Abstract
The primate somatosensory system provides an excellent model system with which to investigate adult neural plasticity. Here, we report immunohistochemical staining data for the GluR1 and GluR2/3 AMPA receptors subunits in somatosensory area 3b 1 week after median nerve compression in adult squirrel monkeys. We find transcortical increases in the staining intensity of GluR1 AMPAR subunits and transcortical decreases in GluR2/3 AMPAR subunits. This pattern of change in the staining intensity of these subunits differs from the changes one would expect if the deprived cortical neurons were undergoing homeostatic synaptic scaling, or from ones that would follow N-methyl-d-aspartate (NMDA) receptor-mediated long-term potentiation. Indeed, this pattern of change appears to recapitulate proportions that exist early in development as if the deprived cortex has reverted to an immature state. We suggest that this state represents yet another stage of peripheral nerve injury-induced reorganization in adult primate somatosensory cortex, and may well be essential for subsequent NMDA receptor-mediated plasticity.
Keywords: sensory plasticity, AMPA, GluR1, GluR2/3, reorganization
Introduction
The fact that the adult brain retains a remarkable degree of flexibility to respond to alterations in the pattern of inputs it receives is beyond dispute. While there were scattered earlier reports of adult plasticity (e.g., Brown and Salinger, 1975; Creutzfeldt and Heggelund, 1975), they were largely ignored by the scientific community until the seminal publications of Merzenich, Kaas and colleagues in the early 1980s (Merzenich et al., 1983a,b). Since then research in the general area of adult neural plasticity has exploded. A number of model systems have been employed in studying neural plasticity in the mature brain, with the primate somatosensory system proving quite fertile (e.g., Churchill et al., 1998, 2001; Florence et al., 1998, 2000; Garraghty and Kaas, 1991; Garraghty et al., 2006; Kaas et al., 1999; Merzenich et al., 1984; Pons et al., 1991; Schroeder et al., 1997), particularly given the likelihood of greater potential for translational relevance (e.g., Courtine et al., 2007).
Following the initial set of more phenomenological reports (Merzenich et al., 1983a,b, 1984), attention shifted to a search for the mechanisms of plasticity (e.g., Allard et al., 1991; Garraghty et al., 1991, 1994; Recanzone et al., 1992; Xerri et al., 1994). The early, pioneering experiments of Merzenich et al. (1983a,b) showed that transection and ligation of the median nerve, which deafferentates the thumb side of the glabrous surface of the hand, was followed by a reorganization of the cortical map in which the deprived cortex regained responsiveness to tactile stimulation of adjacent skin surfaces on the hand with intact innervation. Moreover, this reorganization was shown to proceed in no fewer than two stages, an immediate, unmasking stage in which neurons in deprived cortex expressed new receptive fields directly after the nerve transection, and a more protracted second stage in which neurons throughout the remaining deprived cortex regained responsiveness to tactile stimulation over the ensuing days to weeks (Merzenich et al., 1983b).
The immediate plasticity is assumed to reflect the unmasking of tonically suppressed inputs due to a relaxation in afferent-driven inhibition, and we have reported reductions in GABAA receptor binding in layer IV of deprived area 3b cortex within hours of nerve transection (Garraghty et al., 2006; Wellman et al., 2002), a finding that is consistent with a down-regulation of inhibition. We have shown that the latter stage of reorganization is prevented if N-methyl-D-aspartate (NMDA) glutamatergic receptors are blocked (Garraghty and Muja, 1996). The unmasking phase of reorganization, on the other hand, occurs whether NMDA receptors are blocked or not (Myers et al., 2000). These latter observations have prompted us to draw parallels between this nerve injury-induced model of neural plasticity and NMDA receptor-dependent long-term potentiation (LTP) in the hippocampus (Garraghty and Muja, 1996; Garraghty et al., 2006; Myers et al., 2000). Consistent with this idea, we have shown that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors are up-regulated 1–2 months after median nerve transection (Garraghty et al., 2006), which parallels reports involving hippocampal LTP (e.g., Maren et al., 1993; Tocco et al., 1992).
Our earlier report involved the use of receptor autoradiography, and consequently, could provide no information regarding possible changes in the subunit composition of the receptors being quantified. In the present experiments, we have quantified the immunohistochemical staining of GluR1 and GluR2/3 AMPA receptor subunits in deprived cortex 1 week following median nerve compression to determine whether early changes in AMPA receptor subunit expression contribute to the second phase of reorganization.
Materials and Methods
Nerve compression
We report data from three adult squirrel monkeys (Saimiri sciureus). Animals were maintained on isoflurane anesthesia 2–4% throughout the surgery. The arm was shaved between the wrist and elbow on the ventral surface of the forearm. An initial incision was made along the midline of the ventral forearm around 70 mm from the wrist. Under microscopic view, the median nerve was located and isolated by blunt dissection and elevated. Hemostats were then applied to the nerve at approximately a 45° angle and held in place for 30 s. This was repeated three times distally over the span of a few mm. The hemostat was then rotated 90°, and applied four times to create a crossing pattern to ensure complete compression of the nerve. The nerve was then repositioned, the skin was sutured over the incision site, and the monkey was allowed to recover. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC).
