Abstract
Androgens may regulate the male skeleton either directly by stimulation of the androgen receptor (AR) or indirectly by aromatization of androgens into estrogens and, thereafter, by stimulation of the estrogen receptors (ERs). To directly compare the effect of ER activation on bone in vivo with the effect of AR activation, 9-month-old orchidectomized wild-type and ER-inactivated mice were treated with the nonaromatizable androgen 5α-dihydrotestosterone, 17β-estradiol, or vehicle. Both ERα and AR but not ERβ activation preserved the amount of trabecular bone. ERα activation resulted both in a preserved thickness and number of trabeculae. In contrast, AR activation exclusively preserved the number of trabeculae, whereas the thickness of the trabeculae was unaffected. Furthermore, the effects of 17β-estradiol could not be mediated by the AR, and the effects of 5α-dihydrotestosterone were increased rather than decreased in ER-inactivated mice. ERα, but not AR or ERβ, activation resulted in preserved thickness, volumetric density, and mechanical strength of the cortical bone. ERα activation increased serum levels of insulin-like growth factor I, which were positively correlated with all the cortical and trabecular bone parameters that were specifically preserved by ERα activation but not by AR activation, suggesting that insulin-like growth factor I might mediate these effects of ERα activation. Thus, the in vivo bone-sparing effect of ERα activation is distinct from the bone-sparing effect of AR activation in adult male mice. Because these two pathways are clearly distinct from each other, one may speculate that a combined treatment of selective ER modulators and selective AR modulators might be beneficial in the treatment of osteoporosis.
Sex steroids are important not only for the maintenance of the female skeleton, but also for the male skeleton. The relative contribution of androgens versus estrogens in the regulation of the male skeleton is unclear. Testosterone replacement therapy increases bone mineral density (BMD) in hypogonadal men (1), but several clinical studies indicate that BMD is correlated more to serum levels of estradiol than to serum levels of testosterone in males (2-4). A previous clinical study, which directly compared estrogen versus testosterone effects on bone, showed that estrogens play the dominant role in the regulation of bone resorption markers, whereas both estrogens and testosterone contribute to the maintenance of markers for bone formation (5).
The effects of testosterone can be exerted either directly by means of the androgen receptor (AR) or indirectly by aromatization to estrogens and further by estrogen receptor (ER)α and/or ERβ. All three sex steroid receptors are expressed both in growth-plate cartilage and in bone (6-11). Functional studies using sex steroid receptor-inactivated animal models have demonstrated that ERα but not ERβ is important for the regulation of appendicular longitudinal skeletal growth in male mice (12-14), and a recent report indicates that AR-inactivated male mice have unaffected bone length (15).
Orchidectomy (orx) decreases the amount of trabecular and cortical bone in adult rodents. We and others have shown that the trabecular bone-sparing effect of estrogens is present in orx ERβ-/- but not in ERα-/- or ERα-/-β-/- mice, demonstrating that ERα but not ERβ mediates the trabecular bone-sparing effect of estrogens in male mice (16-18). Interestingly, orx resulted in decreased trabecular volumetric BMD (tvBMD) in ERα-/-β-/- mice, indirectly demonstrating that a testicular factor, probably acting by means of the AR, is also of importance for the maintenance of tvBMD in male mice (16).
It was recently demonstrated in vitro that the ERs and the AR transmit the antiapoptotic signal in osteoblasts with the same efficiency irrespective of whether the ligand is an estrogen or an androgen (19). To directly compare in vivo in males the effect of ER activation on bone with the effect of AR activation, orx WT and ER-inactivated mice were treated with the nonaromatizable androgen 5α-dihydrotestosterone (DHT), 17β-estradiol, or vehicle. We here demonstrate that the in vivo effect of ER activation on bone is clearly distinct from the effect of AR activation in adult male mice.
Materials and Methods
Animals. Male and female double heterozygous (ERα+/-β+/-) mice were mated, resulting in WT, ERα-/-ERβ+/+ (ERα-/-), ERα+/+ERβ-/- (ERβ-/-), and ERα-/-ERβ-/- (ERα-/-β-/-) offspring as described (12). Animals had free access to fresh water and food pellets (B&K Universal AB, Sollentuna, Sweden), consisting of cereal products (76.9% barley, wheat feed, wheat feed, and maize germ), vegetable proteins (14.0% hipro soya), and vegetable oil (0.8% soya oil). At 9 months of age, mice were orchidectomized or sham-operated and treated for 4 weeks with 17β-estradiol (0.05 μg/day) or DHT (45 μg/day), administered by means of s.c. silastic implants (Silclear Tubing, Degania Silicone, Jordan Valley, Israel) in the cervical region (20). Vehicle animals received empty implants. 17β-Estradiol and DHT were obtained from Sigma. The Ethics Committee of Göteborg University approved this study.
