Summary
As a major output station of the basal ganglia, the globus pallidus internal segment (GPi) projects to the thalamus and brainstem nuclei thereby controlling motor behavior. A less well known fact is that the GPi also projects to the lateral habenula (LHb) which is often associated with the limbic system. Using the monkey performing a saccade task with positionally biased reward outcomes, we found that antidromically identified LHb-projecting neurons were distributed mainly in the dorsal and ventral borders of the GPi and that their activity was strongly modulated by expected reward outcomes. A majority of them were excited by the no-reward-predicting target and inhibited by the reward-predicting target. These reward-dependent modulations were similar to those in LHb neurons, but started earlier than those in LHb neurons. These results suggest that GPi may initiate reward-related signals through its effects on the LHb, which then influences the dopaminergic and serotonergic systems.
Introduction
The internal segment of the globus pallidus (GPi) is the final output station of the basal ganglia through which body movements are controlled (DeLong, 1971). The GPi projects to the motor part of the thalamus which is mutually connected with the motor and premotor cortices and to subcortical motor structures such as the pedunculopontine nucleus (Parent et al., 1999). The former pathway may control learned body movements and the latter pathway may control innate movements. Indeed, lesions of the GPi lead to a variety of movement disorders including slowing of movements (Horak and Anderson, 1984; Mink and Thach, 1991), dyskinesia (Crossman, 1987), and akinesia (Molinuevo et al., 2003). Many GPi neurons change their activity when animals or humans move particular parts of their body (DeLong et al., 1985; Iansek and Porter, 1980). In short, the GPi is instrumental for the basal ganglia to control body movements.
One less well known fact is that the GPi also projects to the lateral habenula (LHb), a small nucleus located above the thalamus at its posterior end close to the midline (Lecourtier and Kelly, 2007). This is puzzling given the dominant motor role of the GPi. However, recent studies have shown that many neurons in the basal ganglia encode non-motor signals, especially in relation to expected rewards (Hikosaka et al., 2006). This raises the possibility that the projection from the GPi to the LHb might be a key to link the basal ganglia and the limbic system providing reward-related information.
Indeed, a recent study from our laboratory has shown that the LHb neurons in monkeys are excited by a visual stimulus that indicates absence of reward and inhibited by a stimulus that indicates presence of reward (Matsumoto and Hikosaka, 2007). This negative reward signal may contribute to the well-known reward coding of dopamine neurons (Christoph et al., 1986; Ji and Shepard, 2007; Matsumoto and Hikosaka, 2007).
To test the hypothesis that the GPi is a source of reward-related information in the LHb, we used the antidromic stimulation method to identify GPi neurons that projected to the LHb. The activity of theses neurons was then examined while the monkey was performing a visually guided saccade task with positionally biased reward outcomes. The results showed that LHb-projecting neurons were located mainly at the borders of the GPi, had firing patterns different from movement-related GPi neurons, and they changed their activity in relation to expected rewards.
Results
We identified LHb-projecting neurons by their antidromic activation from the LHb (Fig. 1) using two rhesus monkeys (Macaca mulatta), D and N. In each experiment we positioned the first electrode at the LHb and made sure that its tip was within the LHb by recording multi-unit activity related to rewards, as described in Matsumoto and Hikosaka (Matsumoto and Hikosaka, 2007). We then lowered a second electrode from the putamen to the GP on the same side as the LHb being recorded, while applying biphasic (negative-positive) pulses of electric currents through the LHb electrode periodically (every 0.5 s).
Figure 1.
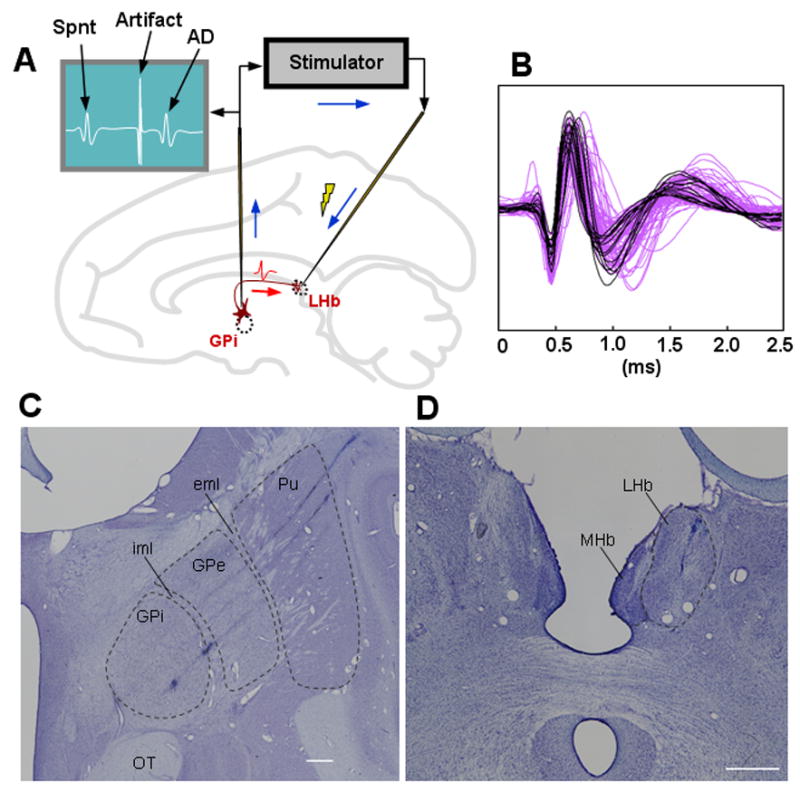
Identification of LHb-projecting GPi neurons. (A) An antidromic spike (AD, drawn in the figure above) of a GPi neuron evoked by an electrical stimulation of the LHb collided with a spontaneous spike (Spnt) of the GPi neuron therefore was not detected by the GPi electrode if the stimulation was delivered soon after the spontaneous spike. (B) Spike shapes of antidromically activated neurons (in purple) and those of presumed motor neurons (in black). (C) Locations of two LHb-projecting GPi neurons are shown as the two electrolytic lesions along the bottom electrode track. Another LHb-projecting neuron was located along the middle track, which was visible in adjacent sections (see Supplementary Fig. S2A). (D) Recording/stimulation site in the right LHb. An electrode tract, which was used for recording and stimulation, is visible in the LHb. iml: internal medullary lamina, eml: external medullary lamina, Pu: putamen, OT: optic tract, LHb: lateral habenula, MHb: medial habenula. Both are coronal sections. White horizontal bars indicate 1 mm.
When an antidromically activated neuron was found, the monkey was asked to perform One-Direction-Rewarded task (1DR; Fig. 2A). In the task, a visual target was presented randomly on the left or right, and the monkey had to make a saccade to it immediately. Correct saccades were signaled by a tone stimulus after the saccade. Saccades to one position were rewarded, whereas saccades to the other position were not rewarded. Thus, the target instructed the saccade direction and also indicated the presence or absence of the upcoming reward. The rewarded position was the same in a block of 24 consecutive trials and was then changed to the other position abruptly for the next block with no external instruction. Both monkeys showed significantly shorter saccade latencies in rewarded trials than in unrewarded trials (Fig. 2B) indicating that they understood the position-reward contingencies.