Immunohistochemistry
One week after nerve compression, animals were anesthetized with isoflurane gas and immediately transcardially perfused with cold 0.9% saline solution followed by 400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Following perfusion the brains were extracted and the left and right somatosensory cortices were dissected out and post-fixed for 2 h in cold fixative (4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Tissue was then cryoprotected overnight in 30% sucrose in phosphate buffer (pH 7.4).
Frozen 40 μm sections of the pre and post-central gyri were cut in a parasagittal plane and collected in staining nets in phosphate buffer. Sections that were clearly within the central sulcus were kept for immunohistochemistry. After sectioning, tissue was washed in immuno-phosphate buffer and then incubated in blocking solution for 30 min and then hydrogen peroxide 0.01% for 15 min at room temperature to reduce endogenous peroxidase activity. Tissue sections were then incubated overnight at 4°C in antisera containing either rabbit phosphor-GluR1 (1:1000 Chemicon) or rabbit GluR2/3 (1:1000 Affinity Bio Reagents), and then washed three times for 10 min in IPB before being incubated in goat anti-rabbit biotinylated antibodies for 1 h at room temperature. Sections were again washed three times for 10 min in PBS, then incubated in ABC solution for 1 h (ABC Elite Kit, Vector), washed again three times for 10 min in acetate–imidazole buffer, and then incubated in acetate–imidazole buffer containing nickel sulfate, 0.5 mg/ml 3,3′-diaminobenzidine-4HCI (DAB, Vector) and 0.01% H2O2 for 8 min. Sections were then washed three times in PBS for 10 min, mounted on gelatin coated glass slides and dried overnight. Once dry, the sections were dehydrated in ascending ethanols, cleared with xylenes, and then cover-slipped. The antibodies used in this experiment bind to specific sites on the GluR1 (Ser831) and GluR2/GluR3 (C terminal) subunits and no cross-reactivity has been reported. Positive and negative controls were generated by omitting the primary or secondary antibody. These sections did not reveal the presence of any binding.
Quantification of receptor subunit staining intensity
Immunohistochemical quantification of tissue sections were carried out at 1480× under bright-field illumination using a microscope (Nikon Eclipse 80i; Nikon Instruments; Melville, NY, USA) and the Stereo Investigator (MBF Bioscience; Williston, VT, USA) software. A tracing grid was constructed with Nissl-stained tissue that consisted of an approximation of the cortical layers I through VI. The 1-mm wide tracing grid was aligned with the median nerve area 3b, 1 mm lateral to the central sulcus spanning across cortical regions corresponding to glabrous median nerve regions of digits 1–3 (Garraghty et al., 2006; Sur et al., 1982). Once the tracing grid was placed, the user moved throughout the predefined cortical layers (II–VI) tracing the somata of user defined neurons. Aside from the tracing grid, layers were generally discernable to the trained observer based on changes in neural phenotype.
Tissue was originally sliced at 40 μm, but following staining procedures and cover-slipping had a final tissue thickness of around 25–30 μm. Per layer, the microscope was focused down through the z-axis to depth of around 15 μm where uniformly stained neurons within the plane of focus were randomly selected and traced (∼30). Additionally, within each layer, neuropil measurements were taken (∼10). These were operationally defined as regions of tissue in which z-axis scrolling from the top to the bottom of the tissue revealed no somata.
Finally white matter measurements were collected (below layer VI ∼5), that consisted of regions in which no antibody binding had occurred. Four to five sections were measured per antibody per hemisphere per animal generating around 120–150 soma measurements and 40–50 neuropil measurements per hemisphere per antibody.
The Stereo Investigator software's luminance function was used to quantify staining intensity of the traced contours (representative neurons, neuropil, and white matter). The luminance function measures the amount of light passing through the tissue per square μm of contour (0 = black and 256 = white), and is used as an indirect measurement of subunit expression. Previous studies by our colleagues using immunohistochemical procedures and similar bright-field densitometry quantification methods show that densitometry/luminance quantification of immunohistochemically labeled tissue is a reliable measure of protein expression (Osborne et al., 2007; Wilber et al., 2007, 2009). Staining intensity data were generated by taking a ratio of the average staining intensity per layer to the average staining intensity of the white matter. Great effort was made to maintain light parameters within the Stereo Investigator software between users and user sessions.
