Abstract
Age-related differences (9- to 15-year-olds) in the neural correlates of mapping from phonology to orthography were examined with functional magnetic resonance imaging (fMRI). Participants were asked to determine if two spoken words had the same spelling for the rime (corresponding letters after the first consonant or consonant cluster). Some of the word pairs had conflicting orthography and phonology (e.g. jazz-has, pint-mint) whereas other pairs had non-conflicting information (e.g. press-list, gate-hate) (see Table 1). There were age-related increases in activation for lexical processing (across conflicting and non-conflicting conditions) in left inferior parietal lobule, suggesting that older children have a more elaborated system for mapping between phonology and orthography that includes connections at different grain sizes (e.g. phonemes, onset-rimes, syllables). In addition, we found that the conflicting conditions had lower accuracy, slower reaction time and greater activation in left inferior frontal gyrus as compared to non-conflicting conditions. Higher accuracy was also correlated with greater activation in left inferior frontal gyrus for the most difficult conflicting condition (e.g. jazz-has). The finding of both a conflict effect and a correlation with accuracy in left inferior frontal gyrus suggests that this region may be involved in resolving the conflict between orthographic and phonological representations.
Introduction
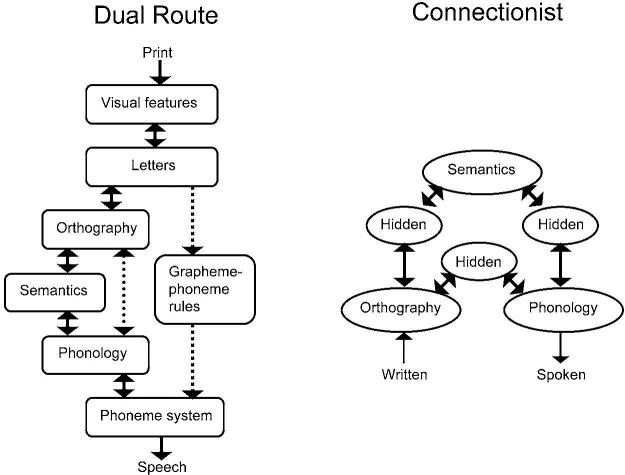
Models of reading development have often been proposed to account for spelling development. In dual-route models of reading, one route involves an indirect sub-lexical grapheme-phoneme rule route and another involves a separate direct lexical route between phonology and orthography at a whole word level (Coltheart, Rastle, Perry, Langdon & Ziegler, 2001). The term indirect refers to the fact that there is an intervening system between orthography and phonology, whereas direct means that there is no intervening system (see Figure 1). In contrast, connectionist models of reading propose that a single indirect route is involved in extracting the statistical regularities between orthography and phonology at different grain sizes (Plaut, McClelland, Seidenberg & Patterson, 1996; Seidenberg & McClelland, 1989). Developmental dual-route models of spelling have proposed that early acquisition is dominated by an indirect grapheme-phoneme rule route, whereas skilled processing relies on the use of a direct lexical route (Frith, 1985). In contrast, developmental spelling models based on connectionist theory propose that acquisition is characterized by greater elaboration of a single indirect route that maps between phonology and orthography (Sprenger-Charolles, Siegel, Bechennec & Serniclaes, 2003). Greater elaboration indicates that mappings are made at different grain sizes including phoneme, onset-rime, syllabic and word-level mappings (Ehri, 1995; Gough & Hillinger, 1980; Marsh, Friedman, Welch & Desberg, 1981; Nunes, Bryant & Bindman, 1997). To the degree that spelling development is similar to reading development, dual-route models predict a shift from reliance on an indirect to a direct route, and therefore, these models predict a developmental decrease in the involvement of an indirect grapheme-phoneme rule route. In contrast, connectionist models predict greater involvement of the single indirect route as it becomes elaborated during reading acquisition. Although models of reading have often been applied to spelling because of some similarities between the two processes, it is clear that in some respects spelling is simply not the reverse of reading (Berninger, Abbott, Jones, Gould, Anderson-Youngstrom, Wolf & Apel, 2006).
Figure 1.
Dual-route versus connectionist models of reading aloud. Dual-route models assume that there is a developmental shift from an indirect grapheme-phoneme rule route (square dotted line) to a direct route from orthography to phonology (circle dotted line). Connectionist models propose that there is a developmental increase in the reliance on an indirect route (hidden units) from orthography to phonology.
Neuroimaging studies with adults performing spelling tasks have shown activation in left inferior frontal gyrus, left inferior parietal cortex (supramarginal gyrus, angular gyrus, and/or inferior parietal lobule) and left inferior temporal/fusiform gyrus. These studies have used a variety of tasks including mentally writing the written word form (Sugishita, Takayama, Shiono, Yoshikawa & Takahashi, 1996), converting Japanese Kana words to Kanji characters and then mentally recalling their visual form (Nakamura, Honda, Okada, Hanakawa, Toma, Fukuyama, Konishi & Shibasaki, 2000), physical writing of a visually presented word (Nakamura et al., 2000), physical writing of a spoken word (Petrides, Alivisatos & Evans, 1995; Tokunaga, Nishikawa, Ikejiri, Nakagawa, Yasuno, Hashikawa, Nishimura, Sugita & Takeda, 1999), and physical writing of a word referring to a visual picture (Katanoda, Yoshikawa & Sugishita, 2001). The most consistent finding across these spelling studies in adults is activation in inferior/superior parietal cortex, a region that has been implicated in mapping between phonological and orthographic representations (Booth, Burman, Meyer, Gitelman, Parrish & Mesulam, 2002, 2003a). The majority of studies have also shown activation in inferior temporal/fusiform gyrus that has been implicated in orthographic processing (Dehaene, Jobert, Naccache, Ciuciu, Poline, Le Bihan & Cohen, 2004), and in inferior frontal gyrus that has been implicated in modulating processes in posterior brain regions (Bitan, Booth, Choy, Burman, Gitelman & Mesulam, 2005) and/or in the hierarchical structuring of cognitive processes (Koechlin & Jubault, 2006).
