Abstract
The current study examined developmental changes in activation and effective connectivity among brain regions during a phonological processing task, using fMRI. Participants, ages 9–15, were scanned while performing rhyming judgments on pairs of visually presented words. The orthographic and phonological similarity between words in the pair was independently manipulated, so that rhyming judgment could not be based on orthographic similarity. Our results show a developmental increase in activation in the dorsal part of left inferior frontal gyrus (IFG), accompanied by a decrease in the dorsal part of left superior temporal gyrus (STG). The coupling of dorsal IFG with other selected brain regions involved in the phonological decision increased with age, while the coupling of STG decreased with age. These results suggest that during development there is a shift from reliance on sensory auditory representations to reliance on phonological segmentation and covert articulation for performing rhyming judgment on visually presented words. In addition, we found a developmental increase in activation in left posterior parietal cortex that was not accompanied by a change in its connectivity with the other regions. These results suggest that maturational changes within a cortical region are not necessarily accompanied by an increase in its interactions with other regions and its contribution to the task. Our results are consistent with the idea that there is reduced reliance on primary sensory processes as task-relevant processes mature and become more efficient during development.
Introduction
The process of mapping visual words onto phonological representations forms the basis of reading acquisition. Phonological processing of visually presented words undergoes major changes while children mature and become fluent readers. The current study uses functional magnetic resonance imaging (fMRI) to examine developmental changes in phonological processing in school-age children performing rhyming judgment on visually presented words. Rhyming judgments in adults activate the left superior temporal, supramarginal and dorsal inferior frontal gyri (Booth et al., 2002a; Crosson et al., 1999; Kareken et al., 2000; Lurito et al., 2000; Paulesu et al., 1996; Pugh et al., 1996; Xu et al., 2001). In children, phonological tasks such as non-word reading, phoneme deletion and rhyming judgment resulted in the activation of left temporo-parietal and frontal regions (Booth et al., 2004; Georgiewa, 1999; Shaywitz et al., 2002; Temple et al., 2001). Developmental changes in activation examined in a number of language tasks showed increases in activation in left inferior frontal gyrus in single word generation tasks (Brown et al., 2005; Gaillard et al., 2003; Holland et al., 2001; Schapiro et al., 2004; Schlaggar et al., 2002; Szaflarski et al., 2006) and in a rhyming task to visually presented words when comparing children to adults (Booth et al., 2004). Developmental increases in activation were also found in left superior temporal gyrus in a verb generation task (Schapiro et al., 2004) and in an auditory narrative comprehension task (Schmithorst et al., 2006). Finally, several studies have also found developmental increases in activation in left inferior parietal lobule in single word generation tasks (Brown et al., 2005; Schapiro et al., 2004).
Developmental increases in activation in regions relevant for task performance may reflect reorganization in cortical areas and formation of new representations (Durston et al., 2006; Johnson, 2000). Alternatively, an increase in activation may reflect a change in strategy, resulting in greater reliance on a specific cognitive process for the performance of the given task. In the latter case, the change is specific to the task, rather than maturation of a cortical system. This pattern, referred to as “process switch” in adult learning studies (Poldrack, 2000), is often accompanied by decreased activation in a different brain region (Raichle et al., 1994). A decrease in activation may thus reflect less engagement of a specific cognitive process in a given task, and consequently reduced activity in the neural substrates associated with that process. Alternatively, a developmental decrease in activation may reflect increasing neural efficiency in a specific brain region while it continues to contribute to the task. In adult learning studies, a mechanism, by which experience results in a reduction in the neural resources required to achieve a cognitive goal, has been suggested to explain the reduction in activation in primed compared to novel stimuli (Dehaene et al., 2004; Wagner et al., 2000) and in practiced items following repeated presentation (Buckner et al., 2000; Poldrack and Gabrieli, 2001; Reber et al., 2005). The developmental decrease in extrastriate activation by words has similarly been suggested to result from improved “tuning” (selectivity) of lower level mechanisms (Brown et al., 2005). During development such a process may result from pruning of neurons and elimination of redundant connections, thereby increasing the signal to noise ratio and strengthening relevant connections in the system (Durston et al., 2006; Johnson, 2000). Examining developmental changes in the interactions among brain regions may help to distinguish between the different potential explanations for increases and decreases in activation.
In the current study, we identify regions that show developmental changes in activation and examine changes in effective connectivity between these regions and regions that are active across ages. While our previous study examined developmental changes in a rhyming task by comparing children to adults (Booth et al., 2004), in the current study, we examine the correlation between activation and age in a sample of children 9–15 years old. This enabled us to deconfound the effect of age from linear changes in performance, the importance of which was emphasized by recent developmental studies (Brown et al., 2005; Casey, 2002).
An additional contribution of the current study is the examination of developmental changes in a task that increases the demands on the mapping between orthography and phonology by independently manipulating orthographic and phonological similarity between words. In the non-conflicting conditions, both orthography and phonology of the words were either similar (lime–dime) or different (staff–gain). In conflicting conditions, words had similar phonology and different orthography (jazz–has) or different phonology and similar orthography (pint–mint). This manipulation encourages the participants to generate the phonological representations of words, rather than rely on orthographic information to perform the rhyming judgment. In addition, it enables a comparison between conditions with high and low demands on the mapping between orthography and phonology (Bitan et al., in press).
Our previous analyses of the same data (Bitan et al., in press) showed the activation for the rhyming task in left inferior frontal and superior temporal gyri as well as greater activation for conflicting compared to non-conflicting conditions in left inferior frontal and left inferior parietal cortices. However, in our previous paper, we did not examine developmental effects. Because these regions have also shown developmental increases in activation in previous studies, we predict that left inferior frontal, left inferior parietal and left superior temporal regions would show developmental increases in activation in the rhyming task, reflecting reorganization and formation of new representations. Alternatively, some of these regions may show a developmental decrease in activation, which may either reflect an increase in the neural efficiency in these regions (Poldrack and Gabrieli, 2001; Reber et al., 2005) or a shift away from cognitive processes involving these regions (Raichle et al., 1994).
Materials and methods
Participants
Thirty-six healthy children (ages 9–15, mean=11.7), 22 females, participated in the study. Children were all right handed (mean=78, range 50–90) according to the 9 item Likert-scale questionnaire (−90 to 90, positive scores indicate right hand dominance). All children were native English speakers, with normal hearing and normal or corrected-to-normal vision. All children were free of neurological diseases or psychiatric disorders and were not taking medication affecting the central nervous system. Children were recruited from the Chicago metropolitan area. Parents of children were given an interview to ensure that they did not have a history of intelligence, reading, attention or oral-language deficits. Children were given standardized intelligence tests (Wechsler Abbreviated Scale of Intelligence (WASI) (The Psychological Corporation, 1999)), and reading tests from the Woodcock Johnson battery (Woodcock et al., 2001). The results per age group are presented in Table 1. The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research Institute approved the informed consent procedures.
Table 1.
Distribution of sex, IQ and reading level in younger and older participants
| Age group (years) | Males | Female | FSIQ | Word ID | Word attack |
|---|---|---|---|---|---|
| <12 | 8 | 9 | 114 | 112 | 111 |
| >12 | 6 | 13 | 107 | 111 | 110 |
FSIQ—full scale IQ as measured by WASI; Word ID, Word attack—reading level subtests in the Woodcock Johnson test.
