Abstract
Nicotine intake constitutes a principal mechanism for tobacco addiction. In addition to primary effects on nicotinic acetylcholine receptors, nicotine has cascading effects, which may underlie its neurobehavioral actions. Nicotine induces serotonin (5-HT) release, which has not classically been thought to be involved in tobacco addiction. However, addiction can be characterized more as a disorder of compulsion than a disorder of enjoyment. 5-HT mechanisms play key roles in compulsive disorders. Nicotine-induced 5-HT release may be a key to tobacco addiction. Ketanserin, a 5-HT2a and 2c receptor antagonist, significantly attenuates nicotine effects on attention and memory. These studies were conducted to determine if ketanserin would reduce nicotine self-administration in rats. Male Sprague- Dawley rats (N=12) were given initial food pellet training and then 10 sessions of nicotine self-administration training (0.03 mg/kg/infusion, i.v.). Then the rats were administered ketanserin (1 or 2 mg/kg, s.c.) or the saline vehicle. Ketanserin (2 mg/kg) significantly decreased nicotine self-administration. This did not seem to be due to sedative or amnestic effects of ketanserin. In a second study, the effects of repeated administration of 2 mg/kg ketanserin (N=11) vs. saline injections (N=10) were examined. In the initial phase, the acute effectiveness of ketanserin in significantly reducing nicotine self-administration was replicated. The effect became attenuated during the following several sessions, but the significant effect became re-established during the final phases of this two-week study. 5-HT mechanisms play critical roles in the maintenance of nicotine self-administration. Better understanding of those roles may help lead to new 5-HT-based treatments for tobacco addiction.
Index Words: Nicotine, Self-administration, Serotonin, 5-HT2, Ketanserin, Rat
1. Introduction
Nicotine self-administration and by extension tobacco addiction has been linked to the neural effect of nicotine-induced dopamine release, particularly via activation of dopamine projections from the ventral tegmental area to the nucleus accumbens, but the this projection is just part of a complex circuit underlying motivated behavior in general and addiction in particular (Corrigall, 1991; Di Chiara, 2000; Di Chiara et al., 2004; Koob, 1999; Mansvelder and McGehee, 2002; Picciotto and Corrigall, 2002). A wide variety of neurotransmitter systems are involved in these neural circuits involving the midbrain, limbic system, basal ganglia and frontal cortex. In addition, dopamine is not the only neurotransmitter system affected by nicotine. Nicotine also induces the release of serotonin, norepinephrine, histamine, GABA, glutamate, and acetylcholine, the endogeneous ligand for nicotinic receptors (Kenny et al., 2000; Li et al., 1998; Wonnacott et al., 1989). The roles of other transmitter systems, in addition to dopamine and acetylcholine, in the neural bases of nicotine reinforcement are important areas of investigation. Discovering their involvement in the neural basis of nicotine self-administration will not only provide a more comprehensive understanding of the neural bases of tobacco addiction, but could lead to the development of new approaches to help people quit smoking.
Often in drug development research, clues to new therapeutic avenues can come from serendipitous findings from people taking medications for other reasons. Antipsychotic drugs taken by people with schizophrenia may provide important clues for new types of smoking cessation treatment. People with schizophrenia have some of the highest smoking rates in our society, double to triple the general population rate (Barnes et al., 2006; Dalack et al., 1998; Hughes et al., 1986). Interestingly, lower smoking is seen with patients with schizophrenia who are taking atypical antipsychotic drugs (Barnes et al., 2006), in particular clozapine (McEvoy et al., 1995). Clozapine is a complex drug with actions on a variety of different neurotransmitter receptors. There may be a subcomponent of clozapine actions that underlies its efficacy in reducing smoking. We have found that clozapine’s serotonin 5-HT2 receptor antagonist effects are particularly relevant to blocking nicotine-induced cognitive enhancement (Levin and Rezvani, 2007).