Peripheral Quantitative Computerized Tomography (pQCT). CT was performed with the PQCT XCT RESEARCH M (Version 4.5B, Norland, Fort Atkinson, WI) operating at a resolution of 70 μm as described (13). Trabecular BMD was determined ex vivo, with a metaphyseal pQCT scan of the distal femur. The scan was positioned in the metaphysis at a distance from the distal growth plate corresponding to 4% of the total length of the femur, and the trabecular bone region was defined as the inner 45% of the total cross-sectional area. Cortical bone parameters were determined ex vivo with a middiaphyseal pQCT scan of the femur.
MicroCT. MicroCT analysis was done on the distal femur by using a Skyscan 1072 scanner (Skyscan N.V.) and imaged with an x-ray tube voltage of 50 kV with a 1-mm aluminum filter. The scanning angular rotation was 185° and the angular increment was 0.675°. Pixel size was 4.56 μm and magnification was ×60. Reconstructed datasets were segmented into binary images by using adaptive local thresholding (21). Trabecular bone distal of the growth plate was selected for analysis within a conforming volume of interest (cortical bone excluded), commencing at a distance of 100 μm from the growth plate and extending a further longitudinal distance of 2.3 mm in the proximal direction. The number of slices was 500, each with the same thickness as the pixel size, 4.56 μm. Trabecular thickness and separation were calculated by the sphere-fitting local thickness method (22). Trabecular bone thickness measurement was calibrated by using aluminum foils of 20- and 250-μm thickness (Advent Research Materials, Oxford). For both foils the tolerance of the stated thickness was ±10%. Paired foils of each thickness were scanned, and CT images of them were reconstructed and segmented by using exactly the same steps and parameters as applied to all the mouse femurs in this study. Aluminum provides a suitable material for calibration of microCT measurement of bone-structure thickness. The x-ray opacity of aluminum is slightly greater than that of cortical bone. However, aluminum is materially uniform on a micrometer scale, unlike hydroxyapatite preparations at densities similar to cortical bone, which is important for precise calibration of thicknesses of microns to tens of microns.
Dual X-Ray Absorptiometry. Measurement of areal BMD of the middiaphyseal area of femur ex vivo was performed with the Norland pDEXA sabre (Norland) and the sabre research software (v3.6) as described (13).
Mechanical Testing. The tibia was subjected to mechanical testing by using Mechanical Tester 8841 (Instron, Canton, MA). Three-point bending force was measured by placing the bone horizontally with the anterior surface upward and applying a pressing force vertically to the midshaft of the bone. Each bone was compressed with a constant speed of 2 mm/min until failure. The breaking force (maximal load) was defined as the bending load at failure.
Serum Parameters. Serum osteocalcin levels were measured by using a monoclonal antibody raised against human osteocalcin (Rat-MID osteocalcin ELISA, Osteometer Biotech, Herlev, Denmark). The sensitivity of the osteocalcin assay was 21.1 ng/ml and intra- and interassay coefficients of variation (CVs) were <10%. Serum insulin-like growth factor I (IGF-I) levels were measured by double-antibody, IGF-binding protein-blocked RIA (23).
Statistical Analysis. Data are expressed as mean ± SEM. In general, statistical significance (P ≤ 0.05) was determined by using one-way ANOVA followed by Student-Newman-Keul's multiple-range test. Two-way ANOVA, in which ERα-/- and ERβ-/- were regarded as separate treatments, followed by Student-Newman-Keul's multiple-range test, was used for the analyses of the endosteal and periosteal circumferences. Linear regression analyses were performed with serum IGF-I as the independent variable and cortical or trabecular bone parameters as the dependant variable. Pearson's correlation coefficient (r) was calculated. Student's t tests were used to analyze data from the linear-regression analyses, and P < 0.05 was considered significant.
Results
Effect of Orchidectomy in WT Mice. As expected, the weights of the seminal vesicles and the ventral prostate were reduced by orx in the WT mice (Table 1). Furthermore, orx resulted in a reduction of both tvBMD, as measured by pQCT, and bone volume, as measured by microCT in the metaphyseal region of femur; Figs. 1, 2, 3), and cortical [bone mineral content (BMC), cross-sectional area, thickness, and density as measured by a middiaphyseal pQCT scan of the femur; Table 2] bone parameters. These orx-induced structural bone changes were associated with reduced mechanical strength, as measured by using three-point bending of the tibia (Fig. 4) and with increased serum levels of osteocalcin (Fig. 5A).