Tasks
Rhyming task
Two words were presented visually in a sequential order and the participant had to determine whether the words rhymed and indicate their judgment by pressing one of two buttons. Each word was presented for 800 ms followed by a 200-ms blank interval. A red fixation cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2600-ms interval. Twenty-four word pairs were presented in each one of four lexical conditions that independently manipulated the orthographic and phonological similarity between words. In the two non-conflicting conditions, the two words were either similar in both orthography and phonology (e.g., dime–lime), or different in both orthography and phonology (e.g., staff–gain). In the two conflicting conditions, the two words had either similar orthography but different phonology (e.g., pint–mint), or different orthography but similar phonology (e.g., jazz–has). If the words rhymed the participant pressed a button with the index finger, and if they did not rhyme the participant pressed a different button with the middle finger.
Control conditions
Two perceptual control conditions were used in which two symbol strings were presented visually in sequential order and the participant had to determine whether the strings matched. In the ‘Simple’ condition, the symbol string consisted of a single symbol, while in the ‘Complex’ condition, the symbol string consisted of three different symbols (e.g.,  ) subtending a similar visual angle and visual complexity as the words. Timing parameters were the same as for the lexical conditions. 24 Items were presented in each perceptual condition, with half of them matching. In addition to the perceptual control conditions, 72 fixation trials were included as a baseline. In the fixation condition, a black fixation cross was presented for the same duration as the stimuli in the lexical and perceptual conditions and participants were instructed to press a button when the black fixation cross turned red. Button press in the fixation condition was included to equate the motor response and motor planning components between the experimental and the baseline conditions.
) subtending a similar visual angle and visual complexity as the words. Timing parameters were the same as for the lexical conditions. 24 Items were presented in each perceptual condition, with half of them matching. In addition to the perceptual control conditions, 72 fixation trials were included as a baseline. In the fixation condition, a black fixation cross was presented for the same duration as the stimuli in the lexical and perceptual conditions and participants were instructed to press a button when the black fixation cross turned red. Button press in the fixation condition was included to equate the motor response and motor planning components between the experimental and the baseline conditions.
Stimulus characteristics
All words were monosyllabic words 4–7 letters long and were matched across conditions for written word frequency in adults and children (The Educator's Word Frequency Guide, 1996) and for written and spoken word frequency in adults (Baayen et al., 1995; Bitan et al., in press). The symbols in the control conditions consisted of re-arranged parts of lower case Courier letters. In the Complex condition a symbol did not repeat within any symbol string. Non-matching pairs differed in one symbol, with the position of the non-matching symbol equally distributed across the string. All words and symbols were presented in lower case, at the center of the screen, with a 0.5-letter offset of position between the first and second stimulus.
Experimental procedure
After informed consent was obtained and the standardized intelligence test was administered, participants were invited for a practice session, in which they were trained to minimize head movement in front of a computer screen using an infrared tracking device. In addition, they performed one run of the experimental task in a simulator scanner, in order to make sure they understood the tasks and to acclimatize themselves to the scanner environment. Different stimuli were used in the practice and in the scanning sessions. Scanning took place within a week from the practice session. In the scanning session, two 8.3-min runs of 108 trial each were performed, in which the order of rhyming, perceptual and fixation trials was optimized for event-related design (Burock et al., 1998). The order of stimuli within task was fixed for all subjects. Accuracy of performance in the scanner and reaction time from the onset of the second item in each trial were recorded.
MRI data acquisition
Images were acquired using a 1.5-T GE scanner, using a standard head coil. Head movement was minimized using vacuum pillow (Bionix, Toledo, OH). The stimuli were projected onto a screen and viewed through a mirror attached to the inside of the head coil. Participants' responses were recorded using an optical response box (Current Designs, Philadelphia, PA). The BOLD functional images were acquired using the EPI (echo planar imaging) method. The following parameters were used for scanning: TE = 35 ms, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24; TR = 2000 ms. Two runs, with 240 repetitions each, were administered for the functional images. In addition, structural T1-weighted 3D image were acquired (SPGR, TR = 21 ms, TE = 8 ms, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124), using an identical orientation as the functional images.
Image analysis
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 4 mm maximum displacement. Sinc interpolation was used to minimize timing errors between slices (Henson et al., 1999). The functional images were co-registered with the anatomical image and normalized to the standard T1 template volume (MNI). The data were then smoothened with a 10-mm isotropic Gaussian kernel. Statistical analyses at the first level were calculated using an event-related design, with ‘rhyming task’, ‘perceptual task,’ and the fixation events as three conditions of interest. A high pass filter with a cutoff period of 128 s was applied. Pairs of items were treated as individual events for analysis and modeled using a canonical HRF. Group results were obtained using random effects analyses by combining subject-specific summary statistics across the group as implemented in SPM2 (Penny and Holmes, 2003).
In a recent paper, we presented the main effect of comparing the rhyming task with the perceptual task and comparisons among the different conditions within the rhyming task (Bitan et al., in press). In the current paper we focus on the developmental changes. Because there were no significant differences between the effects of age in any of the 4 rhyming task conditions, the contrast collapsing across all conditions vs. fixation is used for determining the nodes for the effective connectivity analysis. The main effects for all rhyming task conditions vs. fixation and for the complex perceptual condition vs. fixation were tested using a one-sample t-test. Only the complex perceptual condition was included in the analysis because it was better matched with the words in the rhyming task for visual complexity. Developmental changes were determined with a multiple regression analysis using a contrast collapsing all rhyming task conditions vs. fixation, or the complex perceptual condition vs. fixation, from each individual. The participants' age in months was entered as a covariate, together with accuracy of performance in the scanner as a second covariate. This allowed us to examine age-related increases or decreases in activation that were independent of linear accuracy differences.
Because reaction time was negatively correlated with age, and increased time on the task could result in increased activation, a decrease in activation with age could result from decreased time on task. To account for this possibility, we included reaction time as an additional covariate when testing for decreased activation with age. Because our sample included a larger number of girls than boys in the older ages, we carried out an additional analysis to rule out the possibility that the effect of age was due to sex differences. An ANCOVA was performed with sex as a discrete variable and accuracy as a continuous variable. No activation was found that was greater for boys compared to girls. The map of activation that was greater for girls compared to boys, at a threshold of p<0.005, was used as an exclusive mask to look at the effects of increasing age. The results of the age correlation were not changed by using this mask. In addition, regions that showed correlation with age were overlaid on maps showing activation related to the conflict between orthography and phonology. These included regions that showed greater activation for conflicting compared to non-conflicting conditions and regions in which the differential activation for conflicting (vs. non-conflicting) conditions correlated with accuracy on the conflicting conditions. Although this is a qualitative approach, it provides information regarding the overlap between the different analyses. All reported areas of activation were significant using uncorrected p<0.001 at the voxel level and containing a cluster size greater than or equal to 10 voxels. The results of the main effects (rhyming task or perceptual conditions vs. fixation, conflicting vs. non-conflicting) are displayed with a more stringent threshold of uncorrected p<0.0001 to enable the distinction among brain structures that otherwise comprise a single cluster. In all tables, clusters that survive a family-wise error correction for multiple comparisons at p<0.05 are presented in bold.