Nicotine actions on cognitive function have been found in our studies to be reduced by ketanserin, which is an antagonist at 5-HT2A and 5-HT2C receptors. Nicotine-induced improvements in spatial working memory in the radial-arm maze (Levin et al., 2005) and sustained attention in a signal detection operant task (Rezvani et al., 2005) are reversed by ketanserin. The role of 5-HT2 receptors in mediating nicotine actions are clearly seen with cognitive and reinforcing effects, but this action does not seem to extend to all of nicotine’s effects. Damaj et al. did not find ketanserin to attenuate the reduction in activity or antinociceptive effects of nicotine in mice (Damaj et al., 1994).
There has been some work on a possible neural effect that could underlie ketanserin blockade of nicotine effects. Ketanserin blocked nicotine effects of reducing thalamo-cortical high voltage spindles (Jakala et al., 1997). There is evidence that serotonin and serotonergic receptor acting drugs such as ketanserin can act directly on nicotinic receptors. Ketanserin and other serotonergic ligands as well as serotonin itself have been found to block and enhance desensitization of the muscle nicotinic receptor (Garcia-Colunga and Miledi, 1999). It would be interesting to see if this direct action of ketanserin occurs on neuronal nicotinic receptors as well.
The current study examined the interactive role of 5-HT2A and 5-HT 2C receptor systems with nicotine self-administration. Serotonergic systems have been found to play an important role in nicotine dependence (Olausson et al., 2002). In addition to stimulating the release of serotonin (Li et al., 1998),, nicotine may also have direct effects on presynaptic re-uptake of serotonin. There is in vitro evidence that nicotine can increase the activity of the serotonin transporter in frontal cortical neurons (Awtry et al., 2006). Serotonergic projections from the raphe to the VTA inhibit dopaminergic neuronal activity. Serotonin 5-HT2C receptor antagonists enhance mesocorticolimbic dopamine activity and 5-HT2C receptor agonists suppress it and in contrast 5-HT2A receptors appear to play the opposite role with 5-HT2A receptor agonist treatment potentiating nicotine-induced dopamine release (Di Matteo et al., 2002).
Serotonergic and nicotinic systems interact in a variety of ways important for neurobehavioral activity (Seth et al., 2002). Behavioral pharmacology studies have found important roles for 5-HT2 receptor systems in the expression of nicotine effects. The discriminative stimulus effects of nicotine have been found to be reduced by 5-HT2A and/or 5-HT2C receptor agonists (Batman et al., 2005; Zaniewska et al., 2007). The effect of the 5-HT2A/2C receptor agonist DOI in reducing nicotine discrimination was reversed by administration of the 5-HT2A/2C receptor antagonist ketanserin (Batman et al., 2005). The effects of more selective 5-HT2A or 5-HT2C receptor agonists in reducing nicotine discrimination were reversed by the respective receptor antagonists for these receptors (Zaniewska et al., 2007). In addition, the effects of nicotine on locomotion were also Levin et al., 2008 attenuated by 5-HT2A/2C receptor agonist treatment (Batman et al., 2005). The 5-HT2C receptor agonist R0-60-0175 significantly reduced nicotine self-administration, an effect, which was reversed by the 5-HT2C receptor antagonist SB 242,084 (Grottick et al., 2001). In addition, R0-60-0175 also reduced food motivated responding, reduced chronic nicotine-induced sensitization of locomotor hyperactivity and attenuated nicotine-induced improvement of attentional function in the 5-choice serial reaction time task (Quarta et al., 2007).
In the current study we assessed the involvement of serotonergic mechanisms in one of nicotine’s effect, reinforcement as measured by nicotine self-administration. Ketanserin was chosen for investigation because it provides 5-HT2A and 5-HT2C receptor antagonist activity. Individually 5-HT2A and 5-HT2C ligands have both been found to be important for nicotinic action. Combined 5-HT2A and 5-HT2C antagonism with ketanserin has been found to block nicotine-induced cognitive improvement. However, it is not clear what combined 5-HT2A and 5-HT2C receptor blockade would have on nicotine reinforcement. The current study was conducted to determine whether 5-HT2A and 5-HT2C or as well as 5-HT1D blockade with ketanserin may be helpful in reducing nicotine self-administration.