Three neuroimaging studies have examined children during spelling tasks. Although they did not directly examine developmental differences, one study reported that 7- to 18-year-olds showed reliable activation in left inferior frontal gyrus, but inconsistent activation across subjects in left inferior temporal cortex, during a silent spelling task in the auditory modality (B.C.P. Lee, Kuppusamy, Grueneich, El-Ghazzawy, Gordon, Lin & Haacke, 1999). Another study in 10- to 12-year-old children reported activation in, among other regions, bilateral inferior frontal gyrus, left fusiform gyrus, right inferior parietal lobule, right angular gyrus, and right inferior temporal gyrus for an orthographic mapping task (e.g. are bead-feal both real words) compared to various other language tasks (morpheme mapping with phonological shifts, morpheme mapping without phonological shifts, or phoneme mapping) (Richards, Berninger, Nagy, Parsons, Field & Richards, 2005). Only one study has examined developmental differences between adults and children on spelling judgments to spoken words (e.g. are grade-laid spelled the same from the first vowel onwards) as compared to a tone judgment task (Booth, Burman, Meyer, Zhang, Gitelman, Parrish & Mesulam, 2004). Both adults and children showed activation in left inferior frontal gyrus and left fusiform gyrus. Only adults showed activation in bilateral angular gyrus and bilateral superior parietal lobule. When directly comparing the adults to the children, adults showed greater activation in bilateral inferior frontal gyrus and left angular gyrus/left superior parietal lobule – two critical nodes of the network involved in spelling.
The purpose of this study was to differentiate developmental from behavior-related changes in the neural network involved in orthographic and phonological processing during spelling judgments to words presented in the auditory modality. Although one study has directly examined differences between adults and children during a spelling task (Booth et al., 2004), the current study was unique in several dimensions. In order to look more directly at the developmental process, we examined children from 9 to 15 years old rather than comparing adults and children. A larger number of participants also allowed us to examine both age effects while controlling for accuracy differences, and accuracy effects while controlling for age differences. Based on previous neuroimaging research on spelling tasks in adults and children, we expected to see developmental and/or skill effects in left inferior frontal gyrus and left inferior/superior parietal cortex. Because left inferior parietal cortex has been implicated in the indirect mapping between phonological and orthographic representations, a developmental change of activation in this area would be consistent with models of spelling development based on connectionist theories of reading. Developmental connectionist models predict that there should be greater elaboration of this system involved in indirect mapping.
Another unique characteristic of our study is that we used an event-related design so we could examine the effect of task difficulty by comparing activation for word pairs with conflicting (e.g. pint-mint, jazz-has) versus non-conflicting (e.g. gate-hate, press-list) orthographic and phonological information. Although there are no published studies comparing conflicting to non-conflicting pairs during auditory spelling judgments, research shows that spelling and rhyming judgments in the visual modality are generally more difficult for conflicting than for non-conflicting pairs (McPherson, Ackerman & Dykman, 1997; Polich, McCarthy, Wang & Donchin, 1983), suggesting that orthographic and phonological representations interact regardless of the kind of judgment. Neuroimaging research on adults shows that conflict effects in visual spelling and rhyming tasks are reflected in part in the P300 event-related potential (ERP) thought to be generated by the parietal cortex and thought to be involved in stimulus evaluation and categorization (Kramer & Donchin, 1987; Polich et al., 1983). Behavioral research on children with reading disorder shows a larger conflict effect in visual and auditory rhyming tasks as compared to controls (McPherson et al., 1997; Rack, 1985). Taken together, these results suggest that there should be skill-related changes in brain regions involved in integrating or modulating orthographic and phonological information.
Methods
Participants
Thirty healthy children (ages 9–15, mean = 11.7, 22 females) participated in the study. There were seven 9-year-olds, four 11-year-olds, 13 13-year-olds and six 15-year-olds. Children were right-handed native English speakers, with normal hearing and normal or corrected-to-normal vision. No child was taking medication that affects the central nervous system. Parents were given an interview to ensure that their children did not have a history of intellectual, reading, attention or oral-language deficits. Children were given the verbal portion of the Wechsler Abbreviated Intelligence Scale (WASI; M = 113, SD = 14.5) (Wechsler, 1999). We also administered the Wide Range Achievement Test (WRAT; Wilkinson, 1993). All participants scored 95 or greater on the spelling subtest (M = 113, SD = 11.1). The Institutional Review Board at Northwestern University and Evanston Northwestern Healthcare Research Institute approved the informed consent procedures.
Tasks
Two spoken words were presented in a sequential order and a black fixation-cross appeared throughout the trial. The duration of each word was between 500 and 800 msec followed by a brief period of silence, with the second word beginning 1000 msec after the onset of the first. A red fixation-cross appeared on the screen after the second word, indicating the need to make a response during the subsequent 2600-msec interval. If the two words had the same spelling for all letters from the first vowel onwards, the participant was asked to press a button with the index finger; if the two words did not have the same spelling for all letters from the first vowel onwards, the participant was asked to press a different button with the middle finger.
