Summary
Lipid rafts are specialized membrane microdomains that serve as organizing centers for assembly of signaling molecules, influence membrane fluidity and trafficking of membrane proteins, and regulate different cellular processes such as neurotransmission and receptor trafficking. In this article, we provide an overview of current methods for studying lipid rafts and models for how lipid rafts might form and function. Next, we propose a potential mechanism for regulating lipid rafts in the brain via local control of cholesterol biosynthesis by neurotrophins and their receptors. Finally, we discuss evidence that altered cholesterol metabolism and/or lipid rafts play a critical role in the pathophysiology of multiple CNS disorders, including Smith-Lemli-Opitz syndrome, Huntington, Alzheimer's, and Niemman-Pick Type C diseases.
Keywords: Lipid rafts, cholesterol, growth factors (neurotrophins), brain, myelin, neurodegenerative disorders
Introduction
The role of cholesterol in cellular function has come under intense scrutiny with the advent of the lipid raft hypothesis (Simons and Ikonen 1997; Simons and Ikonen 2000; Maxfield and Tabas 2005; Michel and Bakovic 2007). Lipid rafts are commonly defined as cholesterol- and sphingolipid-enriched membrane microdomains that function as platforms that concentrate and segregate proteins within the plane of the bilayer (Simons and Ikonen 1997; Pike 2006). For the purposes of this discussion, we define caveolae as a subset of lipid rafts characterized by the presence of caveolin (Fielding 2001). Since the advent of the raft hypothesis, the list of putative raft-mediated events has grown at a rapid pace. Initially proposed to function primarily in protein sorting events in polarized cells, lipid rafts are now thought to regulate membrane trafficking in both the exocytic and endocytic pathways, cell migration, and a variety of cell signaling cascades (Brown and London 1998; Simons and Toomre 2000). Lipid-lipid interactions are thought to be of fundamental importance to the formation of lipid rafts, with cholesterol playing a special role as the “glue” that holds these domains together (Barenholz 2002). Cell membranes are thought to contain both raft and non-raft domains, and proteins in turn preferentially associate with one type of domain or the other. Thus, the presence of lipid rafts is thought to enhance certain protein-protein interactions (for proteins contained within the same raft for example) while inhibiting others (by segregating raft- and non-raft proteins). The specialized lipid composition of lipid rafts, characterized by an enrichment of cholesterol and sphingolipids, may also influence protein function directly by modulating the properties of the membrane bilayer.
Although the human brain only accounts for about 2% of total body weight, it contains as much as 25% of cholesterol and cholesterol derivatives (Dietschy and Turley 2001; Dietschy and Turley 2004). Given the abundance of cholesterol in the brain, it is only natural to ask what function lipid rafts play in the CNS. Lipid rafts are proposed to be critical for normal functioning of the brain, and are found in both neuronal and glial cells (Tsui-Pierchala et al. 2002; Gielen et al. 2006; Debruin and Harauz 2007). Lipid rafts serve as sites where the regulated intramembrane proteolysis of many transmembrane proteins take place, including amyloid precursor protein, ErbB4, deleted in colorectal cancer, Delta 1 and Jagged 2, and p75 neurotrophin receptor (Landman and Kim 2004; Vetrivel et al. 2005). Other proposed functions of lipid rafts in the nervous system include neuronal signaling, neuronal cell adhesion, and axon guidance (Paratcha and Ibanez 2002; Golub et al. 2004; Kamiguchi 2006; Michel and Bakovic 2007). The presence of various ionotropic receptors and neurotransmitter transporters within rafts suggests a critical role of rafts in controlling neurotransmission (Tillman and Cascio 2003). In this regard, cholesterol content of the rafts has a potential to effectively control the ion conductance and excitability of membranes, trafficking of ionotropic receptors to and from the cell membrane, size and number of some postsynaptic receptor clusters (such as NMDA and GABAa), and neurotransmitter signaling through G-protein coupled receptors (Stetzkowski-Marden et al. 2006; Willmann et al. 2006; Besshoh et al. 2007; Huang et al. 2007).
Lipid rafts consist of both protein and lipid components, and are thought to exist in continuity with non-raft regions of membrane. This has made their identification and characterization particularly challenging. As such, many details of the structural properties of lipid rafts, and indeed their very existence, are hotly debated (Munro 2003; Hancock 2006; Jacobson et al. 2007). We thus begin our discussion with an overview of methods currently used to study lipid rafts as well as models for how lipid rafts form and function. Next, we outline a potential mechanism for regulating lipid rafts in the brain via local control of cholesterol biosynthesis by neurotrophins and their receptors. Finally, we discuss the consequences of deficits in cholesterol production/metabolism on human brain function, which may be linked to alterations in lipid raft function (Moebius et al. 2000; Nwokoro et al. 2001; Maxfield and Tabas 2005).
Methods for studying lipid rafts in cells and artificial membranes
The earliest results supporting the lipid raft model used a biochemical approach to isolate a detergent-resistant membrane (DRM) fraction from polarized epithelial cells. This fraction was enriched in (glyco)sphingolipids, cholesterol, and glycosylphosphatidylinositol (GPI)-anchored proteins (Brown and Rose 1992). Subsequent studies developed non-detergent based raft isolation methods (reviewed in (Pike 2003)). Biochemical fractionation, either with or without detergent, remains by far the most widely used technique to isolate lipid rafts and their associated proteins (Brown 2006). By this criterion, lipid rafts are abundant at the cell surface and are enriched in cholesterol and sphingolipids and contain a variety of proteins. A number of sorting signals that target proteins to DRMs have been identified. These include binding to raft-associated lipids such as sphingolipids or cholesterol, specific regions of the transmembrane domain, GPI-anchors, and other lipid modifications of peripheral membrane proteins such palmitoylation and myristoylation (Brown 2006). Moreover, the association of proteins with lipid rafts can either be constitutive or transient (Lucero and Robbins 2004).
Lipid-lipid interactions are one of the major mechanisms thought to underlie lipid raft formation (Simons and Ikonen 1997; Rietveld and Simons 1998; Simons and Vaz 2004). In particular, DRMs are thought to represent liquid-ordered (Lo) domains, which coexist in the same membrane with liquid disordered (Ld) domains (Schroeder et al. 1994; Brown and London 1998). Lo domains form due to the preferential interactions of sphingolipids and/or phospholipids containing saturated acyl chains with cholesterol (Brown and London 1998; Rietveld and Simons 1998). In this state, lipids are tightly packed such that their order is close to that observed in the gel state, yet can undergo rapid lateral diffusion (Brown and London 1998). The characteristic features of liquid ordered domains, as well as the phase behavior of mixtures of liquid-ordered and liquid-disordered domains, can be easily studied in simple mixtures of purified lipids (London 2002; Silvius 2003; Veatch and Keller 2005). A great attraction of these artificial membrane systems is that the domains that form are often micron-sized in scale and can thus be directly visualized with fluorescent probes that show a preference for one phase (Brown 2001). In addition, their composition can be readily manipulated, enabling studies of the effects of natural sterols, sterol derivatives, and cholesterol precursors on liquid ordered domain formation (Xu and London 2000; Xu et al. 2001; Megha et al. 2006).
Since cholesterol is required for the formation of Lo domains, removal of cholesterol from membranes would likewise be expected to cause the loss of lipid rafts (Simons and Toomre 2000; Edidin 2003; Pike 2003). Thus, cholesterol depletion has also become widely used as an assay to study lipid rafts. In cells, cholesterol depletion can be accomplished through sequestration of cholesterol by cholesterol-binding compounds, removal of cholesterol from the membrane using methyl-beta-cyclodextrin, and/or inhibition of cholesterol biosynthesis. Consistent with cholesterol's role in enabling lipid raft formation, cholesterol depletion often causes a loss of raft proteins from DRMs. In addition, cholesterol depletion has also been used in functional assays; pathways that are inhibited by cholesterol depletion are defined as raft-dependent (Simons and Toomre 2000; Edidin 2003; Pike 2003).
