Abstract
The nuclear factor-κB (NF-κB) signaling pathway has been targeted for therapeutic applications in a variety of human diseases, includuing cancer. Many naturally occurring substances, including curcumin, have been investigated for their actions on the NF-κB pathway because of their significant therapeutic potential and safety profile. A synthetic monoketone compound termed 3,5-bis(2-flurobenzylidene)piperidin-4-one (EF24) was developed from curcumin and exhibited potent anticancer activity. Here, we report a mechanism by which EF24 potently suppresses the NF-κB signaling pathway through direct action on IκB kinase (IKK). We demonstrate that 1) EF24 induces death of lung, breast, ovarian, and cervical cancer cells, with a potency about 10 times higher than that of curcumin; 2) EF24 rapidly blocks the nuclear translocation of NF-κB, with an IC50 value of 1.3 μM compared with curcumin, with an IC50 value of 13 μM; 3) EF24 effectively inhibits tumor necrosis factor (TNF)-α-induced IκB phosphorylation and degradation, suggesting a role of this compound in targeting IKK; and 4) EF24 indeed directly inhibits the catalytic activity of IKK in an in vitro-reconstituted system. Our study identifies IKK as an effective target for EF24 and provides a molecular explanation for a superior activity of EF24 over curcumin. The effective inhibition of TNF-α-induced NF-κB signaling by EF24 extends the therapeutic application of EF24 to other NF-κB-dependent diseases, including inflammatory diseases such as rheumatoid arthritis.
Curcumin, isolated from the rhizomes of the plant Curcuma longa L., is the major component of the spice curry. This compound, freely available in the human food supply, is associated with numerous therapeutic benefits, including chemoprevention and chemotherapy in cancer, and anti-inflammatory, antioxidant, and antiviral activities (Osawa et al., 1995; Commandeur and Vermeulen, 1996; Cheng et al., 2001; Levi et al., 2001). In addition, the pharmacological safety of curcumin is evident by its consumption for centuries at levels up to 100 mg/day by people in certain countries (Satoskar et al., 1986). These beneficial properties have attracted numerous efforts for the development of curcumin as a safe therapeutic agent (Aggarwal et al., 2006; Anand et al., 2007). Recent therapeutic efficacy against pancreatic cancer in a phase II clinical trial further supports the use of curcumin as a lead for the development of a new class of anticancer agents (Dhillon et al., 2006). Unfortunately, due to the low potency and poor absorption characteristics of curcumin, its clinical potential remains limited (Shoba et al., 1998). However, curcumin represents an ideal lead compound for further chemical modifications and optimization (Adams et al., 2004, 2005).
In an attempt to retain curcumin's favorable medicinal properties and safety profile, while increasing its potency, computer-assisted topological searches of the National Cancer Institute database were carried out to identify lead analogs. Two such analogs incorporating a monoketone were identified and exhibited improved cytotoxic effect over curcumin (Adams et al., 2004). These results stimulated a more thorough search for easily prepared and readily functionalized analogs with improved potency. The strategy adopted is captured by the following manipulation of curcumin. Two carbons and an oxygen were removed from the center of the molecule to produce a monoketone, terminal ring substituents were varied, and an extensible heterocyclic six-membered ring including the ketone was installed (see Fig. 1 for an example). Approximately 100 analogs were tested. A subset of 10 analogs was further evaluated in the 60 panel of National Cancer Institute cancer cell lines and in several in vitro antiangiogenesis screens (Adams et al., 2004). EF24 (Fig. 1) surfaced as one of the top candidate compounds. EF24 has shown to induce apoptosis in cancer cells and to inhibit the growth of human breast tumors in a mouse xenograft model with relatively low toxicity and at a dose much less than that of curcumin (Adams et al., 2004, 2005). Studies with various cancer cells have suggested a model that EF24 impairs cell growth by inducing G2/M arrest followed by induction of apoptosis, which is accompanied by caspase-3 activation, phosphatidylserine externalization, and an increased number of cells with a sub-G1 DNA content (Adams et al., 2005). However, the cell signaling pathways that mediate the EF24 effect remain to be elucidated. This study examines the effect of EF24 on a key survival pathway in lung cancer cells mediated by the NF-κB transcription factor.
Fig. 1.
Structures of curcumin and its analog EF24.
NF-κB is maintained in an inactive state in the cytoplasm by the inhibitor of κB (IκB), which masks the nuclear localization signal of NF-κB. Phosphorylation of IκB by the inhibitor of κB kinase (IKK), via the canonical NF-κB pathway, results in subsequent ubiquitination of IκB and proteasomal degradation. It is this degradation of IκB that liberates NF-κB, allowing its localization to the nucleus and the transcriptional activation of its target genes. In contrast, aberrant activation of NF-κB contributes to deregulated growth, resistance to apoptosis, and propensity to metastasize observed in many cancers (Rayet and Gélinas, 1999; Richmond, 2002). The NF-κB effect is in part due to the up-regulation of NF-κB-controlled genes that promote survival function of tumor cells, including c-myc, Bcl-xL, and members of inhibitor of apoptosis family of genes. In addition, many anticancer agents can induce the activation of NF-κB, resulting in reduced therapeutic efficacy (Nakanishi and Toi, 2005). Thus, agents that effectively impair the NF-κB pathway are expected to have significant therapeutic potential.
In this report, we identify the molecular mechanism of action of EF24. We show that treatment with EF24 results in a marked decrease in cellular viability. The dose necessary to reduce viability correlates well with that needed for EF24 to impair the nuclear translocation of NF-κB and to inhibit IKK. EF24 is at least 10 times more potent than curcumin and represents a lead compound for further therapeutic development of a new generation of natural product-derived anticancer agents.