Effective connectivity analysis
To further examine brain regions that show developmental changes in activation, we conducted an effective connectivity analysis in a network with six regions of interest (ROIs), shown in Table 2. The network was limited to 6 nodes in order to simplify the model. These include three left hemisphere regions that were active for all rhyming task conditions across all ages (Table 3): fusiform gyrus (FG), ventral inferior frontal gyrus (IFG), and lateral temporal cortex (LTC). In addition, three left hemisphere age-dependent regions were included that showed a developmental change in activation specific for linguistic stimuli (Table 4). Of these three, dorsal IFG (BA 9), and intraparietal sulcus (IPS) showed a developmental increase in activation whereas anterior superior temporal gyrus (STG) showed a developmental decrease in activation.
Table 2.
ROIs used for the effective connectivity analysis
| ROI | BA | x | y | z |
|---|---|---|---|---|
| Age-dependent regions | ||||
| Dorsal inferior frontal gyrus | 44/9 | −54 | 9 | 36 |
| Anterior superior temporal gyrus/insula | 22/13 | −45 | −12 | −3 |
| Intraparietal sulcus | 7/19 | −30 | −57 | 48 |
| Regions active across ages | ||||
| Lateral temporal cortex | 22 | −51 | −42 | 9 |
| Ventral inferior frontal gyrus | 45/46 | −45 | 30 | 9 |
| Fusiform/inferior temporal gyrus | 19/37 | −45 | −69 | −12 |
For age-dependent regions these voxels served as the center of ROI across all subjects. For regions that were active across ages these voxels served as the group reference for individually defined ROIs.
Table 3.
Regions of activation in the rhyming and perceptual tasks vs. fixation
| Region | BA | H | z score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Words vs. fixation | |||||||
| Inferior occipital/middle occipital/ fusiform gyri* |
18/19/37 | L+R | Inf | 1526 | −45 | −69 | −12 |
| Inferior frontal/precentral gyri* | 45/46/47/44/9 | L | 7.7 | 1613 | −45 | 30 | 9 |
| Superior frontal gyrus | 6/8 | L+R | 6.4 | 381 | −6 | 12 | 54 |
| Inferior frontal gyrus* | 47 | R | 5.8 | 205 | 36 | 24 | 0 |
| Superior/Middle temporal gyri | 22 | L | 5.5 | 83 | −51 | −42 | 9 |
| Thalamus | – | L | 5.3 | 22 | −21 | −30 | 0 |
| Thalamus | – | L | 5.3 | 65 | −9 | −15 | 9 |
| Anterior cingulate* | 24/32 | L+R | 4.9 | 49 | −6 | 3 | 27 |
| Superior parietal lobule | 7 | L | 4.7 | 46 | −27 | −54 | 48 |
| Precentral gyrus | 6 | R | 4.6 | 12 | 42 | −12 | 63 |
| Putamen | – | L | 4.5 | 52 | −18 | 9 | 3 |
| Symbols vs. fixation | |||||||
| Inferior occipital/middle occipital/ fusiform gyri/precuneus/inferior parietal lobule* |
19/37/7/40 | L | Inf | 1300 | −42 | −75 | −9 |
| Inferior/Middle occipital gyri/precuneus* | 18/19/7 | R | Inf | 1823 | 42 | −78 | −9 |
| Inferior frontal/middle frontal gyri/insula* | 46/47/13 | R | 6.5 | 226 | 51 | 33 | 18 |
| Inferior frontal gyrus | 9 | R | 6 | 152 | 48 | 9 | 27 |
| Anterior cingulate* | 32 | L+R | 5.7 | 118 | 6 | 18 | 45 |
| Inferior frontal/precentral gyri* | 9 | L | 4.9 | 36 | −57 | 9 | 36 |
| Postcentral gyrus | 1 | R | 4.4 | 12 | 60 | −21 | 48 |
Clusters are presented with a threshold of uncorrected p<0.0001, and extent of 10 voxels or greater, with clusters significant at the threshold of corrected p<0.05 displayed in bold.
Regions with overlap between activation for words and symbols.
Inf—infinite.
Table 4.
Correlation between activation in rhyming task and age
| Region | BA | H |
z score |
Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Increase with age (accuracy controlled) | |||||||
| Superior parietal lobule | 7 | R | 3.6 | 12 | 24 | −69 | 54 |
| Middle frontal/precentral gyri | 6 | L | 3.5 | 35 | −39 | −6 | 51 |
| Inferior frontal/precentral gyri | 9 | L | 3.5 | 15 | −54 | 9 | 33 |
| Intraparietal sulcus | 40 | L | 3.5 | 15 | −36 | −54 | 54 |
| Postcentral gyrus/ inferior parietal lobule |
40 | L | 3.4 | 10 | −45 | −33 | 45 |
| Decrease with age (accuracy controlled) | |||||||
| Supramarginal/ Postcentral gyri |
40 | L | 3.8 | 28 | −57 | −27 | 18 |
| Superior/Medial frontal gyri | 9/32 | L | 3.4 | 15 | −15 | 39 | 24 |
| Superior temporal gyrus | 22 | L | 3.3 | 15 | −51 | −9 | −3 |
| Decrease with age (RT and accuracy controlled) | |||||||
| Supramarginal/Heschl gyri | 40/41 | L | 4.6 | 110 | −57 | −27 | 18 |
| Superior temporal/ Heschl gyrus |
22/41 | R | 3.9 | 47 | 57 | −27 | 3 |
| Medial frontal gyrus | 9 | L | 3.7 | 31 | −15 | 39 | 24 |
| Superior temporal gyrus/insula | 22/13 | L | 3.2 | 11 | −42 | −12 | −3 |
Clusters are presented with a threshold of uncorrected p<0.001, and extent of 10 voxels or greater.
The nodes chosen for the effective connectivity analysis in this study extend the model used in our previous study of effective connectivity in children during this task (Bitan et al., 2006). Our earlier study included the three left hemisphere regions that were active for lexical conditions across all ages in this study, identifying the fusiform gyrus as an input region and the LTC and ventral IFG as involved in the phonological decision. From the regions that showed age-dependent activation in the current study, the posterior temporoparietal cluster (Heschl/supramarginal gyri) was not included in the model because it was contiguous with the anterior STG under a more liberal threshold. The MFG (BA 6) was not included because the developmental increase in this area was not specific to the linguistic task.
The six regions of interest were specified for each individual, each specified as a 6-mm radius sphere. For the age-dependent regions (dorsal IFG, anterior STG and IPS), a fixed voxel was chosen as the center of the ROI across all individuals. For regions that were active across ages (ventral IFG, LTC and FG), the ROI was centered on the most significant voxel within 30 mm of the group maximum in the ‘rhyming vs. fixation’ activation map, restricted by an anatomical mask of the relevant region (i.e., IFG for IFG, superior and middle temporal gyri for LTC, and fusiform and inferior temporal gyri for FG). A weaker voxel was chosen in individuals where the distance between the centers of different ROIs was less than 26 mm apart. Two subjects were excluded because they had no significant clusters within 30 mm from the group reference voxel (one in LTC and one in ventral IFG), resulting in a sample of 34 subjects for the effective connectivity analysis. Table 5 shows the peak co-ordinates used for the individually defined ROIs.