Twenty-four word pairs were presented in each one of four lexical conditions that independently manipulated the orthographic and phonological similarity between words. In the two non-conflicting conditions, the two words were either similar in both orthography and phonology (O+P+, e.g. gate-hate), or different in both orthography and phonology (O−P−, e.g. press-list). In the two conflicting conditions, the two words had either similar orthography but different phonology (O+P−, e.g. pint-mint), or different orthography but similar phonology (O−P+, e.g. jazz-has). All words were monosyllabic words, 4–7 letters long, and were matched across conditions for written word frequency in adults and children (The educator's word frequency guide, 1996) and for written and spoken word frequency in adults (Baayen, Piepenbrock & Gulikers, 1995).
There were three kinds of control tasks. The simple perceptual control had 24 pairs of single pure tones, ranging from 325 to 875 Hz. The complex perceptual control had 24 pairs of three-tone stimuli, where all the component tones were within the aforementioned frequency range. For both the simple and complex perceptual controls, participants determined whether the stimuli were identical or not by pressing a yes or no button. The third control task involved 72 null events. The participant was instructed to press a button when a black fixation-cross at the center of the visual field turned red. Procedures for presenting the tones and fixation-cross were the same as the word judgment task. The task was administered in two 108 trial runs, in which the order of lexical, perceptual and null trials was optimized for event-related design (Burock, Buckner, Woldorff, Rosen & Dale, 1998).
Experimental procedure
After the standardized tests were administered, participants were given a practice session in a scanner simulator. Scanning took place within 1 week from the practice session.
MRI data acquisition
Images were acquired using a 1.5 Tesla General Electric (GE) scanner, using a standard head coil. The blood oxygen level dependent (BOLD) functional images were acquired using the echo planar imaging (EPI) method. The following parameters were used for scanning: Time of Echo (TE) = 35 msec, flip angle = 90°, matrix size = 64 × 64, field of view = 24 cm, slice thickness = 5 mm, number of slices = 24, Time of Repetition (TR) = 2000 msec. Two runs, with 240 repetitions each, were administered for the functional images. The first four TRs were discarded. In addition, structural T1 weighted 3-D images were acquired (TR = 21 ms, TE = 8 msec, flip angle = 20°, matrix size = 256 × 256, field of view = 22 cm, slice thickness = 1 mm, number of slices = 124). All images were acquired in the axial plane starting at the top of the brain, so coverage of the cerebellum was not complete for either the structural or functional scans.
Image analysis
Data analysis was performed using SPM2 (Statistical Parametric Mapping) (http://www.fil.ion.ucl.ac.uk/spm). The images were spatially realigned to the first volume to correct for head movements. No individual runs had more than 4 mm maximum displacement in the x, y or z dimension and there were no significant differences in average movement across ages (9-year-olds = 1.62, 11-year-olds = 0.77; 13-year-olds = 1.20; 14-year-olds = 1.01). Sixteen children moved less than 1 mm, 10 children moved less than 2 mm, two children moved less than 3 mm and two children moved less than 4 mm. Sinc interpolation was used to minimize timing-errors between slices (Henson, Buchel, Josephs & Friston, 1999). The functional images were co-registered with the anatomical image, and normalized to the standard T1 template volume from the Montreal Neurological Institute (MNI). The data were then smoothed with a 10-mm isotropic Gaussian kernel. The resampled voxels were 3-mm cubed. Statistical analyses at the first level were calculated using an event-related design, with four lexical conditions, two perceptual conditions, and the null events as conditions of interest. A high pass filter with a cutoff period of 128 seconds was applied. All trials were treated as individual events (including inter-stimulus interval) for analysis and modeled using a canonical hemodynamic response function (HRF). Both correct and incorrect responses were included in the analysis. Random-effects analysis of subject-specific summary statistics provided the basis for group inferences.
Using a one-sample t-test, we compared the lexical to null conditions and the perceptual to null conditions in order to identify brain areas that were involved in the linguistic and non-linguistic events, respectively. Any overlap in these maps would indicate that activation in that region is not specifically associated with lexical processing. Although we do not present the data for the complex perceptual control in this paper because it is largely redundant with the simple perceptual control, including it in the event-related model allows for better deconvolution of the hemodynamic response. Multiple regression was used to examine age and accuracy differences. The multiple regression for age (in months) and accuracy was used with the contrast between all lexical conditions versus null, the contrasts between each of the lexical conditions versus null, and the contrast between the perceptual condition versus null. The accuracy measure used in each regression was the accuracy specific to that contrast. By using multiple regression, age correlations were partialed for accuracy and accuracy correlations were partialed for age, so the correlations show variance of brain activation uniquely explained by each variable. Finally, in order to examine brain regions sensitive to the conflict between orthography and phonology, we compared the conflicting to non-conflicting conditions. All reported areas of activation were significant using p < .001 uncorrected at the voxel level and containing a cluster size greater than 15 voxels. Clusters significant at the p < .05 corrected at the cluster level are also indicated.
Results
Behavioral results
Table 2 presents the behavioral performance data. Correlation analyses showed a trend for a correlation between age and accuracy on the lexical conditions (r(29) = 0.34, p < .10), but not for the perceptual or null condition. T-tests between the lexical conditions for accuracy revealed that O−P+ and O+P− had lower accuracy than each of the non-conflicting conditions (ps < .001). In addition, O−P+ had lower accuracy than O+P− (p < .05) and O+P+ had lower accuracy than O−P− (p < .001). In terms of reaction times, O−P+ and O+P− was slower than each of the non-conflicting conditions, and O+P+ was slower than O−P− (ps < .001). Although O−P+ was numerically slower than O+P−, this difference was not statistically significant due to large standard deviations. Because conflicting conditions had the lower accuracy and slower reaction time than non-conflicting conditions, we collapsed across the two conflicting conditions for some of the fMRI analyses.
Table 2.