Results
GluR1 and GluR2/3 staining intensity: control measurements
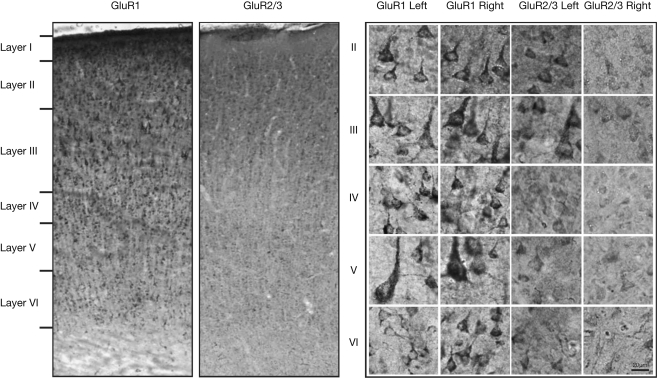
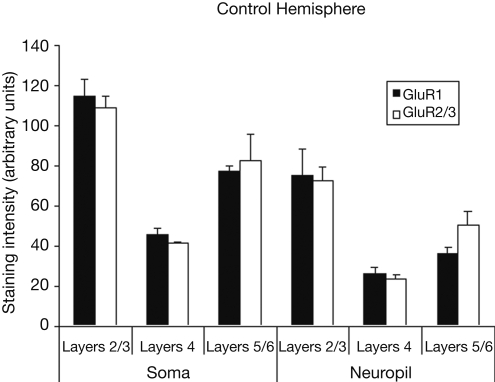
Figure 1 presents photomicrographs of neurons expressing GluR1 and GluR2/3 subunits from layers II–VI of intact and control hemispheres. The left side of the figure shows immunolabeling for both subunits, while the right side of the figure shows higher powered images of labeled neurons within deprived and control hemispheres. This figure demonstrates the comparative increases in GluR1 staining intensity and comparative decreases in GluR2/3 staining intensity in deprived hemisphere (right hemisphere). Figure 2 presents the averages of raw intensity measurements for GluR1 and GluR2/3 AMPA receptor subunits for soma and neuropil in control area 3b median nerve cortex as a function of cortical layer. Statistically, we find that the distributions of GluR1 and GluR2/3 AMPA receptor subunit staining intensities are comparable across the layers of cortex for both cellular and neuropil measurements; and that is there is no main effect for receptor subunit [F (1,24) = 0.023, p > 0.85]. There is, however, a significant difference in the expression of both GluR1 and GluR2/3 receptor subunit staining intensities between the somatic and neuropil measurements across all layers [F (1,24) = 80.08, p < 0.001]. This finding becomes more apparent when averaging somatic staining intensities for GluR1 (47.2 ± 2.28) and GluR2/3 (45.8 ± 1.63) receptor subunits, compared to neuropil measurements which are 27.5 ± 3.45 and 28.2 ± 2.15 for the GluR1 and GluR2/3 subunits respectively. The data as presented in Figure 2 also underscore our finding of equivalent staining intensities of these receptor subunits throughout the median nerve cortex. Finally, we find a significant difference in the staining intensities of GluR1 and GluR2/3 receptor subunits across different layers of the sensory cortex [F (2,24) = 20.93, p < 0.001]. Post-hoc analysis (Scheffé) reveals that there is greater expression of both subunits in layers 2/3 than in either layer 4 or layers 5/6 (both p's < 0.001), whereas the expression does not differ between layer 4 and layers 5/6 (p > 0.35).
Figure 1.
Left: Photomicrographs of GluR1 and GluR2/3 immunostaining in coronal sections through area 3b of a control hemisphere. The laminar pattern of GluR1 and GluR2/3 receptor subunit surface expression maps onto those reported in macaques. Right: Higher-power images of neurons stained with GluR1 and GluR2/3 in the deprived and control hemisphere. Left cortex is control and right cortex is deprived.
Figure 2.
Average staining intensity for GluR1 and GluR2/3 AMPA receptor subunits in the median nerve area of the control hemispheres. Data are grouped by layer(s) (2/3, 4, and 5/6), and by whether the intensity measurements were taken from cell bodies or from neuropil. Staining intensities for GluR1 and GluR2/3 are higher for measurements taken from cell bodies than for measurements taken from neuropil. For both GluR1 and GluR2/3 staining intensities are highest in upper layers of cortex, with weaker staining in layer 4, and more moderate staining throughout layer 5/6.
Effects of deprivation: GluR1 staining intensity
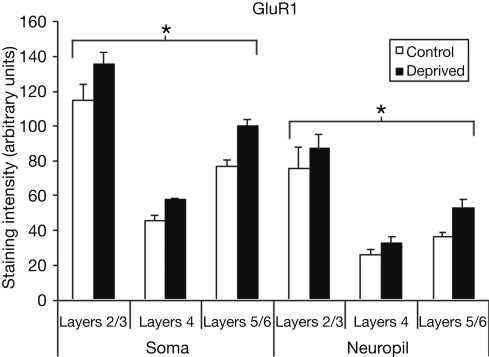
Figure 3 compares the staining intensity means for GluR1 subunits between control and deprived hemispheres. We find that deprivation results in an increase in GluR1 staining intensity in the deprived hemisphere relative to control, with this effect being relatively stable across cortical layers and somatic versus neuropil measures [F (1,12) = 159.65, p < 0.001]. Because this deprivation-induced increase in GluR1 staining intensity is so symmetrically distributed, we also find a significant main effect for layer [F (2,12) = 22.14, p < 0.001], with post-hoc comparisons again showing staining intensity to be higher in layers 2/3 than in layer 4 or layers 5/6 (Scheffé, both p's < 0.05), with no difference between layer 4 and layers 5/6 (p > 0.05). Finally, the difference between somatic and neuropil staining intensities is again present [F (1,12) = 46.51, p < 0.001].