Table 5.
Peak co-ordinates used for individually defined ROIs
| Subject | vIFG |
LTC |
FG |
||||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | x | y | z | |
| 1 | −45 | 24 | −3 | −54 | −33 | 6 | −42 | −45 | −15 |
| 2 | −48 | 27 | 0 | −45 | −51 | 15 | −42 | −45 | −15 |
| 3 | −45 | 27 | −3 | −66 | −33 | 0 | −45 | −75 | −18 |
| 4 | −48 | 30 | 15 | −51 | −42 | 3 | −51 | −78 | −6 |
| 5 | −54 | 21 | 12 | −51 | −51 | −15 | −48 | −78 | −6 |
| 6 | −36 | 30 | 12 | −48 | −39 | 0 | −42 | −66 | −18 |
| 7 | −51 | 36 | 3 | −51 | −60 | 0 | −33 | −78 | −18 |
| 8 | −54 | 15 | −6 | −63 | −33 | 6 | −45 | −60 | −21 |
| 9 | −48 | 42 | 0 | −51 | −51 | 9 | −42 | −54 | −18 |
| 10 | −33 | 24 | 6 | −54 | −42 | 21 | −39 | −42 | −15 |
| 11 | −48 | 12 | 21 | −51 | −57 | −15 | −30 | −78 | −21 |
| 12 | −54 | 42 | 3 | −63 | −30 | 6 | −36 | −51 | −18 |
| 13 | −51 | 42 | 6 | −48 | −42 | 3 | −48 | −54 | −21 |
| 14 | −42 | 27 | 3 | −51 | −54 | −15 | −36 | −81 | −18 |
| 15 | −36 | 30 | −6 | −48 | −48 | 6 | −48 | −78 | −6 |
| 16 | −48 | 27 | 3 | −48 | −69 | 0 | −45 | −51 | −21 |
| 17 | −36 | 33 | −6 | −57 | −42 | 6 | −45 | −75 | −18 |
| 18 | −54 | 24 | 15 | −54 | −48 | 12 | −39 | −42 | −15 |
| 19 | −57 | 39 | 3 | −69 | −21 | 9 | −45 | −51 | −18 |
| 20 | −48 | 30 | 21 | −54 | −42 | 3 | −42 | −69 | −3 |
| 21 | −51 | 42 | 6 | −57 | −60 | −12 | −42 | −69 | −18 |
| 22 | −51 | 33 | 9 | −42 | −66 | 3 | −39 | −42 | −21 |
| 23 | −57 | 27 | 15 | −51 | −60 | −12 | −27 | −87 | −18 |
| 24 | −54 | 33 | 3 | −48 | −48 | 3 | −33 | −66 | −15 |
| 25 | −51 | 45 | 0 | −60 | −15 | 9 | −33 | −69 | −12 |
| 26 | −42 | 30 | 3 | −69 | −30 | 0 | −39 | −57 | −18 |
| 27 | −45 | 45 | 6 | −54 | −54 | 6 | −42 | −78 | −18 |
| 28 | −48 | 39 | 9 | −45 | −63 | −3 | −27 | −93 | −18 |
| 29 | −51 | 30 | 21 | −57 | −42 | 21 | −45 | −66 | −18 |
| 30 | −54 | 33 | 0 | −51 | −45 | 3 | −45 | −75 | −6 |
| 31 | −57 | 30 | 18 | −60 | −51 | 15 | −39 | −69 | −18 |
| 32 | −39 | 30 | 0 | −45 | −60 | 3 | −42 | −78 | −18 |
| 33 | −45 | 42 | 3 | −51 | −57 | −12 | −45 | −69 | −6 |
| 34 | −42 | 30 | 0 | −60 | −33 | 21 | −36 | −51 | −15 |
vIFG—inferior frontal gyrus pars triangularis; LTC (lateral temporal cortex)—superior and middle temporal gyri; FG—fusiform and inferior temporal gyri.
Effective connectivity analysis was examined using the Dynamic Causal Modeling (DCM) tool in SPM2 (Friston et al., 2003; Penny et al., 2004). DCM is a nonlinear systems identification procedure that uses Bayesian estimation to make inferences about effective connectivity between neural systems and how it is affected by experimental conditions. In DCM, three sets of parameters are estimated: the direct influence of stimuli on regional activity; the intrinsic or latent connections between regions in the absence of modulating experimental effects; and the changes in the intrinsic connectivity between regions induced by the experimental design (i.e., modulatory effects) (Mechelli et al., 2003). The validity of the effective connectivity analysis depends on the choice of regions accurately representing the nodes in the network involved in the task. Our analysis adopted a two-stage procedure that is formally identical to the summary statistic approach used in random effects analysis of neuroimaging data. The parameters from the subject-specific, first level DCM models were taken to a second, between-subject level using the random effects approach (Bitan et al., 2005). Subject-specific DCMs were fully and reciprocally connected (resulting in 30 connections) with modulatory (bilinear) effects of the rhyming task specified on coupling among all regions, except for the effects on the fusiform gyrus. DCM requires a specification of the direct influence of the stimuli on a region. In the current model, the fusiform gyrus was specified as the region receiving input for the rhyming task.
The second level analysis was done on the bilinear effects on the coupling between the three age-dependent regions (i.e., dorsal IFG, anterior STG and IPS) and two regions involved in the phonological decision (Bitan et al., 2005) that were active across ages (i.e., ventral IFG and LTC). To test the effect of age on the bilinear effects while controlling for the effect of accuracy, a GLM analysis was conducted separately for each age-dependent region, resulting in a model of 2 paired regions (ventral IFG, LTC) by 2 directions (in, out), with 2 covariates (age in months and accuracy of performance in the scanner). For regions that showed a significant three-way interaction of age by paired region by direction, a follow-up analysis was performed within each direction, and the results are reported at a significance level of p<0.05 corrected for 2 comparisons. The correlation of each bilinear term with age, while accuracy is controlled, is reported at the level of p<0.05 corrected for 4 comparisons (four correlations per region).
Results
Behavioral results
Here we report behavioral results collapsed for conflicting and non-conflicting conditions, because they show a significant difference in accuracy and reaction time (Bitan et al., in press), and this distinction is relevant for the fMRI analysis presented below. (For a detailed statistical analysis of the behavioral results for specific conditions, see Bitan et al., in press). The accuracy of performance in the scanner was 86% on average for all rhyming task conditions, with an average of 78% and 93% on conflicting and non-conflicting conditions, respectively. Average performance on the perceptual control conditions was 93%. The reaction time across rhyming task conditions was 1384 ms (1461 and 1307 ms for conflicting and non-conflicting, respectively) and 1137 ms for the perceptual conditions (Bitan et al., in press). Fig. 1 shows the correlation of age with accuracy of performance and reaction time in the rhyming task. Age was significantly correlated with accuracy of performance in both the rhyming and perceptual tasks (r=0.46, 0.37 respectively; p<0.05). Age was also negatively correlated with reaction time on both the rhyming and perceptual tasks (r=−0.48, −0.39; respectively, p<0.05).