Mean accuracy (ACC in %), reaction time (RT in ms) and their standard deviations (in parentheses)
| Lexical | O−P+ | O+P− | O+P+ | O−P− | Perceptual | Null | |
|---|---|---|---|---|---|---|---|
| ACC | 79 (7.1) | 63 (16.2) | 69 (10.5) | 87 (9.4) | 95 (6.2) | 94 (9.7) | 97 (5.5) |
| RT | 1660 (351) | 1785 (389) | 1741 (393) | 1614 (367) | 1501 (326) | 1223 (263) | 1319 (298) |
Note: Lexical is all linguistic conditions combined.
Brain activation results
Figure 2 and Table 3 show the regions of activation for lexical versus null conditions and for the perceptual versus null conditions. Both the lexical and perceptual conditions showed bilateral activation in Heschl's gyri and superior temporal gyri, extending bilaterally into middle temporal gyrus for the lexical condition. The extent of activation in the temporal lobes for the lexical condition was larger in the left hemisphere, whereas the extent of activation for the perceptual condition was larger in the right hemisphere. Both conditions showed activation in left inferior frontal gyrus, but activation was more extensive for the lexical conditions with additional activation in right inferior frontal gyrus and insula. Both conditions showed activation in medial frontal and cingulate gyri, but this activation was more extensive for the lexical condition. Both conditions also showed activation in left inferior parietal lobule, but the perceptual condition additionally showed activation in right inferior parietal lobule. Only the lexical condition showed activation in visual association cortices including bilateral lingual gyrus, bilateral cuneus and left fusiform gyrus. For the remainder of the results, we will focus on left hemisphere regions of interest. Please see tables for a full listing of activations.
Figure 2.
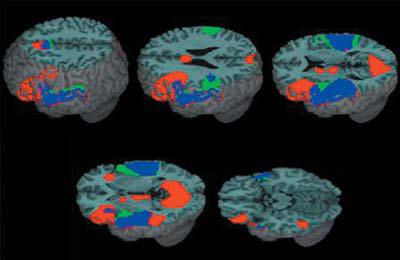
Activation for the lexical versus null conditions (red), for the perceptual versus null conditions (green) and their overlap (blue). Major clusters for the lexical versus null conditions included bilateral inferior frontal gyri, bilateral superior/middle temporal gyri, left fusiform gyrus, left inferior parietal lobule, bilateral medial frontal gyri, and bilateral cuneus.
Table 3.
Regions of activation for the main effects of lexical versus null conditions and perceptual versus null conditions
| Region | H | BA | z-score | voxels | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| Lexical – Null | Superior+middle temporal gyri/Heschl's gyrus/ | L | 22/41/42 | Inf | 2950 | −54 | −18 | 3 |
| Fusiform gyrus/ | 37 | |||||||
| Inferior parietal lobule/ | 40 | |||||||
| Inferior frontal gyrus | 9/44/45/47 | |||||||
| Superior+middle temporal gyri/ Heschl's gyrus | R | 22/41/42 | Inf | 1203 | 63 | −27 | 9 | |
| Medial frontal gyrus/Cingulate gyrus/Thalamus | L | 8/6/32 | 6.15 | 468 | −6 | 18 | 48 | |
| Inferior frontal gyrus/Insula | R | 47/13 | 5 | 81 | 33 | 27 | −3 | |
| Lingual gyri/Cuneus/Posterior cingulate/Parahippocampal gyri | L/R | 17/18/19/30 | 4.94 | 1274 | 18 | −57 | 0 | |
| Perceptual – Null | Superior temporal gyrus/Heschl's gyrus/ | L | 22/41/42 | Inf | 1769 | −54 | −21 | 3 |
| Inferior frontal gyrus/ | 44 | |||||||
| Inferior parietal lobule | 40 | |||||||
| Superior temporal gyrus/Heschl's gyrus/ | R | 22/41/42 | Inf | 1633 | 54 | −21 | 6 | |
| Inferior parietal lobule | 40 | |||||||
| Inferior frontal gyrus | L | 45/47 | 4.15 | 77 | −36 | 27 | 6 | |
| Medial frontal gyrus/Cingulate gyrus | L | 6/32 | 5.46 | 269 | 0 | 6 | 57 |
Note: H = hemisphere, L = left, R = right; BA = Brodmann Area; Inf = Infinite. Significance level is p <.05 corrected at the cluster level. Only clusters with volume greater than 15 voxels are listed.
Figure 3 and Table 4 show the regions where age is positively correlated with activation for the lexical versus null condition, and where age and accuracy are positively correlated with activation for the perceptual versus null condition. For the lexical condition, activation was positively correlated with age in left inferior/superior parietal lobule and left precuneus. There was also a positive correlation with age in left inferior/superior parietal lobule (z = 4.57, x = −33, y = −57 and z = 48, voxels = 85) when we calculated the same analyses partialing for raw accuracy scores on the standardized spelling test (Wilkinson, 1993). For the perceptual condition, activation was positively correlated with age in left superior parietal lobule and was positively correlated with accuracy in left inferior parietal lobule. There was also a positive correlation with age in left superior parietal lobule (z = 3.60, x = −33, y = −60 and z = 54, voxels = 50) when we calculated the same analyses partialing for raw accuracy scores on the standardized spelling test (Wilkinson, 1993). There was no overlap between the lexical and perceptual conditions in the parietal clusters showing correlation of activation with either accuracy or age.
Figure 3.