Figure 2.
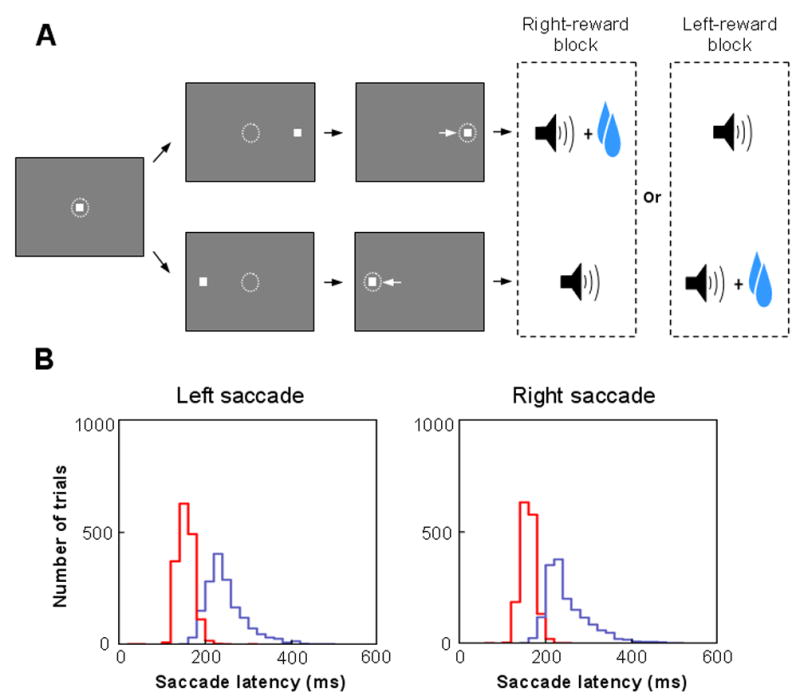
Behavioral task and animals’ performance. (A) Sequence of events in the one-direction-rewarded version of the visually guided saccade task (1DR). The monkey first fixated at the central spot (the dotted circle indicates the eye position). As the fixation point disappeared, a target appeared randomly on the right or left and the monkey was required to make a saccade to it immediately. Correct saccades in one direction were followed by a tone and juice reward; saccades in the other direction followed by a tone alone. The rewarded direction was fixed in a block of 24 trials, and was changed in the following block. (B) Distribution of saccade latencies in rewarded trials (in red) and in unrewarded trials (in blue) (data from monkey D). Saccades in the first trials after the changes in position-reward contingency have been excluded.
Properties of LHb-projecting GPi neurons
A total of 74 GPi neurons were activated antidromically from the LHb. The antidromic latency ranged from 1.3 to 6.8 ms (2.6 ± 1.0 ms, mean ± SD, see Supplementary Fig. S4A–C). The antidromic collisions occurred with a mean latency of 3.2 ± 1.1 ms. The threshold current used for antidromic activation ranged from 5 to 260 μA (115 ± 68 μA, see Supplementary Fig. S4D–F). Fig. 1D shows the LHb stimulating site where an electrode track is visible in the LHb.
Antidromically activated neurons were found usually at the border between the external segment of the globus pallidus (GPe) and the GPi and the ventral border of the GPi (Supplementary Fig. S3). As the electrode was advanced, the characteristic activity of GPe neurons (high frequency tonic firing and occasional pauses) faded and then the characteristic activity of GPi neurons (high frequency tonic firing with no pause) grew larger. In this relatively quiet GPe-GPi border region antidromically activated neurons were found, often in a cluster of 2–3 neurons. A second group of antidromically activated neurons were found at the ventral border of the GPi where the characteristic GPi neurons became sparse. In the following we will call these antidromically activated neurons ‘LHb-projecting GPi neurons’.
To verify the location, we made electrolytic micro-lesions at the recording sites of LHb-projecting GPi neurons. Two electrolytic micro-lesion sites are visible in Fig. 1C along the bottom of the three electrode penetrations: one being at the GPe-GPi border (anatomically called the internal medullary lamina) and the other in the middle of the GPi which corresponds to a structure called the accessory medullary lamina. Other LHb-projecting neurons were also located in the internal medullary lamina, which are visible in adjacent sections (Supplementary Fig. S2). None of the LHb-projecting neurons were found at the border between the putamen and the GPe, a region called the external medullary lamina.
We found that the LHb-projecting neurons were electrophysiologically different from other GPi neurons that were not activated antidromically and were presumed to be related to body movements (see Supplementary Note A). The LHb-projecting GPi neurons had a mean baseline firing rate of 33 ± 20 spikes/s, which is lower than that of the presumed motor GPi neurons (77 ± 16 spikes/s, see Supplementary material) (p<0.01, ANOVA). The duration of spikes of the LHb-projecting GPi neurons, defined as the time interval between the first and second negative peaks of the spike, was generally longer (0.55 ± 0.1 ms; purple waveforms in Fig 1B) than that of the motor GPi neurons (0.46 ± 0.04 ms; black waveforms in Fig 1B) (p<0.01, ANOVA).
About two thirds of LHb-projecting GPi neurons (49/74, 66%) showed significant modulations of their firing rate after the onset of the target. One example is shown in Fig. 3A. The neuron increased its activity phasically after the appearance of the saccade target indicating the absence of upcoming reward, and decreased after the appearance of the target indicating the presence of upcoming reward. The increase and decrease depended on the reward contingency, regardless of target position. We call this type of modulation ‘reward negative type’. This neuron was antidromically activated (Fig. 3C) from the LHb where we recorded the multi-unit activity shown in Fig. 3B. Interestingly, neurons in the LHb where the stimulation was applied showed similar reward-related changes in activity (Fig. 3B). For both the GPi neuron and the LHb multiunit, the responses to the target were phasic and did not continue until the outcome (reward or no reward) and their responses to the outcome were weak, if any.
Figure 3.
