Abstract
Background
Metabolic syndrome (MS) is associated with dyslipidemia, and insulin resistance (IR) may be a main determinant of this dyslipidemia.
Objective
To determine how lipoprotein particle concentration and size are related to MS and IR in a population based-sample of Alaska Eskimos.
Design
Participants underwent a physical exam, personal interview, collection of biological specimens, and diagnostic tests.
Setting
This study was conducted in the Norton Sound Region of Alaska.
Participants
1,158 Inupiat Eskimo adults (women = 653, men = 505).
Main Outcome Measures
Lipoprotein particle profile was evaluated by nuclear magnetic resonance (NMR) and related to presence of MS and level of IR.
Results
Participants with MS (women = 105, men = 52) had a) significantly higher concentrations of all VLDLs and a larger VLDL size (women, p = 0.007; men, p = 0.0001); b) higher concentrations of small LDL (women, p < 0.0001; men, p = 0.09) and lower concentrations of large LDL (women, p < 0.0001), leading to a smaller overall LDL size (women, p < 0.0001; men, p < 0.05); c) significantly lower concentrations of large HDL (both genders, p < 0.0001) and an increase in intermediate (women, p < 0.05) and small HDL (women, p < 0.0001; men, p <0.004). Lipoprotein profile with increasing HOMA-IR resembled that of individuals with MS.
Conclusions
In this population MS is characterized by lipoprotein distribution and size abnormalities independent of obesity, age, and other cardiovascular risk factors, including lipid concentration. IR seems the major determinant.
Keywords: lipoprotein particle distribution, insulin resistance, metabolic syndrome, GOCADAN Study, Alaska Eskimos
INTRODUCTION
Metabolic syndrome (MS) is associated with dyslipidemia characterized by increased triglycerides, low HDL-cholesterol (C), and small LDL particles (1–2). Insulin resistance (IR) may be a main determinant of these lipid abnormalities (3). Each lipoprotein class consists of a continuous spectrum of particles with different size, density, metabolism, and atherogenic impact (4). The relationship of MS and particularly IR to the size and density of lipoprotein particles has been only partly investigated. Although some data relate MS and IR to LDL subfractions (5–7), more information is needed concerning VLDL and HDL subfractions in individuals with MS or IR. Previous studies were limited by small sample sizes, because the usual laboratory methods to measure lipoprotein subclasses require large sample volumes, are laborious, time-consuming (8–9), and unsuitable for large population samples.
Proton nuclear magnetic resonance (NMR), a new, rapid, cost-effective method (10–11) that measures the full spectrum of lipoprotein subfraction distribution and size at the same time on a small fresh or frozen sample, is suitable for use in large cohorts. In the current study, this method was used to investigate, for the first time, how the full spectrum of lipoprotein subclass distribution and size is related to MS and IR in a population based-sample age 18 and older. This study was undertaken to elucidate the role of MS and IR in cardiovascular disease, a topic of much debate (12).
METHODS
Study population
A total of 1,214 predominantly Inupiat Eskimos (537 men and 677 women) ≥ 18 years old from nine villages (including the town of Nome) in the Norton Sound Region of Alaska were examined in 2000–2004 for cardiovascular disease (CVD) and associated risk factors as part of the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study (13). Recruitment was conducted by family. In seven of the nine villages, an average of 82.6% of residents > 18 years participated.
Each participant underwent a physical examination, personal interview, collection of biological specimens, and other diagnostic tests. Data on blood measures were available for 1,160 participants. Of these participants, 3.3% (n = 32) had diabetes according to 1998 World Health Organization criteria (14) and were excluded from the analyses, for a cohort of 636 women and 524 men. Permission was granted by each community to conduct the study; written informed consent was obtained from all participants.
Physical and metabolic measurements
Anthropometry (height, weight, BMI, waist circumference) was performed with participants fasting, according to standard procedures. Abdominal obesity was defined using Adult Treatment Panel III (ATP III) criteria (1). Seated blood pressure was measured three times under standard conditions, with the mean value used as the final measure. Smoking and alcohol intake habits were evaluated via questionnaire; participants were categorized as current, former, and never-smokers (13).
Samples of whole blood, plasma, serum, and urine were collected from each participant and stored at −80° C. All laboratory methods have been published (13). LDL-C was calculated by the Friedewald formula (15).
Definitions of metabolic syndrome and IR
MS was defined according to ATP III criteria (1) as meeting three or more of the following criteria: waist circumference 102 cm for men, 88 cm for women; triglycerides ≥ 150 mg/dL (0.59 mmol/L); HDL-C < 40 mg/dL (1.30 mmol/L) for men, < 50 mg/dL (1.04 mmol/L) for women; arterial hypertension (systolic blood pressure ≥ 130 mmHg, diastolic blood pressure ≥ 85 mmHg); fasting glucose ≥ 100 mg/dL (5.56 mmol/L).
Insulin resistance was estimated by HOMA index calculated as follows:
Fasting plasma glucose (mmol/L)*fasting plasma insulin (mU/L)/22.5 (16).