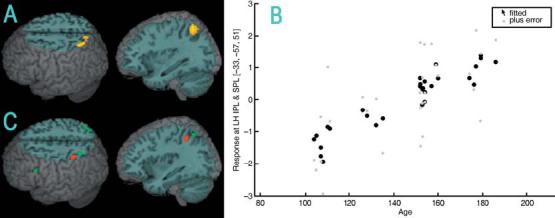
(A) Increasing age correlated with greater activation for the lexical versus null conditions in left inferior/superior parietal lobule. (B) Scatter plot of the age (in months) correlation for the lexical minus null conditions in the most active voxel in left inferior/superior parietal lobule (IPL & SPL). (C) For the perceptual versus null conditions, greater activation correlated with increasing accuracy (red) in left inferior parietal lobule and increasing age (green) in left superior parietal lobule.
Table 4.
Regions of activation for positive age and accuracy (acc) correlations for the lexical versus null conditions and the perceptual versus null conditions
| Region | H | BA | z-score | voxels | x | y | z | ||
|---|---|---|---|---|---|---|---|---|---|
| Lexical – Null | |||||||||
| Age | Inferior+superior parietal lobules/Precuneus | L | 40/7 | 4.17 | 162 | −33 | −57 | 51 | |
| Acc | Insula/Superior temporal gyrus | R | 13/22 | 3.46 | 35 | 36 | −21 | 3 | |
| Perceptual – Null | |||||||||
| Age | Superior parietal lobule | L | 7 | 3.75 | 20 | −33 | −60 | 51 | |
| Superior parietal lobule | R | 7 | 3.43 | 24 | 36 | −60 | 57 | ||
| Acc | Inferior parietal lobule | L | 40 | 3.9 | 51 | −42 | −48 | 48 | |
| Inferior frontal gyrus | R | 45 | 3.53 | 22 | 51 | 24 | 18 | ||
Note: See Table 3 note. Significance level is p < .05 corrected for bolded regions and p <. 001 uncorrected for unbolded regions. Only clusters with volume greater than 15 voxels are listed.
Figure 4 and Table 5 show regions where activation was positively correlated with accuracy for the O−P+ (the most difficult condition) versus null conditions and for the main effect of conflicting versus non-conflicting conditions. Activation for these two contrasts overlap in left inferior frontal gyrus, but not left inferior parietal lobule. However, activation in left inferior parietal lobule for the conflicting versus non-conflicting conditions does overlap the region where age is correlated with activation for the lexical versus null conditions (see Figure 3).
Figure 4.
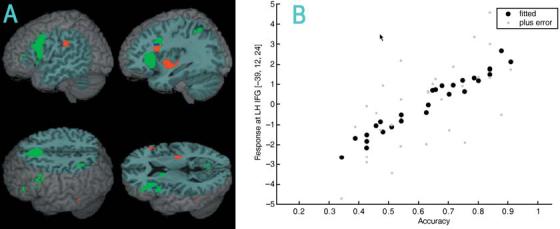
(A) Increasing accuracy (red) correlated with greater activation for the O−P+ (the most difficult conflicting condition) versus null conditions in left inferior frontal gyrus and in left inferior parietal lobule. Accuracy–activation correlations for the O−P+ versus null conditions in left inferior frontal gyrus overlap with the conflicting versus non-conflicting conditions (blue). Other regions more active for the conflicting versus non-conflicting conditions (green) included left inferior parietal lobule and left medial frontal gyrus. (B) Scatter plot of the accuracy–activation correlation for the O−P+ versus null conditions in the most active voxel in left inferior frontal gyrus (IFG).
Table 5.
Regions of activation for positive accuracy correlations for the O−P+ (the most difficult condition) versus null conditions and for the main effect of conflicting versus non-conflicting (non) conditions
| Region | H | BA | z-score | voxels | x | y | z | |
|---|---|---|---|---|---|---|---|---|
| (O−P+) – Null | Inferior frontal gyrus | L | 45 | 3.9 | 77 | −39 | 12 | 24 |
| Inferior parietal lobule | L | 40 | 3.58 | 17 | −51 | −30 | 30 | |
| Insula | L | 13 | 4.15 | 80 | −36 | −3 | −3 | |
| Insula | R | 13 | 3.75 | 48 | 33 | −21 | 6 | |
| Thalamus | L | 3.65 | 18 | −6 | −18 | 3 | ||
| Putamen | R | 3.58 | 38 | 21 | 3 | 3 | ||
| Conflict – Non | Inferior frontal gyrus | L | 45/9 | 5.35 | 556 | −39 | 21 | 6 |
| Inferior parietal lobule | L | 40 | 3.54 | 19 | −36 | −51 | 48 | |
| Medial frontal gyrus | L | 8 | 6.22 | 360 | −6 | 24 | 45 | |
| Lingual gyrus | R | 18 | 3.67 | 26 | 15 | −57 | 0 |
Note: See Table 3 note. Significance level is p < .05 corrected for bolded regions and p < .001 uncorrected for unbolded regions. Only clusters with volume greater than 15 voxels are listed.
We did not find correlations of accuracy with activation in left fusiform gyrus in our whole-brain analysis, unlike a previous study with adults performing an auditory spelling task (Booth et al., 2003a). In order to determine whether there was a trend for brain–behavior correlations, we used a mask of the fusiform cluster found in the lexical versus null conditions (p < .05 uncorrected). Using this mask, we found that activation was positively correlated with accuracy in left fusiform gyrus for the lexical versus null conditions (BA 37; x = 39, y = −27, z = −18; voxels = 20; p < .05 uncorrected).
Discussion
In the current study, children (9- to 15-year-olds) performed a spelling judgment task of spoken words. As a group, they showed activation in a language network involving bilateral inferior frontal gyrus, bilateral superior/middle temporal gyrus, left fusiform gyrus and left inferior parietal lobule. This is consistent with previous neuroimaging studies that implicate in particular left inferior frontal gyrus, left inferior temporal/fusiform gyrus and left inferior parietal cortex in spelling tasks in adults (Katanoda et al., 2001; Nakamura et al., 2000; Petrides et al., 1995; Sugishita et al., 1996; Tokunaga et al., 1999) and children (Booth et al., 2004; B.C.P. Lee et al., 1999; Richards et al., 2005).