Materials and Methods
Materials
Curcumin was purchased from Alfa Aesar (Ward Hill, MA), and its structural analog, EF24, was prepared as reported previously (Adams et al., 2004). Both compounds were dissolved in DMSO, with a stock concentration of 10 mM. TNF-α (Sigma-Aldrich, St. Louis, MO) was resuspended in water to a final concentration of 10 μg/ml. Glutathione transferase (GST)-IκBα (1-54) was purified from expression plasmid as described previously (Mercurio et al., 1997). Antibodies against pS32-IκBα and IκBα were purchased from Cell Signaling Technology Inc. (Danvers, MA). Antibodies against IKKα and IKKβ were purchased from Imgenex (San Diego, CA). Antibodies to Raf-1, and secondary antibody conjugates horseradish peroxidase goat anti-mouse and horseradish peroxidase-goat anti-rabbit antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell Cultures
Cells were maintained in either in RPMI 1640 medium (A549, H157, H460, Calu-1, H358, PC3, 1A9, and MDA-MB231) or Dulbecco's modified Eagle's medium (HeLa), with 10% fetal bovine serum and penicillin/streptomycin in a 37°C incubator with 5% CO2. For Western blot analysis, unless otherwise noted, cells were lysed in 1% NP-40 lysis buffer (1% Nonidet P40, 10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM NaF, 2 mM Na3VO4, 5 mM Na4P2O7, 10 μg/ml aprotonin, 10 μg /ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride).
Cytotoxicity Assay
Cells were plated at 5000 cells/well in 96-well plates. They were treated with test agents in triplicate on the following day and further incubated for 48 h before viability assay was carried out. The sulforhodamine B assay was performed to evaluate cell viability and to obtain the IC50 values (Rubinstein et al., 1990; Skehan et al., 1990). It measures cellular protein content to determine cell density. The mean value and standard error for each treatment were determined, and the cell viability index relative to control (untreated) was calculated. The IC50 is defined as the concentration of agents that decrease viability by 50% in a total cell population (cell viability index, 0.5) compared with control cells (cell viability index, 1) at the end of the incubation period.
High Content Analysis of NF-κB Subcellular Translocation
A549 cells were plated in 96-well plates (BD Biosciences Discovery Labware, Bedford, MA) at 10,000 cells/90 μl/well and grown for 20 h. Test compounds were added to each well and incubated at 37°C. All samples were performed in triplicates. The cells were stimulated with TNF-α as indicated. Reactions were terminated by washing the plates with ice-cold phosphate-buffered saline (PBS) followed by fixation with paraformaldehyde (2%; 100 μl) for 30 min at room temperature. Cells were permeabilized with Triton X-100 (0.1%; 100 μl) for 20 min, washed three times with PBS, and blocked with bovine serum albumin (1%; 100 μl) for 1 h. Rabbit anti-p65 NF-κB antibody (Santa Cruz biotechnology, Inc.) was added and incubated overnight at 4°C. Cells were washed three times in PBS and incubated with goat anti-rabbit IgG with conjugated Alexa Fluor 488 (50 μl; Invitrogen, Carlsbad, CA) along with Hoechst 33342 (1 μg/ml; Promega, Madison, WI). After washing with PBS, cells were imaged with the ImageXpress 5000 with the filter set for fluorescein isothiocyanate (excitation at 490 nm, emission at 525 nm, with dichroic mirror at 505 nm) and 4,6-diamidino-2-phenylindole (excitation at 350 nm, emission at 479 nm, with dichroic mirror at 400 nm) (Molecular Devices, Sunnyvale, CA).
The images were quantified and analyzed using MetaXpress software (Molecular Devices). “Translocation Enhanced” module was used for the NF-κB translocation analysis. The nucleus was defined by Hoechst 33342 staining. The levels of NF-κB translocation were calculated and expressed as the difference between average fluorescence intensity in nucleus and in cytoplasm. After stimulating with TNF-α, the inhibitory effect of test compounds on TNF-α induced NF-κB translocation was expressed as percentage of fluorescence intensity difference (in nucleus and in cytoplasm) in the control wells (TNF-α only) after subtracting background (no TNF-α treatment). Data shown are average values from triplicate samples.
Western Blotting
Cells were lysed in NP-40 buffer (1.0% NP-40, 10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM NaF, 2 mM Na3VO4, 5 mM Na4P2O7, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Equal volumes of cell lysate were subject to electrophoresis on 12.5% SDS-PAGE. Proteins were then electrotransferred to a nitrocellulose membrane (GE Water and Process Technologies, Trevose, PA) as described previously (Zhang et al., 1999). Membranes were blocked in a solution of 5% nonfat dry milk in Tris-buffered saline-Tween 20 buffer (20 mM Tris, pH 7.6, 500 mM NaCl, and 0.5% Tween 20) for 30 min followed by incubation with primary antibody for at least 2 h. The membrane was then washed and treated with the corresponding horseradish peroxidase-conjugated anti-mouse Ig or anti-rabbit Ig as indicated. Immunodetection was performed using West Pico (Pierce Chemical, Rockford, IL) or West Dura (Pierce Chemical) followed by imaging on an Image Station 2000R (Eastman Kodak, Rochester, NY).
Immunoprecipitation
Cells were seeded in 150-mm plates to approximately 70% confluence 1 day before treatment. Cells were treated with increasing doses of EF24 or curcumin with or without TNF-α as indicated and lysed in 1% NP-40 lysis buffer at 4°C. Lysates were clarified by centrifugation (14,000 rpm; 10 min; 4°C). Cytoplasmic extracts were immunoprecipitated with an anti-IKKα antibody (Imgenex) at 4°C overnight. Protein G-Sepharose beads (50% slurry; Pfizer, New York, NY) in lysis buffer were added to each reaction the following day and incubated for an additional 2 h. Protein G beads were then gently spun down and washed two times with NP-40 lysis buffer and one final time with the kinase assay buffer (50 mM HEPES, pH 7.4, 20 mM MgCl2, and 2 mM dithiothreitol). The immunocomplexes were used for IKK kinase assays.
Kinase Assays
IKK Immunocomplex Assay
Immunoprecipitated complexes were added to kinase reaction buffer containing [γ-32P]ATP (20 μCi with 10 μM unlabeled ATP) and GST-IκBα (residues 1–54; 5 μg) in a total volume of 25 μl and incubated at 30°C for 30 min. Reactions were stopped by boiling the kinase solution in a 6× SDS sample buffer for 5 min. The samples were resolved on 12.5% SDS-PAGE. The top portion of the gel was transferred and immunoblotted with anti-IKKβ antibody, whereas the bottom portion was stained with 0.05% Coomassie Blue dye. Radiolabeled phosphate incorporation into GST-IκBα was assessed by the PhosphorImager and quantified with the ImageQuant software (GE Healthcare).