Lipoprotein subfraction profile
Detailed lipoprotein subclassification (type, size, and concentration) was performed on plasma isolated by centrifuge (3,000 rpm, 10 min, 4° C) and stored at −80° C for the NMR spectroscopy (10), using a rapid, automated, commercially available assay (LipoScience Inc., Raleigh, NC, USA). Details of the NMR methodology have been published (10–11).
The data are presented as molar particle concentrations, because the focus of the study is on the occurrence of lipoprotein subclasses and their distribution rather than abnormal lipid composition.
To simplify the data evaluation, the 15 NMR lipoprotein subfractions (V1–V6, IDL, L1–L3, H1–H5) were grouped into three size groups (large, intermediate, and small) for each lipoprotein class. This resulted in the following spectra: large VLDL (V5 + V6, 60–220 nm), intermediate VLDL (V3 + V4, 35–60 nm), and small VLDL (V1 + V2, 27–35 nm); large LDL (L3, 21.3–22.7 nm), intermediate LDL (L2, 19.8–21.2 nm) and small LDL (L1, 18.3–19.7 nm); and large HDL (H4 + H5, 8.8–13 nm), intermediate HDL (H3, 8.2–8.8 nm), and small HDL (H1 + H2, 7.3–8.2 nm) (11). Data on IDL particles (25 nm) are not reported in this study.
Statistical analysis
All data are expressed as mean ± SD. The natural log transformation was applied to variables which were highly skewed. The means of the actual measured data are shown in the tables and figures. However, the transformed variables have been used in all comparisons, analysis of variance (ANOVA), regressions, and correlation analyses. Comparisons between groups were evaluated by Student’s t-test and ANOVA. The p-values were adjusted for the variables known to influence lipid metabolism (age, BMI, systolic blood pressure [SBP], smoking habits) and corrected by the Bonferroni method for multiple comparisons. Data on VLDL particle concentrations were adjusted also for triglyceride levels and data on LDL and HDL particle concentrations were adjusted for LDL-C and HDL-C levels, respectively. Relationships between HOMA-IR index and lipoprotein subclass concentration and size were evaluated using Pearson’s correlation analysis, adjusted for the same covariates as above. All probability values were 2-tailed, and values less than 0.05 were considered statistically significant.
RESULTS
Characteristics of the population are shown (Table 1). Average age was 43 for women and 42 for men, and 18% had impaired glucose regulation (mainly IFG). A large percentage of the population were smokers (63% of the men and 57% of the women were current smokers). Women were significantly more overweight and insulin resistant than the men. However, the women had lower blood pressure levels and higher HDL-C concentrations.
Table 1.
Characteristics of the GOCADAN population, by gender and MS status, N = 1,160
| Women (n = 636) |
Men (n = 524) |
|||||
|---|---|---|---|---|---|---|
| Without MS | With MS | P value | Without MS | With MS | P value | |
| Age (years) | 40.6±15.50 | 47.7±14.85 | <.0001 | 40.9±15.36 | 47.4±15.79 | 0.0037 |
| BMI (kg/m2) | 27.1±5.41 | 34.7±5.67 | <.0001 | 25.6±4.08 | 33.7±5.34 | <.0001 |
| Waist circumference (cm) | 84.7±12.20 | 102.6±12.02 | <.0001 | 84.9±10.02 | 105.3±11.54 | <.0001 |
| Systolic blood pressure (mmHg) | 114.4±13.44 | 128.4±17.79 | <.0001 | 120.1±12.40 | 129.8±11.18 | <.0001 |
| Diastolic blood pressure (mmHg) | 73.2±8.62 | 79.9±9.88 | <.0001 | 77.5±9.14 | 82.6±8.31 | <.0001 |
| Plasma insulin (pmol/L) | 67.0±39.23 | 111.3±79.41 | <.0001 | 56.2±39.44 | 111.3±61.69 | <.0001 |
| HOMA-IR index | 2.2±1.57 | 4.0±3.26 | <.0001 | 1.9±1.51 | 4.0±2.22 | <.0001 |
| Plasma glucose (mmol/L) | 5.0±0.50 | 5.5±0.59 | <.0001 | 5.1±0.55 | 5.6±0.52 | <.0001 |
| Plasma cholesterol (mmol/L) | 5.2±1.06 | 5.5±1.00 | 0.0054 | 5.1±1.04 | 5.3±1.06 | 0.0769 |
| Plasma triglycerides (mmol/L) | 1.2±0.66 | 2.3±0.94 | <.0001 | 1.3±0.74 | 2.5±1.05 | <.0001 |
| LDL cholesterol (mmol/L) | 3.0±0.93 | 3.1±0.91 | 0.0785 | 3.0±0.93 | 3.2±1.00 | 0.2941 |
| HDL cholesterol (mmol/L) | 1.7±0.45 | 1.4±0.42 | <.0001 | 1.5±0.46 | 1.1±0.29 | <.0001 |
| Use of antihypertensive drugs (%) | 7.1 | 33.3 | <0.001 | 8.6 | 35.1 | <0.001 |
| Use of hypolipidemic drugs (%) | 3.0 | 9.8 | .0041 | 2.4 | 17.5 | <0.001 |
| Current smokers (%) | 60.9 | 48.0 | .0210 | 65.6 | 45.6 | .0057 |
| Former smokers (%) | 19.2 | 32.4 | .0052 | 17.3 | 36.9 | .0011 |
| Never smokers (%) | 19.9 | 19.6 | 1.000 | 17.1 | 17.5 | .8534 |
Notes. Data are mean ± SD. The GOCADAN population consists of Alaska Eskimos of the Norton Sound Region. Data are from the baseline exam.