Table 1. Weight of seminal vesicles and ventral prostate.
| Parameter | Sham | orx + V | orx + E | orx + DHT |
|---|---|---|---|---|
| Seminal vesicle weight, mg | ||||
| WT | 347.4 ± 26.8 | 32.6 ± 6.2†† | 42.7 ± 13.6†† | 379.2 ± 25.1** |
| ERα−/−β−/− | 453.8 ± 33.4 | 134.7 ± 21.2† | 211.3 ± 67.6† | 615.0 ± 71.8** |
| Ventral prostate weight, mg | ||||
| WT | 20.0 ± 2.5 | 4.0 ± 0.7†† | 6.2 ± 2.0†† | 16.6 ± 1.8** |
| ERα−/−β−/− | 18.7 ± 2.2 | 6.4 ± 0.3† | 6.5 ± 0.4† | 28.8 ± 4.3††** |
Nine-month-old male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included. n = 7-8 in the WT groups; n = 4-6 in the ERα−/−β−/− groups. Values are given as means ± SEM. **, P < 0.01 vs. orx + V; ††, P < 0.01; †, P < 0.05 vs. sham; ANOVA followed by Student-Newman-Keul's multiple-range test.
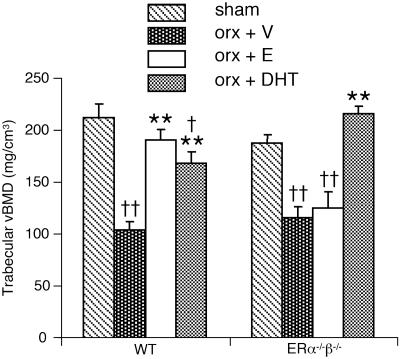
Fig. 1.
tvBMD of the distal metaphyseal area of femur measured by using pQCT. Nine-month-old male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included. n = 7-8 in the WT groups; n = 4-6 in the ERα-/-β-/- groups. Values are given as means ± SEM. **, P < 0.01 vs. orx + V; ††, P < 0.01; †, P < 0.05 vs. sham; ANOVA followed by Student-Newman-Keuls multiple-range test.
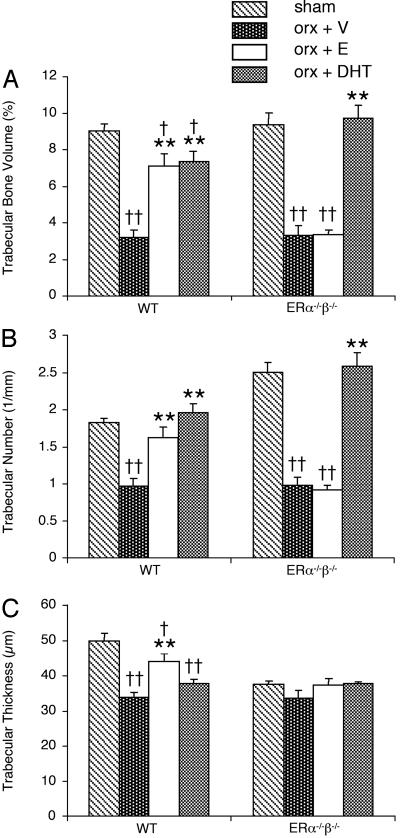
Fig. 2.
Trabecular bone volume (A), trabecular number (B), and trabecular thickness (C) as measured by microCT. Nine-month-old male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included. n = 7-8 in the WT groups; n = 4-6 in the ERα-/-β-/- groups. Values are given as means ± SEM. **, P < 0.01 vs. orx + V; ††, P < 0.01; †, P < 0.05 vs. sham; ANOVA followed by Student-Newman-Keul's multiple-range test.
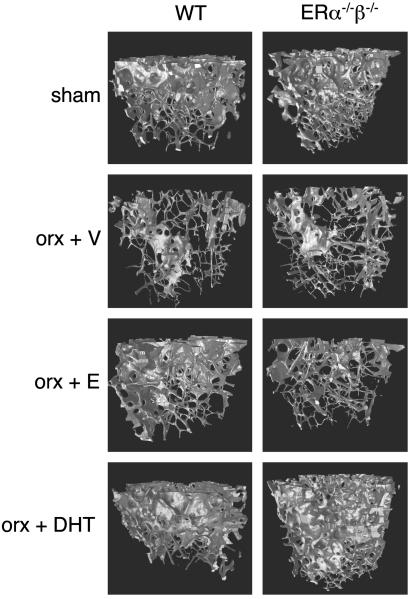
Fig. 3.
Representative microCT scans. Nine-month-old male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included.