In Vitro Recombinant IKKβ Assay
Activated recombinant IKKβ in MOPS buffer (8 mM MOPS-NaOH, pH 7.0, 200 μM EDTA, and 15 mM MgCl2) (Millipore, Billerica, MA) was used to assess the direct effect of EF24 or curcumin on the kinase. The test compounds were incubated in the presence of 40 ng of IKKβ for 15 min at room temperature. The addition of Mg-ATP cocktail (15 mM MgCl2, 100 μM ATP, 8 mM MOPS-NaOH, pH 7.0, 5 mM β-glycerophosphate, 1 mM EGTA, 200 nM sodium orthovanadate, and 200 nM dithiothreitol), 5 μg of purified GST-IκBα, and 0.5 μCi of [γ-32P]ATP in a final volume of 25 μl started the reaction, which was allowed to proceed at 30°C for 15 min. Reactions were terminated and processed as described under IKK immunocomplex assay. In addition, the radiolabeled GST-IκBa protein bands were excised for quantification with a scintillation counter (Beckman LS 6500; Beckman Coulter, Fullerton, CA). For competition assays, total ATP was varied, while the ratio of [γ-32P]ATP to nonradioactive ATP remained constant in the reaction. Test compounds and ATP were incubated in the presence of 20 ng of IKKβ at room temperature. At various time points within the linear range of the enzyme, the reaction was terminated by spotting assay mixture (5 μl) onto P81 phosphocellulose paper (Whatman, Maidstone, UK). The filters were washed three times with 0.75% phosphoric acid and once with acetone. Radioactivity was determined by liquid scintillation counting.
Results
EF24 Exhibits a More Potent Cytotoxic Effect than Curcumin
To evaluate the potency of EF24 in comparison with its parent compound curcumin, we carried out cell viability tests with the sulforhodamine B assay on a panel of lung cancer cells (Fig. 2). Treatment of cells with both EF24 and curcumin led to a significantly decreased viability. Dose-response studies revealed that the IC50 value of EF24 was in the range of 0.7 to 1.3 μM for various cell lines, as summarized in Table 1 and Fig. 2. In contrast, under the same treatment conditions, the IC50 value for curcumin ranged from 15 to 20 μM. These data indicate a more potent cytotoxic effect of EF24 over curcumin for lung cancer cells. This study was extended to include ovarian, breast, prostate, and cervical cancer cells. A similar trend was observed with EF24, exhibiting an IC50 value at least 20-fold lower than curcumin for each cell line, respectively, with the exception of PC3 (10-fold lower) (Table 1). These results are consistent with previous reports, and they demonstrate that this monoketone analog of curcumin, EF24, has a much improved cytotoxic activity over the parent compound (Adams et al., 2004, 2005).
Fig. 2.
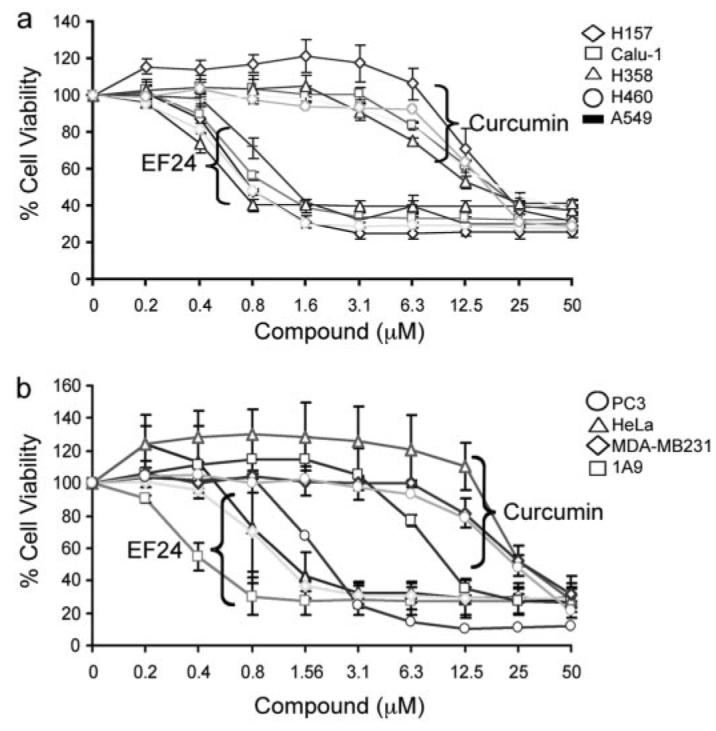
EF24 shows more potent cytotoxic effect than curcumin on cancer cells. Cells were grown in 96-well plates and were treated with EF24 or curcumin as indicated for 48 h. Cell viability was assessed by the sulforhodamine B method and expressed as percentage of control (DMSO). Results with a panel of lung cancer cells are shown in a: A549, H358 (lung adenocarcinoma); H460 (large cell carcinoma); H157 (squamous cell carcinoma); and Calu-1 (lung epidermoid carcinoma). b, results with breast (MDA-MB231), cervical (HeLa), prostate (PC3), and ovarian (1A9) cancer cell lines.
TABLE 1.