Gender significantly influenced lipoprotein particle concentration and size (Table 2); women, although more overweight and more insulin resistant than men, had significantly greater concentrations of large LDL particles (550 ± 187 vs 494 ± 175 nmol, p < 0.0001), significantly lower concentrations of intermediate and small LDL particles (p < 0.0001 for both), and a larger LDL size (p < 0.0001). Women also had greater concentrations of large and intermediate HDL particles (p < 0.0001 and p < 0.006, respectively) and a larger HDL size (p < 0.0001).
Table 2.
Plasma lipoprotein particle concentrations and size, (Alaska Eskimos ages ≥ 18 years, N = 1,128)
| Women (n = 623) | Men (n = 505) | P-Value | |
|---|---|---|---|
| Total VLDL particles (nmol/L) | 58.8 ± 27.2 | 61.5 ± 28.3 | 0.10 |
| Large (nmol/L) | 1.6 ± 2.2 | 1.7 ± 2.1 | 0.40 |
| Intermediate (nmol/L) | 15.8 ± 11.6 | 16.3 ± 12.1 | 0.52 |
| Small (nmol/L) | 41.3 ± 18.7 | 43.5 ± 19.3 | 0.05 |
| VLDL size (nm) | 45.4 ± 9.0 | 45.7 ± 9.6 | 0.82 |
| Total LDL particles (nmol/L) | 1,036 ± 298 | 1,118 ± 336 | <0.0001 |
| Large (nmol/L) | 550 ± 187 | 494 ± 175 | <0.0001 |
| Intermediate (nmol/L) | 92 ± 65 | 119 ± 70 | <0.0001 |
| Small (nmol/L) | 371 ± 245 | 486 ± 269 | <0.0001 |
| LDL size (nm) | 21.7 ± 0.64 | 21.4 ± 0.60 | <0.0001 |
| Total HDL particles (µmol/L) | 29.6 ± 6.6 | 27.1 ± 5.7 | <0.0001 |
| Large (µmol/L) | 7.2 ± 3.9 | 5.1 ± 3.7 | <0.0001 |
| Intermediate (µmol/L) | 2.1 ± 3.1 | 1.8 ± 2.9 | 0.006 |
| Small (µmol/L) | 20.4 ± 5.2 | 20.2 ± 4.7 | 0.60 |
| HDL size (nm) | 9.2 ± 0.5 | 9.0 ± 0.5 | <0.0001 |
Data are mean ± SD.
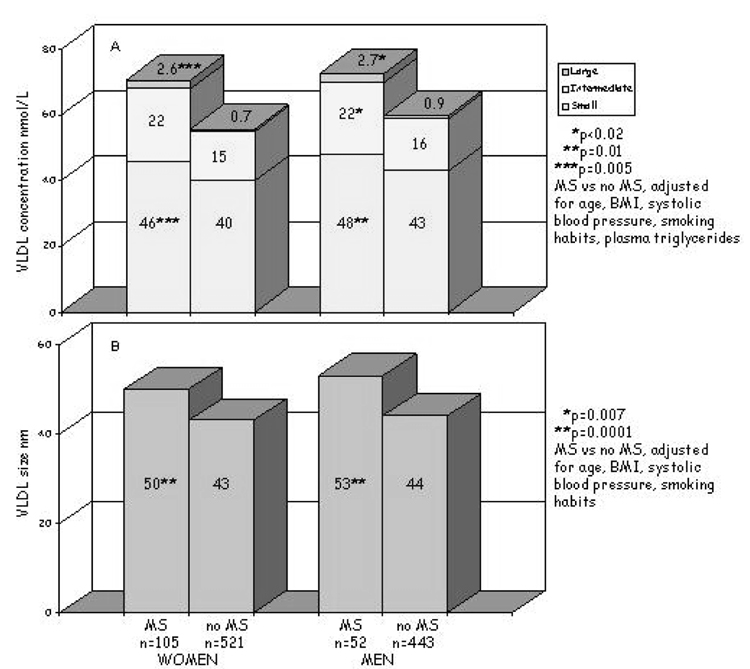
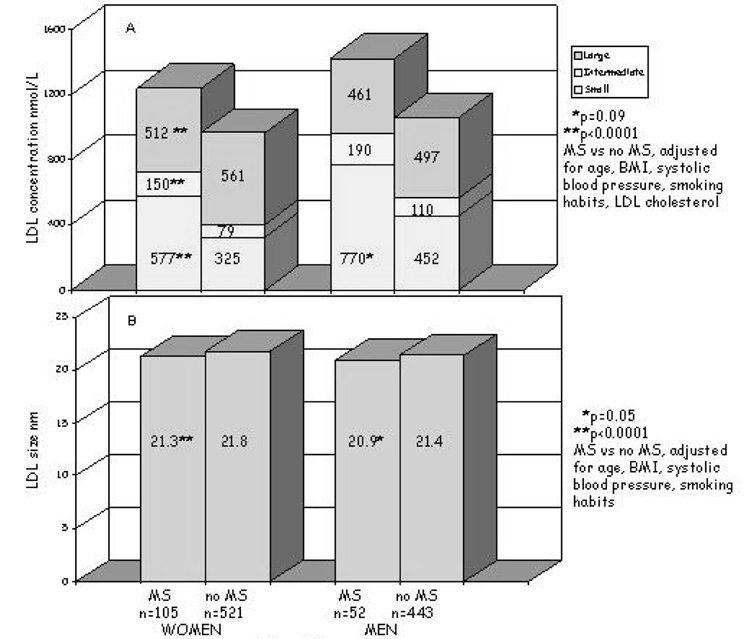
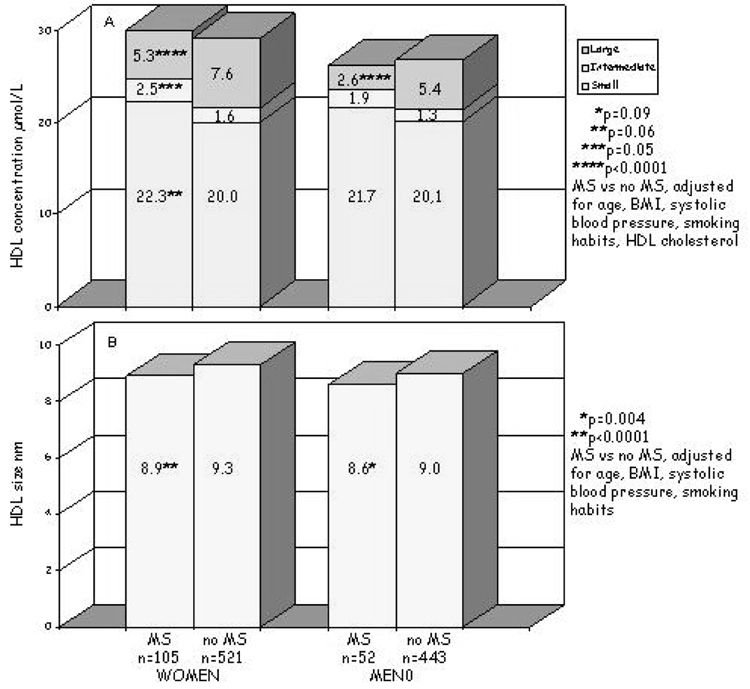