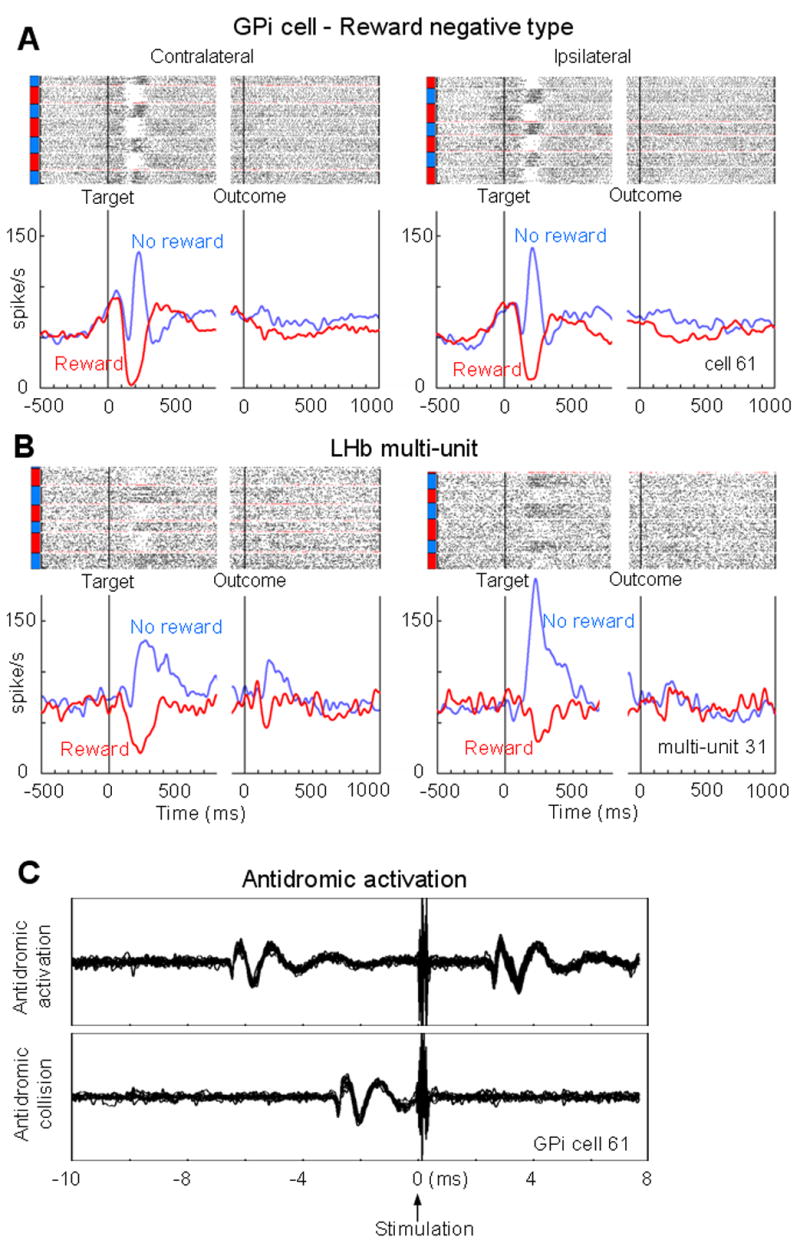
LHb-projecting GPi neuron with negative reward modulations. (A) Changes in spike activity of a GPi neuron while the monkey was performing 1DR task, shown separately for the contralateral target (left) and the ipsilateral target (right). For each saccade direction, rasters of spikes (dots, top) and spike density functions (SDFs; graphs, bottom) are aligned at the onset of target (left) and at the onset of outcome (tone) (right). The rasters are shown in order of occurrence of trials for each direction from bottom to top. Rewarded trials are indicated by red bars on the left side of the raster; unrewarded trials are indicated by blue bars. The gaps in the raster indicate the change of blocks and the raster for the first trial of each block is shown in red. The SDFs are shown for each reward contingency (red, rewarded trials; blue, unrewarded trials). The first trial in each block is not included for the SDF. The GPi neuron showed an excitation in response to a no-reward-predicting target and inhibition to a reward-predicting target. This neuron was antidromically activated from the LHb site where the LHb multi-unit 31 (shown in B) was recorded. (B) The LHb multi-unit activity was similar to the activity of the LHb-projecting GPi neuron in A. (C) Antidromic spikes (top) and collision (bottom) of the LHb-projecting GPi neuron shown in A.
There were also LHb-projecting GPi neurons that responded in an opposite manner to the one described above, increasing their activity in response to the reward-predicting target and decreasing to the no-reward-predicting target. We call this ‘reward positive type’. One example is shown in Fig. 4A. This neuron’s response to the reward-predicting target grew slowly and ended slowly after the outcome.
Figure 4.
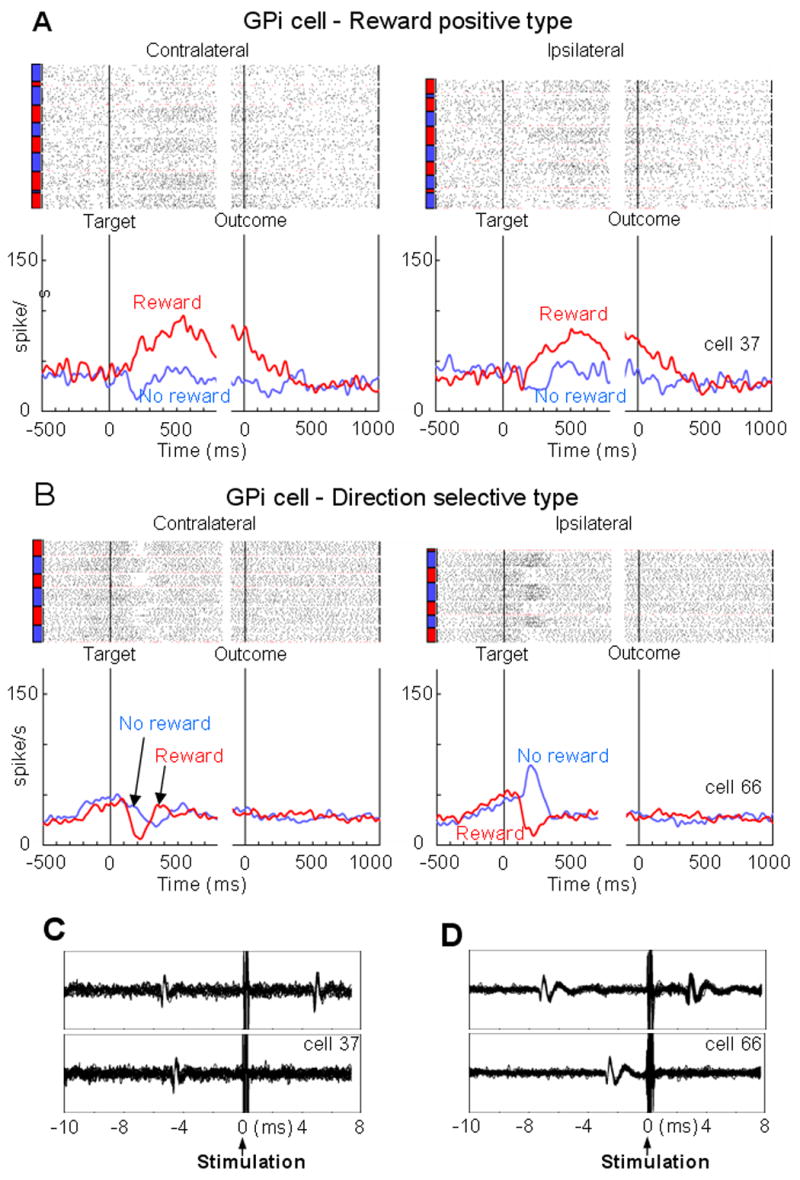
LHb-projecting GPi neurons with positive reward modulation (A) and with direction selectivity (B). (C) and (D) The antidromic activation and collision corresponding to the neurons in A and B, respectively. The convention of the figures is the same as the one in Fig. 3.