Cytotoxicity of curcumin and EF24 against a panel of cancer cell lines IC50 values derived from Fig. 1 are given as the average of at least two separate experiments.
| Cell Line | IC50 |
|
|---|---|---|
| EF24 | Curcumin | |
| μM | ||
| 1A9 (ovarian) | 0.44 ± 0.09 | 9.85 ± 1.20 |
| HeLa (cervical) | 1.23 ± 0.88 | 26.9 ± 7.0 |
| A549 (lung) | 1.31 ± 0.01 | 19.75 ± 4.03 |
| H358 (lung) | 0.66 ± 0.09 | 14.9 ± 3.1 |
| H460 (lung) | 0.79 | 16.9 |
| H157 (lung) | 0.77 ± 0.06 | 19.55 ± 6.71 |
| Calu-1(lung) | 1.03 ± 0.14 | 18.1 ± 2.46 |
| MDA-MB231 (breast) | 1.03 ± 0.40 | 26.55 ± 3.18 |
| PC3 (prostate) | 2.07 | 23.7 |
High Content Analysis Revealed an Effective EF24 Action in Blocking Nuclear Translocation of NF-κB
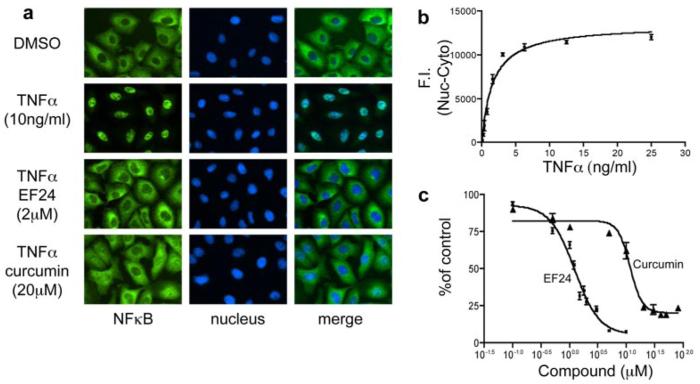
To understand the mechanism of action of EF24 for its improved bioactivity, we examined its effect on the NF-κB signaling pathway, which is suggested to be targeted by curcumin (Singh and Khar, 2006; Lin, 2007). The NF-κB transcription factor mediates a critical survival mechanism in lung cancer cells. To monitor the activation status of NF-κB, we used a high content analysis (HCA) approach to visualize the dynamic movement of the NF-κB p65 subunit between the cytoplasm and nucleus under various experimental conditions. The p65 subunit in A549 cells was detected with an immunofluorescent probe, and its movement was captured through an automated fluorescence microscope (Fig. 3). To validate the HCA assay and to establish experimental conditions for testing the EF24 effect, we initially used a known NF-κB activator, TNF-α, to manipulate the NF-κB movement (Rothe et al., 1995). Although the majority of the p65 subunit was detected in the cytoplasm, the addition of 10 ng/ml TNF-α resulted in complete translocation of the p65 protein into the nucleus, as indicated by overlapping p65 green fluorescent signal with 4,6-diamidino-2-phenylindole-stained blue nuclear signal (Fig. 3a). Time course and dose-response experiments were carried out to establish an EC50 value of 1.5 ng/ml TNF-α, which was used to set the subsequent experimental conditions (Fig. 3b). To examine whether EF24 has any effect on the NF-κB pathway, A549 cells were pretreated with EF24 before TNF-α was added to cause predominant nuclear translocation of NF-κB. Strikingly, EF24 pretreatment retained the NF-κB in the cytoplasm even when the amount of TNF-α used completely relocated the p65 subunit to the nucleus. Curcumin showed a similar effect, albeit with much higher concentration to achieve an effect as EF24 (Fig. 3). Quantification of captured images under various experimental conditions led to the establishment of dose-response curves, which gave rise to an IC50 value of 1.3 and 13 μM for EF24 and curcumin, respectively (Fig. 3c). The 10-fold difference in blocking the NF-κB translocation activity between EF24 and curcumin as revealed by the quantitative HCA is consistent with their difference in cytotoxicity against lung cancer cells. It is likely that EF24 induces cell death in part through interfering with the NF-κB-mediated survival signaling.
Fig. 3.
EF24 impairs TNF-α induced NF-κB nuclear translocation. A549 cells were grown in 96-well plates and treated with TNF-α or control (DMSO) for 30 min before sample processing for the detection of NF-κB as described under Materials and Methods (top two rows of a). The induction of NF-κB nuclear translocation by TNF-α was quantified as shown in b, with an apparent EC50 value of 1.5 ng/ml. The effect of EF24 or cucumin treatment (30 min) on TNF-α-induced nuclear translocation of NF-κB was shown in the lower two rows in a and quantified as shown in c.
EF24 Inhibits TNF-α-Induced IκB Phosphorylation and Subsequent Degradation
When in the cytoplasm, NF-κB is associated with IκB as an inactive protein complex. Nuclear translocation of NF-κB requires dissociation of IκB, which is controlled by phosphorylation of IκB at Ser32 and Ser36 and the subsequent degradation induced by various extracellular signals, including TNF-α (Beg et al., 1993). It is possible that EF24's effect on nuclear translocation of NF-κB is through its action on IκB phosphorylation and/or degradation. To test this model, we first established conditions to monitor the status of IκB phosphorylation and degradation that takes into account their transient nature. A549 cells were treated with TNF-α. Phosphorylation of IκB was visible within 3 min of treatment and reached a high level in approximately 7 min. It was sustained for an additional 6 min (Fig. 4a). IκB phosphorylation was followed by its degradation. Degradation was most notably detected after 20 min of treatment with TNF-α, whereas the control protein Raf-1 remained stable during the entire course of the test. Based on these observations, cells were treated with TNF-α for 10 min for detecting IκB phosphorylation and for 20 min for monitoring IκB stability in subsequent experiments.
Fig. 4.