Although biochemical fractionation and cholesterol depletion remain among the most widely used techniques to define the raft-associated proteins and the function of rafts in cells, a number of questions have been raised about the validity of these approaches (Munro 2003; Shogomori and Brown 2003; Lichtenberg et al. 2005; Zidovetzki and Levitan 2007). In some instances, clustering of raft proteins in microdomains can be detected by electron microscopy. The best studied examples include Ras, influenza hemagglutinin, and components of the high affinity IgE receptor signaling pathway (Wilson et al. 2002; Parton and Hancock 2004; Wilson et al. 2004; Hess et al. 2005)(Takeda et al. 2003). In addition, large-scale lipid phase separation can be observed in plasma membrane blebs (Baumgart et al. 2007). Although these approaches have provided valuable information about lipid rafts and related membrane microdomains, their use is limited to fixed or perturbed cells. In living cells, lipid rafts have been exceptionally difficult to visualize.
Due to the difficulties of directly imaging lipid rafts in live cell membranes, biophysical techniques sensitive to protein and lipid dynamics and domain organization have instead been used to explore their properties (Figure 1). Such techniques can be used to monitor raft-related events with high spatial and temporal resolution without perturbing the integrity of the membrane (Lommerse et al. 2004; Jacobson et al. 2007). One such approach is fluorescence (or Förster) resonance energy transfer (FRET), used to test the hypothesis that raft proteins or lipids are enriched in domains with sub-micron dimensions (reviewed in (Kenworthy 2002; Lommerse et al. 2004; Lagerholm et al. 2005; Rao and Mayor 2005; Silvius and Nabi 2006)). In this technique, proteins or lipids of interest are labeled with fluorophores known as a donor and acceptor. FRET occurs when the donor and acceptor-labeled molecules are within ∼100 Å of one another. The extent of FRET is quantified in terms of the efficiency of energy transfer. This is typically detected by monitoring changes in the fluorescence of the donor fluorophores in the presence or absence of the acceptor or by looking for emission of the acceptor when exciting at the donor wavelength. Alternatively, it can be monitored by following changes in the polarization of fluorescence under conditions where homoFRET, i.e. FRET in which the same type of fluorophore serves as both donor and acceptor, is measured.
Figure 1. Properties of lipid rafts that can be probed by FRET and diffusion-based measurements.
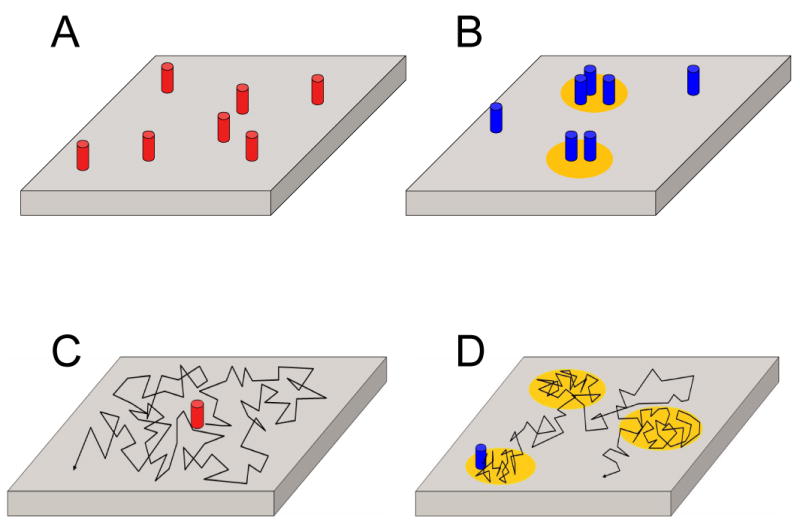
Bulk membrane is depicted in gray, lipid rafts in yellow, raft-associated proteins in blue, and proteins that exist in the absence of rafts in red. The movement of proteins by diffusion within the plane of the membrane is indicated by the black lines. (A) In the absence of lipid rafts, a random protein distribution is expected. This gives rise to either no FRET or a FRET signal that shows a characteristic dependence on the surface density of labeled proteins. A similar result would be expected for proteins that are neither enriched nor excluded from lipid rafts. (B) The association of proteins with lipid rafts brings them within FRET proximity. For FRET to be able to detect such domains, at least one donor-labeled and one acceptor-labeled protein must localize within the same lipid raft. (C) For proteins undergoing classical free diffusion, diffusion is not hindered in any way. (D) One way that lipid rafts can impact protein diffusion is by transiently confining them to liquid ordered domains. This can be detected experimentally by testing for cholesterol-dependent, confined diffusion.
Importantly, the efficiency of FRET is very strongly distance dependent, falling off as the inverse of the 6th power of the distance between the labeled molecules. Thus, FRET provides a convenient way to determine the proximity of two molecules to one another-- for example, their colocalization within the same lipid raft-- over distances smaller than could be measured by conventional fluorescence microscopy (Figure 1). For proteins and lipids in membranes, FRET can occur by chance if the concentration of donors and acceptors is high enough. However, it is possible to distinguish this type of “non-specific” FRET from FRET that occurs as the result of clustering of proteins within lipid rafts, as well as to test various models of domain organization, with the aid of mathematical modeling (Kenworthy and Edidin 1998; Sharma et al. 2004; Kiskowski and Kenworthy 2007).
Based on FRET criteria, some raft proteins show little evidence for clustering in FRET studies (Kenworthy and Edidin 1998; Kenworthy et al. 2000; Glebov and Nichols 2004), Others appear to be clustered in sub-micron domains, as predicted by the lipid raft model (Varma and Mayor 1998; Zacharias et al. 2002; Nichols 2003; Sharma et al. 2004). Interestingly, detailed analysis of such FRET data has shown that only a small fraction of GPI-anchored proteins are found in clusters and that these clusters consist of only a few molecules (Sharma et al. 2004). These findings raise the possibility that raft proteins spend much of their time in a monomeric state and that the fraction of proteins associated with lipid rafts may be highly regulated. It remains to be determined if this type of domain organization is specific to GPI-anchored proteins or a common feature of various types of raft-associated proteins. How the fraction of proteins associated with lipid rafts is regulated also is currently unknown. Further studies of the sub-micron distribution of additional raft proteins should help to resolve these issues. Finally, in addition to providing information about the size and area fraction of lipid rafts, FRET also holds great potential to reveal transient interactions between raft-associated molecules, as illustrated by a recent FRET study of dynamic events that occur during B-cell signaling (Sohn et al. 2006).
Lipid rafts have also been hypothesized to impact the ability of membrane proteins to diffuse freely within the plane of the membrane (Kenworthy 2005) (Figure 1). For example, protein diffusion could be slowed within the specialized liquid-ordered environment of lipid rafts. Other models suggest that proteins may be transiently associated with lipid rafts, especially if the rafts are crosslinked (Dietrich et al. 2002; Shvartsman et al. 2003; Chen et al. 2006). Alternatively, proteins may diffuse as part of a raft complex (Pralle et al. 2000). These effects have been studied using techniques sensitive to lateral diffusion such as fluorescence recovery after photobleaching (FRAP), fluorescence correlation spectroscopy (FCS), and single molecule tracking (reviewed in (Lommerse et al. 2004; Kenworthy 2005; Lagerholm et al. 2005)).
