Abstract
The intestinal protozoan parasite Entamoeba histolytica remains a significant cause of morbidity and mortality worldwide. However, almost nothing is known about the molecules secreted by the parasite that modulate host immune responses or epithelial barrier function in the colon. Herein, we describe the isolation and characterization of a cyclooxygenase (COX)-like enzyme in E. histolytica that is responsible for the biosynthesis of prostaglandin (PG)E2. PGE2 produced by ameba was constitutive but highly dependent on exogenous arachidonic acid substrate. COX-like activity and the immunoreactive protein were localized to the nuclear fraction of E. histolytica. The COX-like protein (72 kDa) was microsequenced and cloned by reverse transcriptase PCR. Ameba COX showed little homology with COX-1/2 enzymes from different species at the nucleotide and amino acid levels. Surprisingly, the arachidonate-binding domain and heme-coordinating and catalytic sites, which are conserved in other species, were absent in ameba. Ameba COX expressed in Escherichia coli demonstrated COX-like enzyme activity in vitro by converting arachidonic acid into PGE2 but not into PGD2 or PGF2α. COX activity was inhibited with 1 mM aspirin but not with indomethacin or COX-1/2-specific inhibitors. Taken together, these studies reveal that E. histolytica produces PGE2, by means of a previously undescribed ancestral COX-like enzyme, which could play a major role in pathogenesis and immune evasion.
The enteric protozoan parasite Entamoeba histolytica is a human pathogen that causes widespread morbidity and mortality and ranks behind only malaria and schistosomiasis as leading causes of parasitic-related deaths (1). Amebic infection of the colon results in dysentery, ulcers, and colitis. Trophozoites can invade the colonic epithelium and disseminate to the liver or other soft organs to form abscesses. Invasion of the colonic epithelium and evasion of host immune defenses have long been intriguing aspects of amebiasis. Before the ameba binds to the epithelium, it must overcome the protective mucous layer. Amebas bind with high affinity to mucins (2), the high molecular weight glycoproteins that provide the protective gel nature to mucus (3). The parasite elaborates a potent yet unidentified mucin secretagogue (4). It is unknown how E. histolytica overcomes luminal barrier function, but it was hypothesized that chronic exposure to mucin secretagogues may eventually deplete the stored mucin pool and thus render the mucus less protective (4).
Macrophages derived from amebic lesions in the liver are deficient in effector and accessory cell functions and cannot kill ameba (5). E. histolytica stimulates prostaglandin (PG)E2 production by macrophages (6, 7), a potent immunomodulating agent that down-regulates cytokine production (IL-1, IL-2, IFN-γ, and tumor necrosis factor α) and inhibits Ia molecule expression, key determinants in controlling disease severity and outcome (8-12). PGs are found in a variety of species of vertebrates and invertebrates (13, 14). In vertebrates they are synthesized by PGH2 synthase, also known as cyclooxygenase (COX) (15, 16). PGs are thought to mediate intestinal response to injury, including vasodilation, enhanced chloride secretion, and enhanced vascular permeability (17). COX is the rate-limiting step in the production of PGs. COX has two isoforms: COX-1 is a constitutive enzyme present in a variety of cells for housekeeping functions, whereas COX-2 is inducible and is found in high levels during intestinal inflammation (18-21), ulcerative colitis, and Crohn's disease. Increased production of PGE2 may exacerbate inflammation and down-regulate immune responses. Nonsteroidal antiinflammatory drugs (NSAIDs) inhibit COX activity and alleviate pain, fever, and swelling associated with increased PGE2 production (22). PGE2 also regulates physiologic responses such as vasodilation and intestinal Cl- and mucin secretion (23-25), processes that may contribute to the pathophysiology of amebiasis.
An unresolved issue in intestinal amebiasis is whether lesions in the colon are induced by parasite adherence and destruction of mucosal epithelial cells, by a host inflammatory response, or by both. At present, it is unknown whether E. histolytica produces immunosuppressive molecules such as PGE2 that may play a role in pathogenesis. Elaboration of this COX-derived product could explain some pathophysiologic symptoms experienced during the intestinal phase of the disease and the immunomodulation that occurs during invasive amebiasis. Herein, we demonstrate that E. histolytica produces PGE2, and we characterize a previously undescribed COX-like enzyme in the parasite.
Materials and Methods
PGE2 Production by E. histolytica. A highly virulent strain of E. histolytica (HM1-I MSS) grown axenically in TYI-S-33 medium (26) in midlog phase (72 h) was chilled on ice for 10 min, harvested by centrifugation at 600 × g for 5 min at 4°C, and washed twice in cold Dulbecco's PBS (pH 7.2; Invitrogen). Trophozoites were resuspended at a concentration of 107/ml in PBS and incubated with 100 μM arachidonic acid (AA) or vehicle at 36.6°C. After centrifugation, PGE2 in the supernatant was extracted with Amprep C2 ethyl columns (Amersham Biosciences) following manufacturer's protocol. PGE2 was quantified by using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI) that uses a monoclonal antibody with very low crossreactivity (<0.01%) to other major PG metabolites.