Lipoprotein subfraction concentrations and particle sizes were compared in men and women with (n = 159) and without (n = 969) MS (Figure 1a). All comparisons were adjusted for the covariates described above. The concentrations of all VLDL-C subfractions were higher in women and men with MS; in these subjects, more VLDL were large or intermediate in size (34.5%) compared with individuals without MS (in which 28% of the VLDL were large or intermediate in size). Consequently, VLDL size was greater in both women and men with MS (p = 0.007, p = 0.0001, respectively). LDL particle concentrations were also higher in women and men with MS (Figure 1b) but, in this case, there was a shift in the distribution: large subfractions were lower (women, p < 0.0001; men, p = 0.12), the intermediate and, particularly, the small particles were higher (almost doubled) (women, p < 0.0001; men, p = 0.09); thus, there was a significant reduction of LDL size (women, p < 0.0001; men, p = 0.05). HDL particle concentrations, as a whole, did not differ in those with and without MS; however, there was a different HDL subfraction distribution in those with MS that was characterized by a decrease in the largest particles (both genders, p < 0.0001), an increase in the intermediate particles (p = 0.05 only for women), and a tendency toward increased small particles (women, p = 0.06; men, p = 0.09 for men) (Figure 1c). This particle distribution was reflected in a significant decrease in HDL size in individuals with MS (women, p < 0.0001; men, p = 0.004) (Figure 1c). Adjusting for alcohol intake did not change the results.
Figure 1.
Figure 1a. Plasma VLDL particle concentrations (A) and size (B) in individuals with metabolic syndrome (MS) and without metabolic syndrome (no MS), by gender. P adjusted for age, BMI, systolic blood pressure, smoking habits, and plasma triglycerides.
Figure 1b. Plasma LDL particle concentrations (A) and size (B) in individuals with metabolic syndrome (MS) and without metabolic syndrome (no MS), by gender. P adjusted for age, BMI, systolic blood pressure, smoking habits, and LDL cholesterol.