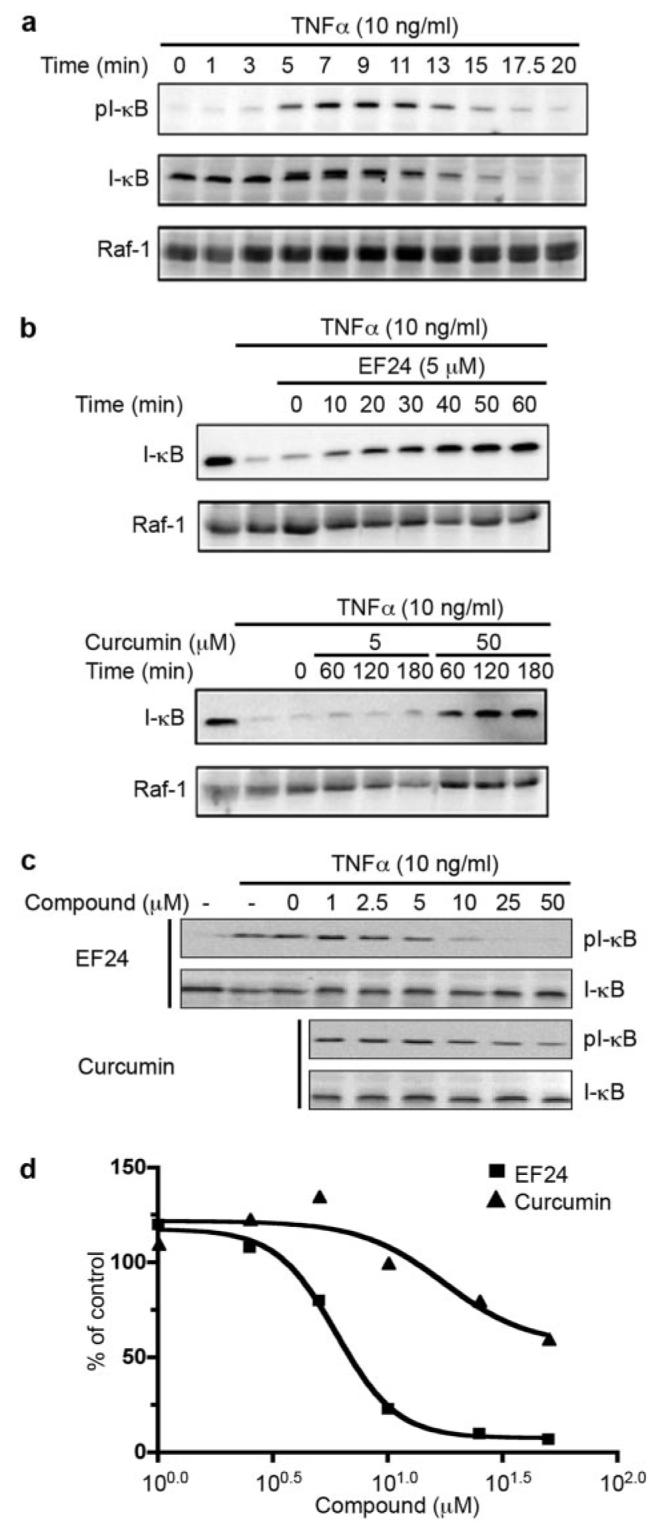
EF24 blocks TNF-α-induced IκB degradation and phosphorylation. a, A549 cells were treated with 10 ng/ml TNF-α. Whole cell lysates were prepared at indicated times and analyzed for the phosphorylation state of IκB with anti-pS32 antibody via Western blotting (top). Then, antibodies on the membrane were stripped and the membrane reprobed for total IκB with antiserum against IκB (middle). Raf-1 was used as a control (bottom). b, A549 cells were pretreated for various times with 5 μM EF24, or 5 or 50 μM curcumin before the addition of TNF-α. Cells were cultured in the presence of 10 ng/ml TNF-α for an additional 20 min, lysed, and analyzed for total IκB levels by Western blot (top). Raf-1 was used as a control (bottom). c, A549 cells were pretreated with compounds (EF24 or curcumin) as indicated for 30 min. TNF-α was added to induce IκB phosphorylation. Cell lysates were prepared after 7 min of treatment and used for probing pS32-IκB followed by probing total IκB with Western blots. Intensity of cross-reacting material bands on Western blots was estimated with a Kodak imaging system. The phosphorylation levels of IκB at S32 are normalized to total IκB and expressed relative to control sample with DMSO treatment (d).
To examine the effect of EF24 on IκB stability, A549 cells were pretreated with 5 μM EF24 or 5 or 50 μM curcumin followed by stimulation with TNF-α. As shown in Fig. 4b, TNF-α alone rapidly induced IκB degradation, whereas pretreatment with 5 μM EF24 for only 10 min was able to effectively block the TNF-α effect, showing accumulated IκB even in the presence of TNF-α. In contrast, curcumin required a much higher concentration (50 μM) and a longer time (60 min) to achieve a level of inhibition similar to EF24. As a control, Raf-1 remained stable during these experimental conditions, suggesting a specific effect of TNF-α on IκB.
Because phosphorylation of IκB proceeds its degradation, we next examined the effect of EF24 on TNF-α-induced phosphorylation of IκB. A549 cells were pretreated with EF24 or curcumin for 30 min before the addition of TNF-α (10 min). Cells were harvested for probing the status of IκB phosphor-ylation with a phosphor-specific antibody, pS32-IκB (Fig. 4c). EF24 inhibited TNF-α-induced IκB phosphorylation in a dose-dependent manner, with an IC50 approximately 10-fold less than that of curcumin (Fig. 4d). These results suggest that EF24 antagonizes the nuclear translocation of NF-κB through its inhibitory action on the phosphorylation of IκB and its subsequent degradation. In support of a selective role of EF24 in inhibiting the IκB kinase signaling, further experiments showed that EF24 is unable to inhibit TNF-α induced activation of c-Jun NH2-terminal kinase and extracellular signal-regulated kinase (Supplemental Fig. S1).