Preparation of E. histolytica Nuclear Proteins (EhNP). Nuclei were prepared from E. histolytica by using a modified protocol as described for mammalian cells (27). Ameba was washed twice in PBS and resuspended in lysis buffer {100 mM Tris (pH 7.4), 7.4 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml E-64, 10 μg/ml 7-amino-1-chloro-3-tosylamido-2-heptanone [Nα-(p-tosyl)lysine chloromethyl ketone], and 0.5% Nonidet P-40 detergent} on ice for 15 min. Nuclei were pelleted by centrifugation at 2,000 × g for 15 min, washed with lysis buffer, and resuspended in sodium phosphate buffer (pH 7.0), and the protein concentration was determined by the bicinchoninic acid method (Pierce).
Protein Sequencing and Identification of COX-Like Protein. Fifty micrograms of EhNP was run in 10% SDS/PAGE gel (prerun with 2 mM mercaptoacetic acid) in duplicates. One portion was used for immunoblot with rabbit polyclonal affinity-purified antibodies against sheep-seminal-vesicle COX-1 (AP-241; Merck Frosst Labs, Kirkland, QC, Canada) to detect the COX-like protein. The corresponding band in the other portion of the gel was stained with 0.1% Coomassie blue, and, after complete destaining, the band was excised and sent for protein microsequencing at the University of Victoria Protein Microchemistry Centre (Victoria, BC, Canada). In brief, protein gel fragments were digested with 1 mg/ml trypsin overnight at room temperature, and the eluted proteins were subjected to reverse-phase HPLC to purify the peptide fragments. The purified peptides were microsequenced from the N terminus in a protein sequencer (Applied Biosystems) according to the manufacturer's instructions. Finally, the protein was identified by Q-star analysis integrated with the system as actinin-like protein (GenBank accession no. AAF20148.1) from E. histolytica.
Cloning and Expression of the COX-Like Gene. Total RNA from E. histolytica was isolated with TRIzol (Invitrogen). Ten micrograms of total RNA was reverse transcribed in 20 μl by using 0.5 μg of oligo (dT), 0.5 mM dNTPs, 40 units of RNaseOUT, 0.01 mM DTT, and 200 units of Moloney murine leukemia virus reverse-transcription enzyme (all from Invitrogen) at 37°C for 1 h. After heat inactivation, COX-like sequences were PCR-amplified by using sense primer (GCAGGATCCCAAGTTGCAAACATGACTGG) and antisense primer (GCAGTCGACTTGATCAAATAAGTCCAAG) and by using plaque-forming unit/polymerase-containing proofreading activity from the reaction mix containing first-strand cDNA. PCR-amplified products were cloned in TA-cloning vector (Promega). Recombinant clones were sequenced from either end and were identified in the GenBank database (accession no. AF208390). To determine the 5′ sequence, RACE was performed by using the 5′ RACE system, Version 2.0 (Invitrogen). The COX-like sequence from the TA-cloning system was directionally subcloned into pQE 30 expression vector (Qiagen, Valencia, CA) at the BamHI and SalI site. His-tagged actinin protein was expressed in Top10F' cells by inducing with 1 mM isopropyl β-d-thio-galactopyranoside for 4 h in cultures at midlog phase. Soluble protein fractions were purified by using nickel-nitrilotriacetate agarose (Qiagen) affinity column and dialyzed (3,500 Mr) several times against PBS at 4°C.
Generation of COX-Like Antibody. New Zealand White rabbits were injected with 150 μg of recombinant COX-like protein in Freund's complete adjuvant s.c. and were subsequently boosted twice with similar amounts of protein in Freund's incomplete adjuvant at 15-day intervals (28). Animals were tested for anti-COX-like antibodies, boosted, and killed 1 week thereafter.
Northern and Southern Blot Analysis. For Northern blot analysis, 10 μg of total RNA was used, and for Southern blot analysis, 15 μg of genomic DNA digested with BamHI, EcoRI, HindIII, and PstI was used as described (29). A 10-kb HindIII fragment of pRW528 containing 17S and 26S rRNA gene from Neurospora crassa was used as a positive control for Northern blot analysis.
Expression of the COX-Like Protein in E. histolytica. The COX-like sequence was subcloned at HindIII and BamHI sites in pHTP-luc expression vector and designated HTP-COX. This construct was used to transfect E. histolytica to overexpress the COX-like enzyme as described (30). After transfection, amebae were allowed to grow for 24 h without antibiotic selection in TYI-S-33 medium and then selected against 6 μg/ml G418. Gradually G418 concentration was increased (12-24 μg/ml) to overexpress the COX protein in E. histolytica.
SDS/PAGE and Western Blot Analysis. Purified active sheep COX-1 (300 ng, Cayman Chemical), sheep COX-2 (300 ng, Cayman Chemical), EhNP (100 μg), and purified ameba COX-like protein (300 ng) were denatured by boiling in sample buffer [64 mM Tris·Cl (pH 6.8), 10.25% glycerol, 2% SDS, and 5.12% 2-mercaptoethanol] containing protease inhibitors (1.5 mM EDTA, 23 μM leupeptin, 14.5 μM pepstatin, 1.53 μM aprotinin, 30 μM 7-amino-1-chloro-3-tosylamido-2-heptanone [Nα-(p-tosyl)lysine chloromethyl ketone], and 1 mM PMSF). Proteins were run in 10% SDS/PAGE, and Western blot analysis was performed by using polyclonal anti-COX-1 antibody (AP-241) or anti-COX-like antibody to detect the COX-like specific proteins.
