invasion assay comparing the invasive ability of the six different subpopulations of pLXSN-Id1 antisense-infected MDA-MB231 cells.
Systemically Targeting Id-1 Reduces Murine Breast Cancer Cell Metastasis in Tumor-Bearing Mice. Further studies were undertaken to determine whether systemically targeting Id-1 expression in vivo could reduce the metastatic phenotype of breast cancer cells. We used CLDC-based nonviral systemic gene transfer to assess the effects of Id-1 gene down-regulation in immunocompetent tumor-bearing mice. It is known that metastatic cancer is a result of not only alterations of gene expression within tumor cells but also the complex interplay of the host environment with the tumor cells (22, 23). Thus, the systemic approach more accurately recapitulates the biologic as well as potential therapeutic settings of metastatic cancer. As our model, we used the 4T1 murine metastatic breast cancer cells, which primarily metastasize to the lungs of syngeneic BALB/c mice. The 4T1 cells, like the human MDA-MB436 and MDA-MB231 cells, express high levels of Id-1 mRNA (Fig. 1a) and protein (unpublished data). These 4T1 mammary carcinoma cells are poorly immunogenic and have growth characteristics that resemble highly invasive human metastatic mammary carcinoma, and the extent of disease is comparable to human stage IV breast cancer (24). Moreover, breast cancer metastasizes more to the lungs (70.3%) of human patients than to the liver (58.4%), bone marrow (53.1%), or brain (14.3%) (25).
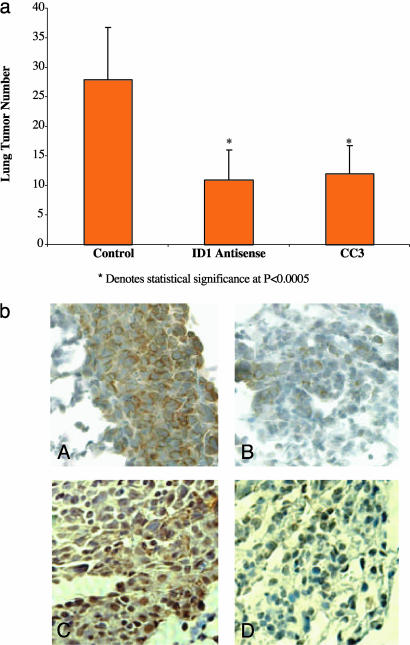
To reduce Id-1 expression in tumor-bearing mice, we injected them i.v. with CLDC containing the full-length antisense Id-1 cDNA in the long-expressing human cytomegalovirus-driven vector (15). We showed that this CLDC-based antisense construct significantly reduced the metastatic spread of 4T1 breast cancer cells in syngeneic BALB/c mice (Fig. 5a). Specifically, a single injection of CLDC containing the antisense Id-1 plasmid, 3 days after i.v. inoculation of 50,000 4T1 cells, significantly reduced the total number of lung metastases compared with tumor-bearing mice treated with CLDC containing the luciferase gene (mock-treated control group). Interestingly, this reduction was comparable to that achieved by injection of the potent metastasis suppressor gene CC3 (16, 26). To determine the effect of systemic delivery of the antisense Id-1 plasmid on Id-1 protein levels in 4T1 lung tumors, tumor-bearing mice were injected with CLDC containing either the antisense Id-1 or control luciferase cDNAs 2 days before killing. By using immunohistochemistry, a high level of Id-1 protein was detected in 4T1 cells from CLDC-luciferase control-treated mice (Fig. 5bA), whereas significantly lower levels of Id-1 protein were detected in 4T1 cells from CLDC-Id-1 antisense-treated mice (Fig. 5bB). The significant and rapid reduction of Id-1 protein level presumably was a result of the fast turnover rate of Id-1 mRNA (10). Moreover, and in agreement with the in vitro data presented in Fig. 4, MT1-MMP levels were significantly reduced in 4T1 cells from CLDC-Id1 antisense-treated mice (Fig. 5bD) in comparison to CLDC-luciferase control-treated mice (Fig. 5bC). High levels of MT1-MMP staining were observed in the cytoplasm of the cancerous 4T1 cells from the control-treated mice, as previously reported for colon cancer cells (27).