Some of the LHb-projecting GPi neurons responded to the target differentially depending on its direction, as shown in Fig. 4B. This neuron was similar to the one in Fig. 3A, but the excitation by the no-reward-predicting target occurred only when the target was presented on the ipsilateral side to the recording site.
Population properties of LHb-projecting GPi neurons
Do the LHb-projecting GPi neurons and the LHb neurons encode expected reward and target positions differently? Fig. 5 suggests an answer to this question. Here we plotted, for each neuron, the difference of response to the target between rewarded and unrewarded trials (abscissa) and contralateral and ipsilateral trials (ordinate), separately for the GPi neurons (Fig. 5A) and the LHb multiunits (Fig. 5B; see Supplementary Fig. S5 for single unit LHb data). For both the GPi neurons and the LHb neurons, reward-dependent activity modulation was larger than position-dependent modulation, but more so among the LHb neurons. The reward-dependent modulation was negative for some GPi neurons (as shown in Fig. 3A) and positive for other GPi neurons (as shown in Fig. 4A). In contrast, the reward-dependent modulation was exclusively negative for the LHb neurons.
Figure 5.

Direction and reward modulations in LHb-projecting GPi neurons (A) and in LHb multiunits (B). For the post-target responses of each neuron, the direction-dependent modulation (vertical axis) and the reward-dependent modulation (horizontal axis) are plotted (see Method for details). Red, blue and green dots indicate the neurons modulated by the reward only, direction only and both reward and direction, respectively (p <0.01, ANOVA). Yellow dots indicate neurons with no modulation (p > 0.01, ANOVA). The marginal histograms show the distributions along the axes. Filled bars indicate neurons with statistically significant post-target responses (p < 0.01, Wilcoxon signed-rank test). Open bars, neurons with no significant post-target responses.
To test this impression, we performed two-way analysis of variance (ANOVA) [target position (contralateral vs. ipsilateral) x reward contingency (reward vs. no reward)] for each neuron. Of the 74 LHb-projecting GPi neurons, 29 neurons showed a reward-only modulation (red dots in Fig. 5A; main effect of reward contingency, p<0.01), 20 neurons also showed direction related modulation on top of reward modulation (green dots; main effect of both reward contingency and target position, p<0.01), and 5 neurons showed a direction-only modulation (blue dots; main effect of target position). About one fourth of the LHb-projecting GPi neurons (n=20) did not show any significant modulation to either reward or position (yellow dots). In contrast, LHb neurons were predominantly modulated by reward contingency only (n=26, red dots in Fig. 5B) or by both reward and position (n=7, green dots).
To further characterize the reward-related property of the LHb-projecting GPi neurons, we categorized them into three groups, namely, 1) negative type, represented by the red and green dots on the left side of Fig 5A, 2) positive type, represented by the red and green dots on the right side of Fig 5A, and 3) reward-unrelated type, represented by the yellow and blue dots in Fig. 5A. Figures 6A and B show the population average spike density functions of the negative and positive groups, respectively. The negative type GPi neurons (n=37, 50%), as a majority, responded with a phasic increase of firing after the onset of the no-reward-predicting target, and with a phasic decrease of firing after the onset of the reward-predicting target (ANOVA, p<0.01, Fig. 6A). Both responses were phasic, and did not continue until the reward outcome. Thus, the response pattern of the negative type GPi neurons was very similar to that of the LHb neurons (Fig. 6C). The positive type GPi neurons (n=12, 16%) acted in an opposite manner, increasing their firing rate after the reward-predicting target and decreasing after the no reward-predicting target (ANOVA, p<0.01, Fig. 6B). The increasing response to the reward-predicting target continued until the reward delivery, as seen in the example neuron shown in Fig. 4A.
Figure 6.
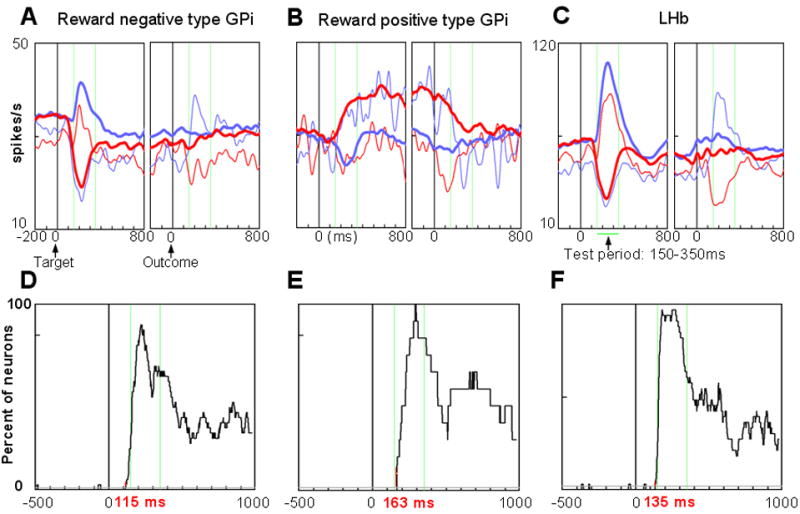
Population activity. (A)–(C) Population responses of LHb-projecting GPi neurons with negative reward modulations (A, n=37), LHb-projecting GPi neurons with positive reward modulations (B, n=12), and LHb neurons (C, n=35). The SDFs are shown for each reward contingency (red, rewarded trials; blue, unrewarded trials). Thick curves indicate activity excluding the first trial in each block. Thin curves indicate activity in the first trial in each block. The two vertical green lines show the time window used to test these responses. (D)–(F) Time-varying proportion of neurons that showed significantly different activity between rewarded trials and unrewarded trials for the three neuron groups corresponding to (A)–(C). The gray lines in (D)–(F) indicate the criterion level (p = 0.01). Time 0 indicates target onset. See Methods for details.
A close examination revealed that the activity of the GPi neurons reflected reward prediction error. Note that during the 1DR task the reward outcome was predictable by the position of the target, except for the first trial of a new block where the position-reward contingency reversed. As represented by the thick blue and red lines in Fig. 6A, the negative type GPi neurons showed no responses to the outcome, except for the first trial (thin blue and red lines in Fig. 6A). The thin blue line indicates the neuronal activity when the monkey had been expecting a reward (as indicated by a decrease in activity after target onset in Fig 6A), but there was no reward unexpectedly (as indicated by an increase in activity after the outcome). The thin red line indicates the neuronal activity when the monkey had been expecting no reward (as indicated by an increase in activity after target onset), but there was a reward unexpectedly (as indicated by a decrease in activity after the outcome). Similar reward prediction-related activity changes were observed in the LHb neurons (Fig. 6C). A further analysis of within-block neural changes is presented in the next section.