COX Activity Assay. COX activity assay was performed on both EhNP and the purified recombinant COX-like enzyme. EhNP or COX-like enzyme was incubated for 1 h at 36.6°C with 100 μM AA in sodium phosphate buffer containing 200 μM tryptophan and 2 μM hematin (Sigma) in 500-μl volume. Reactions involving NSAID, 100 μg of EhNP, or purified active ovine COX-1/2 and COX-like enzyme were preincubated with COX nonspecific inhibitor indomethacin (INDO), aspirin (ASA), COX-1-specific inhibitor SC560, or COX-2 specific inhibitor nimesulide (all from Cayman Chemical) for 30 min at 36.6°C before addition of 100 μM AA. PGE2 was isolated and quantified by enzyme immunoassay (EIA) according to the manufacturer's suggestion (Cayman Chemical). The biosyntheses of PGF2α and PGD2 of the COX-like enzyme were determined by EIA, and peroxidase activity of the recombinant COX-like enzyme and EhNP were determined by COX activity assay kit (Cayman Chemical).
Results
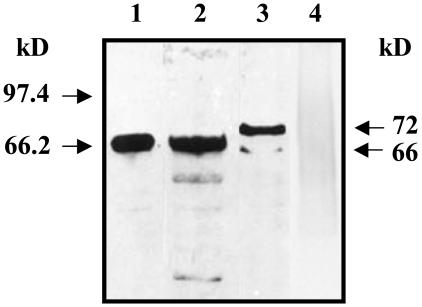
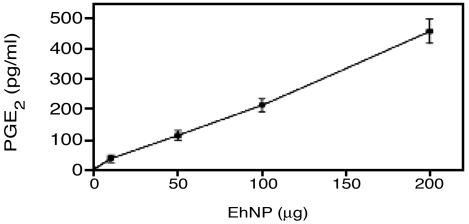
PGE2 Production and Immunodetection of COX. Live trophozoites rapidly took up exogenous AA and produced PGE2 in a time-dependent manner (Table 1). AA was a critical rate-limiting step in the biosynthesis of PGE2. Immunodetection of ameba COX was performed by using affinity-purified polyclonal antibody raised against ovine COX-1. As shown in Fig. 1, the AP-241 antibody detected COX-1 from sheep and U937 human macrophages with a Mr of 68 kDa and protein bands of 72 and 66 kDa from E. histolytica EhNP. Fig. 2 confirms the COX-like activity in the EhNP fraction as PGE2 production increased in a dose-dependent manner with increasing amounts of EhNP. Moreover, there was no immunoreactive COX-1 protein (Fig. 1, lane 4) or PGE2 production from the cytosolic fraction of E. histolytica.
Table 1. PGE2 production by live E. histolytica trophozoites.
| Time, h | PGE2, pg/ml |
|---|---|
| 1 (no AA) | 0 |
| 1 | 28 ± 1 |
| 6 | 57 ± 6 |
| 12 | 74 ± 6 |
Trophozoites (107) were incubated in PBS containing 100 μM AA, and PGE2 levels were quantified by EIA. Ameba viability after 12-h incubation in PBS was 90% by trypan blue exclusion assay. Data represent the mean ± SEM from three independent experiments.
Fig. 1.
Immunoblot analysis of EhNP. Ten nanograms of purified sheep COX-1 (lane 1), 30 μg of U937 human macrophage microsomes (lane 2), 30 μg of EhNP (lane 3), and 100 μg of Eh cytosol proteins (lane 4) were resolved in 10% SDS/PAGE. Affinity-purified polyclonal antibody against sheep COX-1 (AP-241) was used for Western blot analysis.
Fig. 2.
Production of PGE2 by EhNP. EhNP was incubated with 100 μM AA for 1 h at 36.6°C, and PGE2 was measured by enzyme immunoassay. Data represent the mean ± SEM from two independent experiments.