Figure 3.
Comparison of average staining intensities for GluR1 in deprived and control hemispheres. Conventions are the same as in Figure 2. GluR1 staining intensities are higher in the deprived hemisphere in all layers for both cell body and neuropil measurements (*p < 0.001).
Effects of deprivation: GluR2/3 staining intensity
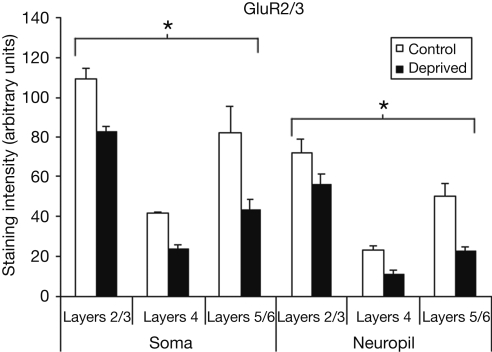
Figure 4 compares the staining intensity means for GluR2/3 subunits between control and deprived hemispheres. For this subunit, we find that deprivation results in a decrease in staining intensity, and that this decrease is relatively stable across cortical layers and somatic versus neuropil measures [F (1,12) = 93.15, p < 0.001]. As we did for the GluR1 subunit, we also find a significant main effect for layer [F (2,12) = 22.14, p < 0.001], with post-hoc comparisons again showing staining intensity to be higher in layers 2/3 than in layer 4 or layers 5/6 (Scheffé, both p's < 0.001), with no difference between layer 4 and layers 5/6 (p > 0.05). Finally, the difference between somatic and neuropil staining intensities is again present [F (1,12) = 78.00, p < 0.001].
Figure 4.
Comparison of average staining intensities for GluR2/3 in deprived and control hemispheres. Conventions are the same as in Figure 2. GluR2/3 staining intensities are lower in the deprived hemisphere in all layers for both cell body and neuropil measurements (*p < 0.001).
Effects of deprivation on AMPA receptors as indexed by the summed staining intensities of GluR1 and GluR2/3 staining
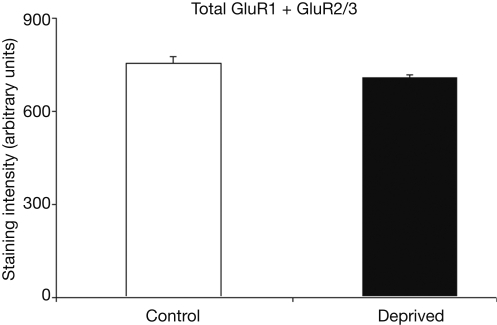
Figure 5 compares the average summed staining for GluR1 and GluR2/3 subunits in the deprived and intact hemispheres. The 7% difference between the deprived and non-deprived measurements is not statistically significant (t = 2.6, p > 0.10). Thus, to the extent that these measures can be used as an estimate of AMPAR expression, these results could suggest that AMPAR trafficking is geared towards changes in functionality through subunit composition as opposed to general scaling 1 week following median nerve compression.
Figure 5.
Average total staining intensities for GluR1 and GluR2/3 in control and deprived hemispheres are comparable. This Figure illustrates that while there are subunit differences in the deprived hemisphere, the summation of these measures indicates a relatively stable total level of combined GluR1 and GluR2/3 expression throughout control and deprived cortex.
Discussion
We report several findings based on quantitative immunohistochemical staining data for AMPA receptor subunits GluR1 and GluR2/3 in area 3b of squirrel monkey somatosensory cortex. (1) In intact area 3b, the staining intensities of these subunits are nearly identical. (2) In control area 3b, both GluR1 and GluR2/3 staining intensities are highest in layers 2/3, and roughly equivalent in layer 4 and layers 5/6. (3) In deprived area 3b, GluR1 staining intensity is increased relative to non-deprived measurements across all cortical layers for both somata and neuropil. (4) In deprived area 3b, GluR2/3 staining intensity is decreased relative to control values across all cortical layers for both somata and neuropil. Bilateral consequences of unilateral sensory manipulations have been rather widely reported (e.g., Calford and Tweedale, 1990, 1991; Clarey et al., 1996; Kozin et al., 1976; Oaklander and Belzberg, 1997; Oaklander and Brown, 2004; Vergara-Aragon et al., 2003), with the ipsilateral effects tending to mirror those found contralaterally (e.g., Calford and Tweedale, 1990; Oaklander and Brown, 2004). In the present study, there are clear differences between the contralateral and ipsilateral hemispheres, suggesting either that there are no changes in the ipsilateral cortex or that they are in the opposite direction of those in the contralateral cortex. We favor the first of these possibilities as we have shown using receptor autoradiography that changes in AMPA receptor binding levels at 1–2 months after nerve injury are confined to the contralateral hemisphere (Garraghty et al., 2006).