Fig. 5.
Systemically targeting Id-1 expression significantly reduced the metastatic spread of 4T1 breast cancer cells in a syngeneic BALB/c mouse model. (a) The 4T1 murine metastatic breast cancer cells, which expressed high levels of Id-1 protein, were first inoculated systemically into BALB/c mice. The tumor-bearing mice were then injected i.v. 3 days after tumor inoculation with various constructs by using CLDC. The graph shows the mean number of lung metastases per mouse treated with the control gene luciferase (18 mice); with Id-1 antisense (17 mice); or with the metastasis suppressor gene CC3 (16 mice) constructs. The graph represents two independent experiments. *, P < 0.0005 compared with control. (b) Immunohistochemistry analysis of Id-1 levels (A and B) as well as MT1-MMP (C and D) in 4T1 breast tumor cells harvested from the lungs of CLDC-injected mice. The protocol was as in a, except the tumor-bearing mice were injected with the corresponding liposome-DNA complexes 2 days before killing. Immunohistochemistry on tumors from CLDC control-treated mice (A and C) and from CLDC-Id-1 antisense-treated mice (B and D) was carried out as described in Methods.
Discussion
Very recently, Id-1 protein expression in 191 patients with lymphnode-negative breast cancer was investigated (19). Patients with strong or moderate Id-1 expression had a significantly shorter overall (P = 0.003) and disease-free (P = 0.01) survival compared with those with absent or low expression. Thus, aberrant expression of Id-1 protein represents a strong independent prognostic marker in node-negative breast cancer. Consistent with our hypothesis that Id-1 is a key regulator in human breast cancer progression, we and others therefore proposed that Id-1 could be a promising candidate for future therapy concepts and that inhibiting Id-1 expression might benefit patients with breast cancer.
Here, these studies demonstrate that using antisense technology to target endogenous Id-1 gene expression can reduce breast cancer cell metastasis to the lungs. Stable infection of human breast cancer cells with antisense Id-1 significantly reduced lung metastasis after their inoculation into nude mice. Therefore, specifically targeting Id-1 in cancer cells alone was sufficient to reduce metastatic spread in mice, when the MDA-MB231 breast cancer cell line was used as our tumor model. Furthermore, systemic CLDC-based injection of an Id-1 antisense plasmid also significantly reduced lung metastasis. In these experiments performed by using syngeneic BALB/c mice, we cannot rule out the possibility that the Id-1 antisense construct may also be delivered to other cell types, including vascular endothelial cells (16). Because expression of Id genes is required also for angiogenesis and neovascularization (28), targeting Id-1 expression in tumor cells as well as endothelial cells from the tumor blood vessels might produce additive or even synergistic antitumor effects.
Taken together, our results indicate that Id-1 protein is a molecular target for blocking breast tumor metastasis. We hypothesize that Id-1 would be a highly effective and selective target for breast cancer therapy. First, Id-1 is not a transcription factor per se. Id-1 has been shown to regulate the activity of several important regulatory proteins involved in transcriptional regulation. Among these proteins are the bHLH transcription factors, Rb, and the Ets family members (2). Through these different interactions, Id-1 protein is central to the pathways regulating proliferation, differentiation, migration, invasion, and cell-cell interaction. Therefore, using Id-1 as a target could affect various aspects of breast carcinogenesis and progression. Second, even though Id-1 is widely expressed during development and tumorigenesis, it is not expressed in most of the mature adult tissues (29). The scarcity of Id-1 expression in adult tissues could be an advantage for systemic therapy because the majority of the normal cells will not be affected. Third, as demonstrated in our studies as well as from the knockout animal model (28), an only partial reduction of Id-1 levels can profoundly affect tumor behavior. Therefore, Id-1 protein is a promising molecular target for breast cancer therapy.