Table 2. Cortical parameters.
| Parameter | Sham | orx + V | orx + E | orx + DHT |
|---|---|---|---|---|
| BMC, mg/mm | ||||
| WT | 1.23 ± 0.03 | 1.07 ± 0.03†† | 1.26 ± 0.05** | 1.01 ± 0.03†† |
| ERα−/− | 1.04 ± 0.04 | 1.00 ± 0.02 | 0.92 ± 0.04 | 0.97 ± 0.03 |
| ERα−/−β−/− | 1.03 ± 0.05 | 0.98 ± 0.08 | 0.98 ± 0.05 | 0.99 ± 0.02 |
| Density, mg/mm3 | ||||
| WT | 1.168 ± 0.006 | 1.128 ± 0.010†† | 1.184 ± 0.007** | 1.111 ± 0.009†† |
| ERα−/− | 1.135 ± 0.010 | 1.134 ± 0.006 | 1.116 ± 0.016 | 1.134 ± 0.010 |
| ERα−/−β−/− | 1.130 ± 0.011 | 1.108 ± 0.020 | 1.100 ± 0.013 | 1.126 ± 0.009 |
| Cross-sectional area, mm2 | ||||
| WT | 1.06 ± 0.02 | 0.94 ± 0.02† | 1.06 ± 0.04** | 0.91 ± 0.02†† |
| ERα−/− | 0.91 ± 0.03 | 0.89 ± 0.01 | 0.82 ± 0.03 | 0.85 ± 0.02 |
| ERα−/−β−/− | 0.91 ± 0.04 | 0.88 ± 0.06 | 0.89 ± 0.03 | 0.88 ± 0.02 |
| Thickness, μm | ||||
| WT | 200 ± 4 | 177 ± 3†† | 200 ± 4** | 171 ± 4†† |
| ERα−/− | 183 ± 5 | 183 ± 2 | 175 ± 5 | 177 ± 5 |
| ERα−/−β−/− | 188 ± 5 | 175 ± 10 | 176 ± 4 | 183 ± 2 |
Cortical bone parameters as determined by using a middiaphyseal pQCT scan of the femur. Nine-month-old WT, ERα−/−, and ERα−/−β−/− male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included. n = 7-8 in the WT and ERα−/− groups; n = 4-6 in the ERα−/−β−/− groups. Values are given as means ± SEM. **, P < 0.01 vs. orx + V; ††, P < 0.01; †, P < 0.05 vs. sham; ANOVA followed by Student-Newman-Keul's multiple-range test.
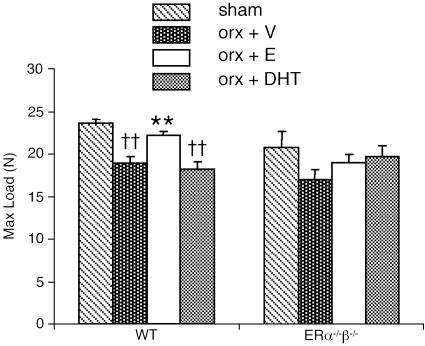
Fig. 4.
Maximal load (N), a measurement of the strength of the bone, as measured by using three-point bending of the tibia. Nine-month-old male mice were orchidectomized and then treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were also included. n = 7-8 in the WT groups; n = 4-6 in the ERα-/-β-/- groups. Values are given as means ± SEM. **, P < 0.01 vs. orx + V; ††, P < 0.01 vs. sham; ANOVA followed by Student-Newman-Keul's multiple-range test.
Fig. 5.
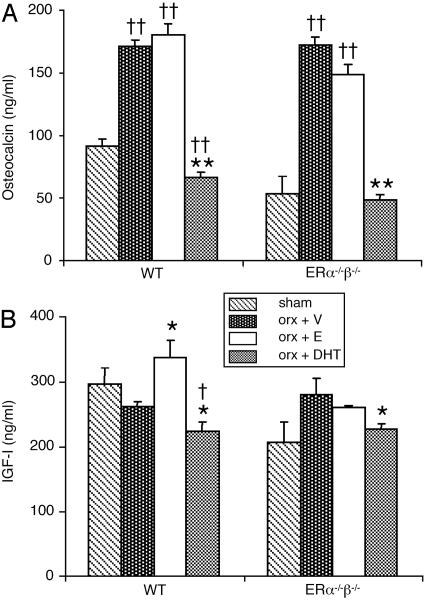
Serum osteocalcin as measured by ELISA (A) and IGF-I as measured by RIA (B). Nine-month-old male mice were orchidectomized and treated with vehicle (V), 17β-estradiol (E), or DHT for 4 weeks. Sham-operated animals were included. n = 7-8 in the WT groups; n = 4-6 in the ERα-/-β-/- groups. Values are given as means ± SEM. **, P < 0.01; *, P < 0.05 vs. orx + V; ††, P < 0.01; †, P < 0.05 vs. sham; ANOVA followed by Student-Newman-Keul's multiple-range test.
Effect of 17β-Estradiol and DHT in Orchidectomized WT Mice. Seminal vesicles and the ventral prostate. The DHT dose used (45 μg/day) was physiological because it completely prevented the orx-induced loss of weight of the seminal vesicles and the ventral prostate in WT mice (Table 1). As expected, 17β-estradiol did not prevent the orx-induced weight loss of the seminal vesicles or the ventral prostate (Table 1).