Control measurements
Our finding of roughly equal staining intensities for Glur1 and Glur2/3 AMPA receptor subunits is in contrast with the data reported by Muñoz et al. (1999) using in situ hybridization of GluR1, 2, and 3 mRNAs. While our methods do not permit us to distinguish between GluR2 and GluR3 subunits, examination of the Muñoz et al. (1999; Figure 1A) data from macaque monkeys finds the summed mRNA expression levels of GluR2 and GluR3 to be considerably higher than that of GluR1. This difference could reflect a New versus Old World primate species difference (though this seems unlikely; see Garraghty et al., 2006), or more likely, the difference between the methods of subunit measurement and quantification. Recognizing the differences in the measure techniques employed, the laminar distributions of GluR1 and GluR2/3 immunostaining intensities reported here are in general agreement with the findings of Muñoz et al. (1999). Their optical density distributions for GluR1–3 (see their Figure 5) roughly map onto our control data layer by layer (see Figure 2). Furthermore, their micrographs of GluR1–3 immunohistochemical staining (their Figure 6B and 6G) generally reflect the staining seen in our images (see Figure 1). Thus, the pattern of GluR1–3 expression in squirrel monkey area 3b generally maps onto that found in Old World macaque monkeys.
Effects of deprivation
One week after median nerve compression, we find increases in immunohistochemical staining intensity for the GluR1 AMPA receptor subunit, and concurrent decreases in staining intensity for the GluR2/3 AMPA receptor subunits throughout all layers of deprived area 3b. This pattern of change differs from ones that have been reported in the emerging literature on homeostatic plasticity, where in, various models of homeostatic AMPA receptor trafficking some investigators have reported increases in both GluR1 and GluR2 subunits (e.g., Hou et al., 2008; Wierenga et al., 2005) while others have reported increases in GluR1 with no changes in GluR2 (e.g., Ju et al., 2004; Sutton et al., 2006; Thiagarajan et al., 2005). These paradigms do provide important examples of AMPAR mechanics at focalized synapses; however, it is difficult to assess any absolute connection between these in vitro cell studies and the more global data generated in our in-vivo model of sensory deprivation/reorganization. Furthermore all of these studies used immature tissue (ED 18 to PND 4), and so the actual relationship to our experiment, which investigates the response of adult neural networks to deprivation and reorganization, remains unknown. With this in mind, recent evidence demonstrates that manifestations of homeostatic plasticity in juveniles and adults have specific nuances that differ from cell cultures (Echegoyen et al., 2007).
Even more relevant to our sensory reorganization model are in-vivo applications concerning experience dependent plasticity. While studies investigating experience dependent glutamate receptor mechanisms share some aspects with our results, they do not fully agree with the pattern of subunit expressions seen in the present study. Wright et al. (2008) used a spared whisker paradigm to study the in-vivo effects of experience dependent depression and its relationship to GluR subunit changes. While they do report significant decreases in GluR2 and GluR3 subunits, there are no significant changes in GluR1 expression (see Supplementary Material Figure 4a; Wright et al., 2008). Reorganization of the median nerve cortex may well involve some of the same experience dependent mechanisms of GluR mediated LTD (Allen et al., 2003), and LTP (Takahashi et al., 2003), but the extent to which reorganization relies on experience dependent mechanisms, exactly how and when it is induced, and what role, if any, homeostatic plasticity plays in its propagation remains unclear. Again, it is important to make distinctions between in-vivo studies that use neonates and adolescent subjects, as opposed to adults, because of the known changes in receptor subunit populations reported during development, as well as, differences that may arise through cross-species studies (mouse versus rat versus monkey).
The increase in Glur1 staining intensity and concomitant decrease in GluR2/3 staining intensity found here in deprived cortex 1 week after nerve compression results in an apparent pattern of subunit expression that is quite comparable to the one shown to exist early in development (e.g., Eybalin et al., 2004; Ho et al., 2007; Kumar et al., 2002). We have suggested previously (Garraghty et al., 2006) that adult monkey somatosensory cortex retains features that are used to shape neural developmental outcomes in other structures. The present result suggests that mature primate somatosensory cortex might also possess mechanisms that permit it to recapitulate developmental processes when challenged to undergo reorganization. Interestingly, genetic screening of adult somatosensory cortex collected from one monkey within hours of nerve injury revealed the up-regulation of a number of genes (e.g., neuroregulin) that play prominent roles in development (Prieto et al., unpublished observations).
A “hidden” stage of reorganization?
The present data reveal no distinct evidence of NMDA receptor-mediated plasticity at this survival duration. The cortical reorganization that follows peripheral nerve injury in monkeys proceeds via an immediate unmasking phase followed by a more protracted secondary stage during which neurons in the initially unresponsive regions of deprived cortex become responsive to novel cutaneous stimulation (Merzenich et al., 1983b; Myers et al., 2000). We have shown previously that this second phase of reorganization can be prevented by blocking NMDA receptors (Garraghty and Muja, 1996), and have drawn some parallels between this secondary phase of plasticity and NMDA receptor-dependent LTP in the hippocampus (Garraghty and Muja, 1996; Garraghty et al., 2006; Myers et al., 2000). Most notably, we have reported an increase in AMPA receptor autoradiographic binding at post-nerve injury survival durations when the putative second phase of reorganization has been completed (Garraghty et al., 2006), but this was not seen at early (i.e., 0–3 days post-nerve injury) survival durations. While there is no direct evidence that subunit expression can be used as a measure of receptor frequency, the increase in the GluR1 subunit staining intensity is balanced reasonably well by the decrease in GluR2/3 staining intensity such that the summed intensities of GluR1 and GluR2/3 staining in the deprived and non-deprived hemispheres are quite comparable. We tentatively conclude that these changes in receptor subunit populations have functional consequences for network activity beyond that which would be associated with increased membrane expression of AMPA receptors found at later stages of reorganization (Garraghty et al., 2006). These qualitative changes in AMPAR composition would have been hidden in our previous studies, and will be explored in further research.