The antidromic activation of GPi neurons from the LHb suggests that the GPi neurons project their axons to the LHb and possibly have synaptic contacts with LHb neurons. However, it does not necessarily indicate that the activity changes in the LHb neurons were initiated by the inputs from the GPi. To investigate this issue, we examined the latency at which each of the three groups of neurons started differentiating their activity depending on the expected reward outcome (see Methods for details). Figure 6D shows the percentage of the negative type GPi neurons that discriminated the reward/no-reward contingency after target onset. The percentage increased abruptly and exceeded the criterion level of significance (indicated by gray line, p = 0.01) at 115 ms after target onset. The discrimination latency for the positive type GPi neurons was 163 ms (Fig. 6E). The discrimination latency for the LHb neurons was 135 ms (Fig. 6F). These results suggest that the negative type GPi neurons, but not the positive type GPi neurons, could initiate the reward-related activity in LHb neurons. This also means that the negative type neurons may have an excitatory influence on the LHb (see Discussion).
Within-block changes of neural and behavioral responses
The changes of neural and the corresponding behavioral responses within a block were examined. Fig. 7A and 7B show the neural responses after the change of the position-reward contingency in different groups of LHb-projecting GPi neurons (upper 4 rows) and LHb neurons (5th row). The reward-negative GPi neurons and LHb neurons showed similar patterns of within-block changes. The similarity includes the post-reward delivery response, where the neurons showed differential responses depending on the presence or absence of reward only on the 1st trial in a block. This demonstrates that these neurons encode reward prediction error. The reward-positive group showed the opposite pattern of post-target response changes, but with no clear 1st-trial-specific post-reward responses. The null group also showed some post-reward responses as the reward-positive neurons, suggesting that the null group may also play some yet unspecified roles. Fig. 7C shows the changes of saccadic latency toward the target.
Figure 7. Within-block changes of neural and behavioral responses.
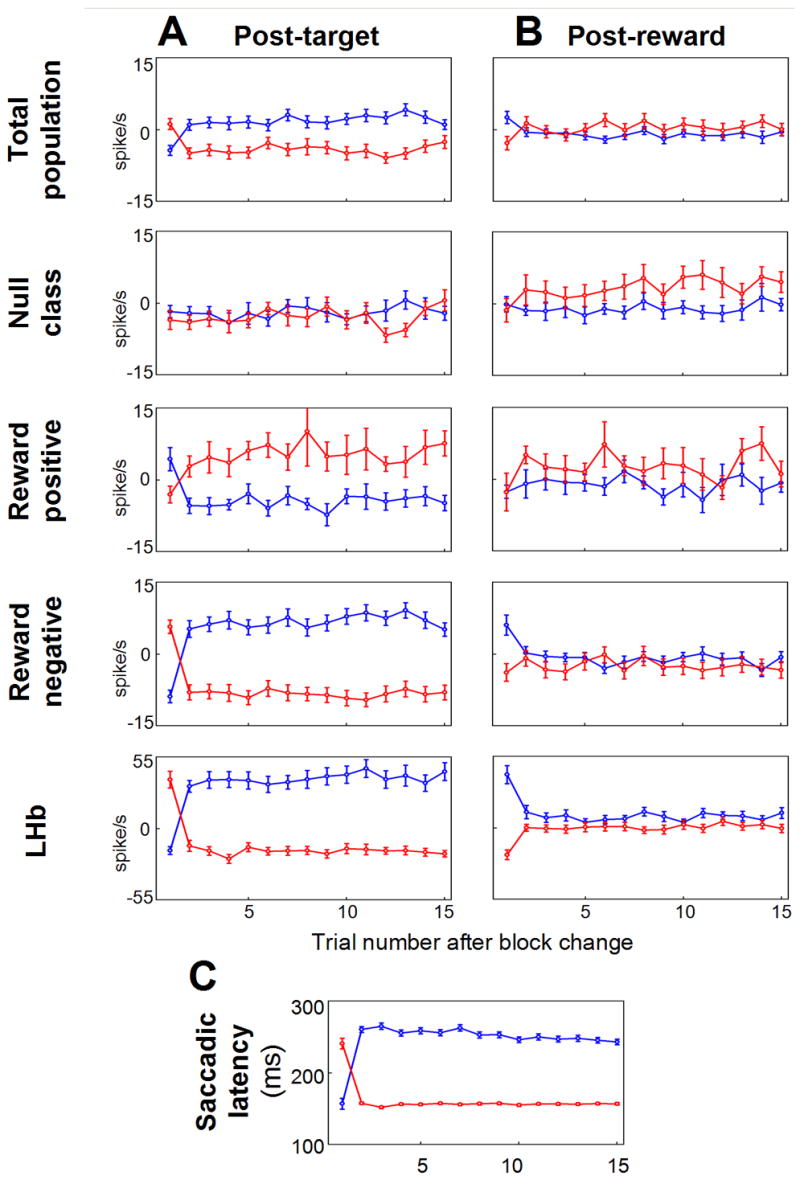
Changes in averaged post-target responses (A), averaged reward on-off responses (B), and averaged saccade latency (C) after the reversal of position-reward contingency are shown. Red and blue circles indicate the data in rewarded and unrewarded trials, respectively. In (A), (B), the data from monkey D and N as well as from ipsilateral and contralateral saccades are combined. Figure C is from monkey D. Error bars indicate s.e.m.
Discussion
We have shown that LHb-projecting GPi neurons changed their activity differentially depending on the expected reward. Their activity was sometimes differential for the direction of the saccade target, but such changes were smaller than their pronounced reward-related activity. The LHb-projecting GPi neurons were different from other GPi neurons in that their firing rates were lower, their spike durations were longer, and they were located peripherally in the GPi. The recording sites were exactly as expected from a series of anatomical studies on the monkey conducted by Parent and his colleagues (Parent et al., 2001). These results suggest that the LHb-projecting neurons are a distinct group of GPi neurons, as suggested anatomically (Parent et al., 2001).
These properties of the LHb-projecting GPi neurons appear similar to ‘border cells’ described by DeLong and his colleagues (DeLong, 1971; Richardson and DeLong, 1991), so called because they were found at the border of the GP. The border cells tended to be activated by the delivery of rewards (as we saw in the LHb-projecting GPi neurons) or aversive airpuffs. However, these authors interpreted the border cells as part of the nucleus basalis of Meynert which projects to the cerebral cortex (Richardson and DeLong, 1991). The border cells were examined further in subsequent studies especially in relation to dopaminergic denervation (Tremblay et al., 1989). While the relationship of the border cells with the LHb is unknown, we speculate that at least a subset of these neurons correspond to the LHb-projecting GPi neurons examined here.
Another line of evidence for the reward processing in the GP came from a series of studies showing that some GPi neurons are sensitive to glucose and change their activity in relation to consummatory behavior (Karadi et al., 1995). Such glucose-sensitive neurons were found in the ventromedial and rostral part of the GPi in the rat and monkey, which roughly matches the location of LHb-projecting neurons.