2. Methods
2.1. Subjects
The procedures used in this study were approved by the Duke University Animal Care and Use Committee and conform to the 1996 edition of the Animal Care Guide. Young adult male Sprague-Dawley albino rats (Taconic Farms, Germantown, N.Y., USA) were singly housed in approved standard laboratory conditions in a Duke University vivarium facility near the testing room to minimize any stress- induced by transporting the rats. The day-night cycle was reversed cycle (lights on 18:00-6:00) so that they were in their active phase during behavioral testing. All rats had ad lib access to water and were fed the same type of rat chow once daily throughout the study to keep them at approximately 85% ad lib weight with food amounts adjusted from 8–16 g per day as they grew to provide a lean healthy growth curve.
2.2. Nicotine Preparation and Administration
Solutions of nicotine ditartrate were prepared weekly in pyrogen-free glassware in sterilized isotonic saline. The doses used were calculated as a function of the nicotine base weight. The pH of the solutions was adjusted to 7.0 using NaOH and then the solutions were passed through a 0.22 •m filter (Millipore Corp, Billerica, MA, USA). All solutions were kept refrigerated in the dark between experiments.
2.3. Nicotine Self-Administration
Chronically indwelling intravenous jugular catheters were implanted i.v. under ketamine anesthesia and were flushed daily with a 0.3 ml solution containing 100U/ml heparinized saline (Baxter Health Corporation, Deerfield, IL, USA), and after sessions the nicotine remaining in each port was drawn out and a sterile lock was infused, consisting of heparinized saline 500U/ml with 0.4 mg Gentamicin as an antibiotic. (American Pharmaceutical Partners, Schaumburg, IL, USA). Phenobarbitol injection tests through the catheter were used to verify patency.
For behavioral training, rats were placed in dual lever test chambers (Med-Associates, VT, USA). Each chamber was equipped with a tone generator, house light, cue light above each lever, and a metal tether to cover the drug delivery line. A Pentium computer programmed with MED-PC software controlled experimental events and data collection. Each catheter was connected to a High-Speed Micro Liter Syringe Pump (Med Associates), and tethers made of polyethylene tubing with huber needles for access to ports and catheters (Instech-Solomon, Plymouth Meeting, PA, USA). During each session, the rats wore Covance infusion harnesses to connect them to the tethers.
Initially, the rats were trained daily with 3 tutor sessions, lasting 15 min., to press the levers for food pellet reinforcers. Half the animals were rewarded for responding on the right lever and half for responding on the left. The cue light over the correct lever was illuminated while the light over the incorrect lever was covered (under the tutor program, both cue lights are turned on, but only one cue light is uncovered, to show which lever is correct.) Responses on the correct lever were rewarded by pressing a button, connected to the control panel, which caused immediate delivery of one 45-mg food pellet and activation of the feedback tone for 0.5 sec. There was no timeout in the tutor sessions. These tutor sessions were followed by 3 daily pellet sessions on a FR-1 schedule, with feedback tone, lasting 45 min. This program activated the correct lever and only the cue light above that lever was illuminated. The training procedure was just before the surgery for catheter implantation, which was just before the onset of nicotine access. This has been added to the methods section.
After the pellet sessions, animals had catheters surgically implanted under ketamine anesthesia to provide access for nicotine self-administration by infusion. A plastic SoloPort was attached intraoperatively to a heparin-coated polyurethane catheter and inserted into a subcutaneous interscapular pocket and sutured to underlying fascia. (Instech-Solomon, PA, USA) The following day, the rats began self-administration sessions with nicotine (0.03mg/kg/infusion, i.v.) as the reinforcer.