The effects of Id-1 targeting on breast tumors appear to be mediated primarily by reduction of the invasive properties acquired by these aggressive cells, although we also observed some influence on the proliferative capacity. This observation supports the notion that Id-1 is one of the genes important in the regulation of “invasive growth.” The basis of this concept is that invasion/migration cannot be dissociated from proliferation and that some genes are able to trigger a cascade of events leading to a general aggressive phenotype in cancer cells (loss of cell-cell interactions, secretion of matrix-degrading enzymes, proliferation, etc.) (30).
To better address the functional insights as to how Id-1 exerts its antimetastatic and antiinvasive functions, we identified the MT1-MMP gene to be significantly down-regulated by targeting Id-1 in breast cancer cells. MT1-MMP is a major MMP because it can degrade extracellular matrix components directly as well as indirectly by activating MMPs. The precise localization of MT1-MMP between cancer cells and surrounding stroma cells has been the subject of controversy. Recently, Dalberg et al. (31) detected MT1-MMP mRNA expression in all invasive breast tumor biopsies investigated and found that it was mainly localized in the tumor cells. Mimori et al. (32) also determined that the highest expression of MT1-MMP mRNA was found in breast cancer specimens showing lymph-node metastasis and/or lymph-vessel invasion. All these data on MT1-MMP indicate that it plays a crucial role during breast cancer progression. However, very little has been known about the regulation of this very important enzyme until recently. Studies on the MT1-MMP promoter indicated that it contains several regulatory sequences, including some E-boxes that were recognized by bHLH transcription factors (33). Therefore, we hypothesize that MT1-MMP expression is regulated by the Id-1-interacting bHLH transcription factors and that MT1-MMP might be a key mediator of the antiinvasion and antimetastasis effects of Id-1 down-regulation in breast tumor cells.
In addition to the potential of Id-1 as a target in breast cancer therapy, we hypothesize that Id-1 targeting could be useful in other types of cancer therapy. Higher levels of Id-1 gene expression have also been detected in tumor cells from other tissue types, compared with normal cells of the same tissue origin (34-39). Moreover, Id-1 expression has been shown to be an unfavorable prognostic marker in early-stage cervical cancer (40), and stronger Id-1 expression was associated with poor differentiation and more aggressive behavior of ovarian tumor cells (41).
This report demonstrates the efficacy of an antisense plasmid-based systemic gene delivery approach by using cationic liposomes to target gene expression in immunocompetent adult tumor-bearing animals. This method could also be a useful alternative to knockout technology in studying the functions of various genes in regulating cancer progression in appropriate animal models. To date, there are still major problems associated with the currently available gene therapy strategies that prevent their routine usage in the clinic (42, 43). The demonstration that antisense Id-1 significantly reduces breast cancer metastasis suggests that a variety of emerging gene-antagonist approaches, including antisense-, RNA interference-, and small molecule-based approaches, may potentially improve breast cancer therapy when targeting Id-1.
Acknowledgments
We thank Dr. John Muschler for critical review of the manuscript and Dr. Andrew P. Smith and Paul Fong for editing. This work was supported by grants from the National Institutes of Health-National Cancer Institute (R01 CA82575 to R.J.D. and R01 CA82548 to P.-Y.D.), the Human Frontier Science Program (R.J.D.), the Oracle Foundation (P.-Y.D.), and the California Breast Cancer Research Program (8WB-0164 to R.J.D. and 1KB-0274, 3IB-0123, 5IB-0111, and 7WB-0026 to P.-Y.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MT1-MMP, membrane-type matrix metalloproteinase 1; bHLH, basic helix-loop-helix; CLDC, cationic liposome-DNA complex; MEC, mammary epithelial cells.