According to Parent (2001), LHb-projecting neurons constitute about 10 % of the total number of neurons in the GPi. Would such a minority of GPi neurons play a significant role in controlling behavior? There are at least two lines of research that may support this view. First, human patients with pallidal lesions often show non-motor symptoms, although the most common symptoms are movement disorders. Even when the patients have no obvious sensorimotor symptoms, they may show a lack of will, motivation, and desire (Miao et al., 2001; Miller et al., 2006), or show psychiatric symptoms resembling depression, schizophrenia, and obsessive-compulsive disorder (Laplane et al., 1989). Second, human brain imaging studies have shown that the GPi is related to reward processing. They report that the ventral striatum (especially the nucleus accumbens) and the dorsal striatum (Knutson and Cooper, 2005) are commonly activated by expected rewards. In many cases, however, the GPi was also activated (Calder et al., 2007; Pessiglione et al., 2007), although in some cases it was not stated so explicitly (Kampe et al., 2001; Kim et al., 2006; Tanaka et al., 2007; Tobler et al., 2007). These motivation- or reward-related observations on the human GP might reflect changes in the state of the LHb-projecting GPi neurons.
If this is true, how might the LHb-projecting GPi neurons participate in controlling behaviors? We found that there are at least two types of neurons in relation to reward: one negative type (excited by no-reward-indicating targets and inhibited by reward-indicating targets) and the other, positive type (excited by reward-indicating targets and inhibited by no-reward-indicating targets). The response pattern of the negative type GPi neurons was similar to that of the LHb neurons (Fig. 6A and 6C), and more critically, the responses to the target started earlier in the negative type GPi neurons than in the LHb neurons. The similarity between the two groups of neurons extends to the responses to the reward prediction error: they were excited by the unexpected absence of reward (i.e., on the first trial after the position-reward contingency) but not by the expected absence of reward, and were inhibited by the unexpected presence of reward. These data, showing that the two groups of neurons having similar phase of activation even at the first trial of a block, are consistent with the idea that the negative type GPi neurons have excitatory connections to LHb neurons. In contrast, the positive type GPi neurons started showing reward sensitivity later than that of LHb neurons and did not clearly encode reward prediction error. They are thus unlikely to initiate the reward-related responses in LHb neurons, but could contribute to the later part of the LHb responses.
The suggested excitatory GPi-LHb connection in this study is somewhat puzzling, because a general consensus seems to be that this connection is GABAergic and inhibitory, in the same way as the other GPi efferents are (Vincent et al., 1982). One possibility is that the excitatory connection is mediated by acetylcholine, as the LHb receives input from the cholinergic nucleus basalis of Meynert and ventro-lateral septum (Herkenham and Nauta, 1977). This is consistent with the finding that some of the LHb projecting neurons in the rostral part of the entopeduncular nuclues (the GPi counter part of the rat) are cholinergic (Moriizumi and Hattori, 1992). Another candidate is glutamate, as a relatively high level of AMPA receptor subtypes was found in the LHb (e.g. Petralia and Wenthold, 1992). It is also possible that the excitatory effect is due to disinhibition such that intra-LHb interneurons, which exert tonic inhibition on LHb neurons, are inhibited by the input from GPi neurons. While the current evidence indicates a possible excitatory connection between the GPi and the LHb, the exact nature of this pathway needs further investigation.
While the negative type GPi neurons had a similar response pattern to that of LHb neurons, a closer examination revealed that many of them were modulated also by the direction of the target (Fig. 5A) unlike LHb neurons (multiunit activity in Fig. 5B, single unit activity in Supplementary Fig. S5). This suggests that sensorimotor signals, which the GPi neurons have, are removed, and instead reward-related signals are extracted presumably by some LHb local connections. We think that these reward-related signals may originate from the dorsal striatum (caudate and putamen), for the following reasons. First, Tremblay (Tremblay and Filion, 1989) showed that border cells in the monkey GP, which might correspond to the LHb-projecting GPi neurons (see above), were strongly excited or inhibited by electrical stimulation in the caudate and putamen. Second, Saleem et al. found a strong projection from the monkey striatum to the LHb, probably via the GPi, after injecting an MRI visible anterograde transsynaptic transport agent manganese in the monkey caudate and putamen (Saleem et al., 2002).
However, it is unlikely that the dorsal striatum is the only source of inputs to the LHb-projecting GPi neurons. The ventral striatum, including the nucleus accumbens and the ventral putamen, projects to the GPi, specifically to its peripheral regions (Haber et al., 1990). Also the dopaminergic innervation of the monkey GP is conspicuously high in the peri-GPi region (Lavoie et al., 1989). These ventral striatal and dopaminergic projections match the anatomical locations of the LHb-projecting neurons. Thus, the LHb-projecting GPi neurons may integrate a number of signals ranging from motivation (via the ventral striatum), reinforcement (via dopamine neurons), and the reward value of a target in a motor context (via the caudate and putamen).
In conclusion, our data suggest that the GPi has two functionally distinct outputs, one involved in motor execution and the other involved in reward evaluation (Fig. 8). The motor execution pathway consists of Striatum→GPi→Thalamus/Brainstem connections. The reward evaluation pathway consists of Striatum→GPi→LHb→Dopamine→Striatum connections. Along this extra-basal ganglia pathway, sensorimotor information is removed and reward information is extracted at the GPi → LHb level. This reward evaluation signal is then used to reinforce/discourage the ongoing action via the dopamine projections to the striatum. The same signal may also be used to control mood and social behaviors via the projections of the LHb to the dorsal and median raphe nuclei which contain serotonin neurons (Kalen et al., 1989).
Figure 8.
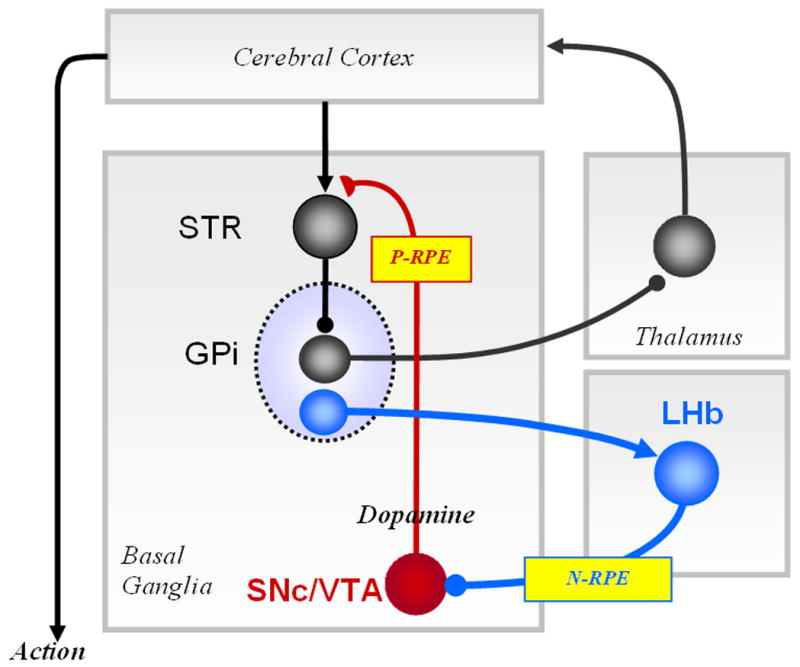
Circuit diagram showing mutual relationship between the lateral habenula (LHb) and the basal ganglia. The GPi has two functionally distinct outputs, one for motor execution (via the motor thalamus or brainstem nuclei) and the other for reward evaluation (via LHb). Note that saccadic eye movements are controlled by the substantia pars reticulata (not shown), instead of the GPi. Excitatory, inhibitory and modulatory connections are illustrated with arrow heads, filled circles and half circles, respectively. LHb-SNc/VTA connection may not be monosynaptic. STR (striatum), SNc (substantia nigra compacta), VTA (ventral tegmental area). Negative reward prediction error (N-RPE) signals, in addition to other signals, are transmitted from the GPi through the LHb to the SNc/VTA which then sends positive reward prediction error (P-RPE) signals to the STR and other structures. See text for further discussion.