Cloning of the COX-Like Sequence. Ameba COX was cloned from the 72-kDa immunoreactive protein band shown in Fig. 1. Initial attempts to clone the COX-like DNA sequence by RT-PCR using degenerate or homologous primers derived from several known COX sequences available from the GenBank database failed. After microsequencing the immunoreactive protein and Q-star analysis, the putative COX protein was identified as actinin-like protein (GenBank accession nos. AAF20148.1 for protein and AF208390 for its mRNA) from E. histolytica. The actinin DNA sequence was 1,640 bp long [1-1,640 bp, with ORF 4-1,620 bp] and it was quoted as a partial cDNA sequence in the database. Therefore, several attempts were made to clone the 5′ sequence by 5′ RACE, but we were unable to amplify any sequences beyond methionine, the second amino acid of AAF20148.1. Despite numerous attempts, we could not obtain amino acid asparagine N (nucleotide sequence: AAC) or any other amino acid as reported previously in the GenBank database. Northern blot analysis also showed that the transcribed COX-like mRNA was 1.6 kb (Fig. 7A). Therefore, we conclude that methionine is the start codon of the COX-like enzyme containing ORF 4-1614 bp, which encodes 537 aa of the actinin protein. Consequently, all numbering will be mentioned considering methionine as the first amino acid and ATG (nucleotide 4 of AF208390) as the start codon for the COX-like enzyme. At the amino acid and nucleotide level (data not shown), ameba COX showed little homology with mammals, trout, or coral as revealed from multiple alignments with the COX-1 enzyme (Fig. 3), and the phylogenetic tree suggests it may be the earliest known COX (Fig. 4). None of the conserved amino acids for COX-1 were conserved in ameba COX. Specifically, the arachi-donate-binding domains Arg-120 and Tyr-355 and the heme coordinating sites His-207 and His-388 were absent. The catalytic site Tyr-385 was also not present. Surprisingly, there was no Ser-530, the target of ASA attack. ASA acetylates Ser-530 and inhibits enzyme function. These amino acid numbers correspond to the human COX-1 enzyme. COX-like enzyme has a Gln- and Glu-rich region at 236-355 aa and 225-313 aa. It also has a putative actinin-binding domain at 11-20 aa and several Ca+2-binding domains at 380-392, 416-428, and 446-458 aa. Several putative N-glycosylation and protein kinase C phosphorylation sites were also present.
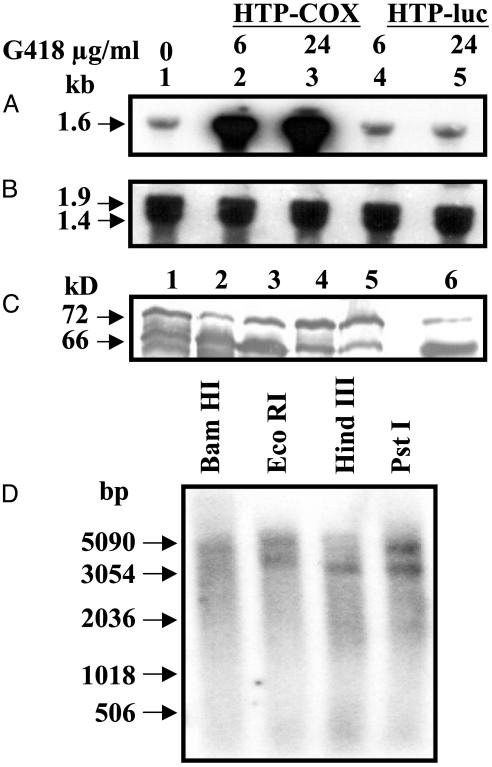
Fig. 7.
Northern, Western, and Southern blot analysis of COX mRNA/protein and genomic DNA from E. histolytica. (A) In vivo overexpression of COX-like mRNA in E. histolytica. Lane 1, wild type; lanes 2 and 3, E. histolytica transfected with HTP-COX plasmid; lanes 4 and 5, E. histolytica transfected with HTP-luc vector control. (B) Ribosomal DNA sequence from N. crassa showing the presence of equal loading of total RNA in each lane. Lanes 1-5 are identical to A. (C) Western blot analysis of the overexpressed COX-like proteins from A. Lanes 1-5 are identical to A; lane 6 is the recombinant COX protein standard. (D) Southern blot analysis of genomic DNA from E. histolytica using BamHI, EcoRI, HindIII, and PstI restriction enzymes.
Fig. 3.
Amino acid sequence alignment of E. histolytica COX with human, trout, and coral COX-1 sequences. Note the putative actinin-binding domain at 11-20 and the Ca+2-binding domain at 380-392, 416-428, and 446-458. Several putative N-glycosylation and protein kinase C phosphorylation sites are 85-88, 97-100, 144-150, 363-366, 405-408, 420-423, and 50-52; and 407-409, 441-443, and 515-517, respectively.
Fig. 4.
Phylogenetic tree showing the relationship of COX-like enzyme with other known COX-1 sequences. Sequences are the same as in Fig. 3.