References
- 1.Benezra, R., Davis, R. L., Lockshon, D., Turner, D. L. & Weintraub, H. (1990) Cell 61, 49-59. [DOI] [PubMed] [Google Scholar]
- 2.Norton, J. D. (2000) J. Cell Sci. 113, 3897-3905. [DOI] [PubMed] [Google Scholar]
- 3.Riechmann, V., van Cruchten, I. & Sablitzky, F. (1994) Nucleic Acids Res. 22, 749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riechmann, V. & Sablitzky, F. (1995) Cell Growth Differ. 6, 837-843. [PubMed] [Google Scholar]
- 5.Schmidhauser, C., Bissell, M. J., Myers, C. A. & Casperson, G. F. (1990) Proc. Natl. Acad. Sci. USA 87, 9118-9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desprez, P. Y., Roskelley, C., Campisi, J. & Bissell, M. J. (1993) Mol. Cell. Differ. 1, 99-110. [Google Scholar]
- 7.Desprez, P. Y., Hara, E., Bissell, M. J. & Campisi, J. (1995) Mol. Cell. Biol. 15, 3398-3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parrinello, S., Lin, C. Q., Murata, K., Itahana, Y., Singh, J., Krtolica, A., Campisi, J. & Desprez, P. Y. (2001) J. Biol. Chem. 276, 39213-39219. [DOI] [PubMed] [Google Scholar]
- 9.Desprez, P. Y., Lin, C. Q., Thomasset, N., Sympson, C. J., Bissell, M. J. & Campisi, J. (1998) Mol. Cell. Biol. 18, 4577-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin, C. Q., Singh, J., Murata, K., Itahana, Y., Parrinello, S., Liang, S. H., Gillett, C. E., Campisi, J. & Desprez, P. Y. (2000) Cancer Res. 60, 1332-1340. [PubMed] [Google Scholar]
- 11.Singh, J., Murata, K., Itahana, Y. & Desprez, P. Y. (2002) Oncogene 21, 1812-1822. [DOI] [PubMed] [Google Scholar]
- 12.Singh, J., Itahana, Y., Parrinello, S., Murata, K. & Desprez, P. Y. (2001) J. Biol. Chem. 276, 11852-11858. [DOI] [PubMed] [Google Scholar]
- 13.Hara, E., Yamaguchi, T., Nojima, H., Ide, T., Campisi, J., Okayama, H. & Oda, K. (1994) J. Biol. Chem. 269, 2139-2145. [PubMed] [Google Scholar]
- 14.Chomczynski, P. & Sacchi, N. (1987) Anal. Biochem. 162, 156-159. [DOI] [PubMed] [Google Scholar]
- 15.Tu, G., Kirchmaier, A. L., Liggitt, D., Liu, Y., Liu, S., Yu, W. H., Heath, T. D., Thor, A. & Debs, R. J. (2000) J. Biol. Chem. 275, 30408-30416. [DOI] [PubMed] [Google Scholar]
- 16.Liu, Y., Thor, A., Shtivelman, E., Cao, Y., Tu, G., Heath, T. D. & Debs, R. J. (1999) J. Biol. Chem. 274, 13338-13344. [DOI] [PubMed] [Google Scholar]
- 17.Wexler, H. (1966) J. Natl. Cancer Inst. 36, 641-645. [DOI] [PubMed] [Google Scholar]
- 18.Thompson, E. W., Paik, S., Brunner, N., Sommers, C. L., Zugmaier, G., Clarke, R., Shima, T. B., Torri, J., Donahue, S., Lippman, M. E., et al. (1992) J. Cell Physiol. 150, 534-544. [DOI] [PubMed] [Google Scholar]
- 19.Schoppmann, S. F., Schindl, M., Bayer, G., Aumayr, K., Dienes, J., Horvat, R., Rudas, M., Gnant, M., Jakesz, R. & Birner, P. (2003) Int. J. Cancer 104, 677-682. [DOI] [PubMed] [Google Scholar]
- 20.Albini, A., Iwamoto, Y., Kleinman, H. K., Martin, G. R., Aaronson, S. A., Kozlowski, J. M. & McEwan, R. N. (1987) Cancer Res. 47, 3239-3245. [PubMed] [Google Scholar]