Experimental Procedures
Three rhesus monkeys (Macaca mulatta), D, S and N, were used as subjects in this study. The monkey S was used only for the anatomical study. All animal care and experimental procedures were approved by the National Eye Institute, Institute Animal Care and Use Committee and complied with the Public Health Service Policy on the humane care and use of laboratory animals.
Behavioral task
Behavioral tasks were the same as the ones described previously (Matsumoto and Hikosaka, 2007). The monkey was seated in a primate chair in a dimly lit room. Visual stimuli were rear-projected by a projector onto a frontoparallel screen 33 cm from the monkey’s eyes. Eye movements were monitored using a scleral search coil system with 1-ms resolution. The monkey was trained to perform a one-direction-rewarded version of the visually guided saccade task (Lauwereyns et al., 2002), 1DR (Fig. 2A). A trial started when a small fixation spot appeared on the screen. After the monkey maintained fixation on the spot for 1000 ms, the fixation spot disappeared and a peripheral target appeared at either the right or left side, 15° from the fixation spot. The monkey was required to make a saccade to the target within 500 ms. Correct and incorrect saccades were signaled by a tone and a beep 200 ms after the saccade, respectively. Within a block of 24 trials, saccades to one fixed direction were rewarded with 0.3 ml of apple juice while saccades to the other direction were not rewarded. The position-reward contingency was reversed in the next block with no external instruction. Even in the unrewarded trials, the monkey had to make a correct saccade; otherwise, the same trial was repeated. In rewarded trials a liquid reward was delivered which started simultaneously with the tone stimulus.
Electrophysiology
One recording chamber was placed over the midline of the parietal cortex, tilted posteriorly by 40°, and was aimed at the habenula; the other recording chamber was placed over the fronto-parietal cortex, tilted laterally by 35°, and was aimed at the GPi. Single-unit recordings and electrical stimulations were performed using tungsten electrodes (Frederick Haer) that were advanced by an oil-driven micro-manipulator (MO-97A, Narishige). The recording and stimulation sites were determined using a grid system, which allowed recordings at every 1 mm between penetrations. The electrode was introduced into the brain through a stainless steel guide tube, which was inserted into one of the grid holes and then to the brain via the dura. For finer mapping of neurons, we also used a complementary grid, which allowed electrode penetrations between the holes of the original grid. The activity of single neurons was recorded using tungsten electrodes (Frederick Haer Company, Bowdoinham, ME, diameter 0.25mm, 1–3 M Ohm). The signal was amplified with a band-pass filter (200 Hz – 5 kHz) (BAK, Mount Airy, MD) and collected at 1 kHz via custom-made window discriminator (MEX). Single neurons were isolated on-line using a custom voltage-time window discrimination software (MEX, LSR/NEI/NIH).
Antidromic activation and collision
For the stimulation of the LHb, the position of the LHb was mapped first by MRI (4.7T, Bruker). The electrophysiological features of the LHb (Matsumoto and Hikosaka, 2007) were also used to locate the LHb. After finding the LHb, the 1DR task was performed and the multi-unit activity of the LHb was recorded. After finishing the recording, the LHb electrode was connected to the stimulator (S88, Grass Technologies). The stimulating electrode together with the micro-manipulator was shielded with insulated aluminum foil grounded to the dura to attenuate the artifact generated by the stimulation. For stimulation we delivered biphasic negative-positive pulse with 0.2 ms per phase duration between the LHb electrode and the guide tube. The default setting of the stimulation current was 200 μA for the monkeys D and S, and 300 μA for monkey N. When an antidromically activated neuron was found, the current was lowered to examine the threshold current.
While the stimulation was triggered automatically every 0.5 s, the GPi electrode was lowered. The electrode traveled through the Putamen, GPe and GPi. During the periodic stimulations and advancement of the electrode, we examined any sign of spikes occurring at a fixed time after stimulation using a custom-made antidromic software (MEX/LSR/NEI/NIH). When a spike was found that occurred consistently with a fixed latency, we tried to isolate the spike from background activity using a voltage-time window discrimination software (MEX/LSR/NEI/NIH), and then triggered the LHb stimulation by the isolated and spontaneously occurring spike detected at the GPi electrode. The collision test was done by changing the latency between the spontaneous GPi spike and the LHb stimulation. If the stimulation-evoked GPi spike disappeared after decreasing the pre-stimulation latency, the spike was considered to be activated antidromically, provided that this collision latency was longer than the antidromic latency by about 0.3 ms (absolute refractory period). Then, the GPi neuron whose spike was activated antidromically was considered to project to the LHb. We then recorded the activity of the LHb-projecting GPi neuron while the monkey was performing the 1DR task. If the GPi neuron remained stable, we recoded the activity of the GPi neuron and the multi-unit activity of LHb neurons simultaneously.
Histology
The monkey S was used for the anatomical study. Using the antidromic activation and collision test described above, LHb-projecting neurons were identified. Upon the identification, an electrolytic micro-lesion was made by passing a negative current of 13 μA for 40 s. A total of 7 lesions were made and all of the lesion sites were identified after staining. After the conclusion of the experiment the animal was deeply anaesthetized with an overdose of pentobarbital sodium, and perfused with 10% formaldehyde. The brain was blocked and equilibrated with 10% sucrose. Frozen sections were cut every 50 μm in the plane parallel to the electrode penetration into the GPi. The sections were stained with cresyl violet.
Statistical analysis
We defined the post-target response as the average discharge rate during 150–350 ms period after the target onset minus the background discharge rate measured during the 1000 ms before the fixation point appeared. The reward response was defined as the average discharge rate during 150–350 ms after the onset of the tone stimulus (which was synchronized with reward onset if reward was present) minus the background discharge rate. We set the time windows such that they included major parts of the excitatory and inhibitory responses of both LHb and LHb-projecting GPi neurons.
To evaluate the relative contribution of reward contingency (reward or no reward) and target position to the post-target response, we first calculated ‘reward modulation’ and ‘direction modulation’, as following.
Reward modulation = post-target responses on rewarded trials – post-target responses on unrewarded trials (both directions combined).
Direction modulation = post-target responses on contralateral trials – post-target responses on ipsilateral trials (rewarded and unrewarded combined).
Using a two-way ANOVA [target position (contralateral vs. ipsilateral) x reward contingency (reward vs. no reward)] (p< 0.01) we classified LHb-projecting GPi neurons into five groups: 1) reward-only type: neurons that showed a main effect of reward contingency only, 2) position-only type: neurons that showed a main effect of target position only, 3) reward & position type: neurons that showed main effects of reward contingency and target position, 4): reward-position interaction type, and 5) unmodulated type: neurons that showed no main effect. Of the 74 neurons, 5 neurons showed significant interaction effect. See Supplementary Note E and Supplementary Fig. S6 for further details.