FRAP measures the diffusion of a population of fluorescently labeled molecules by monitoring the recovery of fluorescence into a small region after subjecting it to a rapid bleaching event, via exchange with molecules in the surrounding region by diffusion (Chen et al. 2006). The speed of recovery is characterized by a diffusion coefficient (units of length2/time, typically cm2/s or μm2/s), and the extent of recovery provides a measure of the fraction of molecules that are free to diffuse or mobile fraction. Differences in these parameters between raft and non-raft proteins, or changes as the result of cholesterol depletion are used to probe for the association of proteins with lipid rafts versus non-raft domains (Niv et al. 2002; Kenworthy et al. 2004). On the basis of such experiments, caveolae have been shown to be quite stable and immobile (van Deurs et al. 2003). On the other hand, under steady state conditions, raft proteins can diffuse relatively rapidly, suggesting lipid rafts are dynamic structures (Kenworthy et al. 2004). Interestingly, in polarized epithelial cells, the diffusion of non-raft proteins, but not raft proteins, is highly temperature dependent (Meder et al. 2006). This suggest that the plasma membrane of these cells consists of dispersed non-raft domains separated by a connected raft phase at low temperatures, and conversely, dispersed raft domains separated by connected non-raft domains at 37° C (Meder et al. 2006). However, because FRAP is a population-based measurement, it cannot provide detailed information about the mechanisms that lead to such changes in either bulk diffusion coefficients or mobile fractions at the level of single molecules.
Single particle tracking follows the behavior of individual protein and lipid molecules and thus gain insights into the ways in which their diffusion is regulated. Here, very sensitive detectors are required to enable the visualization of single molecules and tracing of their movements. Interpretation of the resulting trajectories also requires considerable analysis (Ritchie and Kusumi 2003; Chen et al. 2006). Nevertheless the outcomes of such experiments can be extremely informative. One question that can be addressed, for example, is whether the relative diffusion of a pair of proteins is correlated in a manner consistent with their confinement to rapidly diffusing, small domains (Vrljic et al. 2002). Other single particle tracking studies have addressed the question of how GPI-anchored proteins, a class of raft-associated molecules, behave upon crosslinking by multivalent antibodies, a stimulus known to induce intracellular signaling pathways by poorly understood mechanisms (Chen et al. 2006; Suzuki et al. 2007; Suzuki et al. 2007). Impressively, such studies allow one to trace out the signaling pathways involved by monitoring the recruitment of individual signaling proteins to sites of crosslinking. Protein diffusion during T-cell signaling, another example of a signaling event thought to involve lipid rafts, has also been investigated using single molecule techniques. This approach revealed an unexpected role of protein-protein networks in regulating the enrichment or exclusion of proteins from sites of T-cell signaling (Douglass and Vale 2005).
FCS monitors the movement of small numbers of fluorescent molecules through a very small observation volume (Elson 2004). Autocorrelation analysis of the resulting fluorescence fluctuations over time provides information about the number of molecules passing through the volume and their characteristic residence time, which is related to their diffusion coefficient. In the lipid raft field, FCS has proven to be especially useful in studies in artificial membranes, but can also be applied to cells (Kahya and Schwille 2006). A variation on FCS called cross correlation jointly monitors the movement of two different colors of fluorescent molecules simultaneously. Using this technique, it is possible to test for cross-correlations indicative of molecule diffusing together as a complex. This type of analysis holds great potential for elucidating molecular interactions that occur during events such as cell signaling mediated by raft-associated proteins and lipids (Larson et al. 2005).
In addition to these approaches, new methods are also being brought to bear to study lipid rafts in cells, including techniques sensitive to membrane ordering (Gaus et al. 2006; Davey et al. 2007) and superresolution techniques such as fluorescence photoactivation localization microscopy that enable localization of molecules with sub-diffraction precision (Hess et al. 2007). Thus, the arsenal of biophysical tools to investigate lipid rafts and related membrane domains is continuing to grow.
Molecular mechanisms underlying raft formation and function
Lipid rafts are hypothesized to function by a process of compartmentalization (Simons and Ikonen 1997; Pierce 2002; Manes et al. 2003; Pike 2003; Parton and Hancock 2004). In this model, interactions of molecules within the same raft are enhanced, while interactions of non-raft and raft molecules are inhibited due to their spatial segregation.
But, how does segregation of proteins into raft and non-raft domains occur? As already introduced, the most commonly cited model suggests that lipid rafts correspond to Lo domains that coexist with Ld domains. In turn, different raft proteins exhibit varying “affinities” for rafts, reflected by the extent to which they associate with Lo domains (Brown 2006). To test this idea, recent in vitro studies have begun to examine the partitioning of peptides and/or proteins into Lo versus Ld domains (Hammond et al. 2005; Vidal and McIntosh 2005). However, partitioning is not the only mechanism by which lateral compartmentalization of proteins could be achieved, and it is not yet clear that this is the most appropriate model to describe the behavior of raft proteins and lipids in cell membranes. For example, some proteins expected to associate with Lo domains on the basis of their association with DRMs in cells show little partitioning when studied in liposomes (Shogomori et al. 2005). With the recent development of methods to examine lipid domain segregation in plasma membrane blebs, it should be possible to begin to examine this model in more detail (Baumgart et al. 2007; Sengupta et al. 2007).
Other models have focused on explaining how rafts can be functional if they are small and transient structures. One of these, the lipid shell model, suggests that individual “raft” proteins are surrounded by a shell of raft lipids, giving rise to their characteristic association with detergent-resistant membrane fractions (Anderson and Jacobson 2002; Jacobson et al. 2007). The lipid shell model allows for the existence of raft proteins as monomers, yet also suggests the shells could function to target proteins to pre-existing domains/stable domains such as caveolae, or form larger domains via regulated self-assembly and/or crosslinking. Yet another model is suggested by the observations that the clustering of certain raft proteins occurs in a concentration-independent fashion (Sharma et al. 2004). This indicates that the fraction of proteins associated with domains is constant over a wide range of expression levels, a result that implies these domains are actively regulated by the cell. The possibility that protein-protein or protein-lipid interactions stabilize small transient domains and/or induce the formation of larger, longer-lived entities has been proposed by a number of groups (Kusumi et al. 2004; Kusumi and Suzuki 2005; Hancock 2006; Jacobson et al. 2007). Finally, the possibility that cell membranes exist close to a phase boundary and thus small changes in lipid composition could drive raft assembly or disassembly has also been suggested (Keller et al. 2004; Veatch and Keller 2005).
To date, most studies have focused on the role of cholesterol in driving the formation of lipid rafts. It is clear however that the presence of cholesterol in lipid rafts should have physical consequences on the membrane as well, by influencing membrane thickness, elasticity, and even curvature (Allende et al. 2004; Bacia et al. 2005). These structural consequences of cholesterol enrichment may in turn impact the ability of certain proteins to associate with lipid rafts, as illustrated by studies of the sorting of peptides between liquid ordered and liquid disordered domains in vitro (McIntosh et al. 2003). Protein activity may additionally be modulated by the specialized lipid environment of rafts (Pike 2003).
In summary, current models differ in their proposed details of the molecular mechanisms underlying lipid raft formation. Nevertheless, most models agree that cholesterol is a critical regulator of lipid raft function. Thus, local regulation of cholesterol levels via control of cholesterol biosynthesis could represent a powerful mechanism to control raft-dependent events such as signaling and trafficking. Below, we discuss evidence that such a regulatory mechanism may in fact occur in the brain, through a feedback loop that links regulation of the cholesterol biosynthesis machinery and growth factor function.
Regulation of CNS cholesterol biosynthesis: a novel mechanism for regulation of lipid rafts?