Trabecular bone. The capacity of 17β-estradiol and DHT to preserve the trabecular and cortical bone was then compared. The tvBMD was first measured in the metaphyseal region of the distal femur by using pQCT. Both 17β-estradiol and DHT prevented orx-induced reduction in tvBMD in WT mice (Fig. 1). To investigate whether 17β-estradiol and DHT prevent the orx-induced loss of trabecular bone by similar alterations in the trabecular bone microarchitecture, 3D microCT analyses of the metaphyseal region of distal femur were performed. Treatment with 17β-estradiol prevented orx-induced reduction in the trabecular bone volume as a result of both preserved number and preserved thickness of trabeculae in WT mice (Figs. 2 and 3). DHT treatment also preserved the trabecular bone volume but, in contrast to 17β-estradiol treatment, it was only caused by preserved trabecular number, whereas the trabecular thickness was unaffected by DHT treatment (Figs. 2 and 3). Thus, even though both 17β-estradiol, by activation of ERs, and DHT, by activation of the AR, exert trabecular bone-sparing effects, it is clear that the underlying mechanisms of action differ as the trabecular thickness is increased by 17β-estradiol but not by DHT.
Cortical bone. Cortical bone parameters were first measured by a middiaphyseal pQCT scan of the femur. Cortical BMC was preserved by 17β-estradiol but not by DHT in orx WT mice (Table 2). The stimulatory effect of 17β-estradiol was due both to an increased cortical volumetric BMD and to an increased cortical cross-sectional area, the latter as a result of an effect on cortical thickness in orx mice (Table 2). DHT did not have any effect on cortical bone parameters in these 10-month-old orx mice (Table 2). The effects on cortical bone were also analyzed by a middiaphyseal dual x-ray absorptiometry scan of the femur, confirming the pQCT findings that 17β-estradiol but not DHT preserved the amount of cortical bone in orx WT mice (cortical areal BMD: ovx plus 17β-estradiol, +12.1 ± 2.7% over vehicle, P < 0.05; orx plus DHT, +1.1 ± 5.2% over vehicle, not significant; ANOVA, followed by Student-Newman-Keul's multiple-range test).
Mechanical strength. Mechanical strength, as measured by using three-point bending of the tibia and given as maximal load, was preserved by 17β-estradiol, but not by DHT, in orx WT mice (Fig. 4). The qualitative bone parameter elastic modulus was not affected by 17β-estradiol or DHT treatment in either genotype (data not shown).
Serum osteocalcin and IGF-I. DHT, but not 17β-estradiol, prevented the increase in osteocalcin that followed orchidectomy (Fig. 5A). 17β-Estradiol increased, whereas DHT slightly decreased, serum levels of IGF-I in orx WT mice (Fig. 5B). Furthermore, serum IGF-I levels were positively correlated with all the cortical (BMC, cortical volumetric BMD, cross-sectional area, thickness, maximal load) and trabecular (trabecular thickness) bone parameters that were specifically preserved by 17β-estradiol but not by DHT (Table 3).
Table 3. Correlation with serum IGF-I and different bone parameters.
| Compartment | Measurement | r |
|---|---|---|
| Trabecular | Volumetric BMD | 0.06 |
| Trabecular thickness | 0.40** | |
| Trabecular number | −0.22 | |
| Cortical | BMC | 0.59*** |
| Volumetric BMD | 0.53*** | |
| Cross-sectional area | 0.58*** | |
| Thickness | 0.45*** | |
| Maximal load | 0.40** | |
| Elastic modulus | −0.1 |
Correlations of all animals (n = 50) included in the study were calculated by using Pearson's correlation coefficient (r). **, P < 0.01; ***, P < 0.001.
Effect of 17β-Estradiol and DHT in Orchidectomized ERα-/-β-/- Mice. Orx resulted in reduced trabecular bone parameters, whereas the cortical bone parameters were mainly unaffected by orx in ERα-/-β-/- mice (Figs. 1, 2, 3 and Table 2).
Effect of 17β-estradiol. The effect of 17β-estradiol on tvBMD is mediated by ERα as no effect was seen in ERα-/-β-/- or ERα-/- mice (Fig. 1, data not shown, and ref. 16). Furthermore, no effect of 17β-estradiol was seen on any other trabecular or cortical bone parameter in orx ERα-/-β-/- mice (Figs. 2, 3, 4 and Table 2). Statistical analyses performed independently on results from ERα+/+ and ERα-/- mice, respectively, showed that the ERα-mediated increase in cortical thickness of femur in orx mice was caused by a decrease in the endosteal circumference (-4.9 ± 1.6%; P < 0.05 two-way ANOVA, followed by Student-Newman-Keul's multiple-range test), whereas the periosteal circumference was not affected. Serum levels of osteocalcin and IGF-I were also not altered by 17β-estradiol in ERα-/-β-/- mice (Fig. 5).