Figure 1c. Plasma HDL particle concentrations (A) and size (B) in individuals with metabolic syndrome (MS) and without metabolic syndrome (no MS), by gender. P adjusted for age, BMI, systolic blood pressure, smoking habits, and HDL cholesterol.
To evaluate whether the abnormalities in lipoprotein subfraction distribution and size in individuals with MS could be linked to the IR typical of this syndrome, the population was stratified by tertile of HOMA-IR index (< 1.47, 1.47–2.40, > 2.40). Differences in lipoprotein subfraction concentration and size were evaluated separately by gender, adjusted as reported before (Table 3 and Table 4). Increasing tertiles of HOMA-IR in women were characterized by the same pattern of lipoprotein particle distribution and size observed in both genders with MS (Table 3). In men, the trend was similar, but statistical significance was reached for differences in small VLDL (p < 0.05), VLDL size (p = 0.0002), and large and intermediate HDL (p = 0.01 for both). Comparisons of lipoprotein subfractions by tertile of IR were repeated in those without MS and similar changes were seen (data not shown).
Table 3.
Lipoprotein particle concentrations and size in women (Alaska Eskimos ages ≥ 18 years), by tertile of HOMA–IR
| HOMA-IR | ||||
|---|---|---|---|---|
| < 1.47 (n = 207) |
1.47–2.40 (n = 208) |
> 2.40 (n = 208) |
P Adjusted* | |
| VLDL (nmol/L) | ||||
| Large | 0.6 ± 1.4 | 0.8 ± 1.5 | 1.6 ± 2.3 | 0.003 |
| Intermediate | 13.8 ± 11.7 | 15.5 ± 10.9 | 17.8 ± 12.1 | 0.62 |
| Small | 38.8 ± 17.9 | 41.0 ± 19.5 | 43.9 ± 18.9 | 0.18 |
| VLDL size (nm) | 42.3 ± 7.1 | 44.1 ± 9.6 | 47.2 ± 8.9 | <0.001 |
| LDL (nmol/L) | ||||
| Large | 558 ± 178 | 565 ± 187 | 535 ± 193 | 0.02 |
| Intermediate | 74 ± 52 | 82 ± 51 | 117 ± 77 | 0.0001 |
| Small | 310 ± 199 | 332 ± 201 | 459 ± 289 | 0.0006 |
| LDL size (nm) | 21.8 ± 0.6 | 21.8 ± 0.6 | 21.5 ± 0.7 | 0.01 |
| HDL (µmol/L) | ||||
| Large | 8.0 ± 3.9 | 7.5 ± 3.8 | 6.2 ± 3.6 | 0.0005 |
| Intermediate | 1.8 ± 3.1 | 1.4 ± 2.7 | 2.1 ± 3.4 | 0.91 |
| Small | 19.2 ± 4.8 | 20.3 ± 5.3 | 21.6 ± 5.3 | 0.02 |
| HDL size (nm) | 9.4 ± 0.5 | 9.3 ± 0.4 | 9.0 ± 0.5 | 0.0002 |
Data are mean ± SD.
Adjusted for age, BMI, systolic blood pressure, smoking habits, plasma triglycerides (VLDL subfractions), LDL-cholesterol (LDL subfractions), and HDL-cholesterol (HDL subfractions).
Lipoprotein particles were measured using nuclear magnetic resonance.
Table 4.
Lipoprotein particle concentrations and size in men (Alaska Eskimos ages ≥ 18 years), by tertile of HOMA–IR
| HOMA-IR Index | ||||
|---|---|---|---|---|
| < 1.47 (n = 168) |
1.47–2.40 (n = 168) |
> 2.40 (n = 169) |
P Adjusted* | |
| VLDL (nmol/L) | ||||
| Large | 0.7 ± 1.6 | 1.0 ± 1.8 | 1.4 ± 2.7 | 0.28 |
| Intermediate | 13.4 ± 11.1 | 17.0 ± 13.1 | 18.4 ± 12.0 | 0.21 |
| Small | 40.5 ± 17.9 | 44.7 ± 20.3 | 45.6 ± 18.9 | 0.05 |
| VLDL size (nm) | 41.9 ± 8.5 | 45.9 ± 9.4 | 47.3 ± 10.1 | <0.0002 |
| LDL (nmol/L) | ||||
| Large | 489 ± 162 | 511 ±175 | 479 ± 187 | 0.36 |
| Intermediate | 99 ± 50 | 112 ± 65 | 146 ± 82 | 0.06 |
| Small | 410 ± 208 | 465 ± 260 | 583 ± 307 | 0.37 |
| LDL size (nm) | 21.5 ± 0.5 | 21.4 ± 0.6 | 21.2 ± 0.6 | 0.42 |
| HDL (µmol/L) | ||||
| Large | 6.2 ± 3.9 | 4.8 ± 3.4 | 4.3 ± 3.2 | 0.01 |
| Intermediate | 1.2 ± 3.1 | 1.3 ± 2.4 | 1.7 ± 3.0 | 0.01 |
| Small | 19.7 ± 4.6 | 20.2 ± 4.2 | 20.7 ± 5.1 | 0.79 |
| HDL size (nm) | 9.1 ± 0.5 | 8.9 ± 0.5 | 8.8 ± 0.5 | 0.33 |
Data are mean ± SD.