A lever press on the active side resulted in the activation of the feedback tone for 0.5 sec, the immediate delivery of one 50 µl infusion of nicotine in less than 1 sec. Each infusion was immediately followed by a one-min. timeout in which the house and cue lights went out and responses were recorded but not reinforced. Two levers were available to be pressed in every session of nicotine self-administration. Only one caused the delivery of nicotine the other did not and served as a control. The benchmark infusion dose of nicotine was (0.03 mg/kg/infusion, i.v.).
In study 1, acute doses of ketanserin (1 and 2 mg/kg, s.c.) and the control saline vehicle solution were given in a repeated measures counterbalanced order twice (N=12). The injections were given ten min. before the onset of the session. Consecutive injections were at least 2 days apart. In study 2, in a separate set of rats, after a pretraining of five sessions the rats were randomly assigned to ketanserin and saline control treatment groups based on the average number of nicotine infusions in the presessions. The effective ketanserin dose from the first study (2 mg/kg, s.c.) was given repeatedly before testing for ten days over the course of two weeks (5 days/week). Saline vehicle-treated controls (N=10) and ketanserin-treated rats (N=11) matched for preketanserin nicotine self-administration were administered injections on the same schedule.
2.4. Statistical Analysis
The self-administration data were assessed by analysis of variance. In study 1, a repeated measures design was used with each rat receiving both of the ketanserin doses and the saline vehicle. The baseline for each animal under the saline treatment condition is used as the control as is standard for repeated measures analysis of variance. In study 2, there was a mixed design with a between subjects factor of ketanserin administration (0 vs. 2 mg/kg) and a repeated measures within subject factor of repeated dosing and testing. A blocking factor of pretest infusion rate (above or below the pretest median) was used in the analysis of the ketanserin effect. An alpha level of P<0.05 (two-tailed) was used as a cutoff for statistical significance.
3. Results
3.1. Study 1
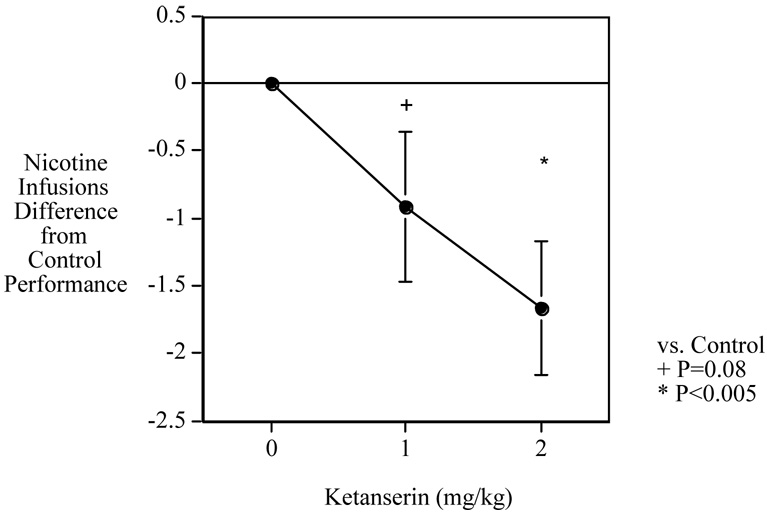
The average number of food pellets earned per session was 60.1±5.4 (mean±S.E.M.). During the 10 sessions of training with nicotine reinforcement the rats averaged 6.09±0.84 infusions per session. The main effect of ketanserin on nicotine self-administration was significant (F(2,22)=5.45, P<0.025). As shown in Fig. 1, comparisons of each dose with control showed that the lower 1 mg/kg ketanserin did not quite cause a significant (P=0.08) decrease in nicotine self-administration, a decrease of 25.5%. The higher 2 mg/kg ketanserin dose did cause a clearly significant (P<0.005) reduction in nicotine self-administration, a decrease of 46.5%. The mean±S.E.M. for the rats with the different ketanserin doses were: Saline=3.58±0.87, Ketanserin 1 mg/kg=2.67±0.87, and Ketanserin 2 mg/kg=1.92±0.95.
Fig. 1. Acute Ketanserin Effects on Nicotine Self-Administration.