Effect of DHT. All the DHT effects seen in orx WT mice were also present in orx ERα-/-β-/- mice. Comparison of the effect of DHT in WT versus ERα-/-β-/- mice demonstrated that DHT produced a greater effect in ERα-/-β-/- mice than in WT mice for the weight of the ventral prostate, the trabecular bone volume, the tvBMD, and the trabecular number (P < 0.05, Student's t test; Table 1 and Figs. 1 and 2). A similar but not significant tendency was also seen for the weight of seminal vesicles (P = 0.073; Table 1).
Discussion
The role of estrogens in the regulation of the adult skeleton in females is well established, whereas the relative importance of estrogens versus androgens in the regulation of bone metabolism in adult males remains unclear. We and others have demonstrated that orx-induced trabecular bone loss can be prevented by testosterone, DHT, and 17β-estradiol in adult rodents (16, 17, 20, 24-26). The present study, by using both pQCT and microCT, confirms our previous reports that ERα but not ERβ is of importance for the trabecular bone-sparing effect of estrogens in male mice (16, 17). Furthermore, the trabecular bone-sparing effect of estradiol is only mediated by ERα and not by the AR. In addition, we directly show that the AR-activator DHT increased the amount of trabecular bone independent of ERs. These findings, together with recently published data by Sims et al. (18), clearly demonstrate that in contrast to what has been concluded from in vitro studies (19, 26), no functional cross-reactivity exists between ERs and AR in vivo.
pQCT analyses of tvBMD demonstrated that the trabecular bone-sparing effect of AR activation and ERα activation was of the same magnitude. However, more detailed analysis of the trabecular bone microarchitecture, with high-resolution microCT, demonstrated that ER but not AR activation preserved the trabecular thickness, whereas AR activation only preserved the number of trabeculae (Fig. 6). These findings show that AR activation and ER activation regulate the amount of trabecular bone by different mechanisms of action. This notion is further supported by the different results of 17β-estradiol and DHT treatment for 4 weeks on serum levels of osteocalcin. One possible explanation for this is that the estrogen effect may be mediated not only by decreased bone turnover, but also by increased bone formation, whereas DHT treatment only decreases the bone turnover. In contrast to ovariectomized mice, limited studies have been done on the effect of estradiol on serum levels of osteocalcin and bone turnover in orchidectomized mice. In this regard, we have found that estradiol treatment of orchidectomized mice did not reduce serum levels of osteocalcin (16, 17). Our present findings on serum osteocalcin levels are consistent with our previous data. Moreover, we have found no evidence for reduced bone formation in 17β-estradiol-treated orchidectomized mice as studied by histomorphometric analyses (17). Thus, neither serum levels of osteocalcin nor bone formation, parameters measured by dynamic histomorphometry, were reduced by estradiol treatment in orchidectomized mice. One limitation of the present study is that no marker of bone resorption was measured.
Fig. 6.
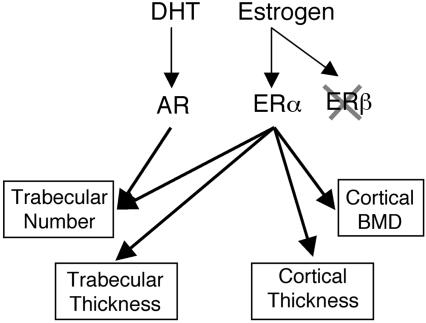
Differential effects on bone of ERα and AR activation in adult male mice. Activation of ERα preserves the trabecular number, trabecular thickness, cortical thickness, and cortical density, whereas activation of AR preserves only the trabecular number after orx.
An interesting difference between the effect of ER activation and AR activation was also seen on the cortical bone parameters. 17β-Estradiol preserved the amount of cortical bone (cortical cross-sectional area and thickness), the density of the cortical bone, and the mechanical strength of the cortical bone. In contrast, no effect by DHT treatment was seen on any of these cortical bone parameters. The ERα-induced increase in cortical thickness compared with orx mice was caused by a reduced endosteal circumference, whereas the periosteal circumference was unaffected. We and others have recently demonstrated that endocrine, liver-derived IGF-I is an important regulator of the amount of cortical bone and the mechanical strength of cortical bone (27, 28). In the present study, ER activation, but not AR activation, resulted in increased serum levels of IGF-I, which in turn were positively correlated with several cortical bone parameters, suggesting that the effect of 17β-estradiol on these cortical bone parameters might be mediated by an induction of the amount of endocrine IGF-I. Thus, we propose that ER activation, but not AR activation, results in increased serum levels of IGF-I, which in turn increase the amount of cortical bone in orx mice.