Cholesterol plays a wide variety of roles in the CNS, most notably as a precursor for steroid hormones and myelin, in addition to its potential role in forming lipid rafts (Simons and Ikonen 2000; Dietschy and Turley 2001; Fielding 2001). Cholesterol biosynthesis starts with acetyl-CoA as a substrate and involves at least 20 enzymes (Dietschy and Turley 2001; Dietschy and Turley 2004). The rate-limiting enzyme in cholesterol biosynthesis is 3-hydroxy-3-methylglutaryl-coenzyme A reductase (Hmgcr; EC 2.3.3.10) which catalyzes conversion of Hmg-CoA to mevalonic acid. In addition to cholesterol biosynthesis, the cholesterogenic pathway plays a pivotal role in the synthesis of many bioactive compounds, including four key intermediates: mevalonate, farnesyl pyrophosphate, squalene, and lanosterol. Mevalonate is the first committed intermediate in the synthesis of dolichols that are carriers for addition of carbohydrate chains to glycoproteins. Farnesyl pyrophosphate is an important precursor for nonsterol isoprenoids geranylgeranyl pyrophosphate, heme A, and ubiquinone. The first dedicated step in de novo cholesterol biosynthesis begins with formation of squalene and ends with the reduction of 7-dehydrocholesterol by 7-dehydrocholesterol reductase (Dhcr7; EC 1.3.1.21) into cholesterol (Bae et al. 1999).
All of brain cholesterol is synthesized locally, with the highest rate of synthesis occuring during first postnatal weeks in humans and rodents (Jurevics and Morell 1995; Jurevics et al. 1997), and this time window corresponds to the peak of myelination process. Myelin, produced by oligodendrocytes, ensheathes axons and is critical for the conduction of action potential along the neurons (Miller 2002). It has high lipid and cholesterol content, which are critical for its insulating function. Disturbances in lipid homeostasis result in altered CNS structure and function: deficient cholesterol biosynthesis in oligodendrocytes delays myelination (Saher et al. 2005), while altered biosynthesis of other lipids (e.g. galactolipids) give rise to defects in myelination (Marcus and Popko 2002). These findings indicate that development and maintenance of myelin membranes require a complex interplay of lipids and proteins, and membrane rafts may play a critical role in this process (Gielen et al. 2006; Debruin and Harauz 2007)(Colognato et al. 2002).
While there is clear role of oligodendrocytes in myelin formation, the respective roles of neurons and glial cells in cholesterol biosynthesis in the adult nervous system are poorly understood (Pfrieger 2003). Although the prevailing view is that in the adult nervous system astrocytes produce brain-derived cholesterol (Pfrieger 2003), there have been suggestions that neuronal cells, in addition to glial cells, are also capable of cholesterol synthesis (Saito et al. 1987; Suzuki et al. 2007). A detailed map of the expression of cholesterogenic enzymes in the adult nervous system is not yet available. However, our recent study revealed that adult cortical, cholinergic and hippocampal neurons express high transcript levels of Hmgcr and Dhcr7 (Korade et al. 2006). The co-expression of the first and last enzymes of the cholesterogenic pathway in cortical, hippocampal, and cholinergic neurons (Korade et al. 2006) suggests that these neurons may require high levels of endogenous cholesterol production for their normal function. There are also regional differences in the distribution profile of cholesterol-synthesizing enzymes (Ness 1994; Bae et al. 1999), sterol-sensing factors, intracellular transporters, lipoprotein receptors, and cholesterol shuttle proteins (Stockinger et al. 1998; Ong et al. 2000; Ong et al. 2004). Considering the complexity of adult nervous system, it is not surprising that different brain regions show marked differences in cholesterol content (Zhang et al. 1996). In particular, hippocampal and cortical neurons show different intrinsic concentrations of cholesterol (Ko et al. 2005). This difference is also present in the detergent resistant membranes, suggesting that hippocampal neurons are also enriched in membrane rafts. Furthermore, the data suggest that cholesterol is a potent regulator of in vitro neuronal morphology: increased cholesterol levels lead to less complex dendritic arborization (Ko et al. 2005). Support for this view also comes from an independent study by Pooler et al., in which they report that a Hmgcr inhibitor, pravastatin, enhanced neurite outgrowth, neurite length, and neurite branching in cultured hippocampal neurons (Pooler et al. 2006).
In addition to building evidence for regional differences in cholesterogenic enzyme expression, several recent lines of evidence suggest that their expression may be dynamically regulated by neurotrophic factors. Neurotrophic factors exert multiple biological functions in the nervous system. They are essential for the development of the brain; they promote survival and differentiation and are important for maintenance of synaptic contacts (Chao 2003). A recent study by Suzuki et al, (Suzuki et al. 2007), showed that cortical and hippocampal neurons grown in culture for several days increase amount of cholesterol and this is due to increased expression level of cholesterol-synthesizing enzymes. Importantly, these investigators found that brain-derived neurotrophic factor (BDNF) regulates expression of cholesterogenic enzymes specifically in neurons but not in glial cells. Furthermore, this de novo synthesized cholesterol was incorporated into lipid rafts and BDNF stimulation lead to increased level of caveolin-2 and presynaptic proteins in rafts but not in nonrafts. The neurotrophic effects on the cholesterol/lipid raft system appear to be mediated through both the specific (TrkB) (Suzuki et al. 2007) and common (p75) neurotrophin receptors (Korade et al. 2006). Blocking TrkB prevented increase in cholesterol (Suzuki et al. 2007). Our recent studies revealed that p75 is a potent regulator of cholesterogenic enzyme expression in neuronal cultures and several human neuroblastoma cell lines (Korade et al. 2006).
Overexpression of GDNF in neuronal progenitor cells was shown to regulate four enzymes of the cholesterol-biosynthesis pathway but it is not known if these changes lead to alterations of lipid rafts (Pahnke et al. 2004). Similarly, microarray analysis of NGF-induced gene expression in PC12 cells revealed changes in cholesterol biosynthesis enzymes (Lee et al. 2005).
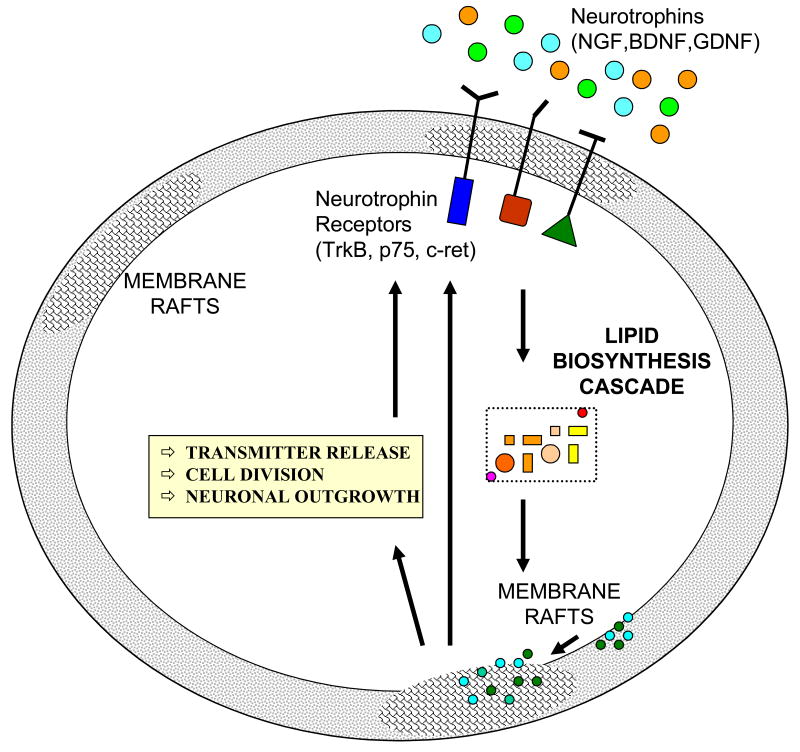
All of these studies revealed that neurotrophins and their receptors are involved in regulation of cholesterol metabolism. A simplified model of this regulation is shown in Figure 2. Binding of neurotrophins leads to activation of their respective receptors and transcriptional regulation of lipidogenic enzymes in the nervous tissue. The altered lipid biosynthesis will lead to altered organization/composition of lipid rafts and changes in neurotransmitter release, cell division, and neuronal outgrowth. The growth factor-membrane raft interaction is a two-way street. Not only do growth factors regulate membrane raft structure, but membrane rafts are also essential for growth factor signaling. Growth factor receptor tyrosine kinases EGFR, Trk and PDGFR are enriched in lipid rafts and other receptors, normally located outside the rafts (e.g. Ret), can be recruited to the raft by GPI-anchored co-receptors (Paratcha and Ibanez 2002; Tsui-Pierchala et al. 2002; Kamiguchi 2006). Therefore, disruption of rafts is likely to impair signaling via these systems. Much more work is needed to define the consequence of this altered cholesterol biosynthesis on lipid raft composition and function.