Dose-effect function of acute ketanserin attenuating nicotine self-administration in rats (mean± S.E.) difference in performance after ketanserin injection vs. performance of each rat after control saline injection (N=12).
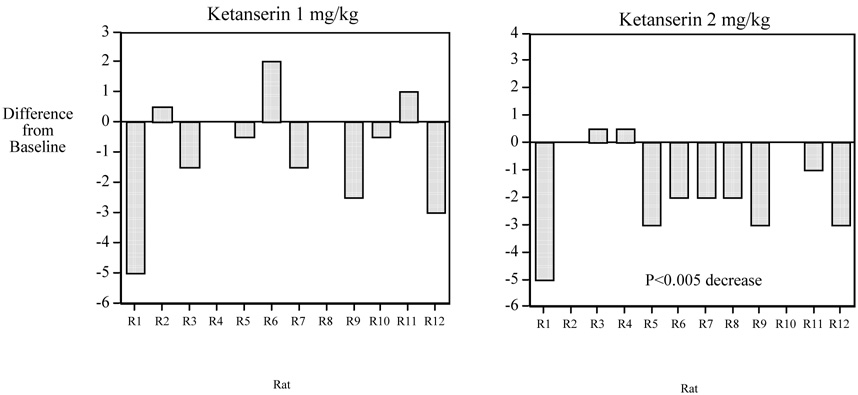
Two-thirds of the subjects showed reductions in nicotine self-administration with 2 mg/kg of ketanserin (Fig. 2). The ketanserin effect seemed to be specific to nicotine self-administration inasmuch as the number of responses on the incorrect lever was not significantly affected by ketanserin.
Fig. 2. Ketanserin Effects on Nicotine Self-Administration in Rats.
Individual animal data for acute ketanserin effects on nicotine self-administration (N=12). Two-thirds of the subjects showed reductions in nicotine self-administration with 2 mg/kg of ketanserin vs. their performance after control saline injections.
3.2. Study 2
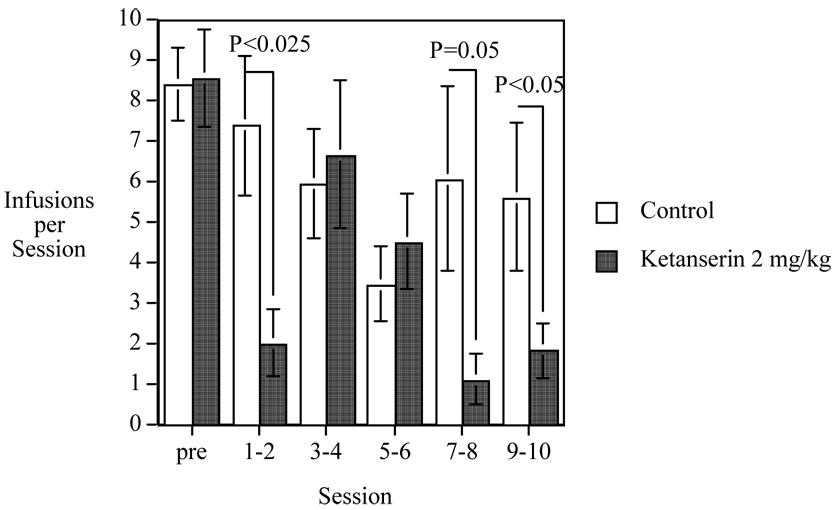
The effect of repeated ketanserin administration on nicotine was assessed. There was a significant interaction of ketanserin treatment × session block (F(4,76)=3.07, P<0.025). Fig. 3 depicts the effect of ketanserin over the course of administration. The analysis of the simple main effects showed that during the first session block (sessions 1–2) there was a significant (P<0.005) ketanserin-induced reduction in nicotine self-administration. This effect was not seen during the middle sessions of the study (sessions 3–6) until it was reestablished during the later stages of testing. During sessions 7–8 there was not quite significant (P=0.054) ketanserin-induced decrease relative to control. During sessions 9–10, the final phase of the study, there was a clearly significant (F(1,17)=5.62, P<0.05) ketanserin-induced reduction in nicotine selfadministration. The controls showed some indication of variation of infusion number through ten-session injection period but this was not significant (P=0.40). In contrast, the ketanserin-treated subjects did show a significant (F(4,40)=3.46, P<0.025) effect of session block with a significant (P<0.025) quadratic trend across sessions reflecting the lessening of the effect in the middle sessions and its re-establishment during the later sessions.