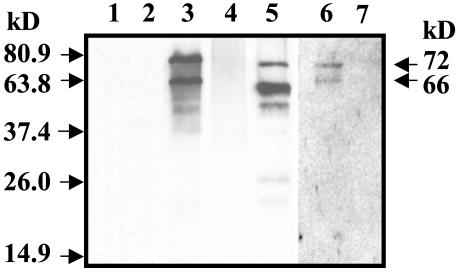
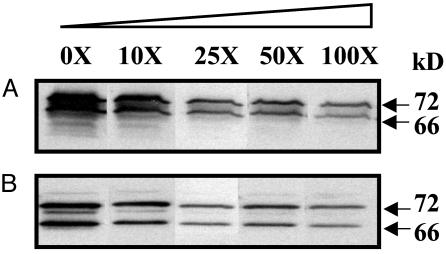
Expression and Characterization of COX-Like Protein in Escherichia coli. Ameba COX-like protein was weakly recognized by commercially available mammalian-specific anti-COX antibodies. Therefore, the COX-like recombinant protein expressed in vitro by using pQE30 plasmid in the Top10F′ cell was used to generate antibodies. As shown in Fig. 5, COX-like polyclonal antibody detected the 72- and 66-kDa protein bands (as in Fig. 1) in EhNP and in the recombinant COX-like protein along with smaller protein fragments because of leaky expression in E. coli. The smaller protein fragments with His tag at the 5′ end were purified by Ni-agarose chromatography and thus were detected by COX-like antibody as it was raised against the full-length protein. The COX-like antibody did not detect active ovine COX-1 and COX-2 enzymes (Fig. 5) under conditions where ameba COX proteins were immunodominant; however, under prolonged exposure, the COX-like antibody weakly detected ovine COX-1 (lane 6). In competition experiments by using 100-fold excess EhNP or COX-like recombinant protein, immunoreactivity of amebae COX was inhibited 65% (Fig. 6).
Fig. 5.
Immunoblot analysis of EhNP and recombinant COX-like protein. Polyclonal antibodies against the COX-like protein detected the 72- and 66-kDa protein bands in EhNP and recombinant COX-like protein. Lane 1, 300 ng of active sheep COX-1 enzyme; lane 2, 300 ng of sheep COX-2 enzyme; lane 3, 100 μg of EhNP; lane 4, 100 μg of Eh cytosol protein; lane 5, 50 μg of recombinant ameba COX-like protein; lane 6, 300 ng of active sheep COX-1 enzyme; lane 7, 300 ng of sheep COX-2 enzyme.
Fig. 6.
Inhibition of COX-like antibody binding in the presence of excess EhNP or COX-like protein. One hundred micrograms of EhNP (A) and 50 μg of recombinant COX-like proteins (B) were resolved in 10% SDS/PAGE and competed with excess proteins as indicated. Excess proteins (0×-100×) were preincubated with polyclonal antibodies against the COX-like protein for 30 min before probing the blot.
Because various NSAIDs are known to inhibit mammalian COX activity, COX-like enzyme activity was compared with active ovine COX-1 and COX-2. As shown in Table 2, COX-like enzyme activity was significantly inhibited only with 1 mM ASA (similar results were obtained for EhNP). As expected, COX nonspecific (ASA and INDO), COX-1-specific (SC560), and COX-2-specific (nimesulide) inhibitors were highly active at inhibiting enzyme activity of their specific COX isoforms. Interestingly, INDO, another COX nonspecific inhibitor, did not inhibit ameba COX-like activity. A consistent and unexplained finding was the slight increase in PGE2 production observed with the COX-like enzyme in the presence of nimesulide. These data strongly suggest that ameba COX-like enzyme is pharmacologically different from mammalian COX with respect to different NSAID sensitivities. Furthermore, there was no peroxidase activity in EhNP and the COX-like enzyme, unlike its mammalian counterpart. Another major finding was that ameba COX could synthesize only PGE2 but not PGF2α or PGD2 from AA substrate.
Table 2. Comparison of the sensitivity of E. histolytica COX-like enzyme with ovine COX-1/2 to NSAIDs.
| PGE2 production, pg/ml
|
|||
|---|---|---|---|
| COX inhibitors | COX-like | Ovine COX-1 | Ovine COX-2 |
| None | 48 ± 3 | 169 ± 12 | 139 ± 3 |
| 50 μM INDO | 48 ± 3 | 22 ± 6* (87%) | 49 ± 8* (65%) |
| 400 μM ASA | 50 ± 4 | 30 ± 9* (82%) | 41 ± 3* (70%) |
| 1 mM ASA | 1 ± 1* | 0.0* (100%) | 0.0* (100%) |
| 40 μM SC560 | 48 ± 3 | 39 ± 2* (77%) | 148 ± 5 |
| 40 μM nimesulide | 69 ± 4 | 123 ± 4 (27%) | 49 ± 3* (65%) |
Active ovine COX-1/2 (2 units each) and COX-like (4.80 μg) enzymes were incubated with NSAIDs for 30 min at 36.6°C prior to COX activity assay as described in Materials and Methods. PGE2 production was quantified by EIA. Data represent the mean ± SEM from two independent experiments. *, P < 0.05 by Student's t test when compared with homologous controls. Values in parentheses indicate percentage inhibition from homologous controls.
Expression of Ameba COX-Like mRNA in Vivo. Fig. 7A shows the basal and overexpressed 1.6-kb COX-like mRNA transcript in E. histolytica by Northern blot analysis. This mRNA transcript correlates well with the full-length COX-like mRNA described above. COX-like mRNA expression levels increased as the plasmid copy number increased in the presence of high concentrations of G418 (Fig. 7B). In the vector control, expression levels of COX-like mRNA were similar to basal levels. There was an excellent correlation between COX-like mRNA levels and COX-like protein expression (Fig. 7C) as detected by Western blot analysis. In particular, the 66-kDa protein was increased 7-fold as compared with wild-type or vector control. Overexpression of the COX-like enzyme did not induce any phenotypic or morphological changes in ameba.