Figure 2. Proposed feed-forward and feedback regulation of the lipid raft – neurotrophin signaling cascade.
Binding of neurotrophins leads to activation of their respective receptors and transcriptional regulation of lipidogenic enzymes in the nervous tissue. The altered lipid biosynthesis will lead to altered organization/composition of lipid rafts and changes in neurotransmitter release, cell division, and neuronal outgrowth. Finally, altered microdomain structure will also affect the insertion and signaling of the neurothrophin receptors, thus completing the lipid-raft/neurotrophin regulatory cycle.
Human conditions with altered cholesterol biosynthesis: consequences of raft dysfunction?
Cholesterol is not only important for normal brain function but also has been linked to CNS pathophysiology. Cholesterol disturbances in the CNS appear to lead to brain abnormalities (microcephaly, macrocephaly and mental retardation). Here, we discuss evidence that altered cholesterol metabolism and/or lipid rafts play a critical role in the pathophysiology of multiple CNS disorders, including Smith-Lemli-Opitz syndrome (Jira et al. 2003), Huntington's (Valenza et al. 2005), Alzheimer's (Wellington 2004; Wolozin 2004), and Niemman-Pick Type C (Vance et al. 2005) diseases. However, at the present time it is unclear which of these disturbances are mediated through raft-dependent mechanisms.
Reduced or absent Dhcr7 leads to Smith-Lemli-Opitz syndrome (SLOS), which is characterized by developmental deformities, incomplete myelination, and mental retardation (Jira et al. 2003). In SLOS, the tissue cholesterol and total sterol levels are markedly reduced while the concentrations of 7-dehydrocholesterol are greatly elevated (Jira et al. 2003). This elevation in 7-dehydrocholesterol inhibits the activity of Hmgcr, thus further exacerbating the cholesterol deficit (Honda et al. 1998; Fitzky et al. 2001). Importantly, as 7-dehydrocholesterol can incorporate into lipid rafts and alters the protein composition within it (Keller et al. 2004), at least some of the disturbances seen in SLOS can be due to the altered function of the lipid rafts. The greatly increased level of 7-dehydrocholesterol leads to increased membrane fluidity and decreased intermolecular packing of phospholipids fatty acyl chains (Vainio et al. 2006; Rog et al. 2007; Samuli Ollila et al. 2007). Membrane X-ray diffraction studies suggest that the minor difference between the structure of cholesterol and 7-dehydrocholesterol leads to significant differences in membrane lipid organization and dynamics (Xu et al. 2001). 7-dehydrocholesterol differs from cholesterol in a double bond at the 7th position in the sterol ring, and this appears to be an important structural difference that translates into a functional difference: depletion of cholesterol from hippocampal membranes and replenishment with 7-dehydrocholesterol does not restore ligand-binding activity of the serotonin 1A receptor, despite the recovery of the overall membrane order (Singh et al. 2007). Based on the clinical symptoms of SLOS and our own findings of high neuronal expression of cholesterogenic enzymes (Korade et al. 2006), we propose that altered neuronal synthesis of cholesterol may be an important contributing factor to the SLOS pathophysiology, and that these events may involve altered membrane raft events.
In Huntington's disease, the expanded glutamate tract in the huntingtin protein leads to reduced cholesterol biosynthesis and neuronal cell death (Valenza et al. 2005). Specifically, the expression level of cholesterol biosynthesis genes was downregulated in human postmortem striatum and cortex as well as in brain tissue from HD mouse (Valenza et al. 2005). Interestingly, the application of exogenous cholesterol to striatal neurons expressing mutant huntingtin prevented their death (Valenza et al. 2005). The reduction of both cholesterol and BDNF in HD brains suggests a causal relationship between BDNF signaling and cholesterol homeostasis, and it is likely that one of the critical sites of disturbance is the signal transmission at the lipid rafts (Zuccato et al. 2005).
Alzheimer's disease is characterized by accumulation of amyloid beta peptide (Aβ1-42) derived by presenilin 1/gamma secretase cleavage of amyloid precursor protein (APP) (Price et al. 1998; Sisodia and St George-Hyslop 2002). Cholesterol metabolism is altered in the brain of patients with AD and the critical evidence of the importance of normal cholesterol homeostasis in AD comes from epidemiological studies (Wellington 2004). These revealed that the prevalence of diagnosed AD is reduced in individuals taking statins (inhibitors of cholesterol biosynthesis) for long time periods (Wolozin 2004). Experimental evidence revealed that statins are capable of reducing amyloid β42 deposition in animal models of AD (Refolo et al. 2001). The analysis of temporal cortex from AD subjects showed loss of lipid rafts (Molander-Melin et al. 2005), while hippocampi showed reduced cholesterol content in the rafts (Ledesma et al. 2003). In addition the inhibition of cholesterol transport alters PS1 localization and APP processing in neuronal cells (Runz et al. 2002).
Finally, Niemann-Pick type C (NPC) disease is characterized by accumulation of unesterified cholesterol and other lipids in endosomal vesicles, which lead to a set of CNS abnormalities that share clinical and histopathological similarities between NPC and AD: progressive dementia, neurofibrillary tangles, tau hyperphosphorylation, increased APP and abnormal accumulation of Aβ42 in the hippocampus and cerebral cortex (Vance 2006). Cultured striatal neurons from NPC KO mice do not respond to BDNF although they express TrkB on their cell membrane (Henderson et al. 2000). This is demonstrated by the absence of autophosphorylation of TrkB receptor and defective neurite outgrowth in NPC KO neurons (Henderson et al. 2000). This study corroborates the link between neurotrophins and cholesterol. The common clinical symptoms between AD and NPC suggest a set of shared pathophysiological events, and these most likely involve the cholesterol biosynthesis/lipid raft signaling system.
Perspectives
In the nervous tissue various molecular complexes are assembled in lipid rafts, thus mediating a diversity of downstream cellular events such as neurotransmitter processing and release, cell division, cell adhesion, and neuronal outgrowth. Most prominently, neurotrophin receptors are embedded in lipid rafts and BDNF, NGF and GDNF signaling depends on the normal functioning of lipid membrane microdomains. However, it appears that the lipid raft-neurotrophin interaction undergoes a dual feed-forward and feedback regulation: recent evidence suggests that the neurotrophin system is capable of regulating the lipid and cholesterol content of the rafts themselves. These findings, combined with evidence of altered lipid biosynthesis and neurotrophin disturbances in multiple CNS disorders suggest that lipid rafts are likely to play a critical role in the pathophysiology of multiple CNS disorders, including Smith-Lemli-Opitz syndrome, Huntington, Alzheimer's, and Niemman-Pick Type C diseases. Novel methods to study lipid rafts will aid in resolving composition of rafts and their role in nervous system function.