EF24 Directly Inhibits the IKKβ Kinase Activity
Because phosphorylation of IκB is catalyzed by IKK in the canonical NF-κB signaling pathway, the above-mentioned results imply a more potent EF24 action over curcumin on the IKK protein complex. To examine whether EF24 and curcumin differentially target the cellular IKK complex for effective NF-κB inhibition, we monitored the kinase activity of IKK upon compound treatment and compared the efficacy of EF24 and curcumin. A549 cells were pretreated with increasing doses of either a compound or vehicle for 1 h before the addition of 10 ng/ml TNF-α for 10 min. Cells were lysed, and heterotrimeric IKK complexes were immunoprecipitated with an antibody to IKKα. The kinase activity of the immunoprecipitated IKK complex was determined by its ability to phosphorylate recombinant GST-IκB. Although pretreatment of cells with EF24 was able to completely neutralize TNF-α-activated IKK complex activity, pretreatment with curcumin required a much higher dose to achieve a similar effect (Fig. 5). Although this cell-based immunocomplex kinase assay allows the detection of permeabilized compound effect on intracellular IKK complex activity, it is unable to distinguish a direct effect of compound on a specific kinase from an indirect effect. It is possible that EF24 directly targets the IKKβ activity, a well defined kinase for IκB. To test this notion, a reconstituted in vitro kinase assay was used with recombinant IKKβ and other defined components in the reaction. Increasing concentrations of EF24 or curcumin were incubated with active recombinant IKKβ for 15 min before the addition of substrate, GST-IκB, and [γ-32P]ATP. Incorporation of radiolabeled 32P to GST-IκB was detected by radiography and quantified by scintillation counting of excised GST-IκB protein bands (Fig. 6). It is striking that EF24 effectively inhibited the ability of IKKβ to phosphorylate its physiological substrate IκB, with an estimated IC50 value of 1.9 μM. Interestingly, EF24 also impaired the autokinase activity of IKKβ with a similar potency (data not shown). These data strongly support a direct role of EF24 in the inhibition of IKKβ. In contrast, curcumin shows a much weaker effect on IKKβ in the in vitro kinase assay, with an apparent IC50 value above 20 μM (Fig. 6). It is clear that the structural change of curcumin to EF24 has drastically enhanced its inhibitory effect on IKKβ catalytic activity. Thus, this in vitro kinase assay with recombinant IKKβ reveals IKKβ as a direct target of EF24.
Fig. 5.
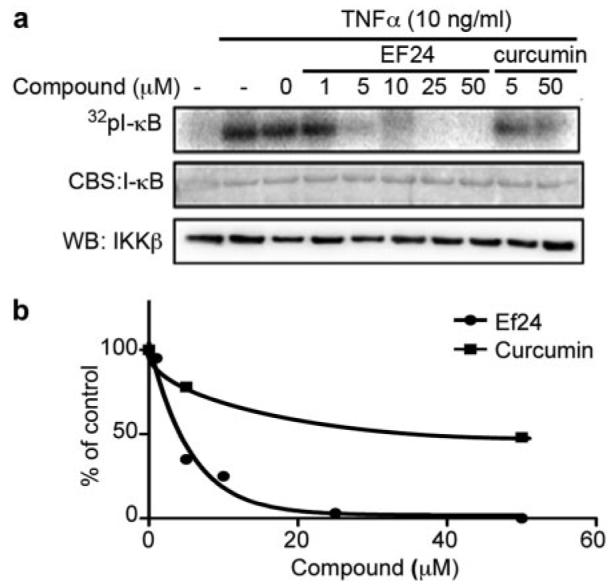
EF24 inhibits the kinase activity of endogenous IKK complex A549 cells were pretreated with EF24 or curcumin at indicated doses for 1 h followed by 10 ng/ml TNF-α stimulation for 10 min. Cell lysates were prepared for immunoprecipitation of the IKK complex with an antibody raised against IKKα. The isolated IKK complex was used in an in vitro kinase assay with GST-IκB as a substrate. Samples were resolved on a SDS-PAGE and stained for total IκB protein with Coomassie Blue (a, middle). The gel was dried to reveal radiolabeled IκB with a PhosphorImager (a, top). The amount of IKKβ in the immunocomplex used in each reaction was revealed by Western blot. As a negative control, the inactive IKK complex without TNF-α treatment is shown in left lane. Quantified result from a is plotted relative to vehicle control (b).
Fig. 6.
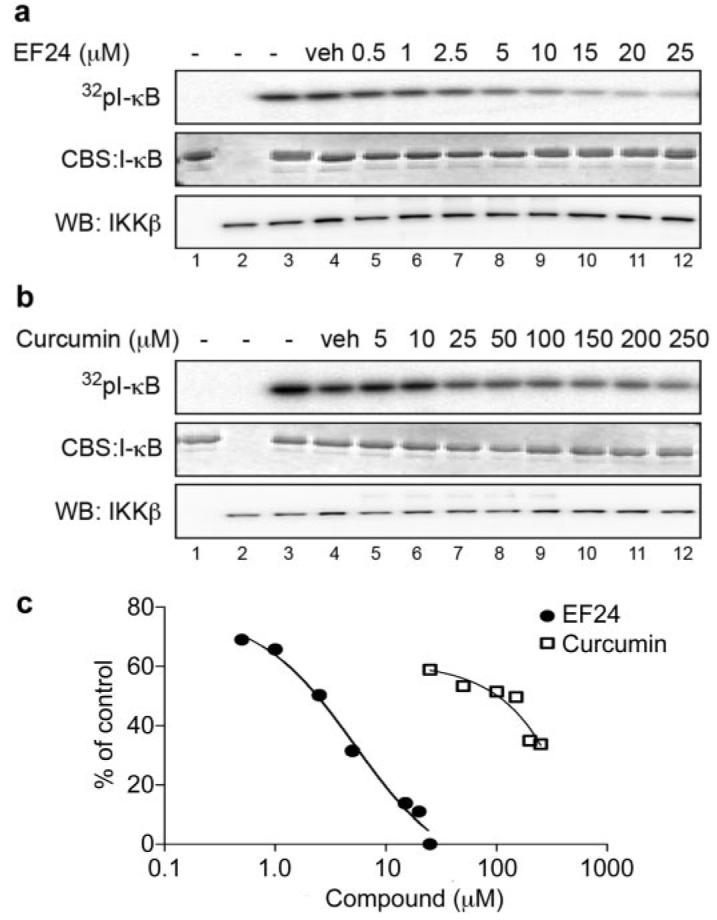
EF24 inhibits IKKβ kinase activity in a reconstituted system. Recombinant IKKβ was incubated with increasing concentrations of EF24 (a) or curcumin (b) for 15 min. Addition of Mg/[γ-32P] cocktail with purified GST-IκB started the reactions, which were continued for 15 min at 30°C. Proteins were separated by SDS-PAGE and processed for radiolabeled GST-IκB (top), total GST-IκB (middle), and IKKβ (bottom) as described in legend to Fig. 5. Controls include reactions without IKKβ (lane 1) or without IκB (lane 2).