Fig. 1.
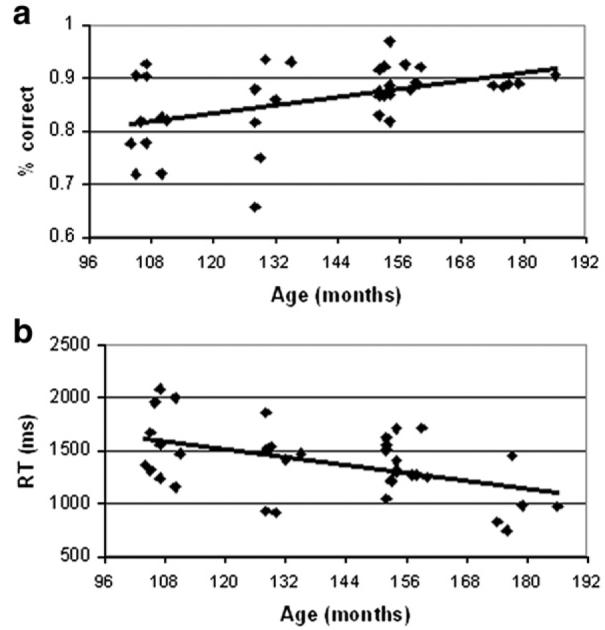
Correlation of performance in the scanner in the rhyming task with age of participants. (a) Accuracy and (b) reaction time are presented.
fMRI results
Main effects
Fig. 2 and Table 3 present regions that were active for the rhyming task and the complex perceptual task compared to fixation. Both perceptual and rhyming tasks activated the bilateral fusiform/ventral occipital cortex (BAs 18, 19, 37), anterior cingulate (BA 32), and bilateral inferior frontal cortex (BA 47/13 on the right, BA 9 on the left). Activation for rhyming task was also found in a large portion of the left inferior frontal gyrus, in left superior temporal gyrus, and in left posterior parietal cortex.
Fig. 2.
Regions of activation in the rhyming (red) and perceptual (blue) tasks, and their overlap (purple). Activation is displayed at the threshold of uncorrected p<0.0001.
Correlation with age in the rhyming task
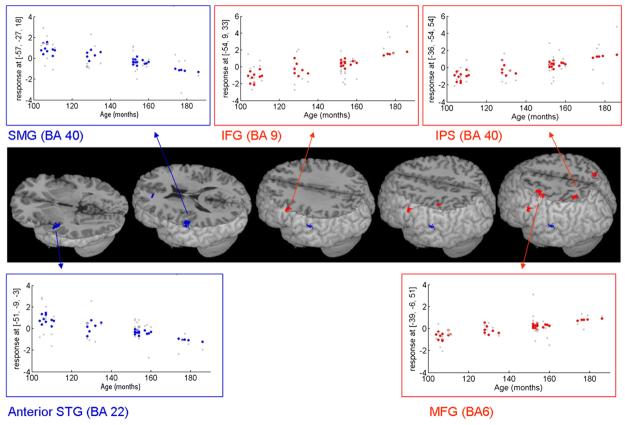
Fig. 3 and Table 4 present regions that showed an increase or decrease in activation with age for the rhyming task, when linear effects of accuracy were controlled. Increase in activation was found in left inferior frontal gyrus (BA 9), in left middle frontal gyrus (BA6) and in bilateral posterior parietal cortices (BA 40/7). A decrease in activation with age was found in the anterior part of left superior temporal gyrus (BA 22) and in a posterior cluster in left supramarginal gyrus. To account for the possible confound of time on task, reaction time was included as an additional covariate in the examination of decreased activation with age. Table 4 shows that when reaction time and accuracy are both controlled a decrease in activation with age is still found in the anterior part of left superior temporal gyrus. In addition the posterior cluster was found bilaterally in Heschl gyri (BA 41) extending into supramarginal gyrus on the left and into posterior superior temporal gyrus on the right.
Fig. 3.
Regions showing age-related changes in activation for the rhyming task when linear effects of accuracy are controlled. Red—increased activation with age; blue—decreased activation with age. Plots show the correlation of signal intensity (y axis) and age in months (x axis) in each region. IFG—inferior frontal gyrus (−54, 9, 33), IPS—intraparietal sulcus (−36, −54, 54), STG—superior temporal gyrus (−51, −9, −3), SMG—supramarginal gyrus (−57, −27, 18), MFG—middle frontal gyrus (−39, −6, 51). BA—Brodmann area.
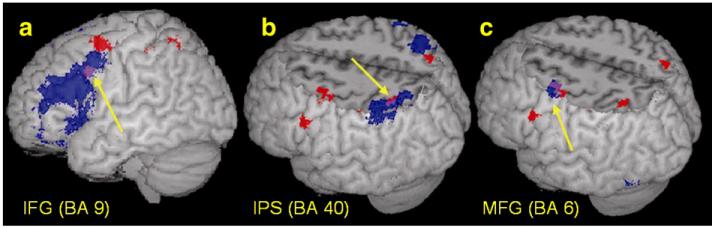
Age correlation and sensitivity to the conflict
Regions that show a developmental change in activation were overlaid on regions that were involved in the conflict between orthography and phonology (Figs. 4a, b). Although this is a qualitative approach, it shows that some of the developmental changes occur in regions engaged in the resolution of this conflict. Table 6 presents regions that showed greater activation in conflicting compared to non-conflicting conditions, and regions in which the differential activation for conflicting (compared to non-conflicting) conditions was correlated with accuracy in the conflicting conditions. Fig. 4a shows that the developmental increase in activation in left inferior frontal gyrus (BA 9) was found in a region that shows greater activation for conflicting compared to non-conflicting conditions. Fig. 4b shows the overlap between the developmental increase in activation in the posterior parietal cortices and the correlation between the differential activation for conflicting conditions and accuracy in the conflicting conditions. Although the clusters are distributed across anatomical structures (the intraparietal sulcus and inferior parietal lobule), these clusters overlap in the left hemisphere, and will be interpreted together.
Fig. 4.
Developmental increase in activation in the rhyming task (red) overlaid on other maps (blue). (a) Blue=conflicting vs. non-conflicting in inferior frontal gyrus (BA9; displayed at the threshold of uncorrected p<0.0001); (b) blue=correlation between the differential activation for conflicting (compared to non-conflicting conditions) and accuracy in conflicting conditions in intraparietal sulcus (BA 40) and (c) blue=the developmental increase in activation in perceptual condition in middle frontal gyrus (BA 6). Overlap is always purple.
Table 6.