Acknowledgments
Supported by R01 GM72846 to AKK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allende D, Vidal A, McIntosh TJ. Jumping to rafts: gatekeeper role of bilayer elasticity. Trends Biochem Sci. 2004;29:325–330. doi: 10.1016/j.tibs.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- Bacia K, Schwille P, Kurzchalia T. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. Proc Natl Acad Sci U S A. 2005;102:3272–3277. doi: 10.1073/pnas.0408215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Lee JN, Fitzky BU, Seong J, Paik YK. Cholesterol biosynthesis from lanosterol. Molecular cloning, tissue distribution, expression, chromosomal localization, and regulation of rat 7-dehydrocholesterol reductase, a Smith-Lemli-Opitz syndrome-related protein. J Biol Chem. 1999;274:14624–14631. doi: 10.1074/jbc.274.21.14624. [DOI] [PubMed] [Google Scholar]
- Barenholz Y. Cholesterol and other membrane active sterols: from membrane evolution to “rafts”. Prog Lipid Res. 2002;41:1–5. doi: 10.1016/s0163-7827(01)00016-9. [DOI] [PubMed] [Google Scholar]
- Baumgart T, Hammond AT, Sengupta P, Hess ST, Holowka DA, Baird BA, Webb WW. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besshoh S, Chen S, Brown IR, Gurd JW. Developmental changes in the association of NMDA receptors with lipid rafts. J Neurosci Res. 2007;85:1876–1883. doi: 10.1002/jnr.21336. [DOI] [PubMed] [Google Scholar]
- Brown DA. Seeing is believing: visualization of rafts in model membranes. Proc Natl Acad Sci USA. 2001;98:10517–10518. doi: 10.1073/pnas.191386898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 2006;21:430–439. doi: 10.1152/physiol.00032.2006. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lagerholm BC, Yang B, Jacobson K. Methods to measure the lateral diffusion of membrane lipids and proteins. Methods. 2006;39:147–153. doi: 10.1016/j.ymeth.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Chen Y, Thelin WR, Yang B, Milgram SL, Jacobson K. Transient anchorage of cross-linked glycosyl-phosphatidylinositol-anchored proteins depends on cholesterol, Src family kinases, caveolin, and phosphoinositides. J Cell Biol. 2006;175:169–178. doi: 10.1083/jcb.200512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4:833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Davey AM, Walvick RP, Liu Y, Heikal AA, Sheets ED. Membrane order and molecular dynamics associated with IgE receptor cross-linking in mast cells. Biophys J. 2007;92:343–355. doi: 10.1529/biophysj.106.088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruin LS, Harauz G. White matter rafting--membrane microdomains in myelin. Neurochem Res. 2007;32:213–228. doi: 10.1007/s11064-006-9137-4. [DOI] [PubMed] [Google Scholar]
- Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K. Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J. 2002;82:274–284. doi: 10.1016/S0006-3495(02)75393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12:105–112. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–283. doi: 10.1146/annurev.biophys.32.110601.142439. [DOI] [PubMed] [Google Scholar]
- Elson EL. Quick tour of fluorescence correlation spectroscopy from its inception. J Biomed Opt. 2004;9:857–864. doi: 10.1117/1.1779234. [DOI] [PubMed] [Google Scholar]
- Fielding CJ. Caveolae and signaling. Curr Opin Lipidol. 2001;12:281–287. doi: 10.1097/00041433-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fitzky BU, Moebius FF, Asaoka H, Waage-Baudet H, Xu L, Xu G, Maeda N, Kluckman K, Hiller S, Yu H, Batta AK, Shefer S, Chen T, Salen G, Sulik K, Simoni RD, Ness GC, Glossmann H, Patel SB, Tint GS. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Opitz/RSH syndrome. J Clin Invest. 2001;108:905–915. doi: 10.1172/JCI12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaus K, Zech T, Harder T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol Membr Biol. 2006;23:41–48. doi: 10.1080/09687860500466857. [DOI] [PubMed] [Google Scholar]
- Gielen E, Baron W, Vandeven M, Steels P, Hoekstra D, Ameloot M. Rafts in oligodendrocytes: evidence and structure-function relationship. Glia. 2006;54:499–512. doi: 10.1002/glia.20406. [DOI] [PubMed] [Google Scholar]
- Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol. 2004;6:238–243. doi: 10.1038/ncb1103. [DOI] [PubMed] [Google Scholar]
- Golub T, Wacha S, Caroni P. Spatial and temporal control of signaling through lipid rafts. Curr Opin Neurobiol. 2004;14:542–550. doi: 10.1016/j.conb.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Hammond AT, Heberle FA, Baumgart T, Holowka D, Baird B, Feigenson GW. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci U S A. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LP, Lin L, Prasad A, Paul CA, Chang TY, Maue RA. Embryonic striatal neurons from niemann-pick type C mice exhibit defects in cholesterol metabolism and neurotrophin responsiveness. J Biol Chem. 2000;275:20179–20187. doi: 10.1074/jbc.M001793200. [DOI] [PubMed] [Google Scholar]
- Hess ST, Gould TJ, Gudheti MV, Maas SA, Mills KD, Zimmerberg J. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci U S A. 2007;104:17370–17375. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess ST, Kumar M, Verma A, Farrington J, Kenworthy A, Zimmerberg J. Quantitative electron microscopy and fluorescence spectroscopy of the membrane distribution of influenza hemagglutinin. J Cell Biol. 2005;169:965–976. doi: 10.1083/jcb.200412058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Tint GS, Honda A, Nguyen LB, Chen TS, Shefer S. 7-Dehydrocholesterol down-regulates cholesterol biosynthesis in cultured Smith-Lemli-Opitz syndrome skin fibroblasts. J Lipid Res. 1998;39:647–657. [PubMed] [Google Scholar]
- Huang P, Xu W, Yoon SI, Chen C, Chong PL, Liu-Chen LY. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73:534–549. doi: 10.1016/j.bcp.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- Jira PE, Waterham HR, Wanders RJ, Smeitink JA, Sengers RC, Wevers RA. Smith-Lemli-Opitz syndrome and the DHCR7 gene. Ann Hum Genet. 2003;67:269–280. doi: 10.1046/j.1469-1809.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64:895–901. doi: 10.1046/j.1471-4159.1995.64020895.x. [DOI] [PubMed] [Google Scholar]
- Jurevics HA, Kidwai FZ, Morell P. Sources of cholesterol during development of the rat fetus and fetal organs. J Lipid Res. 1997;38:723–733. [PubMed] [Google Scholar]
- Kahya N, Schwille P. Fluorescence correlation studies of lipid domains in model membranes. Mol Membr Biol. 2006;23:29–39. doi: 10.1080/09687860500489099. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H. The region-specific activities of lipid rafts during axon growth and guidance. J Neurochem. 2006;98:330–335. doi: 10.1111/j.1471-4159.2006.03888.x. [DOI] [PubMed] [Google Scholar]
- Keller RK, Arnold TP, Fliesler SJ. Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J Lipid Res. 2004;45:347–355. doi: 10.1194/jlr.M300232-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy A. Peering inside lipid rafts and caveolae. Trends Biochem Sci. 2002;27:435–437. doi: 10.1016/s0968-0004(02)02178-3. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK. Fleeting glimpses of lipid rafts: how biophysics is being used to track them. J Investig Med. 2005;53:312–317. doi: 10.2310/6650.2005.53608. [DOI] [PubMed] [Google Scholar]
- Kenworthy AK, Edidin M. Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 Å using imaging fluorescence resonance energy transfer. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskowski MA, Kenworthy AK. In silico characterization of resonance energy transfer for disk-shaped membrane domains. Biophys J. 2007;92:3040–3051. doi: 10.1529/biophysj.106.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Zou K, Minagawa H, Yu W, Gong JS, Yanagisawa K, Michikawa M. Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J Biol Chem. 2005;280:42759–42765. doi: 10.1074/jbc.M509164200. [DOI] [PubMed] [Google Scholar]