Genomic Organization of the COX-Like Gene. By Southern blot analysis (Fig. 7D), we detected 5,051-bp BamHI, 6,065- and 5,208-bp EcoRI, 4,095-bp HindIII, and 5,951- and 4,466-bp PstI fragments from the E. histolytica genome. In particular, HindIII had only one restriction site at position 158 of the COX-like cDNA sequence and detected a 4,095-bp fragment from the ameba genome. Although BamHI, EcoRI, and PstI enzymes did not have any restriction sites within the COX-like cDNA, EcoRI and PstI detected two similar-sized fragments (5.2 and 4.0 kb and 5.9 and 4.4 kb, respectively) of equal intensity. Based on the intensity of different-sized bands, the data suggest the presence of a multicopy gene family containing COX-like sequence.
Discussion
A COX-Like Enzyme in Ameba. Herein we show that ameba produces PGE2 through a COX-like enzyme in the presence of exogenous AA. The COX-like protein was localized on the nuclear envelope, unlike mammalian COXs, which are membrane-bound and present on the luminal surfaces of the endoplasmic reticulum and the inner and outer membranes of the nuclear envelope (31, 32). PGE2 plays a major role during inflammation by modulating the host immune system and functions differently through its four EP receptors. Recently, mammalian nuclear EP1-4 receptors were discovered (33). PG transporter hPGT was also identified and thought to be a mediator for PGE2 to interact with its nuclear receptor (34).
Ameba COX-like enzyme differs significantly from other known COX enzymes. Sheep COX-1 (pI 6.7) is uniformly glycosylated at three sites (Asn-68, Asn-144, and Asn-410) and appears as a single band on SDS/PAGE with a molecular mass of ≈67 kDa. Native mammalian COX-2, on the other hand, is more heterogeneously glycosylated at an additional site (Asn-588). Multiple molecular species of COX-2 can be readily observed with SDS/PAGE with a molecular mass of 68-72 kDa (35). N-glycosylation may play a role in the maturation of COXs, but deglycosylation of the mature enzyme does not affect activity (35). Moreover, mammalian COX-1 and -2 appear as homodimers in solution after detergent solubilization, whether glycosylated or not (36, 37). The 72- and 66-kDa COX-like protein had a pI of 4.6, whereas sheep COX-1 had pI of 6.7 (two-dimensional SDS/PAGE, data not shown), which suggest that these two COX enzymes differ significantly from each other. The predicted mRNA size and average protein size are 1,611 nt and 62.9 kDa, which are very close to the experimental observation (Figs. 1 and 7A). From the recombinant COX-like enzyme, it was evident that the 72- and 66-kDa proteins are variants of the same protein, as polyclonal antibodies against the COX-like protein detected both bands in EhNP and recombinant COX-like protein. This could be due to different degrees of glycosylation of the same COX-like protein in vivo (38), analogous to mammalian COX-2. The multiple alignment of COX-like protein and DNA sequence with COX sequences from various other species also showed less homology (Fig. 3).
The COX-like enzyme catalyzed AA to PGE2 as EhNP did in a similar fashion. PGE2 biosynthesis depended on purified enzyme concentration, and enzyme activity was only inhibited by high concentrations of ASA. These results indicate a pharmacological profile that is completely different from other known COX enzymes. All catalytically important conserved amino acid sequences (Arg-120, Tyr-355, His-207, His-388, Tyr-385, and Ser-530) of human COX-1 were absent in the COX-like enzyme. In Plasmodium falciparum, conversion of AA to PG was not inhibited by 3 mM ASA, indicating that the serine acetylation site is also absent in malaria parasites (39). INDO inhibits by sterically hindering AA binding sites Arg-120 and Tyr-355 (40). Ameba COX-like enzymes lack these conserved sites and, therefore, are resistant to high concentrations of INDO.
Sequence comparisons between COX isoforms from the same species showed 60-65% sequence identity, whereas sequence identity among orthologs from different species varied from 85% to 90% (21). When compared with sequences from other proteins, particularly other heme-dependent peroxidases, significant levels of similarity were detected. COX enzymes are members of the mammalian heme-dependent peroxidase family (41), which include myeloperoxidase and thyroid peroxidase. The COX catalytic domain shares a great deal of structural homology with mammalian myeloperoxidase (42, 43), consistent with the sequence comparisons. Structural homology between the COX catalytic domain and nonmammalian heme-dependent peroxidases is also detectable (44, 45). The authors argue (44) that the COX enzyme evolved from heme-dependent peroxidases like myeloperoxidase. COX, myeloperoxidase, and several nonmammalian peroxidases retain another structural feature: a calcium-binding motif [(V-X-)G-X-D-X-S or G-X-D-X-G] that occurs adjacent to the heme pocket. COX-like enzyme also has three calcium-binding motifs. It is highly possible that ameba COX-like enzyme is the earliest form on an evolutionary scale and all other COX or peroxidases evolved from this enzyme. The phylogenetic tree suggests that probably all COX enzymes originated from a common ancestor, and that divergence occurred at a very early stage of evolution after division of the animal kingdom into vertebrates and invertebrates.