Age-related increases in activation were found in left inferior parietal lobule for all lexical conditions combined. The age-related increases in our study are consistent with a previous study of adults and children that showed developmental increases in left inferior parietal cortex during cross-modal tasks that require mapping between orthography and phonology (Booth et al., 2004). It has been previously suggested that the inferior parietal cortex is involved in extracting statistical regularities between orthography and phonology (Booth et al., 2002, 2003a). One of the central skills associated with reading and spelling acquisition is the development of accurate mapping between orthographic and phonological word forms (Booth, Perfetti & MacWhinney, 1999). This development may be associated with greater elaboration of the connections between these systems at different levels including phoneme, onset-rime, syllabic and word-level mappings for both reading (Ehri, 1995; Gough & Hillinger, 1980; Marsh et al., 1981) and spelling (Nunes et al., 1997). The finding of developmental increases in left inferior parietal cortex is also consistent with connectionist models which argue that a single indirect mechanism is involved in mapping between orthographic and phonological representations (Plaut et al., 1996; Seidenberg & McClelland, 1989).
The left inferior parietal region showing age-related increases in activation in our study also overlapped the region where conflicting conditions produced greater activation than non-conflicting conditions, presumably because conflicting conditions make more demands on the mapping between phonology and orthography (Bitan, Burman, Chou, Dong, Cone, Cao, Bigio & Booth, in press). Finally, we found that increasing accuracy was correlated with greater activation in left inferior parietal lobule for the most difficult conflicting condition (e.g. jazz-has). This finding is consistent with a previous study that showed that accuracy in adults on a spelling task was positively correlated with activation in left inferior parietal cortex (Booth et al., 2003a). The greater activation for higher skill children could reflect that they are more effective at recruiting a system for mapping between orthographic and phonological representations.
Age-related increases in activation were found for bilateral superior parietal and accuracy-related increases in activation were evident in left inferior parietal lobule, both for the perceptual conditions. However, the clusters for these two conditions did not overlap with the activation for the age-related increases in left inferior parietal lobule for the lexical conditions, suggesting that the perceptual activation is not related to linguistic processing. It is interesting that our perceptual tone judgment task presented in the auditory modality produced activation in an area typically associated with spatial attention and visuo-spatial processing (Carpenter, Just, Keller, Eddy & Thulborn, 1999; Gitelman, Nobre, Parrish, LaBar, Kim, Meyer & Mesulam, 1999). However, there is emerging evidence that the superior parietal lobule may be involved in auditory attention, particularly to frequency information (Shomstein & Yantis, 2006; Zatorre, Mondor & Evans, 1999). It may be that there were age- and accuracy-related increases in activation in this area because attentional processing is more advanced in older children or is more effectively recruited in higher accuracy children.
The change of activation in inferior parietal cortex in adults when learning new relationships between orthography and phonology seems to be similar to the developmental and skill-related differences found in the current study with children. Studies have shown that learning to associate new orthographic information with phonological information modulates activation in left inferior parietal cortex (H.S. Lee, Fujii, Okadu, Tsukiura, Umetsu, Suzuki, Nagasaka, Takahashi & Yamadori, 2003). For example, Japanese speakers learning to make associations between Korean letters and speech sounds showed greater functional connectivity of this region over learning with posterior inferior temporal gyrus (Hashimoto & Sakai, 2004). Another study compared the neural correlates of three training approaches on learning nonwords (Sandak, Mencl, Frost, Rueckl, Katz, Moore, Mason, Fulbright & Constable, 2004). Both phonological (rhyming judgments) and orthographic (letter judgments) training produced greater activation in left angular gyrus as compared to semantic training, consistent with the involvement of this region in mapping between letters and sounds.
Activation in left inferior frontal gyrus was correlated with increasing accuracy in the most difficult lexical condition (e.g. jazz-has). This finding is consistent with studies in children that have shown brain–behavior correlations between accuracy and activation in the inferior frontal gyrus during language tasks (Gaillard, Sachs, Whitnah, Ahmad, Balsamo, Petrella, Braniecki, McKinney, Hunter, Xu & Grandin, 2003; Turkeltaub, Garaeu, Flowers, Zefirro & Eden, 2003). The accuracy-related increase in activation in left inferior frontal gyrus in our study overlapped with a cluster that showed greater activation for the conflicting compared to the non-conflicting condition, further suggesting that the accuracy-related increase is associated with lexical processing and not some non-linguistic process. Other studies have also found more activation in left inferior frontal gyrus for conflicting compared to non-conflicting conditions during spelling and rhyming tasks in the visual modality (Bitan et al., in press). Greater activation in left inferior frontal gyrus in higher accuracy children in our spelling task could be due to their more effective modulation of posterior systems involved in phonological and orthographic processing (Bitan et al., 2005). We did not find a correlation between age and activation in inferior frontal gyrus, unlike previous studies (Holland, Plante, Byars, Strawsburg, Schmithorst & Ball, 2001), most of which did not partial out the effects of accuracy (Brown, Lugar, Coalson, Miezin, Petersen & Schlaggar, 2005; Schlaggar, Brown, Lugar, Visscher, Miezin & Petersen, 2002).