We, and others, have previously described the reorganizational changes in primate cortical somatotopy that follow peripheral nerve injury as proceeding in two stages, one immediate and one more protracted (e.g., Garraghty and Muja, 1996; Merzenich et al., 1983b; Myers et al., 2000). It is important to note that this characterization was based solely on electrophysiological mapping data. The present findings suggest the existence of yet another stage of reorganization during which there is a shift in AMPA receptor phenotype indicative of a return to immature AMPAR function. In their elegant study of the time-course of cortical reorganization after sciatic nerve injury in adult rats, Cusick et al. (1990) noted what they thought might be an intermediate stage of reorganization that intervened between the immediate unmasking phase and a later wave of plasticity (see Figure 2; Cusick et al., 1990). They suggested that “early and late cortical changes both involve local synaptic reorganization, but that late, slowly developing cortical changes do not occur unless neurons and synapses are appropriately conditioned by a ‘priming’ sequence of earlier changes” (emphasis added; Cusick et al., 1990, p. 357). While the time-course of reorganization in rat cortex after sciatic nerve injury is substantially longer than that in monkey cortex after median nerve injury, the intermediate stage of plasticity suggested here could reflect such a “priming sequence” that sets the stage for the subsequent NMDA receptor-mediated reorganization (cf., Hagemann et al., 1998). We posit that this intermediate stage reflects a return to a pseudo-developmental state, much as has been reported to accompany cortical infarct both in terms of human clinical and brain imaging observations, and in terms of protein expression measures in animal models (for review, see Cramer and Chopp, 2000). The hallmark of this recapitulation of ontology is the return to earlier forms of synaptic transmission, which include Ca++ permeable AMPARs, and a peak of cortical excitatory potential seen 7–10 days post injury in the peri-infract cortical regions (Hagemann et al., 1998). Such changes in deprived primate cortex at this post-nerve injury survival duration would presumably facilitate, or be essential for the NMDA receptor-dependent phase of reorganization (Garraghty and Muja, 1996).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke; Grant number: NS37348.
References
- Allard T., Clark S. A., Jenkins W. M., Merzenich M. M. (1991). Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J. Neurophysiol. 66, 1048–1058 [DOI] [PubMed] [Google Scholar]
- Allen C. B., Celikel T., Feldman D. E. (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat. Neurosci. 6, 291–299 10.1038/nn1012 [DOI] [PubMed] [Google Scholar]
- Brown D., Salinger W. (1975). Loss of X-cells in lateral geniculate nucleus with monocular paralysis: neural plasticity in the adult cat. Science 189, 1011–1012 10.1126/science.1220007 [DOI] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. (1990). Interhemispheric transfer of plasticity in the cerebral cortex. Science 249, 805–807 10.1126/science.2389146 [DOI] [PubMed] [Google Scholar]
- Calford M. B., Tweedale R. (1991). Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens. Mot. Res. 8, 249–260 [DOI] [PubMed] [Google Scholar]
- Churchill J. D., Arnold L. L., Garraghty P. E. (2001). Somatotopic reorganization in the brainstem and thalamus following peripheral nerve injury in adult primates. Brain Res. 910, 142–152 10.1016/S0006-8993(01)02703-2 [DOI] [PubMed] [Google Scholar]
- Churchill J. D., Muja N., Myers W. A., Besheer J., Garraghty P. E. (1998). Somatotopic consolidation: a third phase of reorganization after peripheral nerve injury in adult squirrel monkeys. Exp. Brain Res. 118, 189–196 10.1007/s002210050271 [DOI] [PubMed] [Google Scholar]
- Clarey J. C., Tweedale R., Calford M. B. (1996). Interhemispheric modulation of somatosensory receptive fields: evidence for plasticity in primary somatosensory cortex. Cereb. Cortex 6, 196–206 10.1093/cercor/6.2.196 [DOI] [PubMed] [Google Scholar]
- Courtine G., Bunge M. B., Fawcett J. W., Grossman R. G., Kaas J. H., Lemon R., Maier I., Martin J., Nudo R. J., Ramon-Cueto A., Rouiller E. M., Schnell L., Wannier T., Schwab M. E., Edgerton V. R. (2007). Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat. Med. 13, 561–566 10.1038/nm1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer S. C., Chopp M. (2000). Recovery recapitulates ontogeny. Trends Neurosci. 23, 265–271 10.1016/S0166-2236(00)01562-9 [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O., Heggelund P. (1975). Neural plasticity in visual cortex of adult cats after exposure to visual patterns. Science 188, 1025–1027 10.1126/science.1145187 [DOI] [PubMed] [Google Scholar]