Further kinetics studies showed both a decrease in Km and Vmax values when EF24 was present in the IKKβ kinase assay; however, Lineweaver-Burk plots are not parallel (Supplemental Fig. S2; data not shown). This suggests that EF24 may be acting as a mixed type inhibitor with respect to ATP. These data further strengthen our conclusion that EF24 directly acts on and inhibits IKKβ.
Discussion
The monoketone analog of curcumin, EF24, has been shown to induce cell cycle arrest and apoptosis in many cancer cell lines, with potency much higher than that of curcumin. Consistent with previous reports, EF24 exhibited IC50 values 10 to 20 times lower than that of curcumin in a panel of non–small-cell lung cancer cells with different genetic background as well as in ovarian, cervical, breast, and prostate cancer cells. However, the molecular mechanism underlying the enhanced therapeutic efficacy remains to be defined. Here, we present evidence that supports a direct action of EF24 on the NF-κB survival signaling pathway. The amount of EF24 required for suppression of lung cancer A549 cell growth has been correlated with its ability to prevent the nuclear translocation of p65 subunit of NF-κB, to block IκB phosphorylation and its subsequent degradation, and to inhibit the catalytic activity of the IKK protein complex (Fig. 7). Curcumin, in contrast, exhibited at least 10 times lower potency in all of the above-mentioned assays. Importantly, in an in vitro-reconstituted kinase assay, EF24 has been shown to inhibit the kinase activity of IKKβ. Thus, direct targeting of IKKβ and its mediated survival signaling may partially explain the improved therapeutic potency of EF24 over curcumin.
Fig. 7.
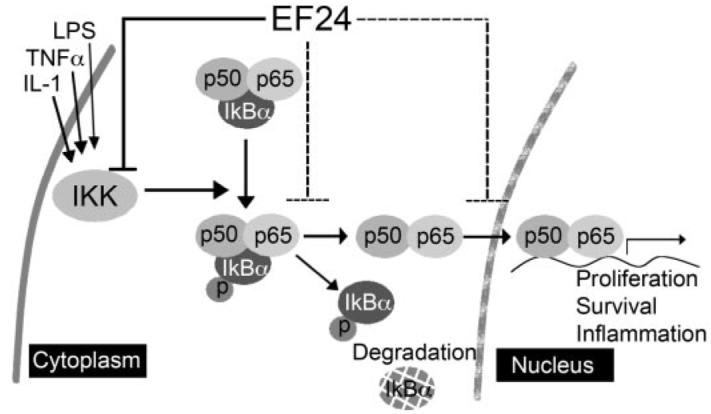
Working model for the EF24 mode of action. EF24 efficiently blocks the cytokine-induced nuclear translocation of NF-κB, which is important for cell survival and inflammation signaling. This EF24 activity is at least in part due to its inhibitory effect on IκB degradation and possibly through a direct inhibitory effect on the kinase activity of IKKβ.
Although the NF-κB signaling pathway has been implicated as one of the curcumin targets, a direct inhibitory effect of curcumin on the catalytic activity of a purified IKK protein has not been shown (Bharti et al., 2003; Deeb et al., 2004). Our research identifies EF24 as a new chemical class of IKKβ inhibitors derived from the natural product curcumin. However, whether EF24 serves as an irreversible inhibitor of IKKβ requires further investigation. With the recognition of a new IKKβ inhibitor, EF24 may serve as a lead compound for further chemical optimization in search for more potent and efficacious IKKβ inhibitors (Karin, 2006). It should be noted that curcumin has been reported to be an inhibitor of several other kinases as well, in particular protein kinase C, epidermal growth factor receptor tyrosine kinase, and mammalian target of rapamycin serine/threonine kinase (Lin, 2007). It remains to be determined whether EF24 may be active against other kinases. Currently, we have ruled out the possibility that EF24 acts as a general inhibitor of TNF-α signaling by monitoring the phosphorylation status of two additional downstream targets of the TNF-α pathway, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase 1/2. Neither of these kinases was found to be suppressed when EF24 was present (Supplemental Fig. S1).
Why is EF24 a more effective inhibitor of IKKβ than curcumin? This question cannot be answered definitively in the present work, but a possible explanation can be outlined based on structural differences and the commonly accepted mechanism of kinase inhibition. Curcumin exists in the enol form both in the solid state and in solution (Mague et al., 2004; Payton et al., 2007). In addition, in the crystal lattice, the molecule adopts an extended planar form that spans 19 Å in the longest direction (i.e., H–H). The X-ray structure of EF24, in contrast, depicts a nonplanar molecule with a propeller arrangement of the terminal phenyl rings that incorporates a reasonable degree of flexibility and a long dimension of 15 Å (J. P. Snyder and A. Sun, unpublished). Assuming that both curcumin and EF24 occupy at least partially the ATP binding site of IKKβ kinase as the origin of their inhibition, the shorter and more flexible EF24 would seem to be more adaptable to the globular binding pocket. For comparison, we refer to roscovitine, a compound that potently blocks a number of cyclin-dependent kinases (Meijer and Raymond, 2003). Roscovitine is a nonplanar flexible molecule with a long dimension of 14 Å in a low-energy conformation. The properties match those of EF24 and suggest that a compact and potentially mobile kinase ligand is best suited to the ATP site. The mixed type competitive inhibitor of EF24 with respect to ATP partially supports this notion. Ultimately, this hypothesis can be tested by determination of the X-ray structures of curcumin and EF24 bound to IKKβ.