- Korade Z, Mi Z, Portugal C, Schor NF. Expression and p75 neurotrophin receptor dependence of cholesterol synthetic enzymes in adult mouse brain. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Koyama-Honda I, Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta. 2005;1746:234–251. doi: 10.1016/j.bbamcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting microdomains in intact cell membranes. Annu Rev Phys Chem. 2005;56:309–336. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- Landman N, Kim TW. Got RIP? Presenilin-dependent intramembrane proteolysis in growth factor receptor signaling. Cytokine Growth Factor Rev. 2004;15:337–351. doi: 10.1016/j.cytogfr.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Larson DR, Gosse JA, Holowka DA, Baird BA, Webb WW. Temporally resolved interactions between antigen-stimulated IgE receptors and Lyn kinase on living cells. J Cell Biol. 2005;171:527–536. doi: 10.1083/jcb.200503110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma MD, Abad-Rodriguez J, Galvan C, Biondi E, Navarro P, Delacourte A, Dingwall C, Dotti CG. Raft disorganization leads to reduced plasmin activity in Alzheimer's disease brains. EMBO Rep. 2003;4:1190–1196. doi: 10.1038/sj.embor.7400021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Ryu CJ, Hong HJ, Kim J, Lee EH. CDNA microarray analysis of nerve growth factor-regulated gene expression profile in rat PC12 cells. Neurochem Res. 2005;30:533–540. doi: 10.1007/s11064-005-2688-y. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Lommerse PH, Spaink HP, Schmidt T. In vivo plasma membrane organization: results of biophysical approaches. Biochim Biophys Acta. 2004;1664:119–131. doi: 10.1016/j.bbamem.2004.05.005. [DOI] [PubMed] [Google Scholar]
- London E. Insights into lipid raft structure and formation from experiments in model membranes. Curr Opin Struct Biol. 2002;12:480–486. doi: 10.1016/s0959-440x(02)00351-2. [DOI] [PubMed] [Google Scholar]
- Lucero HA, Robbins PW. Lipid rafts-protein association and the regulation of protein activity. Arch Biochem Biophys. 2004;426:208–224. doi: 10.1016/j.abb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Manes S, del Real G, Martinez AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- Marcus J, Popko B. Galactolipids are molecular determinants of myelin development and axo-glial organization. Biochim Biophys Acta. 2002;1573:406–413. doi: 10.1016/s0304-4165(02)00410-5. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- McIntosh TJ, Vidal A, Simon SA. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys J. 2003;85:1656–1666. doi: 10.1016/S0006-3495(03)74595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder D, Moreno MJ, Verkade P, Vaz WL, Simons K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci U S A. 2006;103:329–334. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megha, Bakht O, London E. Cholesterol precursors stabilize ordinary and ceramide-rich ordered lipid domains (lipid rafts) to different degrees. Implications for the Bloch hypothesis and sterol biosynthesis disorders. J Biol Chem. 2006;281:21903–21913. doi: 10.1074/jbc.M600395200. [DOI] [PubMed] [Google Scholar]
- Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67:451–467. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- Moebius FF, Fitzky BU, Glossmann H. Genetic defects in postsqualene cholesterol biosynthesis. Trends Endocrinol Metab. 2000;11:106–114. doi: 10.1016/s1043-2760(00)00235-6. [DOI] [PubMed] [Google Scholar]
- Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Mansson JE, Fredman P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J Neurochem. 2005;92:171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- Ness GC. Developmental regulation of the expression of genes encoding proteins involved in cholesterol homeostasis. Am J Med Genet. 1994;50:355–357. doi: 10.1002/ajmg.1320500411. [DOI] [PubMed] [Google Scholar]
- Nichols BJ. GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol. 2003;13:686–690. doi: 10.1016/s0960-9822(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Niv H, Gutman O, Kloog Y, Henis YI. Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J Cell Biol. 2002;157:865–872. doi: 10.1083/jcb.200202009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwokoro NA, Wassif CA, Porter FD. Genetic disorders of cholesterol biosynthesis in mice and humans. Mol Genet Metab. 2001;74:105–119. doi: 10.1006/mgme.2001.3226. [DOI] [PubMed] [Google Scholar]
- Ong WY, Hu CY, Soh YP, Lim TM, Pentchev PG, Patel SC. Neuronal localization of sterol regulatory element binding protein-1 in the rodent and primate brain: a light and electron microscopic immunocytochemical study. Neuroscience. 2000;97:143–153. doi: 10.1016/s0306-4522(00)00031-2. [DOI] [PubMed] [Google Scholar]
- Ong WY, Sundaram RK, Huang E, Ghoshal S, Kumar U, Pentchev PG, Patel SC. Neuronal localization and association of Niemann Pick C2 protein (HE1/NPC2) with the postsynaptic density. Neuroscience. 2004;128:561–570. doi: 10.1016/j.neuroscience.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Pahnke J, Mix E, Knoblich R, Muller J, Zschiesche M, Schubert B, Koczan D, Bauer P, Bottcher T, Thiesen HJ, Lazarov L, Wree A, Rolfs A. Overexpression of glial cell line-derived neurotrophic factor induces genes regulating migration and differentiation of neuronal progenitor cells. Exp Cell Res. 2004;297:484–494. doi: 10.1016/j.yexcr.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Paratcha G, Ibanez CF. Lipid rafts and the control of neurotrophic factor signaling in the nervous system: variations on a theme. Curr Opin Neurobiol. 2002;12:542–549. doi: 10.1016/s0959-4388(02)00363-x. [DOI] [PubMed] [Google Scholar]
- Parton RG, Hancock JF. Lipid rafts and plasma membrane microorganization: insights from Ras. Trends Cell Biol. 2004;14:141–147. doi: 10.1016/j.tcb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW. Cholesterol homeostasis and function in neurons of the central nervous system. Cell Mol Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SK. Lipid rafts and B-cell activation. Nat Rev Immunol. 2002;2:96–105. doi: 10.1038/nri726. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Pooler AM, Xi SC, Wurtman RJ. The 3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor pravastatin enhances neurite outgrowth in hippocampal neurons. J Neurochem. 2006;97:716–723. doi: 10.1111/j.1471-4159.2006.03763.x. [DOI] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DL, Sisodia SS, Borchelt DR. Genetic neurodegenerative diseases: the human illness and transgenic models. Science. 1998;282:1079–1083. doi: 10.1126/science.282.5391.1079. [DOI] [PubMed] [Google Scholar]
- Rao M, Mayor S. Use of Forster's resonance energy transfer microscopy to study lipid rafts. Biochim Biophys Acta. 2005;1746:221–233. doi: 10.1016/j.bbamcr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Pappolla MA, LaFrancois J, Malester B, Schmidt SD, Thomas-Bryant T, Tint GS, Wang R, Mercken M, Petanceska SS, Duff KE. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- Rietveld A, Simons K. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim Biophys Acta. 1998;1376:467–479. doi: 10.1016/s0304-4157(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Kusumi A. Single-particle tracking image microscopy. Methods Enzymol. 2003;360:618–634. doi: 10.1016/s0076-6879(03)60131-x. [DOI] [PubMed] [Google Scholar]