Regions involved in the conflict between orthography and phonology
| Region | BA | H | z score | Voxels | x | y | z |
|---|---|---|---|---|---|---|---|
| Conflicting>non-conflicting conditions | |||||||
| Medial frontal gyrus/caudate | 8/32 | L+R | 7.1 | 1616 | −6 | 24 | 45 |
| Inferior frontal gyrus/insula | 9/47/13 | L | 6.5 | 1231 | −39 | 6 | 27 |
| Insula | 13 | R | 5.6 | 237 | 33 | 24 | 0 |
| Inferior frontal/middle frontal/precentral gyri | 9/46 | R | 5.3 | 271 | 42 | 12 | 27 |
| Inferior parietal lobule | 40 | L | 4.2 | 31 | −33 | −48 | 42 |
| The difference ‘conflicting–non-conflicting’ correlated with accuracy in conflicting | |||||||
| Inferior/Superior parietal lobules | 40/7 | L | 4.8 | 162 | −45 | −51 | 57 |
| Fusiform gyrus | 36 | R | 4.4 | 53 | 39 | −18 | −12 |
| Inferior frontal gyrus | 47 | R | 4.3 | 45 | 42 | 45 | −6 |
| Superior parietal lobule | 7 | R | 4.2 | 138 | 36 | −60 | 54 |
| Inferior frontal gyrus | 47 | R | 4.0 | 53 | 51 | 3 | 3 |
| Inferior parietal/postcentral gyri | 40/2 | R | 4.0 | 20 | 57 | −36 | 51 |
| Middle occipital gyrus | 19 | R | 3.9 | 14 | 33 | −78 | 3 |
| Inferior temporal gyrus | 37 | L | 3.8 | 32 | −48 | −48 | −15 |
| Middle temporal gyrus | 37 | R | 3.3 | 17 | 57 | −57 | −6 |
Regions with greater activation for conflicting vs. non-conflicting conditions are displayed at a threshold of uncorrected p<0.0001 with clusters significant at the threshold of corrected p<0.05 displayed in bold. Regions that show increased sensitivity to the conflict with increased accuracy in conflicting condition are presented with the standard threshold of uncorrected p<0.001.
Correlation with age in perceptual conditions
An increase of activation with age (when accuracy was controlled) was found for the complex perceptual condition in the left middle frontal gyrus (BA 6) (x=−39, y=0, z=57; z-score = 4.2, 40 voxels). Fig. 4c shows that activation in middle frontal gyrus for both the rhyming and the perceptual tasks increased with age.
Effective connectivity analysis
The effect of age on the bilinear effects, while controlling for accuracy, was examined separately in each age-dependent region, using a GLM analysis of two-paired regions ×2 directions with 2 covariates (age and accuracy). For dorsal IFG a significant main effect of age was found (F(1,31)=5.2, p<0.05), with no interaction of age with direction or paired region. Fig. 5 shows that all bilinear effects on the coupling with dorsal IFG increase with age. Although there is no significant interaction, the correlation with age is strongest for the effect of LTC on dorsal IFG (r=0.41, p corrected for 4 comparisons <0.05). A similar GLM analysis performed on the coupling of anterior STG shows a significant three-way interaction of age by region by direction (F(1,31)=15.1, p<0.01). In a separate analysis within each direction, only the coupling going out from anterior STG shows a significant main effect of age (F(1,31)=5.6, p<0.05 corrected for 2 comparisons) and a significant interaction of age with paired region (F(1,31)=7.7, p<0.05 corrected for 2 comparisons). Fig. 5 shows that the effect of anterior STG on ventral IFG and LTC both decrease with age, but the correlation with age is stronger for the effect on ventral IFG (r=(−0.46), p corrected for 4 comparisons <0.05). A GLM analysis for the coupling of IPS did not reveal any significant main effect or interaction with age. Although for the effect of IPS on LTC there is a trend for positive correlation with age, this effect is not significant (p corrected for 4 comparisons=0.10). Bilinear effects on the connections from fusiform gyrus to age-dependent regions were uncorrelated with age (r=0.04; 0.17; −0.08 for coupling with dIFG, IPS, aSTG, respectively). They are not displayed in Fig. 5 since they were not part of the prediction that focused on the coupling of age-dependent regions with regions involved in the phonological decision.
Fig. 5.
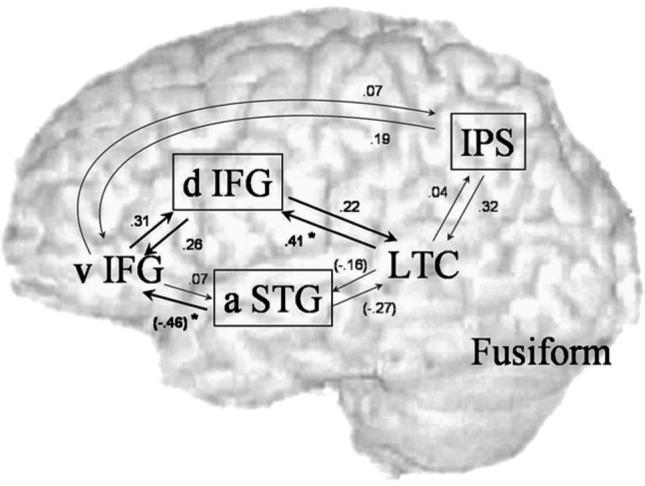
Correlation with age in bilinear effects (controlled for accuracy). Boxed regions—regions showing correlation of activation with age in conventional analysis. Black arrows—significant main effect of age; grey—non-significant correlations with age; star—significant correlation with age when calculated separately for each bilinear effect. d—Dorsal, v—ventral, a—anterior, IFG—inferior frontal gyrus, STG—superior temporal gyrus, LTC—lateral temporal cortex, IPS—intraparietal sulcus.
Discussion
Our behavioral results show that performance on the rhyming task improved with age, both in terms of accuracy and reaction time. Nevertheless, our sample size enabled us to identify developmental changes in activation while controlling for linear differences in performance. Our fMRI results indicate three patterns of age-related changes. Consistent with our prediction, a developmental increase in activation, specific to linguistic stimuli, was found in the dorsal aspect of left inferior frontal gyrus (IFG; BA 9) and in bilateral posterior parietal regions (BA 40/7). However, an age-related decrease in activation, specific to linguistic stimuli, was found in anterior left superior temporal gyrus (STG; BA 22) and bilateral Heschl gyri (BA 41). In addition, a developmental increase in activation for both linguistic and perceptual stimuli was found in left middle frontal gyrus (BA 6). Effective connectivity analysis showed an overall developmental increase in the coupling of dorsal IFG and a decrease in the effect of anterior STG on ventral IFG. There was no significant change in the coupling of left intraparietal sulcus with other regions selected in the model.
Increase in activation in dorsal inferior frontal gyrus
The age-related increase in activation in the dorsal part of left inferior frontal gyrus (BA 9) was found in a region that was more active for conditions that have conflicting orthographic and phonological information (e.g., pint–mint, jazz–has) compared to non-conflicting conditions. Activation in BA 9 has been previously found in tasks that require phonological segmentation and covert articulation (Clark and Wagner, 2003; Demonet et al., 1992; Fiebach et al., 2002; Fiez et al., 1999; Gitelman et al., 2005; Mechelli et al., 2005). In a meta-analysis of 129 language studies, phonological, semantic and syntactic clusters of activation peaks were identified in the frontal and temporal lobes (Vigneau et al., 2006). Among the clusters identified in the meta-analysis, the closest (12 mm) to the developmental increase found in the current study was in lower precentral gyrus (BA 9; −48, 2, 26), and was associated with phonological processing, silent rehearsal and complex mouth and tongue movements. The greater activation in BA 9 in conflicting compared to non-conflicting conditions in our study suggests that conflicting conditions recruit phonological segmentation and covert articulation processes to a greater extent than non-conflicting conditions (Bitan et al., in press).