- Cusick C. G., Wall J. T., Whiting J. H., Jr, Wiley R. G. (1990). Temporal progression of cortical reorganization following nerve injury. Brain Res. 537, 355–358 10.1016/0006-8993(90)90385-O [DOI] [PubMed] [Google Scholar]
- Echegoyen J., Neu A., Graber K. D., Soltesz I. (2007). Homeostatic plasticity studied using in vivo hippocampal activity-blockade: synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE 8, e700. 10.1371/journal.pone.0000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybalin M., Caicedo A., Renard N., Ruel J., Puel J. L. (2004). Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur. J. Neurosci. 20, 2981–2989 10.1111/j.1460-9568.2004.03772.x [DOI] [PubMed] [Google Scholar]
- Florence S. L., Hackett T. A., Strata F. (2000). Thalamic and cortical contributions to neural plasticity after limb amputation. J. Neurophysiol. 83, 3154–3159 [DOI] [PubMed] [Google Scholar]
- Florence S. L., Taub H. B., Kaas J. H. (1998). Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121 10.1126/science.282.5391.1117 [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Arnold L. L., Wellman C. L., Mowery T. M. (2006). Receptor autoradiographic correlates of deafferentation-induced reorganization in adult primate somatosensory cortex. J. Comp. Neurol. 497, 636–645 10.1002/cne.21018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty P. E., Hanes D. P., Florence S. L., Kaas J. H. (1994). Pattern of peripheral deafferentation predicts reorganizational limits in adult primate somatosensory cortex. Somatosens. Mot. Res. 11, 109–117 10.3109/08990229409028864 [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Kaas J. H. (1991). Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc. Natl. Acad. Sci. U.S.A. 88, 6976–6980 10.1073/pnas.88.16.6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty P. E., LaChica E. A., Kaas J. H. (1991). Injury-induced reorganization of somatosensory cortex is accompanied by reductions in GABA staining. Somatosens. Mot. Res. 8, 347–354 [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Muja N. (1996). NMDA receptors and plasticity in adult primate somatosensory cortex. J. Comp. Neurol. 367, 319–326 [DOI] [PubMed] [Google Scholar]
- Hagemann G., Redecker C., Neumann-Haefelin T., Freund H.-J., Witte O. W. (1998). Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Ann. Neurol. 44, 255–258 10.1002/ana.410440217 [DOI] [PubMed] [Google Scholar]
- Ho M. T., Pelkey K. A., Topolnik L., Petralia R. S., Takamiya K., Xia J., Huganir R. L., Lacaille J. C., McBain C. J. (2007). Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J. Neurosci. 27, 11651–11662 10.1523/JNEUROSCI.2671-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q., Zhang D., Jarzylo L., Huganir R. L., Man H. Y. (2008). Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc. Natl. Acad. Sci. U.S.A. 105, 775–780 10.1073/pnas.0706447105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W., Morishita W., Tsui J., Gaietta G., Deerinck T. J., Adams S. R., Garner C. C., Tsien R. Y., Ellisman M. H., Malenka R. C. (2004). Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 7, 244–253 10.1038/nn1189 [DOI] [PubMed] [Google Scholar]
- Kaas J. H., Florence S. L., Jain N. (1999). Subcortical contributions to massive cortical reorganizations. Neuron 22, 657–660 10.1016/S0896-6273(00)80725-4 [DOI] [PubMed] [Google Scholar]
- Kozin F., McCarty D. J., Sims J., Genant H. (1976). The reflex sympathetic dystrophy syndrome I. Clinical and histologic studies: evidence for bilaterality, response to corticosteroids and articular involvement. Am. J. Med. 60, 321–331 10.1016/0002-9343(76)90747-6 [DOI] [PubMed] [Google Scholar]
- Kumar S. S., Bacci A., Kharazia V., Huguenard J. R. (2002). A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J. Neurosci. 22, 3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Tocco G., Standley S., Baudry M., Thompson R. F. (1993). Postsynaptic factors in the expression of long-term potentiation (LTP): increased glutamate receptor binding following LTP induction in vivo. Proc. Natl. Acad. Sci. U.S.A. 90, 9654–9658 10.1073/pnas.90.20.9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J., Nelson R. J., Sur M., Felleman D. (1983a). Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience 8, 33–55 10.1016/0306-4522(83)90024-6 [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Kaas J. H., Wall J. T., Sur M., Nelson R. J., Felleman D. J. (1983b). Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience 10, 639–665 10.1016/0306-4522(83)90208-7 [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Nelson R. J., Stryker M. P., Cynader M. S., Schoppmann A., Zook J. M. (1984). Somatosensory cortical map changes following digit amputation in adult monkeys. J. Comp. Neurol. 224, 591–605 10.1002/cne.902240408 [DOI] [PubMed] [Google Scholar]