Significance of COX-Like Enzyme in Ameba. PGE2 production by E. histolytica can exert multiple functions in the pathogenesis of intestinal amebiasis and survival of ameba in tissues. PGE2 is a potent mucin secretagogue (25) that can overcome luminal barrier function by causing hypersecretion and, thus, depletion of the protective mucus barrier (45). PGE2 can directly initiate pathogenesis by stimulating the production of IL-8 from human colonic cells, which is a potent chemoattractant and activator of neutrophils (46, 47). This stimulation can lead to local nonspecific mucosal epithelial cell damage that can facilitate mucosal invasion by trophozoites. In amebic liver granulomas, the local production of PGE2 by E. histolytica can down-regulate effector and accessory cell functions of infiltrated immune cells (macrophages and T cells). In experimental amebiasis, liver granuloma macrophages are suppressed for effector functions, which are partially restored in the presence of INDO (6).
In summary, our results demonstrate a previously undescribed COX-like enzyme in E. histolytica that is distinct from other known COX enzymes. It also provides a long-awaited answer to the question of whether E. histolytica synthesizes PGE2. Finally, the expressed COX-like enzyme has some unusual catalytic activity that makes it a very good research subject from both evolutionary and mechanistic points of view. The interrelationship between peroxidase and oxygenase activities of the COX-like enzyme during PGE2 biosynthesis remains a subject of investigation.
Acknowledgments
We thank Dr. Gary O'Neil (Merck Frosst Labs) for providing the affinity-purified polyclonal antibody against sheep COX-1 (AP 241), Dr. Armando Jardim (McGill University) for advice, and Dr. Barbara Mann (University of Virginia, Charlottesville) for providing the pHTP-luc and pRW528 plasmids. This study was supported by a grant from the Canadian Institutes of Health Research.
Abbreviations: COX, cyclooxygenase; PG, prostaglandin; AA, arachidonic acid; EhNP, Entamoeba histolytica nuclear proteins; NSAID, nonsteroidal antiinflammatory drug; ASA, aspirin; INDO, indomethacin.
References
- 1.Walsh, J. A. (1986) Rev. Infect. Dis. 8, 228-238. [DOI] [PubMed] [Google Scholar]
- 2.Chadee, K., Petri, W. A., Innes, D. J. & Ravdin, J. I. (1987) J. Clin. Invest. 80, 1245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moncada, D. & Chadee, K. (2002) in Infections of the Gastrointestinal Tract, eds. Blaser, M. J., Guerrant, R. L., Smith, P. D., Greenberg, H. B. & Ravdin, J. I. (Lippincott Williams and Wilkins, Philadelphia), pp. 57-79.
- 4.Chadee, K., Keller, K., Forstner, J., Innes, D. J. & Ravdin, J. I. (1991) Gastroenterology 100, 986-997. [DOI] [PubMed] [Google Scholar]
- 5.Denis, M. & Chadee, K. (1988) Infect. Immun. 56, 3126-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, W. & Chadee, K. (1992) Immunology 76, 242-250. [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Ramirez, B., Escalante, B., Rosales-Encina, J. L. & Talamas-Rohana, P. (1997) Prostaglandins 53, 411-421. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel, S. L., Chensue, S. W. & Phan, S. H. (1986) J. Immunol. 136, 186-192. [PubMed] [Google Scholar]
- 9.Anastassiou, E. D., Paliogianni, F., Balow, J. P., Yamada, H. & Boumpas, D. T. (1992) J. Immunol. 148, 2845-2852. [PubMed] [Google Scholar]
- 10.Kunkel, S. L., Spengler, M., May, M. A., Spengler, R., Larrick, J. & Remick, D. (1988) J. Biol. Chem. 263, 5380-5384. [PubMed] [Google Scholar]
- 11.Betz, M. & Fox, B. S. (1991) J. Immunol. 146, 108-113. [PubMed] [Google Scholar]
- 12.Figueiredo, F., Uhing, R. J., Okomogi, K., Gettys, T. W., Johnson, S. P., Adams, D. O. & Prpic, V. (1990) J. Biol. Chem. 265, 12317-12323. [PubMed] [Google Scholar]
- 13.Bundy, G. L. (1985) Adv. Prostaglandin Thromboxane Leukotriene Res. 14, 229-262. [PubMed] [Google Scholar]
- 14.Gerwick, W. H. (1999) in Comprehensive Natural Products Chemistry, ed. Sankawa, U. (Elsevier Science, Oxford), pp. 207-254.