- 21.Polette, M. & Birembaut, P. (1998) Int. J. Biochem. Cell Biol. 30, 1195-1202. [DOI] [PubMed] [Google Scholar]
- 22.Bissell, M. J. & Radisky, D. (2001) Nat. Rev. Cancer 1, 46-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berns, A. (2001) Nature 410, 1043-1044. [DOI] [PubMed] [Google Scholar]
- 24.Pulaski, B. A., Terman, D. S., Khan, S., Muller, E. & Ostrand-Rosenberg, S. (2000) Cancer Res. 60, 2710-2715. [PubMed] [Google Scholar]
- 25.Urano, Y., Fukushima, T., Kitamura, S., Mori, H., Baba, K. & Aizawa, S. (1986) Jpn. J. Cancer Clin. Suppl., 205-223. [PubMed]
- 26.Shtivelman, E. (1997) Oncogene 14, 2167-2173. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi, M., Tsunoda, T., Seiki, M., Nakamura, Y. & Furukawa, Y. (2002) Oncogene 21, 5861-5867. [DOI] [PubMed] [Google Scholar]
- 28.Lyden, D., Young, A. Z., Zagzag, D., Yan, W., Gerald, W., O'Reilly, R., Bader, B. L., Hynes, R. O., Zhuang, Y., Manova, K. & Benezra, R. (1999) Nature 401, 670-677. [DOI] [PubMed] [Google Scholar]
- 29.Benezra, R., Rafii, S. & Lyden, D. (2001) Oncogene 20, 8334-8341. [DOI] [PubMed] [Google Scholar]
- 30.Fujita, N., Jaye, D. L., Kajita, M., Geigerman, C., Moreno, C. S. & Wade, P. A. (2003) Cell 113, 207-219. [DOI] [PubMed] [Google Scholar]
- 31.Dalberg, K., Eriksson, E., Enberg, U., Kjellman, M. & Backdahl, M. (2000) World J. Surg. 24, 334-340. [DOI] [PubMed] [Google Scholar]
- 32.Mimori, K., Ueo, H., Shirasaka, C. & Mori, M. (2001) Oncol. Rep. 8, 401-403. [DOI] [PubMed] [Google Scholar]
- 33.Lohi, J., Lehti, K., Valtanen, H., Parks, W. C. & Keski-Oja, J. (2000) Gene 242, 75-86. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama, H., Kleeff, J., Wildi, S., Friess, H., Buchler, M. W., Israel, M. A. & Korc, M. (1999) Am. J. Pathol. 155, 815-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kebebew, E., Treseler, P. A., Duh, Q. Y. & Clark, O. H. (2000) Surgery 128, 952-957. [DOI] [PubMed] [Google Scholar]
- 36.Langlands, K., Down, G. A. & Kealey, T. (2000) Cancer Res. 60, 5929-5933. [PubMed] [Google Scholar]
- 37.Takai, N., Miyazaki, T., Fujisawa, K., Nasu, K. & Miyakawa, I. (2001) Cancer Lett. 165, 185-193. [DOI] [PubMed] [Google Scholar]
- 38.Hu, Y. C., Lam, K. Y., Law, S., Wong, J. & Srivastava, G. (2001) Clin. Cancer Res. 7, 2213-2221. [PubMed] [Google Scholar]
- 39.Polsky, D., Young, A. Z., Busam, K. J. & Alani, R. M. (2001) Cancer Res. 61, 6008-6011. [PubMed] [Google Scholar]
- 40.Schindl, M., Oberhuber, G., Obermair, A., Schoppmann, S. F., Karner, B. & Birner, P. (2001) Cancer Res. 61, 5703-5706. [PubMed] [Google Scholar]
- 41.Schindl, M., Schoppmann, S. F., Strobel, T., Heinzl, H., Leisser, C., Horvat, R. & Birner, P. (2003) Clin. Cancer Res. 9, 779-785. [PubMed] [Google Scholar]
- 42.Nishikawa, M. & Huang, L. (2001) Hum. Gene Ther. 12, 861-870. [DOI] [PubMed] [Google Scholar]
- 43.Greco, O., Scott, S. D., Marples, B. & Dachs, G. U. (2002) Front. Biosci. 7, d1516-d1524. [DOI] [PubMed] [Google Scholar]