Fig. 3. Chronic Ketanserin Effects on Nicotine Self-Administration.
Chronic ketanserin effects on nicotine self-administration (mean± S.E.) Controls (N=10) and Ketanserin 2 mg/kg (N=11). The pre session average is the mean for the five sessions of training on nicotine self-administration before the randomization of the rats into matched treatment groups.
4. Discussion
The serotonin 5-HT2 receptor antagonist ketanserin significantly reduced nicotine self-administration in rats. The first study identified an acute dose of 2 mg/kg of ketanserin that significantly reduced nicotine self-administration. This dose has not been seen previously to cause sedative effects or to cause cognitive impairment. However, it has been shown to significantly attenuate nicotine-induced improvements in working memory and attentional performance (Levin et al., 2005; Rezvani et al., 2005). The second study in a separate set of rats replicated the finding that acute ketanserin significantly reduces nicotine self-administration. In addition, it extended this finding with tests of the effectiveness of chronic ketanserin administration. Interestingly, there was a biphasic effect of chronic ketanserin. After the initial suppression of nicotine self-administration, ketanserin was not found to be effective for several sessions. Finally, during the final phase of the ten-session test series the effectiveness of ketanserin in significantly reducing nicotine self-administration was re-established.
Other studies have found serotonergic interactions with nicotine self-administration. It is interesting that other studies have found that the discriminative stimulus effects of nicotine were reduced by 5-HT2A and/or 5-HT2C receptor agonists (Batman et al., 2005; Zaniewska et al., 2007), that the effect of the 5-HT2A/2C receptor agonist DOI in reducing nicotine discrimination was reversed by administration of the 5-HT2A/2C receptor antagonist ketanserin (Batman et al., 2005) and that 5-HT2C receptor agonist treatment reduced nicotine self-administration (Grottick et al., 2001). It may be that an optimal serotonergic tone is needed to support nicotine self-administration and that either increases or decreases from this tone can effectively reduce nicotine self-administration.
Principally, research on mechanisms of nicotine reinforcement and tobacco smoking addiction have focused on nicotinic acetylcholinergic receptors as the immediate target of nicotine and dopaminergic projections from the ventral tegmental area to the nucleus accumbens as the reward pathway critical for the action of a variety of abused drugs. However, abused drugs in general and nicotine in particular engage a variety of interconnected neural systems, which may be critical substrates for their abuse potential. Serotonergic mechanisms, particularly 5-HT2C receptors, are key in controlling the activity of dopamine neurons of the ventral tegmental area (Di Matteo et al., 2002). Manipulations of serotonergic systems may provide a way to reduce drug-taking behavior.
Other actions of ketanserin may also underlie the effectiveness of ketanserin in reducing nicotine self-administration. In addition to ketanserin effects on 5-HT2A and 5-HT2C receptors ketanserin also binds to 5-HT1D receptors (Varnas et al., 2001; Wurch et al., 1998), which may also be relevant to nicotine self-administration given their presence in the nucleus accumbens (Bonaventure et al., 1997). Ketanserin also has effects at α-1 adrenergic receptors (Marwood, 1994). This action may also contribute to ketanserin-induced reduction in nicotine self-administration. Prazocin, an α-1 adrenergic receptor antagonist has been found to reduce nicotine self-administration (Villegier et al., 2007).