The tvBMD and the trabecular bone volume were not correlated with serum IGF-I. However, microCT analyses showed that the thickness of the trabeculae, but not the number of trabeculae, was correlated with serum IGF-I; and because it was the thickness of the trabeculae that was specifically increased by 17β-estradiol, but not by DHT, one may speculate that 17β-estradiol increases the trabecular thickness by an induction of serum IGF-I levels.
The above-mentioned results of sex steroid receptor specificity in bone, derived from the experiment by using replacement therapy with 17β-estradiol or DHT to orx mice, are supported by our observations of the effect of orx in ER double-inactivated mice. Orx of these mice resulted in reduced tvBMD but unaffected cortical bone parameters, indicating that the trabecular bone is, at least in part, maintained by activation of the AR in gonadally intact, ER double-inactivated male mice, whereas AR activation in these mice does not influence cortical bone.
For some androgen-regulated parameters the effect of DHT was greater in ERα-/-β-/- mice than in WT mice, which may be because of increased AR expression in ERα-/-β-/- mice. AR expression was not analyzed in the present study. Sims et al. (18), however, have shown that AR expression was increased in ERα-inactivated mice.
In conclusion, ER activation results in preserved thickness and number of trabeculae and preserved thickness and volumetric density of cortical bone; these effects of 17β-estradiol cannot be mediated by means of the AR (Fig. 6). In contrast, AR activation results in a specific preservation of the number of trabeculae, whereas the thickness of the trabeculae and the cortical bone parameters are unaffected (Fig. 6). 17β-Estradiol increased serum levels of IGF-I, which were positively correlated with all the cortical and trabecular bone parameters that were specifically preserved by 17β-estradiol but not by DHT, suggesting that IGF-I might be involved in the mechanism behind these effects of 17β-estradiol. Thus, the bone-sparing effect of ER activation in vivo is clearly distinct from the effect of AR activation in adult gonadectomized male mice (Fig. 6). Because these two pathways are clearly distinct from each other, one may speculate that a combined treatment of selective ER modulators and selective AR modulators might be beneficial in the treatment of osteoporosis.
Acknowledgments
We thank Anette Hansevi and Maud Petersson for excellent technical assistance. We also thank the SWEGENE Center for Bio-Imaging (CBI) and Göteborg University for technical support regarding image analysis. This study was supported by the Swedish Medical Research Council, the Swedish Foundation for Strategic Research, European Commission Grant QLK4-CT-2002-02528, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, Petrus and Augusta Hedlunds Foundation, the Swedish Cancer Fund, Karo Bio AB, and Katholieke Universiteit Leuven Grant OT/01/39.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ER, estrogen receptor; AR, androgen receptor; BMD, bone mineral density; tvBMD, trabecular volumetric BMD; BMC, bone mineral content; orx, orchidectomy; DHT, 5α-dihydrotestosterone; IGF-I, insulin-like growth factor I; CT, computerized tomography; pQCT, peripheral quantitative computerized tomography.
References
- 1.Katznelson, L., Finkelstein, J. S., Schoenfeld, D. A., Rosenthal, D. I., Anderson, E. J. & Klibanski, A. (1996) J. Clin. Endocrinol. Metab. 81, 4358-4365. [DOI] [PubMed] [Google Scholar]
- 2.Slemenda, C. W., Longcope, C., Zhou, L., Hui, S. L., Peacock, M. & Johnston, C. C. (1997) J. Clin. Invest. 100, 1755-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillberg, P., Johansson, A. G. & Ljunghall, S. (1999) Calcif. Tissue Int. 64, 209-213. [DOI] [PubMed] [Google Scholar]
- 4.Ongphiphadhanakul, B., Rajatanavin, R., Chanprasertyothin, S., Piaseu, N. & Chailurkit, L. (1998) Clin. Endocrinol. (Oxford) 49, 803-809. [DOI] [PubMed] [Google Scholar]