The NF-κB pathway has been found to be activated in lung cancer, with various chemotherapeutic regiments adding to already elevated NF-κB activation in tumors and representative cell lines (Mukhopadhyay et al., 1995; Wang et al., 1996). Lung cancer A549 cells may have developed a certain dependence on the up-regulated NF-κB pathway for sustained survival. This mechanism may involve up-regulated NF-κB-controlled survival genes, such as Bcl-XL and inhibitors of apoptosis. Thus, inhibition of IKKβ and its mediated NF-κB signaling by EF24 likely lead to the suppression of survival mechanism and the induction of cell death. In contrast, it has been reported that up-regulated NF-κB also has a transcription independent function through suppression of tumor suppressor gene PTEN expression (Vasudevan et al., 2004). Activated p65 in nucleus has been shown to sequester the transcriptional coactivators CBP/p300, leading to the suppression of PTEN expression. PTEN is a negative regulator of Akt, a major survival kinase in A549 cells (Stambolic et al., 1998). In addition to inhibiting NF-κB-controlled expression of survival genes, EF24-mediated IKK inhibition may result in the release of p65-sequestered cAMP response element-binding protein-binding protein/p300 and the subsequent activation of PTEN. In support of our model, Selvendiran et al. (2007) demonstrated that EF24 induces the expression of PTEN in ovarian cancer cells, which mediates EF24-triggered G2/M cell cycle arrest and apoptosis.
Because the IKK protein complex plays a vital role in cellular responses to many environmental signals under both physiological and pathological conditions, IKK inhibitors are known to have significant therapeutic value (Karin, 2006). It is expected that the EF24 class of agents may not only have a role as potential cancer therapeutics but also may have important applications in various IKK/NF-κB dysregulated autoimmune and inflammatory diseases, including rheumatoid arthritis.
Supplementary Material
Acknowledgments
We thank members of the Fu, Khuri, Sun, and Liotta laboratories for assistance and enlightening discussions.
This work was supported in part by National Institutes of Health Grants R01-GM53165 (to H.F.)., P01-CA116676 (to F.R.K., H.F., and S.S.), P50-CA128613 (to S.S., F.R.K., J.P.S., and H.F.), and Emory University Research Committee. F.R.K., S.S., and H.F. are Georgia Cancer Coalition Distinguished Scholars. H.F. is a Georgia Research Alliance Distinguished Investigator. Y.D. is Emory Drug Development and Pharmacogenomics Academy Fellow and recipient of Emory Head and Neck Cancer Specialized Programs of Research Excellence P50-CA128613 career development award. S.L.T. is a recipient of American Association for the Advancement of Science/Packard Fellowship and Emory Facilitating Academic Careers in Engineering and Science program fellow.
ABBREVIATIONS
- NF-κB
nuclear factor-κB
- EF24
3,5-bis(2-flurobenzylidene)piperidin-4-one
- IκB
inhibitor of κB
- IKK
inhibitor of κB kinase
- DMSO
dimethyl sulfoxide
- TNF
tumor necrosis factor
- NP-40
Nonidet P40
- PBS
phosphate-buffered saline
- PAGE
polyacrylamide gel electrophoresis
- GST
glutathione transferase
- MOPS
3-(N-morpholino)propanesulfonic acid
- HCA
high content analysis
- PTEN
phosphatase with sequence homology to tensin
Footnotes
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
References
- Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, Snyder JP, Liotta DC, Jones DP, Shoji M. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollings-head MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shoji M. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) downregulates expression of cell proliferation, antiapoptotic and metastatic gene products through suppression of IκBα kinase and AKT activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- Commandeur JN, Vermeulen NP. Cytotoxicity and cytoprotective activities of natural compounds. The case of curcumin. Xenobiotica. 1996;26:667–680. doi: 10.3109/00498259609046741. [DOI] [PubMed] [Google Scholar]
- Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Chapman RA, Dulchavsky SA, Gautam SC. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther. 2004;3:803–812. [PubMed] [Google Scholar]
- Dhillon N, Wolff RA, Abbruzzese JL, Hong DS, Camacho LH, Li L, Braiteh FS, Kurzrock R. Phase II clinical trial of curcumin in patients with advanced pancreatic cancer. J Clin Oncol. 2006;24(18 Suppl) Abstract 14151. [Google Scholar]
- Karin M. NF-kappaB and cancer: mechanisms and targets. Mol Carcinog. 2006;45:355–361. doi: 10.1002/mc.20217. [DOI] [PubMed] [Google Scholar]
- Levi MS, Borne RF, Williamson JS. A review of cancer chemopreventive agents. Curr Med Chem. 2001;8:1349–1362. doi: 10.2174/0929867013372229. [DOI] [PubMed] [Google Scholar]
- Lin JK. Molecular targets of curcumin. Adv Exp Med Biol. 2007;595:227–243. doi: 10.1007/978-0-387-46401-5_10. [DOI] [PubMed] [Google Scholar]
- Mague JT, Alworth WL, Payton FL. Curcumin and derivatives. Acta Crystallogr C. 2004;60:608–610. doi: 10.1107/S0108270104015434. [DOI] [PubMed] [Google Scholar]
- Meijer L, Raymond E. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc Chem Res. 2003;36:417–425. doi: 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2 cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay T, Roth JA, Maxwell SA. Altered expression of the p50 subunit of the NF-kappa B transcription factor complex in non-small cell lung carcinoma. Oncogene. 1995;11:999–1003. [PubMed] [Google Scholar]
- Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- Osawa T, Sugiyama Y, Inayoshi M, Kawakishi S. Antioxidative activity of tetrahyrocurcuminids. Biosci Biotechnol Biochem. 1995;59:1609–1612. doi: 10.1271/bbb.59.1609. [DOI] [PubMed] [Google Scholar]
- Payton F, Sandusky P, Alworth WL. NMR study of the solution structure of curcumin. J Nat Prod. 2007;70:143–146. doi: 10.1021/np060263s. [DOI] [PubMed] [Google Scholar]
- Rayet B, Gélinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- Richmond A. Nf-kappa B, chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]
- Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- Selvendiran K, Tong L, Vishwanath S, Bratasz A, Trigg NJ, Kutala VK, Hideg K, Kuppusamy P. EF24 induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by increasing PTEN expression. J Biol Chem. 2007;282:28609–28618. doi: 10.1074/jbc.M703796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Singh S, Khar A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med Chem. 2006;6:259–270. doi: 10.2174/187152006776930918. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-kappa B prevents apoptosis. Mol Cell Biol. 2004;24:1007–1021. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen J, Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc Natl Acad Sci U S A. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.