Ketanserin and similar 5-HT2 antagonists may have broader use in combating drug abuse. Filip et al found that ketanserin significantly attenuates cocaine-induced locomotor activation (Filip et al., 2001). Ketanserin reduces MDMA-induced dopamine release (Nash, 1990). Ketanserin attenuates the discriminative stimulus effects of methamphetamine (Munzar et al., 1999). Ketanserin and similar drugs may be useful in attenuating psychotomimetic effects of lysergic acid diethylamide (LSD) given that it blocks the 5-HT2 receptor site of action of LSD (Munzar et al., 1999).
In conclusion, the results of this study show that the 5-HT2 receptor antagonist ketanserin significantly reduces nicotine self-administration in rats. Previously, we found that ketanserin blocks nicotine effects of memory and attentional improvement (Levin et al., 2005; Rezvani et al., 2005). Drugs acting on 5-HT2 or possibly 5-HT1D receptors may be a new effective means for smoking cessation.
Acknowledgements
This research was supported by an unrestricted grant from Philip Morris USA and by NIDA grant DA015756.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awtry TL, Frank JG, Werling LL. In vitro regulation of serotonin transporter activity by protein kinase A and nicotinic acetylcholine receptors in the prefrontal cortex of rats. Synapse. 2006;59:342–349. doi: 10.1002/syn.20251. [DOI] [PubMed] [Google Scholar]
- Barnes M, Lawford BR, Burton SC, Heslop KR, Noble EP, Hausdorf K, Young RM. Smoking and schizophrenia: Is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Australian & New Zealand J. Psychiat. 2006;40:575–580. doi: 10.1080/j.1440-1614.2006.01841.x. [DOI] [PubMed] [Google Scholar]
- Batman AM, Munzar P, Beardsley PM. Attenuation of nicotine's discriminative stimulus effects in rats and its locomotor activity effects in mice by serotonergic 5-HT2A/2C receptor agonists. Psychopharmacology. 2005;179:393–401. doi: 10.1007/s00213-004-2035-z. [DOI] [PubMed] [Google Scholar]
- Bonaventure P, Schotte A, Cras P, Leysen JE. Autoradiographic mapping of 5-HT1B- and 5-HT1D receptors in human brain using 3H]alniditan, a new radioligand. Receptors & Channels. 1997;5:225–230. [PubMed] [Google Scholar]
- Corrigall WA. Understanding brain mechanisms in nicotine reinforcement. Br. J. Addiction. 1991;86:507–510. doi: 10.1111/j.1360-0443.1991.tb01798.x. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am. J. Psychit. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glennon RA, Martin BR. Involvement of the serotonergic system in the hypoactive and antinociceptive effects of nicotine in mice. Brain Res. Bull. 1994;33:199–203. doi: 10.1016/0361-9230(94)90252-6. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47 Suppl 1:227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Cacchio M, Di Giulio C, Esposito E. Role of serotonin2C receptors in the control of brain dopaminergic function. Pharmacol. Biochem. Behav. 2002:71. doi: 10.1016/s0091-3057(01)00705-5. [DOI] [PubMed] [Google Scholar]
- Filip M, Nowak E, Papla I. On the role of serotonin2A/2C receptors in the sensitization to cocaine. J. Physiol. Pharmacol. 2001;52:471–481. [PubMed] [Google Scholar]
- Garcia-Colunga J, Miledi R. Blockage of mouse muscle nicotinic receptors by serotonergic compounds. Exp. Physiol. 1999;84:847–864. [PubMed] [Google Scholar]