- 15.Smith, W. L. & Marnett, L. J. (1991) Biochim. Biophys. Acta 1083, 1-17. [DOI] [PubMed] [Google Scholar]
- 16.Vane, J. R., Bakhle, Y. S. & Botting, R. M. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 97-120. [DOI] [PubMed] [Google Scholar]
- 17.Eberhart, C. E. & Dubois, R. N. (1995) Gastroenterology 109, 285-301. [DOI] [PubMed] [Google Scholar]
- 18.Eckmann, L., Stenson, W. F., Savidge, T. C., Lowe, D. L., Barrett, K. E., Fierer, J., Smith, J. R. & Kagnoff, M. F. (1997) J. Clin. Invest. 100, 296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riehl, T., Cohn, S., Tessner, T., Schloemann, S. & Stenson, W. F. (2000) Gastroenterology 118, 1106-1116. [DOI] [PubMed] [Google Scholar]
- 20.Singer, I. I., Kawka, D. W., Schloemann, S., Tessner, T., Riehl, T. & Stenson, W. F. (1998) Gastroenterology 115, 297-306. [DOI] [PubMed] [Google Scholar]
- 21.Smith, W. L. & Dewitt, D. L. (1996) Adv. Immunol. 62, 167-215. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, J. A. & Warner, T. D. (1999) Br. J. Pharmacol. 128, 1121-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willis, A. L. (1987) in Prostaglandins and Related Lipids (CRC, Boca Raton, FL), Vols. 1 & 2, pp. 260. [Google Scholar]
- 24.Whittle, B. J. R. & Vane, J. R. (1987) in Physiology of the Gastrointestinal Tract, ed. Johnson, L. R. (Raven, New York), 2nd Ed., Vol. 1, pp. 143-180. [Google Scholar]
- 25.Belley, A. & Chadee, K. (1999) Gastroenterology 117, 1352-1362. [DOI] [PubMed] [Google Scholar]
- 26.Diamond, L. S., Harlow, D. R. & Cunnick, C. C. (1978) Trans. R. Soc. Trop. Med. Hyg. 72, 431-432. [DOI] [PubMed] [Google Scholar]
- 27.Spencer, A. G., Woods, J. W., Arakawa, T., Singer, I. I. & Smith, W. L. (1998) J. Biol. Chem. 273, 9886-9893. [DOI] [PubMed] [Google Scholar]
- 28.Campbell, D., Mann, B. J. & Chadee, K. (2000) Eur. J. Immunol. 30, 423-430. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd Ed.
- 30.Purdy, J. E., Mann, B. J., Pho, L. T. & Petri, W. A., Jr. (1994) Proc. Natl. Acad. Sci. USA 91, 7099-7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neill, G. P., Mancini, J. A., Kargman, S., Yergey, J., Kwan, M. Y., Falgueyret, J. P., Abramovitz, M., Kennedy, B. P., Ouellet, M., Cromlish, W., et al. (1994) Mol. Pharmacol. 45, 245-254. [PubMed] [Google Scholar]
- 32.Spencer, A. G., Thuresson, E., Otto, J. C., Song, I., Smith, T., DeWitt, D. L., Garavito, R. M. & Smith, W. L. (1999) J. Biol. Chem. 274, 32936-32942. [DOI] [PubMed] [Google Scholar]
- 33.Bhattacharya, M., Varma, D. R. & Chemtob, S. (1999) Gene Ther. Mol. Biol. 4, 323-338. [Google Scholar]
- 34.Lu, R., Kanai, N., Bao, Y. & Schuster, V. L. (1996) J. Clin. Invest. 98, 1142-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otto, J., Dewitt, D. & Smith, W. (1993) J. Biol. Chem. 268, 18234-18242. [PubMed] [Google Scholar]
- 36.Smith, W., Garavito, R. & DeWitt, D. (1996) J. Biol. Chem. 271, 33157-33160. [DOI] [PubMed] [Google Scholar]
- 37.Smith, W. L., DeWitt, D. L. & Garavito, R. M. (2000) Annu. Rev. Biochem. 69, 145-182. [DOI] [PubMed] [Google Scholar]
- 38.Percival, M. D., Bastien, L., Griffin, P. R., Kargman, S., Ouellet, M. & O'Neill, G. P. (1997) Protein Expression Purif. 9, 388-398. [DOI] [PubMed] [Google Scholar]
- 39.Kilunga, K. B., Eguchi, N., Urade, Y., Yamashita, K., Mitamura, T., Tai, K., Hayaishi, O. & Horii, T. (1998) J. Exp. Med. 188, 1197-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callan, O. H., So, O. Y. & Swinney, D. C. (1996) J. Biol. Chem. 271, 3548-3554. [DOI] [PubMed] [Google Scholar]
- 41.Zeng, J. & Fenna, R. E. (1992) J. Mol. Biol. 226, 185-207. [DOI] [PubMed] [Google Scholar]
- 42.Garavito, R. M., Picot, D. & Loll, P. J. (1994) Curr. Opin. Struct. Biol. 4, 529-535. [Google Scholar]
- 43.Picot, D., Loll, P. J. & Garavito, R. M. (1994) Nature 367, 243-249. [DOI] [PubMed] [Google Scholar]
- 44.Garavito, R. M. & Mulichak, A. M. (2003) Annu. Rev. Biophys. Biomol. Struct. 32, 183-206. [DOI] [PubMed] [Google Scholar]
- 45.Moncada, D., Keller, K. & Chadee, K. (2003) Infect. Immun. 71, 838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, Y. & Chadee, K. (1998) J. Immunol. 161, 3746-3752. [PubMed] [Google Scholar]
- 47.Yu, Y. & Chadee, K. (1997) Gastroenterology 112, 1536-1547. [DOI] [PubMed] [Google Scholar]