- Muñoz A., Woods T. M., Jones E. G. (1999). Laminar and cellular distribution of AMPA, kainate, and NMDA receptor subunits in monkey sensory-motor cortex. J. Comp. Neurol. 407, 472–490 [DOI] [PubMed] [Google Scholar]
- Myers W. A., Churchill J. D., Muja N., Garraghty P. E. (2000). Role of NMDA receptors in adult primate cortical somatosensory plasticity. J. Comp. Neurol. 418, 373–382 [DOI] [PubMed] [Google Scholar]
- Oaklander A. L., Belzberg A. J. (1997). Unilateral nerve injury down-regulates mRNA for Na+ channel SCN10A bilaterally in rat dorsal root ganlia. Mol. Brain Res. 52, 162–165 10.1016/S0169-328X(97)00239-8 [DOI] [PubMed] [Google Scholar]
- Oaklander A. L., Brown J. M. (2004). Unilateral nerve injury produces bilateral loss of distal innervation. Ann. Neurol. 55, 639–644 10.1002/ana.20048 [DOI] [PubMed] [Google Scholar]
- Osborne M. C., Verhovshek T., Sengelaub D. R. (2007). Androgen regulates trkB immunolabeling in spinal motoneurons. J. Neurosci. Res. 85, 303–309 10.1002/jnr.21122 [DOI] [PubMed] [Google Scholar]
- Pons T. P., Garraghty P. E., Ommaya A. K., Kaas J. H., Taub E., Mishkin M. (1991). Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252, 1857–1860 10.1126/science.1843843 [DOI] [PubMed] [Google Scholar]
- Recanzone G. H., Merzenich M. M., Jenkins W. M., Grajski K. A., Dinse H. R. (1992). Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J. Neurophysiol. 67, 1031–1056 [DOI] [PubMed] [Google Scholar]
- Schroeder C. E., Seto S., Garraghty P. E. (1997). Emergence of radial nerve dominance in median nerve cortex after median nerve transection in an adult squirrel monkey. J. Neurophysiol. 77, 522–526 [DOI] [PubMed] [Google Scholar]
- Sur M., Nelson R. J., Kaas J. H. (1982). Representations of the body surface in cortical areas 3b and 1 of squirrel monkeys: comparisons with other primates. J. Comp. Neurol. 211, 177–192 10.1002/cne.902110207 [DOI] [PubMed] [Google Scholar]
- Sutton M. A., Ito H. T., Cressy P., Kempf C., Woo J. C., Schuman E. M. (2006). Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125, 785–799 10.1016/j.cell.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Svoboda K., Malinow R. (2003). Experience strengthening transmission by driving AMPA receptors into synapses. Science 299, 1585–1588 10.1126/science.1079886 [DOI] [PubMed] [Google Scholar]
- Thiagarajan T. C., Lindskog M., Tsien R. W. (2005). Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47, 725–737 10.1016/j.neuron.2005.06.037 [DOI] [PubMed] [Google Scholar]
- Tocco G., Maren S., Shors T. J., Baudry M., Thompson R. F. (1992). Long-term potentiation is associated with increased [3H]AMPA binding in rat hippocampus. Brain Res. 573, 228–234 10.1016/0006-8993(92)90767-4 [DOI] [PubMed] [Google Scholar]
- Vergara-Aragon P., Gonzalez C. L., Whishaw I. Q. (2003). A novel skilled-reaching impairment in paw supination on the “good” side of the hemi-Parkinson rat improved with rehabilitation. J. Neurosci. 23, 579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman C. L., Arnold L. L., Garman E E., Garraghty P. E. (2002). Acute reductions in GABAA receptor binding in layer IV of adult primate somatosensory cortex after peripheral nerve injury. Brain Res. 954, 68–72 10.1016/S0006-8993(02)03343-7 [DOI] [PubMed] [Google Scholar]
- Wierenga C. J., Ibata K., Turrigiano G. G. (2005). Postsynaptic expression of homeostatic plasticity at neocortical synapses. J. Neurosci. 25, 2895–2905 10.1523/JNEUROSCI.5217-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber A. A., Southwood C. J., Sokoloff G., Steinmetz J. E., Wellman C. L. (2007). Neonatal maternal separation alters adult eyeblink conditioning and glucocorticoid receptor expression in the interpositus nucleus of the cerebellum. Dev. Neurobiol. 67, 1751–1764 10.1002/dneu.20549 [DOI] [PubMed] [Google Scholar]
- Wilber A. A., Southwood C. J., Wellman C. L. (2009). Brief neonatal maternal separation alters extinction of conditioned fear and corticolimbic glucocorticoid and NMDA receptor expression in adult rats. Dev. Neurobiol. 69, 73–87 10.1002/dneu.20691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N., Glazewski S., Hardingham N., Phillips K., Pervolaraki E., Fox K. (2008). Laminar analysis of the role of GluR1 in experience-dependent and synaptic depression in barrel cortex. Nat. Neurosci. 11, 1140–1142 10.1038/nn.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C., Stern J. M., Merzenich M. M. (1994). Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J. Neurosci. 14, 1710–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]