Those neurons that showed significant reward modulations (i.e., reward-only type and reward & position type) were further classified into 1) positive type, if their reward modulation had positive values, and 2) negative type, if their reward modulation had negative values. To further verify this classification of negative type and positive type, we compared this with the area under the curve (AUC) values of the ROC (receiver-operator-characteristic). All the positive type neurons had average AUC larger than 0.5, and the negative type neurons had average AUC less than 0.5.
We determined the latency of reward-dependent modulation for each of the three groups of neurons: negative type GPi neurons, positive type GPi neurons, and LHb neurons. First, we quantified for each neuron, at each time point after target onset, how much its activity is different between rewarded trials and unrewarded trials. For this purpose we computed spike density function (SDF) for each trial. Based on the trial-by-trial SDFs, we computed an ROC (receiver-operator-characteristic) value at every 1 ms bin, starting from 1000 ms before target onset till 1000 ms after target onset. Using the two-tailed permutation test, we determined whether the ROC value comparing the rewarded and unrewarded trials was significantly separated from the ROC value based on the shuffled data (p< 0.01, with 1000 permutations). If the significant difference held true for 25 consecutive time bins (25 ms), we judged that the neuron showed significant reward-dependent modulation during the 25 ms period. This method efficiently eliminated occasional blips that reached the significance level (on average, 1% of the examined period is expected to be significant by definition.). Then, for each group of neurons, we counted the number of neurons, at each time bin, that showed reward-dependent modulation. The latency of the reward-dependent modulation for each group of neurons was determined at the time point when the number of neurons that showed the reward-dependent modulation significantly exceeded the control variation level (an upper 1% standard deviation level based on the data during the 1000 ms pre-target period) for at least 25 consecutive time bins (25 ms, because of the same reason described above).
Supplementary Material
Acknowledgments
We are grateful to K.G. Thompson and B.G. Cumming for their help in statistical analysis, M. Johnson, E. Bromberg-Martin and M. Yasuda for helpful comments, M. Matsumoto for providing the single unit LHb data. We also thank M. Smith for his help in histology. This work was supported by the intramural research program of the National Eye Institute.
Footnotes
AUTHOR CONTRIBUTIONS
S.H. and O.H. jointly performed the experiments, conducted the data analyses and wrote the manuscript. O.H. organized this project.
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–3428. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman AR. Primate models of dyskinesia: the experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience. 1987;21:1–40. doi: 10.1016/0306-4522(87)90322-8. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–427. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- Haber SN, Wolfe DP, Groenewegen HJ. The relationship between ventral striatal efferent fibers and the distribution of peptide-positive woolly fibers in the forebrain of the rhesus monkey. Neuroscience. 1990;39:323–338. doi: 10.1016/0306-4522(90)90271-5. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal Ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophysiol. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Iansek R, Porter R. The monkey globus pallidus: neuronal discharge properties in relation to movement. J Physiol. 1980;301:439–455. doi: 10.1113/jphysiol.1980.sp013216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalen P, Strecker RE, Rosengren E, Bjorklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989;492:187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U. Reward value of attractiveness and gaze. Nature. 2001;413:589. doi: 10.1038/35098149. [DOI] [PubMed] [Google Scholar]
- Karadi Z, Faludi B, Lenard L, Czurko A, Niedetzky C, Vida I, Nishino H. Glucose-sensitive neurons of the globus pallidus: II. Complex functional attributes. Brain Res Bull. 1995;37:157–162. doi: 10.1016/0361-9230(94)00268-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Is Avoiding an Aversive Outcome Rewarding? Neural Substrates of Avoidance Learning in the Human Brain. PLoS Biol. 2006;4:e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Laplane D, Levasseur M, Pillon B, Dubois B, Baulac M, Mazoyer B, Tran Dinh S, Sette G, Danze F, Baron JC. Obsessive-compulsive and other behavioural changes with bilateral basal ganglia lesions. A neuropsychological, magnetic resonance imaging and positron tomography study. Brain. 1989;112(Pt 3):699–725. doi: 10.1093/brain/112.3.699. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature. 2002;418:413–417. doi: 10.1038/nature00892. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Smith Y, Parent A. Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J Comp Neurol. 1989;289:36–52. doi: 10.1002/cne.902890104. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007 doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Miao J, Galluzzi S, Beltramello A, Giubbini R, Zanetti O, Frisoni GB. Bilateral pallidal lesions following major haemorrhage: description of a case. J Neurol. 2001;248:806–808. doi: 10.1007/s004150170098. [DOI] [PubMed] [Google Scholar]
- Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, Kleber HD. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- Molinuevo JL, Marti MJ, Blesa R, Tolosa E. Pure akinesia: an unusual phenotype of Hallervorden-Spatz syndrome. Mov Disord. 2003;18:1351–1353. doi: 10.1002/mds.10520. [DOI] [PubMed] [Google Scholar]
- Moriizumi T, Hattori T. Choline acetyltransferase-immunoreactive neurons in the rat entopeduncular nucleus. Neuroscience. 1992;46:721–728. doi: 10.1016/0306-4522(92)90158-x. [DOI] [PubMed] [Google Scholar]
- Parent M, Levesque M, Parent A. The pallidofugal projection system in primates: evidence for neurons branching ipsilaterally and contralaterally to the thalamus and brainstem. J Chem Neuroanat. 1999;16:153–165. doi: 10.1016/s0891-0618(99)00008-3. [DOI] [PubMed] [Google Scholar]
- Parent M, Levesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. The Journal of comparative neurology. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Richardson RT, DeLong MR. Electrophysiological studies of the functions of the nucleus basalis in primates. Adv Exp Med Biol. 1991;295:233–252. doi: 10.1007/978-1-4757-0145-6_12. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Pauls JM, Augath M, Trinath T, Prause BA, Hashikawa T, Logothetis NK. Magnetic resonance imaging of neuronal connections in the macaque monkey. Neuron. 2002;34:685–700. doi: 10.1016/s0896-6273(02)00718-3. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Schweighofer N, Asahi S, Shishida K, Okamoto Y, Yamawaki S, Doya K. Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS ONE. 2007;2:e1333. doi: 10.1371/journal.pone.0001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J Neurophysiol. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Filion M. Responses of pallidal neurons to striatal stimulation in intact waking monkeys. Brain Res. 1989;498:1–16. doi: 10.1016/0006-8993(89)90394-6. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Filion M, Bedard PJ. Responses of pallidal neurons to striatal stimulation in monkeys with MPTP-induced parkinsonism. Brain Res. 1989;498:17–33. doi: 10.1016/0006-8993(89)90395-8. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Kimura H, McGeer EG. A histochemical study of GABA-transaminase in the efferents of the pallidum. Brain Res. 1982;241:162–165. doi: 10.1016/0006-8993(82)91239-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