- Rog T, Pasenkiewicz-Gierula M, Vattulainen I, Karttunen M. What happens if cholesterol is made smoother: importance of methyl substituents in cholesterol ring structure on phosphatidylcholine-sterol interaction. Biophys J. 2007;92:3346–3357. doi: 10.1529/biophysj.106.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runz H, Rietdorf J, Tomic I, de Bernard M, Beyreuther K, Pepperkok R, Hartmann T. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. J Neurosci. 2002;22:1679–1689. doi: 10.1523/JNEUROSCI.22-05-01679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saher G, Brugger B, Lappe-Siefke C, Mobius W, Tozawa R, Wehr MC, Wieland F, Ishibashi S, Nave KA. High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 2005;8:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Saito M, Benson EP, Rosenberg A. Metabolism of cholesterol and triacylglycerol in cultured chick neuronal cells, glial cells, and fibroblasts: accumulation of esterified cholesterol in serum-free culture. J Neurosci Res. 1987;18:319–325. doi: 10.1002/jnr.490180208. [DOI] [PubMed] [Google Scholar]
- Samuli Ollila OH, Rog T, Karttunen M, Vattulainen I. Role of sterol type on lateral pressure profiles of lipid membranes affecting membrane protein functionality: Comparison between cholesterol, desmosterol, 7-dehydrocholesterol and ketosterol. J Struct Biol. 2007;159:311–323. doi: 10.1016/j.jsb.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Schroeder R, London E, Brown D. Interactions between saturated acyl chains confer detergent resistance on lipids and glycosylphosphatidylinositol (GPI)-anchored proteins: GPI-anchored proteins in liposomes and cells show similar behavior. Proc Natl Acad Sci U S A. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Hammond AT, Ostermeyer-Fay AG, Barr DJ, Feigenson GW, London E, Brown DA. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- Shvartsman DE, Kotler M, Tall RD, Roth MG, Henis YI. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts. J Cell Biol. 2003;163:879–888. doi: 10.1083/jcb.200308142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- Silvius JR, Nabi IR. Fluorescence-quenching and resonance energy transfer studies of lipid microdomains in model and biological membranes. Mol Membr Biol. 2006;23:5–16. doi: 10.1080/09687860500473002. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin(1A) receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358:495–499. doi: 10.1016/j.bbrc.2007.04.135. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, St George-Hyslop PH. gamma-Secretase, Notch, Abeta and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- Sohn HW, Tolar P, Jin T, Pierce SK. Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci U S A. 2006;103:8143–8148. doi: 10.1073/pnas.0509858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetzkowski-Marden F, Recouvreur M, Camus G, Cartaud A, Marchand S, Cartaud J. Rafts are required for acetylcholine receptor clustering. J Mol Neurosci. 2006;30:37–38. doi: 10.1385/JMN:30:1:37. [DOI] [PubMed] [Google Scholar]
- Stockinger W, Hengstschlager-Ottnad E, Novak S, Matus A, Huttinger M, Bauer J, Lassmann H, Schneider WJ, Nimpf J. The low density lipoprotein receptor gene family. Differential expression of two alpha2-macroglobulin receptors in the brain. J Biol Chem. 1998;273:32213–32221. doi: 10.1074/jbc.273.48.32213. [DOI] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Edidin M, Kusumi A. Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J Cell Biol. 2007;177:731–742. doi: 10.1083/jcb.200609175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki KG, Fujiwara TK, Sanematsu F, Iino R, Edidin M, Kusumi A. GPI-anchored receptor clusters transiently recruit Lyn and G alpha for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J Cell Biol. 2007;177:717–730. doi: 10.1083/jcb.200609174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Kiyosue K, Hazama S, Ogura A, Kashihara M, Hara T, Koshimizu H, Kojima M. Brain-derived neurotrophic factor regulates cholesterol metabolism for synapse development. J Neurosci. 2007;27:6417–6427. doi: 10.1523/JNEUROSCI.0690-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Leser GP, Russell CJ, Lamb RA. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc Natl Acad Sci U S A. 2003;100:14610–14617. doi: 10.1073/pnas.2235620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman TS, Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Encinas M, Milbrandt J, Johnson EM., Jr Lipid rafts in neuronal signaling and function. Trends Neurosci. 2002;25:412–417. doi: 10.1016/s0166-2236(02)02215-4. [DOI] [PubMed] [Google Scholar]
- Vainio S, Jansen M, Koivusalo M, Rog T, Karttunen M, Vattulainen I, Ikonen E. Significance of sterol structural specificity. Desmosterol cannot replace cholesterol in lipid rafts. J Biol Chem. 2006;281:348–355. doi: 10.1074/jbc.M509530200. [DOI] [PubMed] [Google Scholar]
- Valenza M, Rigamonti D, Goffredo D, Zuccato C, Fenu S, Jamot L, Strand A, Tarditi A, Woodman B, Racchi M, Mariotti C, Di Donato S, Corsini A, Bates G, Pruss R, Olson JM, Sipione S, Tartari M, Cattaneo E. Dysfunction of the cholesterol biosynthetic pathway in Huntington's disease. J Neurosci. 2005;25:9932–9939. doi: 10.1523/JNEUROSCI.3355-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13:92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- Vance JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006;580:5518–5524. doi: 10.1016/j.febslet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16:193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- Veatch SL, Keller SL. Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Cheng H, Kim SH, Chen Y, Barnes NY, Parent AT, Sisodia SS, Thinakaran G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J Biol Chem. 2005;280:25892–25900. doi: 10.1074/jbc.M503570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal A, McIntosh TJ. Transbilayer peptide sorting between raft and nonraft bilayers: comparisons of detergent extraction and confocal microscopy. Biophys J. 2005;89:1102–1108. doi: 10.1529/biophysj.105.062380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrljic M, Nishimura SY, Brasselet S, Moerner WE, McConnell HM. Translational diffusion of individual Class II MHC membrane proteins in cells. Biophys J. 2002;83:2681–2692. doi: 10.1016/S0006-3495(02)75277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington CL. Cholesterol at the crossroads: Alzheimer's disease and lipid metabolism. Clin Genet. 2004;66:1–16. doi: 10.1111/j.0009-9163.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Willmann R, Pun S, Stallmach L, Sadasivam G, Santos AF, Caroni P, Fuhrer C. Cholesterol and lipid microdomains stabilize the postsynapse at the neuromuscular junction. Embo J. 2006;25:4050–4060. doi: 10.1038/sj.emboj.7601288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. FcepsilonRI signaling observed from the inside of the mast cell membrane. Mol Immunol. 2002;38:1259–1268. doi: 10.1016/s0161-5890(02)00073-1. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, Samelson LE, Yang LH, Kotula PG, Oliver JM. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell. 2004;15:2580–2592. doi: 10.1091/mbc.E03-08-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B. Cholesterol, statins and dementia. Curr Opin Lipidol. 2004;15:667–672. doi: 10.1097/00041433-200412000-00007. [DOI] [PubMed] [Google Scholar]
- Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Appelkvist EL, Kristensson K, Dallner G. The lipid compositions of different regions of rat brain during development and aging. Neurobiol Aging. 1996;17:869–875. doi: 10.1016/s0197-4580(96)00076-0. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Liber D, Ramos C, Tarditi A, Rigamonti D, Tartari M, Valenza M, Cattaneo E. Progressive loss of BDNF in a mouse model of Huntington's disease and rescue by BDNF delivery. Pharmacol Res. 2005;52:133–139. doi: 10.1016/j.phrs.2005.01.001. [DOI] [PubMed] [Google Scholar]