Reading acquisition is marked by the greater elaboration of orthographic representations involving increases in the number of lexical representations, in the precision of these representations and in the interconnectivity between these representations (Perfetti, 1992). Adults show greater activation than children in left fusiform gyrus when presented with words in the visual modality (Booth et al., 2003b) and adults also show greater selective activation than children in left fusiform gyrus when processing visual word forms as compared to auditory word forms (Booth, Burman, Van Santen, Harasaki, Gitelman, Parrish & Mesulam, 2001). This pattern suggests a greater functional elaboration of this system in response to task demands. Unlike a previous study with adults performing an auditory spelling task (Booth et al., 2003a), we did not find significant accuracy-related differences in left fusiform gyrus using whole-brain analysis, although we did find a weak correlation with accuracy when we used a mask of the fusiform cluster found in the lexical versus null contrast. Another study with adolescents and adults has also shown a correlation of skill with activation in left fusiform gyrus when reading words (Brem, Bucher, Halder, Summers, Dietrich, Martin & Brandeis, 2006). Altogether, these findings suggest a developmental increase in the functional specification of fusiform activity with more accurate spelling and reading.
In conclusion, this study implicates a network involving left inferior frontal gyrus, left fusiform gyrus and left inferior parietal lobule in spelling tasks. The age- and accuracy-related increases in activation in left inferior parietal lobule suggest that this region becomes more elaborated with development and skill for making mappings between phonology and orthography. The accuracy-related increases in activation in left inferior frontal cortex for the difficult conflicting condition suggests that this region is involved in modulating orthographic and phonological representations.
Table 1.
Lexical rhyme conditions varying in their degree of phonological–orthographic consistency
| Similar orthography | Dissimilar orthography | |
|---|---|---|
| Similar | O+P+ | O−P+ |
| Phonology | gate-hate | *jazz-has |
| Dissimilar | O+P− | O−P− |
| Phonology | *pint-mint | press-list |
Note:
Phonological–orthographic conflicting conditions, in which phonological information (whether the two words rhyme) conflicts with orthographic information (whether the two words are spelled the same beginning with the first vowel).
Acknowledgements
This research was supported by grants from the National Institute of Child Health and Human Development (HD042049) and by the National Institute of Deafness and Other Communication Disorders (DC06149) to James R. Booth.
References
- Baayen RH, Piepenbrock R, Gulikers L. The celex lexical database (Version Release 2) [CD-ROM] Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1995. [Google Scholar]
- Berninger VW, Abbott RD, Jones J, Gould L, Anderson-Youngstrom M, Wolf BJ, Apel K. Early development of language by hand: composing, reading, listening, and speaking connections; three letter-writing modes; and fast mapping in spelling. Developmental Neuropsychology. 2006;29(1):61–92. doi: 10.1207/s15326942dn2901_5. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM. Shifts of effective connectivity within a language network during rhyming and spelling. Journal of Neuroscience. 2005;25:5397–5403. doi: 10.1523/JNEUROSCI.0864-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou TL, Dong L, Cone NE, Cao F, Bigo JD, Booth JR. The interaction of orthographic and phonological information in children: an fMRI study. Human Brain Mapping. doi: 10.1002/hbm.20313. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. NeuroImage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. The relation between brain activation and lexical performance. Human Brain Mapping. 2003a;19:155–169. doi: 10.1002/hbm.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Choy J, Gitelman DR, Parrish TR, Mesulam MM. Modality-specific and -independent developmental differences in the neural substrate for lexical processing. Journal of Neurolinguistics. 2003b;16:383–405. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Zhang L, Gitelman DR, Parrish TR, Mesulam. MM. Development of brain mechanisms for processing orthographic and phonological representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TR, Mesualm MM. The development of specialized brain systems in reading and oral-language. Child Neuropsychology. 2001;7(3):119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Booth JR, Perfetti CA, MacWhinney B. Quick, automatic, and general activation of orthographic and phonological representations in young readers. Developmental Psychology. 1999;35:3–19. doi: 10.1037/0012-1649.35.1.3. [DOI] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D. Evidence for developmental changes in the visual word processing network beyond adolescence. NeuroImage. 2006;29:822–837. doi: 10.1016/j.neuroimage.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Keller TA, Eddy W, Thulborn K. Graded functional activation in the visuo-spatial system with the amount of task demand. Journal of Cognitive Neuroscience. 1999;11:9–24. doi: 10.1162/089892999563210. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108(1):204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Jobert A, Naccache L, Ciuciu P, Poline JB, Le Bihan D, Cohen L. Letter binding and invariant recognition of masked words: behavioral and neuroimaging evidence. Psychological Science. 2004;15(5):307–313. doi: 10.1111/j.0956-7976.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- The educator's word frequency guide. Touchstone Applied Science Associates, Inc.; Brewster, NY: 1996. [Google Scholar]
- Ehri LC. Phases of development in learning to read words by sight. Journal of Research in Reading. 1995;18(2):116–125. [Google Scholar]