Adjusted for age, BMI, systolic blood pressure, smoking habits, plasma triglycerides (VLDL subfracions), LDL-cholesterol (LDL subfractions), and HDL-cholesterol (HDL subfractions).
Lipoprotein particles were measured using nuclear magnetic resonance.
Correlations among HOMA-IR index and lipoprotein particle concentration and size were also evaluated adjusting for the same variables as before (Table 5). There was a significant direct correlation between HOMA-IR index, large VLDL concentration, and VLDL size, while intermediate and small VLDL were inversely related to IR. For LDL there was a significant direct correlation with both intermediate and small particles, while for HDL, HOMA-IR index was significantly and positively correlated only with the concentration of intermediate-size HDL (p < 0.006). Adjusting for alcohol intake did not change the results.
Table 5.
Pearson’s coefficient between HOMA-IR index and nuclear magnetic resonance lipoprotein subfractions
| r* | P | |
|---|---|---|
| VLDL (nmol/L) | ||
| Large | 0.10 | 0.006 |
| Intermediate | −0.09 | 0.02 |
| Small | −0.l7 | <0.0001 |
| VLDL size (nm) | 0.20 | <0.0001 |
| LDL (nmol/L) | ||
| Large | −0.05 | 0.07 |
| Intermediate | 0.12 | <0.0001 |
| Small | 0.10 | 0.001 |
| LDL size (nm) | −0.05 | 0.09 |
| HDL (µmol/L) | ||
| Large | −0.005 | 0.89 |
| Intermediate | 0.10 | 0.006 |
| Small | −0.04 | 0.25 |
| HDL size (nm) | −0.04 | 0.34 |
Partial Pearson’s coefficients were calculated adjusting for age, BMI, systolic blood pressure, smoking habits, plasma triglycerides (VLDL subfractions), LDL-cholesterol (LDL subfractions), and HDL-cholesterol (HDL subfractions).
DISCUSSION
In this study we have evaluated the impact of MS and IR on plasma lipoprotein subclass distribution and size. To our knowledge, this has been the first demonstration in a large population-based sample and independent of confounding factors (i.e., age, BMI, systolic blood pressure, and smoking habits) that MS is associated with a spectrum of lipoprotein distribution and size abnormalities, as measured by NMR spectroscopy. This relationship is also independent of plasma lipid concentrations and, therefore, these abnormalities may represent an additive cardiovascular risk for individuals with MS. Because similar relations were observed with IR, our results suggest that IR may be a major determinant of these lipoprotein abnormalities.
These results extend and reinforce on a larger scale data obtained by previous studies using traditional methods for lipoprotein fractionation. It is known that MS is associated with an increase in plasma triglycerides, low HDL-C concentration, and increased levels of small, dense LDL (2).
Less information is available on other lipoprotein subclass alterations. Our data have shown that in both genders there is an increase in all VLDL particle concentrations and in VLDL size, probably due to an increase of the largest particles. These changes may contribute to the higher cardiovascular risk of individuals with MS. According to Colhoun et al. (17), a larger VLDL size was significantly associated with coronary calcification in nondiabetic individuals. Also in a prospective study (18), large VLDL were positively associated with coronary artery disease (CAD) severity, independently of plasma triglyceride levels.
The current study shows that in both genders MS is associated with a decrease in large and an increase in small LDL subfraction concentrations, with a subsequent reduction in LDL size, independent of LDL-C levels. The relevance of an increase in small LDL as a cardiovascular risk factor has been supported by cross-sectional (19–23) and prospective studies (24–26).
Data on HDL subclass distribution show that individuals with MS have fewer large and more intermediate and small HDL particles. In our population, differences in HDL subfraction distribution seem less prominent in men but this finding may have been influenced by the sample: fewer men in this sample had MS. These HDL particle abnormalities may also contribute to the high cardiovascular risk of people with MS. An association between small HDL particles and severity of coronary disease has been reported (27–29). Moreover, both small HDL particles and larger VLDL size were found to be significant predictors of type 2 diabetes in the IRAS study (7), reinforcing the role of these lipoprotein abnormalities from a pathophysiologic and clinical point of view.
The other main finding of our study is the relationship between IR and abnormalities in lipoprotein distribution and size. Previous studies support the relationship between IR and LDL particle concentration and size (6–7, 30). Less information exists on the relationship between IR and VLDL/HDL particles. In our study, increasing IR was associated with VLDL and HDL particle concentrations and size abnormalities similar to the ones observed in subjects with MS, with a shift toward a worse profile in terms of cardiovascular risk. A similar relationship was also seen in those with IR but no MS. These results are similar to those of other studies using traditional methods (31) or NMR (30). The present study extends these observations to a larger population-based sample.