The age-related increase in activation in dorsal IFG (BA 9) is consistent with previous findings of greater activation in adults compared to children in left BA 9/44 in a rhyming task to visually presented words (Booth et al., 2004), and with a developmental increase in activation in BA 44, in single word generation tasks (Schapiro et al., 2004; Schlaggar et al., 2002). The increase in activation in the current study may suggest that during development school-age children show greater reliance on phonological segmentation and covert articulation for making rhyming judgments. Alternatively, the increase in activation may reflect maturation of the cortical system involved in phonological segmentation, including reorganization and formation of new representations, but with no change in the contribution of this region to the phonological task. The results of our effective connectivity analysis support the former interpretation. The coupling of dorsal IFG with the two regions that are involved in integration and making the phonological decision, i.e., ventral IFG and LTC (Bitan et al., 2005), increased with age. These results suggest that the increase in activation in dorsal IFG does not reflect only maturation of the cortical system but also growing contribution of this region to performance on the rhyming task.
Decreases in activation in superior temporal gyrus
Our results show an age-related decrease in activation in the dorsal aspect of the superior temporal gyrus, in two clusters (which are contiguous with a more liberal threshold). When linear effects of accuracy and reaction time were controlled, decreased activation with age was found in the anterior part of left superior temporal gyrus (STG) on the border of the insula (BA 22/13), and in posterior bilateral clusters in Heschl gyri (BA 41) extending into supramarginal gyrus on the left and into superior temporal gyrus on the right. Both left hemisphere clusters are distinct from the more posterior lateral temporal cortex (LTC) which was active across all ages and is presumably involved in integration of abstract phonological information (Bitan et al., 2005). Heschl gyri is primary auditory cortex, sensitive to acoustic parameters (Binder et al., 2000; Howard et al., 2000; Inui et al., 2006). Two nearby clusters, identified in a recent meta-analysis (Vigneau et al., 2006) (planum temporale, 9 mm; −60, −27, 9; and STG, 14 mm, −50, −38, 12), were associated with unimodal auditory processing such as consonant perception (Joanisse and Gati, 2003) and voice onset time processing (Jancke et al., 2002). The anterior STG cluster, which also showed a developmental decrease in activation in the current study, is located near an auditory phonological cluster (14 mm, −56, −12, −3) identified in a meta-analysis (Vigneau et al., 2006). This cluster was sensitive to human voice and speech sounds (Belin et al., 2002) and was activated by listening to syllables (Poeppel et al., 2004; Sekiyama et al., 2003), pseudo-words (Binder et al., 2000), and detection of rhymes (Booth et al., 2002b).
Our results show a developmental decrease in activation in the dorsal aspect of left superior temporal gyrus, implicated in sensory acoustic processing and in auditory phonological processing. One interpretation for these findings may be that maturation and experience resulted in improved neural efficiency in this region, and that the generation of auditory phonological representations in older children requires less neural activity. However, in terms of connectivity, this interpretation would predict a preservation or even an increase in the effect of anterior STG on brain regions involved in the phonological decision (i.e., LTC and ventral IFG). Instead, our results show an age-related decrease in the effect of anterior STG on these regions, suggesting an alternative account for the decrease in activation in anterior STG. Altogether these results suggest that during development, there is less engagement of auditory phonological representations when making a phonological judgment on visually presented words. Instead, older children presumably rely on abstract phonological representations, associated with LTC, and on phonological segmentation and covert articulation associated with dorsal IFG.
Support for this interpretation can be found in a study that examined the effect of age of acquisition on activation to visually presented words (Fiebach et al., 2003). Reading early acquired words resulted in greater activation in the temporal operculum (−44, −10, 9; 12 mm from the anterior STG in the current study), while reading late acquired words resulted in greater activation in lateral inferior frontal areas. The authors suggest that early learned words are represented in the brain in a more auditory sensory manner than late learned words (Fiebach et al., 2003). These findings support the conclusion of the current study that the reduced involvement of the dorsal STG reflects age-related reduction in the engagement of auditory processing in reading visually presented words. Our results are also consistent with the findings of word generation studies showing developmental decreases in activation in the middle portion of the left insula (BA 13; −41, −13, −2; 2 mm from the anterior STG cluster in the current study) (Brown et al., 2005) and in left posterior insula (Szaflarski et al., 2006). The authors suggest that these regressive changes in activation reflect narrowing of activation from a relatively large and diffuse set of lower level brain regions that compensate for less efficient processing in language-specific areas, to regions that are specifically related to the task. Higher level regions in frontal and parietal cortex may be recruited to provide top-down guidance for the selection of the lower level mechanisms for specific task performance (Bitan et al., 2006; Brown et al., 2005; Johnson, 2000).
Increase in activation in intraparietal sulcus
The age-related increase in activation in left intraparietal sulcus (BA 40) was found in a region in which the differential activation for conflicting compared to non-conflicting conditions was correlated with the accuracy in conflicting conditions. The correlation with accuracy suggests that the cognitive processes associated with this region are critical for performance in the conflicting conditions. We have suggested that conflicting pairs require repetitive mapping between orthography and phonology, which presumably involves the left inferior parietal lobule (Bitan et al., in press). Previous studies have also showed that left inferior parietal lobule is involved in tasks that require mapping between orthographic and phonological representations (Booth et al., 2002a; Clark and Wagner, 2003; Demonet et al., 1992; Fiebach et al., 2002; Fiez et al., 1999; Mechelli et al., 2005). The cluster in the intraparietal sulcus that shows a developmental increase in activation in the current study is also close (9 mm) to another cluster in superior/inferior parietal lobules that was more active for an orthographic compared to the phonological judgment task (Bitan et al., in press).
The age-related increase in the activation in left intraparietal sulcus is consistent with similar findings in different parts of the posterior parietal cortex. In single word generation tasks to both visually and auditory presented words, a developmental increase in activation was found in left inferior parietal lobule (BA 40) (Brown et al., 2005) and precuneus (BA 7/19) (Schapiro et al., 2004). The results of the effective connectivity analysis show that despite the developmental increase in activation, there was no significant change in the coupling between IPS and other brain regions involved in the rhyming task. One possible interpretation of these results is that the positive correlation with age was too weak to reach significance; alternatively, there may a change in connectivity with regions which are not part of this model. A third, more intriguing, explanation for these results is that the cortical system involved in orthographic processing and in mapping of orthography to phonology matures and becomes more elaborated during development, but there is no change in its interaction with other regions that reflects its contribution to the rhyming task. The increase in activation may thus reflect growing connectivity within the region, rather than connectivity between regions.
Increase in activation common to linguistic and perceptual stimuli
Our results show a developmental increase in activation in left middle frontal gyrus (BA 6). This cluster overlapped with a region showing a developmental increase in activation for the perceptual task, suggesting that it was not specific for linguistic processing. BA 6 has been implicated in previous studies of visual and verbal working memory (Crosson et al., 1999; Fiez et al., 1996; Jonides et al., 1993; Petrides et al., 1993). Working memory may be required in the current rhyming task to hold the first stimulus in memory while processing the second stimulus in a pair. The age-related increase in activation in BA 6 may reflect the maturation of working memory mechanisms, not specific to language processing.