- Grottick AJ, Corrigall WA, Higgins GA. Activation of 5-HT(2C) receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology. 2001;157:292–298. doi: 10.1007/s002130100801. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am. J. Psychiat. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jakala P, Puolivali J, Bjorklund M, Koivisto E, Riekkinen P., Jr Activation of acetylcholine receptors and 5-HT2 receptors have additive effects in the suppression of neocortical high-voltage spindles in aged rats. Psychopharmacology. 1997;132:270–280. doi: 10.1007/s002130050345. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Neal MJ. Evidence for a complex influence of nicotinic acetylcholine receptors on hippocampal serotonin release. J. Neurochem. 2000;75:2409–2414. doi: 10.1046/j.1471-4159.2000.0752409.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann. N. Y. Acad. Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Levin ED, Icenogle L, Farzad A. Ketanserin attenuates nicotine-induced working memory improvement in rats. Pharmacol. Biochem. Behav. 2005;82:289–292. doi: 10.1016/j.pbb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem. Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J. Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Marwood JF. Influence of alpha 1-adrenoceptor antagonism of ketanserin on the nature of its 5-HT2 receptor antagonism. Clin. Exp. Pharmacol. Physiol. 1994;21:955–961. doi: 10.1111/j.1440-1681.1994.tb02657.x. [DOI] [PubMed] [Google Scholar]
- McEvoy J, Freudenreich O, McGee M, Vanderzwaag C, Levin ED, Rose J. Clozapine decreases smoking in patients with chronic schizophrenia. Biol. Psychiat. 1995;37:550–552. doi: 10.1016/0006-3223(94)00365-A. [DOI] [PubMed] [Google Scholar]
- Munzar P, Laufert MD, Kutkat SW, Novakova J, Goldberg SR. Effects of various serotonin agonists, antagonists, and uptake inhibitors on the discriminative stimulus effects of methamphetamine in rats. J. Pharmacol. Exp. Therap. 1999;291:239–250. [PubMed] [Google Scholar]
- Nash JF. Ketanserin pretreatment attenuates MDMA-induced dopamine release in the striatum as measured by in vivo microdialysis. Life Sci. 1990;47:2401–2408. doi: 10.1016/0024-3205(90)90484-9. [DOI] [PubMed] [Google Scholar]
- Olausson P, Engel JA, Soderpalm B. Involvement of serotonin in nicotine dependence: Processes relevant to positive and negative regulation of drug intake. Pharmacol. Biochem. Behav. 2002;71:757–771. doi: 10.1016/s0091-3057(01)00673-6. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J. Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta D, Naylor CG, Stolerman IP. The serotonin 2C receptor agonist Ro-60-0175 attenuates effects of nicotine in the five-choice serial reaction time task and in drug discrimination. Psychopharmacology. 2007;193:391–402. doi: 10.1007/s00213-007-0802-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Caldwell DP, Levin ED. Nicotinic-serotonergic drug interactions and attentional performance in rats. Psychopharmacology. 2005;179:521–528. doi: 10.1007/s00213-004-2060-y. [DOI] [PubMed] [Google Scholar]
- Seth P, Cheeta S, Tucci S, File SE. Nicotinic-serotonergic interactions in brain and behaviour. Pharmacol. Biochem. Behav. 2002;71:795–805. doi: 10.1016/s0091-3057(01)00715-8. [DOI] [PubMed] [Google Scholar]
- Varnas K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the post mortem human brain using [(3)H]GR 125743. Brain Res. 2001;915:47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine selfadministration in rat. Psychopharmacology. 2007;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG. Presynaptic modulation of transmitter release by nicotinic receptors. In: Nordberg A, Fuxe K, Holmstedt B, Sundwall A, editors. Progress In Brain Research. Vol. 79. Elsevier Science Publishers B.V.; 1989. pp. 157–163. [DOI] [PubMed] [Google Scholar]
- Wurch T, Colpaert FC, Pauwels PJ. Chimeric receptor analysis of the ketanserin binding site in the human 5-Hydroxytryptamine1D receptor: importance of the second extracellular loop and fifth transmembrane domain in antagonist binding. Mol. Pharmacol. 1998;54:1088–1096. doi: 10.1124/mol.54.6.1088. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegalinski E, Filip M. Effects of the serotonin 5-HT2A and 5-HT2C receptor ligands on the discriminative stimulus effects of nicotine in rats. Eur. J. Pharmacol. 2007;571:156–165. doi: 10.1016/j.ejphar.2007.05.067. [DOI] [PubMed] [Google Scholar]