Our data also provide additional information on gender differences in lipoprotein distribution. Women in our study, although more obese and insulin resistant than the men, had lower levels of small LDL. This gender difference in atherogenic LDL subclass profile has been shown by Freedman et al. (32) in the Framingham Offspring Study using NMR and in other smaller studies (23, 33–34). Changes in lipoprotein profile with MS and IR were greater in women than in men. This may be due to the higher percentage of women in our sample with MS and IR.
This study was limited by the following factors. The population studied was all Alaska Eskimos; the homogeneity of this population is an advantage for examining metabolic relationships. This population has high levels of cardiovascular disease, and the lipoprotein subclass distribution abnormalities found in this population may explain, at least in part, this increased risk. However, these observations must be confirmed in other populations. This study was also limited by its cross sectional design. Relations between MS and lipoprotein subfractions need to be examined in a longitudinal analysis, which will be possible after the second GOCADAN exam is completed. Finally, the data were obtained using NMR methodology. This method has the advantage of quickly providing simultaneously a quantification of both size and concentration of different lipoprotein subclasses without requiring physical separation. Lipoprotein subclass profile determined by NMR and established methods have been shown to correspond well (10–11, 35–36).
In conclusion, our data show that MS is characterized by a spectrum of VLDL, LDL, and HDL distribution and size abnormalities, independent of age, BMI, SBP, smoking, lipid concentrations, and that IR may be a major determinant of these lipoprotein subfraction abnormalities. These abnormalities may explain the high cardiovascular risk of people with MS beyond the traditional cardiovascular risk factors, which include abnormalities in lipid concentrations. Measures are needed in this and other populations to prevent and reverse the lifestyle changes that lead to these abnormalities and subsequent cardiovascular disease.
ACKNOWLEDGMENTS
This work was supported by grant # HL064244-07 from the National Heart, Lung and Blood Institute. The authors acknowledge the assistance and cooperation of the Eskimo communities of the Norton Sound region, Alaska, without whose support this study would not have been possible. We thank Rachel Schaperow, MedStar Research Institute, Hyattsville, MD, for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.National Cholesterol education program. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) executive summary. Bethesda MD: National Institutes of Health. US Dept. of Health and Human Services; 2001. NIH Publication No-3670. [Google Scholar]
- 2.Ginsberg HN, Zhang Y-L, Hernandez-Ono A. Metabolic Syndrome: Focus on Dyslipidemia. Obesity. 2006;14 Suppl 1:41S–49S. doi: 10.1038/oby.2006.281. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Insulin resistance, insulin resistance syndrome, and cardiovascular disease. Panminerva Med. 2005;47:201–210. [PubMed] [Google Scholar]
- 4.Musliner TA, Krauss RM. Lipoprotein subspecies and risk of coronary disease. Clin. Chem. 1988;34 suppl:B78–B83. [PubMed] [Google Scholar]
- 5.Rizzo M, Berneis K. Small, dense low density lipoproteins and the metabolic syndrome. Diabetes Metab. Res. Rev. 2007;23:14–20. doi: 10.1002/dmrr.694. [DOI] [PubMed] [Google Scholar]
- 6.Mykkänen L, Haffner SM, Rainwater DL, Karhapää P, Miettinen H, Laakso M. Relationship of LDL size to insulin sensitivity in normoglycemic men. Arterioscler. Thromb. Vasc. Biol. 1997;17:1447–1453. doi: 10.1161/01.atv.17.7.1447. [DOI] [PubMed] [Google Scholar]
- 7.Festa A, Williams K, Hanley AJG, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 8.Caslake MJ, Packard CJ. The use of ultracentrifugation for the separation of lipoproteins. In: Rifai N, Warnick GR, Dominiczak MN, editors. Handbook of lipoprotein testing. 2nd ed. Washington, DC: AACC Press; 2000. pp. 609–623. [Google Scholar]
- 9.Rainwater DL, Moore PH, Jr, Shelley WR, Dyer TD, Slifer SH. Characterization of a composite gradient gel for the electrophoretic separation of lipoproteins. J Lipid Res. 1997;38:1261–1266. [PubMed] [Google Scholar]
- 10.Otvos JD, Jeyarajah EJ, Bennet DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38:1632–1638. [PubMed] [Google Scholar]
- 11.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48:171–180. [PubMed] [Google Scholar]
- 12.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal. Joint statement from the American Diabetes Association and the European association for the Study of Diabetes. Diabetologia. 2005;48:1684–1699. doi: 10.1007/s00125-005-1876-2. [DOI] [PubMed] [Google Scholar]