Conclusions
The current study examined developmental changes in the neural correlates of a phonological judgment task, as reflected in activation in specific brain regions and connectivity between them. Consistent with previous studies that showed both progressive and regressive changes during development; our results show language-specific increases in the activation in left dorsal inferior frontal gyrus accompanied by decreases in activation in dorsal superior temporal regions. The coupling of these regions with brain regions involved in the phonological decision followed a similar pattern of increase for dorsal IFG and a decrease for anterior STG. These results suggest that while children of all ages in this study generate abstract phonological representations when performing rhyming judgments on visually presented words, there is a shift from reliance on auditory phonology in younger children to greater reliance on phonological segmentation and covert articulation in older children. These results support the notion that as task-relevant processes mature there is a reduction in reliance on low-level auditory processes that serve as compensatory mechanisms in earlier ages. Our results also show an age-related increase in activation in a posterior parietal region with, however, no change in the coupling of this region with other brain regions. These findings may suggest that although the cortical system involved in mapping between orthography and phonology matures, there is no change in the contribution of this system to performing the rhyming task. Our findings demonstrate the contribution of effective connectivity analysis to the understanding of developmental changes in activation in specific brain regions. Although we did not find evidence for a decrease in activation accompanied by an increase in connectivity, this pattern may nevertheless be found in different brain regions or in different tasks and would suggest that experience resulted in growing in neural efficiency.
Acknowledgments
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to James R. Booth.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
References
- Baayen RH, Piepenbrock R, Gulikers L. The CELEX Lexical Database (Version Release 2) [CD-ROM] Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1995. [Google Scholar]
- Belin P, Zatorre RJ, Ahad P. Human temporal-lobe response to vocal sounds. Cogn. Brain Res. 2002;13:17–26. doi: 10.1016/s0926-6410(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb. Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam M-M. Shifts of effective connectivity within a language network during rhyming and spelling. J. Neurosci. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam M-M, Booth JR. Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou T, Lu D, Cone NE, Cao F, Bigio JD, Booth JR. The interaction between orthographic and phonological information in children: an fMRI study. Human Brain Mapping. doi: 10.1002/hbm.20313. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Modality independence of word comprehension. Hum. Brain Mapp. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. J. Cogn. Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb. Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Casey B. NEUROSCIENCE: windows into the human brain. Science. 2002;296:1408–1409. doi: 10.1126/science.1072684. [DOI] [PubMed] [Google Scholar]
- Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia. 2003;41:304–317. doi: 10.1016/s0028-3932(02)00163-x. [DOI] [PubMed] [Google Scholar]
- Crosson B, Rao SM, Woodley SJ, Rosen AC, Bobholz JA, Mayer A, Cunningham JM, Hammeke TA, Fuller SA, Binder JR, Cox RW, Stein EA. Mapping of semantic, phonological, and orthographic verbal working memory in normal adults with functional magnetic resonance imaging. Neuropsychology. 1999;13:171–187. doi: 10.1037//0894-4105.13.2.171. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words—behavioral and neuroimaging evidence. Psychol. Sci. 2004;15:307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev. Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J. Cogn. Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY, Hernandez AE. Distinct brain representations for early and late learned words. NeuroImage. 2003;19:1627–1637. doi: 10.1016/s1053-8119(03)00227-1. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. J. Neurosci. 1996;16:808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez J, Balota D, Raichle M, Petersen S. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum. Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P. fMRI during word processing in dyslexic and normal reading children. NeuroReport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam M-M. Language network specializations: an analysis with parallel task designs and functional magnetic resonance imaging. NeuroImage. 2005;26:975–985. doi: 10.1016/j.neuroimage.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Henson R, Buchel C, Josephs O, Friston K. The slice-timing problem in event-related fMRI. NeuroImage. 1999;9:S125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J. Comp. Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Inui K, Okamoto H, Miki K, Gunji A, Kakigi R. Serial and parallel processing in the human auditory cortex: a magnetoencephalographic study. Cereb. Cortex. 2006;16:18–30. doi: 10.1093/cercor/bhi080. [DOI] [PubMed] [Google Scholar]
- Jancke L, Wustenberg T, Scheich H, Heinze HJ. Phonetic perception and the temporal cortex. NeuroImage. 2002;15:733–746. doi: 10.1006/nimg.2001.1027. [DOI] [PubMed] [Google Scholar]
- Joanisse MF, Gati JS. Overlapping neural regions for processing rapid temporal cues in speech and nonspeech signals. NeuroImage. 2003;19:64–79. doi: 10.1016/s1053-8119(03)00046-6. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: elements of an interactive specialization framework. Child Dev. 2000;71:75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working-memory in humans as revealed by PET. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Lowe M, Chen SHA, Lurito J, Mathews V. Word rhyming as a probe of hemispheric language dominance with functional magnetic resonance imaging. Neuropsychiatry Neuropsychol. Behav. Neurol. 2000;13:264–270. [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SH, Mathews VP. Comparison of rhyming and word generation with fMRI. Hum. Brain Mapp. 2000;10:99–106. doi: 10.1002/1097-0193(200007)10:3<99::AID-HBM10>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Noppeney U, Friston KJ. A dynamic causal modeling study on category effects: bottom-up or top-down mediation? J. Cogn. Neurosci. 2003;15:925–934. doi: 10.1162/089892903770007317. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Ralph MAL, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes A. Random effects analysis. In: Frackowiak RSJ, Friston KJ, Frith CD, editors. Human Brain Function. Academic Press; San Diego: 2003. pp. 843–850. [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ. Comparing dynamic causal models. NeuroImage. 2004;22:1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal-cortex in memory processing. Proc. Natl. Acad. Sci. U. S. A. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D, Guillemin A, Thompson J, Fritz J, Bavelier D, Braun AR. Auditory lexical decision, categorical perception, and FM direction discrimination differentially engage left and right auditory cortex. Neuropsychologia. 2004;42:183–200. doi: 10.1016/j.neuropsychologia.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues—a theoretical review. NeuroImage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JDE. Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb. Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Gitelman DR, Parrish TB, Mesulam MM. Priming effects in the fusiform gyrus: changes in neural activity beyond the second presentation. Cereb. Cortex. 2005;15:787–795. doi: 10.1093/cercor/bhh179. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK. BOLD fMRI signal increases with age in selected brain regions in children. NeuroReport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296:1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. NeuroImage. 2006;29:254–266. doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiyama K, Kanno I, Miura S, Sugita Y. Auditory-visual speech perception examined by fMRl and. PET. Neurosci. Res. 2003;47:277–287. doi: 10.1016/s0168-0102(03)00214-1. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, Holland SK. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann. Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. NeuroReport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cereb. Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, M. KS, M. N. Woodcock-Johnson III. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega-Bermudez F, Pietrini P, Reeves-Tyer P, DiCamillo P, Theodore W. Conjoint and extended neural networks for the computation of speech codes: the neural basis of selective impairment in reading words and pseudowords. Cereb. Cortex. 2001;11:267–277. doi: 10.1093/cercor/11.3.267. [DOI] [PubMed] [Google Scholar]