- 5.Falahati-Nini, A., Riggs, B. L., Atkinson, E. J., O'Fallon, W. M., Eastell, R. & Khosla, S. (2000) J. Clin. Invest. 106, 1553-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal, O., Kindblom, L. G. & Ohlsson, C. (1999) J. Bone Miner. Res. 14, 923-929. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson, L. O., Chrysis, D., Pajulo, O., Boman, A., Holst, M., Rubinstein, J., Ritzen, E. M. & Savendahl, L. (1999) J. Clin. Endocrinol. Metab. 84, 370-373. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson, O., Chrysis, D., Pajulo, O., Boman, A., Holst, M., Rubinstein, J., Martin, E., Ritzen, E. & Sävendahl, L. (2003) J. Endocrinol. 177, 319-326. [DOI] [PubMed] [Google Scholar]
- 9.Wiren, K. M., Chapman Evans, A. & Zhang, X. W. (2002) J. Endocrinol. 175, 683-694. [DOI] [PubMed] [Google Scholar]
- 10.Onoe, Y., Miyaura, C., Ohta, H., Nozawa, S. & Suda, T. (1997) Endocrinology 138, 4509-4512. [DOI] [PubMed] [Google Scholar]
- 11.Arts, J., Kuiper, G. G., Janssen, J. M., Gustafsson, J.-Å., Lowik, C. W., Pols, H. A. & van Leeuwen, J. P. (1997) Endocrinology 138, 5067-5070. [DOI] [PubMed] [Google Scholar]
- 12.Vidal, O., Lindberg, M. K., Hollberg, K., Baylink, D. J., Andersson, G., Lubahn, D. B., Mohan, S., Gustafsson, J.-Å. & Ohlsson, C. (2000) Proc. Natl. Acad. Sci. USA 97, 5474-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windahl, S. H., Vidal, O., Andersson, G., Gustafsson, J.-Å. & Ohlsson, C. (1999) J. Clin. Invest. 104, 895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims, N. A., Dupont, S., Krust, A., Clement-Lacroix, P., Minet, D., Resche-Rigon, M., Gaillard-Kelly, M. & Baron, R. (2002) Bone (NY) 30, 18-25. [DOI] [PubMed] [Google Scholar]
- 15.Kawano, H., Sato, T., Yamada, T., Matsumoto, T., Sekine, K., Watanabe, T., Nakamura, T., Fukuda, T., Yoshimura, K., Yoshizawa, T., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 9416-9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg, M. K., Moverare, S., Skrtic, S., Alatalo, S., Halleen, J., Mohan, S., Gustafsson, J.-Å. & Ohlsson, C. (2002) J. Bone Miner. Res. 17, 555-562. [DOI] [PubMed] [Google Scholar]
- 17.Vandenput, L., Ederveen, A. G., Erben, R. G., Stahr, K., Swinnen, J. V., Van Herck, E., Verstuyf, A., Boonen, S., Bouillon, R. & Vanderschueren, D. (2001) Biochem. Biophys. Res. Commun. 285, 70-76. [DOI] [PubMed] [Google Scholar]
- 18.Sims, N. A., Clement-Lacroix, P., Minet, D., Fraslon-Vanhulle, C., Gaillard-Kelly, M., Resche-Rigon, M. & Baron, R. (2003) J. Clin. Invest. 111, 1319-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kousteni, S., Bellido, T., Plotkin, L. I., O'Brien, C. A., Bodenner, D. L., Han, L., Han, K., DiGregorio, G. B., Katzenellenbogen, J. A., Katzenellenbogen, B. S., et al. (2001) Cell 104, 719-730. [PubMed] [Google Scholar]
- 20.Vandenput, L., Boonen, S., Van Herck, E., Swinnen, J. V., Bouillon, R. & Vanderschueren, D. (2002) J. Bone Miner. Res. 17, 2080-2086. [DOI] [PubMed] [Google Scholar]
- 21.Canny, J. (1986) IEEE Trans. Pattern Anal. Machine Intelligence 185, 679-698. [PubMed] [Google Scholar]
- 22.Hildebrand, T. & Ruegsegger, P. (1997) J. Microsc. 185, 67-75. [Google Scholar]
- 23.Blum, W. F. & Breier, B. H. (1994) Growth Regul. 4, Suppl. 1, 11-19. [PubMed] [Google Scholar]
- 24.Wakley, G. K., Schutte, H. D., Jr., Hannon, K. S. & Turner, R. T. (1991) J. Bone Miner. Res. 6, 325-330. [DOI] [PubMed] [Google Scholar]
- 25.Vanderschueren, D., Van Herck, E., Suiker, A. M., Visser, W. J., Schot, L. P. & Bouillon, R. (1992) Endocrinology 130, 2906-2916. [DOI] [PubMed] [Google Scholar]
- 26.Kousteni, S., Chen, J. R., Bellido, T., Han, L., Ali, A. A., O'Brien, C. A., Plotkin, L., Fu, Q., Mancino, A. T., Wen, Y., et al. (2002) Science 298, 843-846. [DOI] [PubMed] [Google Scholar]
- 27.Sjogren, K., Sheng, M., Moverare, S., Liu, J. L., Wallenius, K., Tornell, J., Isaksson, O., Jansson, J. O., Mohan, S. & Ohlsson, C. (2002) J. Bone Miner. Res. 17, 1977-1987. [DOI] [PubMed] [Google Scholar]
- 28.Yakar, S., Rosen, C. J., Beamer, W. G., Ackert-Bicknell, C. L., Wu, Y., Liu, J. L., Ooi, G. T., Setser, J., Frystyk, J., Boisclair, Y. R. & LeRoith, D. (2002) J. Clin. Invest. 110, 771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]