- Frith U. Beneath the surface of developmental dyslexia. In: Patterson JCMKE, Coltheart M, editors. Surface dyslexia: Neuropsychological and cognitive studies of phonological recoding. Erlbaum; London: 1985. pp. 301–330. [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam MM. A large-scale distributed network for covert spatial attention: further anatomical delineation based on strigent behavioral and cognitive controls. Brain. 1999;122:1093–1106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- Gough PB, Hillinger ML. Learning to read: an unnatural act. Bulletin of the Orton Society. 1980;30:179–196. [Google Scholar]
- Hashimoto R, Sakai KL. Learning letters in adulthood: direct visualization of cortical plasticity for forming a new link between orthography and phonology. Neuron. 2004;42(2):311–322. doi: 10.1016/s0896-6273(04)00196-5. [DOI] [PubMed] [Google Scholar]
- Henson R, Buchel C, Josephs O, Friston K. The slice-timing problem in event-related fMRI. NeuroImage. 1999;9:S125. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M. A functional MRI study on the neural substrates for writing. Human Brain Mapping. 2001;13(1):34–42. doi: 10.1002/hbm.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Jubault T. Broca's area and the hierarchical organization of human behavior. Neuron. 2006;50:963–974. doi: 10.1016/j.neuron.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Donchin E. Brain potentials as indices of orthographic and phonological interaction during word matching. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1987;13(1):76–86. doi: 10.1037//0278-7393.13.1.76. [DOI] [PubMed] [Google Scholar]
- Lee BCP, Kuppusamy K, Grueneich R, El-Ghazzawy O, Gordon RE, Lin W, Haacke EM. Hemispheric language dominance in children demonstrated by functional magnetic resonance imaging. Journal of Child Neurology. 1999;14(2):78–82. doi: 10.1177/088307389901400203. [DOI] [PubMed] [Google Scholar]
- Lee HS, Fujii T, Okuda J, Tsukiura T, Umetsu A, Suzuki M, Nagasaka T, Takahashi S, Yamadori A. Changes in brain activation patterns associated with learning of Korean words by Japanese: an fMRI study. NeuroImage. 2003;20(1):1–11. doi: 10.1016/s1053-8119(03)00254-4. [DOI] [PubMed] [Google Scholar]
- Marsh G, Friedman MP, Welch V, Desberg P. A cognitive-developmental theory of reading acquisition. In: Waller TG, MacKinnon GE, editors. Reading research: Advances in theory and practice. Academic Press; New York: 1981. pp. 199–221. [Google Scholar]
- McPherson BW, Ackerman PT, Dykman RA. Auditiory and visual rhyme judgements reveal differences and similarities between normal and disabled adolescent readers. Dyslexia. 1997;3:63–77. [Google Scholar]
- Nakamura K, Honda M, Okada T, Hanakawa T, Toma K, Fukuyama H, Konishi J, Shibasaki H. Participation of the left posterior inferior temporal cortex in writing and mental recall of Kanji orthography: a functional MRI study. Brain. 2000;123(5):954–967. doi: 10.1093/brain/123.5.954. [DOI] [PubMed] [Google Scholar]
- Nunes T, Bryant P, Bindman M. Morphological spelling strategies: developmental stages and processes. Developmental Psychology. 1997;33(4):637–649. doi: 10.1037//0012-1649.33.4.637. [DOI] [PubMed] [Google Scholar]
- Perfetti CA. The representation problem in reading acquisition. In: Gough P, Ehri L, Treiman R, editors. Reading acquisition. Erlbaum; Hillsdale, NJ: 1992. pp. 145–174. [Google Scholar]
- Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: computational principles in quasi regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Polich J, McCarthy G, Wang WS, Donchin E. When words collide: orthographic and phonological interference during word processing. Biological Psychology. 1983;16(3–4):155–180. doi: 10.1016/0301-0511(83)90022-4. [DOI] [PubMed] [Google Scholar]
- Rack JP. Orthographic and phonetic coding in developmental dyslexia. British Journal of Psychology. 1985;76(3):325–340. doi: 10.1111/j.2044-8295.1985.tb01956.x. [DOI] [PubMed] [Google Scholar]
- Richards T, Berninger V, Nagy W, Parsons AC, Field KM, Richards A. Brain activation during language task contrasts in children with and without dyslexia: inferring mapping processes and assessing reponse to spelling instruction. Educational and Child Psychology. 2005;22:62–80. [Google Scholar]
- Sandak R, Mencl W, Frost SJ, Rueckl JG, Katz L, Moore DL, Mason SA, Fulbright RK, Constable RT. The neurobiology of adaptive learning in reading: a contrast of different training conditions. Cognitive, Affective and Behavioral Neuroscience. 2004;4(1):67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuro-anatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Yantis S. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. Journal of Neuroscience. 2006;26(2):435–439. doi: 10.1523/JNEUROSCI.4408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger-Charolles L, Siegel LS, Bechennec D, Serniclaes W. Development of phonological and orthographic processing in reading aloud, in silent reading, and in spelling: a four-year longitudinal study. Journal of Experimental Child Psychology. 2003;84(3):194–217. doi: 10.1016/s0022-0965(03)00024-9. [DOI] [PubMed] [Google Scholar]
- Sugishita M, Takayama Y, Shiono T, Yoshikawa K, Takahashi Y. Functional magnetic resonance imaging (fMRI) during mental writing with phonograms. NeuroReport: An International Journal for the Rapid Communication of Research in Neuroscience. 1996;7(12):1917–1921. doi: 10.1097/00001756-199608120-00009. [DOI] [PubMed] [Google Scholar]
- Tokunaga H, Nishikawa T, Ikejiri Y, Nakagawa Y, Yasuno F, Hashikawa K, Nishimura T, Sugita Y, Takeda M. Different neural substrates for Kanji and Kana writing: a PET study. NeuroReport: An International Journal for the Rapid Communication of Research in Neuroscience. 1999;10(16):3315–3319. doi: 10.1097/00001756-199911080-00012. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Garaeu L, Flowers DL, Zefirro TA, Eden G. Development of the neural mechanisms for reading. Nature Neuroscience. 2003;6(6):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. The Psychological Corporation; Toronto: 1999. [Google Scholar]
- Wilkinson GS. Wide range achievement test. 3rd edn. Wide Range; Wilmington, DE: 1993. [Google Scholar]
- Zatorre RJ, Mondor TA, Evans AC. Auditory attention to space and frequency activates similar cerebral systems. Neuroimage. 1999;10(5):544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]