- 13.Howard BV, Devereux RB, Cole SA, Davidson M, Dyke B, Ebbesson SO, Epstein SE, Robinson DR, Jarvis B, Kaufman DJ, Laston S, MacCluer JW, Okin PM, Roman MJ, Romenesko T, Ruotolo G, Swenson M, Wenger CR, Williams-Blangero S, Zhu J, Saccheus C, Fabsitz RR, Robbins DC. A genetic and epidemiologic study of cardiovascular disease in Alaska Natives (GOCADAN): design and methods. Int J Circumpolar Health. 2005;64:206–221. doi: 10.3402/ijch.v64i3.17985. [DOI] [PubMed] [Google Scholar]
- 14.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1977;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrikson DS. Estimation of the concentration of lo-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 16.Mattews DR, Hoster JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Colhoun HM, Otvos JD, Rubens MB, Taskinen MR, Underwood SR, Fuller JH. Lipoprotein subclasses and particle sizes and their relationship with coronary artery calcification in men and women with and without type 1 diabetes. Diabetes. 2005;51:1949–1956. doi: 10.2337/diabetes.51.6.1949. [DOI] [PubMed] [Google Scholar]
- 18.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18:1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 19.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–1921. [PubMed] [Google Scholar]
- 20.Tornvall P, Karpe F, Carlson LA, Hamsten A. Relationships of low density lipoprotein subfractions to angiographically defined coronary artery disease in young survivors of myocardial infarction. Atherosclerosis. 1991;90:67–80. doi: 10.1016/0021-9150(91)90245-x. [DOI] [PubMed] [Google Scholar]
- 21.Campos H, Genest JJ, Jr, Blijlevens E, McNamara JR, Jenner JL, Ordovas JM, Wilson PW, Schaefer EJ. Low density lipoprotein particle size and coronary artery disease. Arterioscler Thromb. 1992;12:187–195. doi: 10.1161/01.atv.12.2.187. [DOI] [PubMed] [Google Scholar]
- 22.Coresh J, Kwiterovich PO, Jr, Smith HH, Bachorik PS. Association of plasma triglyceride concentration and LDL particle diameter, density, and chemical composition with premature coronary artery disease in men and women. J Lipid Res. 1993;34:1687–1697. [PubMed] [Google Scholar]
- 23.Griffin BA, Freeman DJ, Tait GW, Thomson J, Caslake MJ, Packard CJ, Shepherd J. Role of plasma triglyceride in the regulation of plasma low density lipoprotein (LDL) subfractions: relative contribution of small, dense LDL to coronary heart disease risk. Atherosclerosis. 1994;106:241–253. doi: 10.1016/0021-9150(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 25.Stampfer MJ, Krauss RM, Ma J, Blanche PJ, Holl LG, Sacks FM, Hennekens CH. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. JAMA. 1996;276:882–888. [PubMed] [Google Scholar]
- 26.Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation. 1997;95:69–75. doi: 10.1161/01.cir.95.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Wilson HM, Patel JC, Skinner ER. The distribution of high-density lipoprotein subfractions in coronary survivors. Biochem Soc Trans. 1990;18:1175–1176. doi: 10.1042/bst0181175. [DOI] [PubMed] [Google Scholar]
- 28.Cheung MC, Brown BG, Wolf AC, Albers JJ. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J Lipid Res. 1991;32:383–394. [PubMed] [Google Scholar]
- 29.Johansson J, Carlson LA, Landou C, Hamsten A. High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb. 1991;11:174–182. doi: 10.1161/01.atv.11.1.174. [DOI] [PubMed] [Google Scholar]
- 30.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52:453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- 31.Tilly-Kiesi M, Knudsen P, Groop L, Taskinen MR. Hyperinsulinemia and insulin resistance are associated with multiple abnormalities of lipoprotein subclasses in glucose-tolerant relatives of NIDDM patients. Botnia Study Group. J Lipid Res. 1996;37:1569–1578. [PubMed] [Google Scholar]
- 32.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D'Agostino RB, Wilson PW, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 33.Tan CE, Forster L, Caslake MJ, Bedford D, Watson TDG, McConnel M, Packard CJ, Shepherd J. Relations between plasma lipids and postheparin plasma lipids and postheparin plasma lipases and VLDL and LDL subfraction patterns in normolipidemic men and women. Ateroscler Thromb Vasc Biol. 1995;15:1839–1848. doi: 10.1161/01.atv.15.11.1839. [DOI] [PubMed] [Google Scholar]
- 34.Nikkilä M, Pitkäjärvi, Koivula T, Solakivi T, Lehtimäki, Laippala P, Jokela H, Lehtomäki E, Seppä K, Sillanaukee P. Women have a larger and less atherogenic low density lipoproetin particle size than men. Atherosclerosis. 1996;119:181–190. doi: 10.1016/0021-9150(95)05645-9. [DOI] [PubMed] [Google Scholar]
- 35.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–1937. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 36.Tsai MY, Georgopoulos A, Otvos JD, Ordovas JM, Hanson NQ, Peacock JM, Arnett DK. Comparison of ultracentrifugation and nuclear magnetic resonance spectroscopy in the quantification of triglyceride-rich lipoproteins after an oral fat load. Clin Chem. 2004;50:1201–1204. doi: 10.1373/clinchem.2004.032938. [DOI] [PubMed] [Google Scholar]