Abstract
Classically, it has been thought that high-affinity nicotinic receptors containing β2 subunits are the most important receptor subtypes for nicotinic involvement in cognitive function and nicotine self-administration, while low affinity α7-containing nicotinic receptors have not been thought to be important. In the current study, we found that knockout of either β2 or α7 subunits caused significant deficits in spatial discrimination in mice. The character of the impairment in the two knockouts was different. The β2 knockout preferentially impaired cognition in males while the α7 caused impairment regardless of sex. Both β2 and α7-containing nicotinic receptors also are important for nicotine self-administration, also in different ways. Most animal model studies of nicotine self-administration are relatively short-term whereas the problem of tobacco addiction is considerably longer-term. To better model the impact of nicotinic receptor subtypes on nicotine self-administration over the long term, we studied the impact of genetic knockout of low affinity α7 receptors vs. high affinity β2 containing nicotinic receptors. Mice with knockouts of either of these receptors and their wildtype counter parts were given free access to a choice of nicotine-containing and nicotine-free solution in their home cages on a continuous basis over a period of five months. During the first few weeks, the β2-containing nicotinic receptor knockout mice showed a significant decrease in nicotine consumption relative to wildtype mice, whereas the α7 knockout mice did not significantly differ from wildtype controls at the beginning of their access to nicotine. Interestingly, in the longer-term after the first few weeks of nicotine access, the β2 knockout mice returned to knockout mouse levels of nicotine consumption, whereas the α7 knockout mice developed an emergent decrease in nicotine consumption. The α7 receptor knockout-induced decrease in nicotine consumption persisted for the five-month period of the study. Both α7 and β2 containing nicotinic receptors play critical roles in cognitive function and nicotine self-administration. Regarding cognitive function, β2-containing receptors are important for maintaining normal sex differences in spatial learning and memory, whereas α7 receptors are important for cognitive function regardless of sex. Regarding nicotine self-administration high affinity β2-containing nicotinic receptors are important for consumption during the initial phase of nicotine access, but it is the α7 nicotinic receptors that are important for the longer-term regulation of nicotinic consumption.
Keywords: Nicotinic, α7 knockout, β2 knockout, Nicotine, Learning, Memory, Radial-arm Maze, Self-administration
Introduction
Nicotine receptor knockouts or null mutants have been very useful for determining the roles of the different nicotinic receptor subtypes in neurobehavioral function as well as response to nicotine. In general, β2-containing nicotinic receptors have been found to be involved in more neurobehavioral functions than α7 nicotinic receptors in studies using receptor knockouts [10,25] or pharmacological manipulations [11]. β2-containing nicotinic receptors have been found to be important for nicotine self-administration as well as cognitive function. Animals lacking β2-containing nicotinic receptors have been shown to be insensitive to the nicotine cue [28]. Also it has been demonstrated that substitution to the nicotine discriminative stimulus required high intrinsic activity at β2- but not at β4- or α7-containing receptors [29]. Until recently α7 receptors nicotinic receptors have not appeared to be substantially involved in either nicotine reinforcement or cognitive function [13]. The current studies determined in greater detail the involvement of both β2 and α7-containing nicotinic receptors in cognitive function and nicotine self-administration.
The neural substrates of nicotine self-administration have been the focus of considerable study because it is believed that this will point to the neural bases of tobacco addiction and better treatments for smoking cessation. Several animal models have been developed to study different aspects of nicotine reinforcement. These include intravenous (IV) nicotine self-administration, oral nicotine self-administration, conditioned place preference and locomotor activity sensitization. Each model provides a piece in the puzzle of tobacco addiction. One piece missing from the range of models is the comparison of the differential bases of induction of nicotine self-administration from the long-term persistence of self-administration.
Animal models of nicotine self-administration have shown the importance of high affinity nicotinic receptors, particularly α4β2 receptors, in the foundation of nicotine self-administration. In rats, application of the α4β2 nicotinic antagonist DHβE into the ventral tegmental area (VTA) substantially interferes with nicotine self-administration [11]. In mice, genetic manipulations have shown the decrease in nicotine self-administration with knockouts of the β2 receptor subunit [10,25]. In contrast, application of an α7 antagonist to the ventral tegmental area (VTA) in rats or knockout of nicotinic α7 receptors in mice have been found to have little or no impact on nicotine self-administration [11]. However, definitive conclusions concerning the relative involvement of different nicotinic receptor subtypes should be held in abeyance because of the limitations of the models in which the receptor involvement are being conducted.
Nicotinic α4β2 receptors provide the generalization of discriminative stimulus properties to nicotine, whereas α7 receptors does not seem to play a role [29]. Animals lacking α7 nicotinic receptors learn to discriminate nicotine as well as wild-type animals [30]. Thus, it has been suggested that the α7 receptors may play little role in nicotine reinforcement [29].
There is some evidence in the literature supporting the involvement of α7 nicotinic receptors in drug abuse. Both α4β2 and α7 nicotinic receptors in the ventral striatum are involved in cocaine-induced dopamine release [33,34]. Recently it has been found that both α7 and α4β2 nicotinic receptors are necessary for development of sensitization to cocaine effects on dopamine release in the ventral striatum [33].
There are a variety of limitations to animal models of nicotine self-administration. One of the most relevant is the time limitation. Humans self-administer nicotine through tobacco use for years and decades, whereas nicotine self-administration is only studied over a period of week in the typical rodent study. There may be very different mechanisms in the short vs. long-term phases of nicotine self-administration. In the current study, we examined nicotine self-administration through drinking water in mice with knockout of either α7 or β2 receptor subunits during a period of five months.
In addition to the roles nicotinic receptors play in tobacco addiction, they also are critically involved in a wide variety of neurobehavioral functions. Prominently among these, nicotinic systems have also been found to be crucial for cognitive function [20]. In particular, nicotine has been shown in a variety of studies to significantly improve memory. Pharmacological studies have pointed to the importance of both α4β2 and α7 nicotinic receptors in the basis of memory function. Infusion of either DHβE, an α4β2 antagonist, or MLA, an α7 antagonist, into the hippocampus significantly impairs working memory in the radial-arm maze [1–3,5,16,18,23,26]. In addition, systemic administration of either α4β2 or α7 agonists can improve working memory accuracy on the radial-arm maze [17,19]. Mice with β2 null mutations show cognitive impairments [9,31]. Pharmacological manipulations show the importance of β2 containing nicotinic receptors for nicotine-induced improvements in cognitive function [12]. To date fewer significant behavioral effects have been found in α7 null mutant mice [14,24], though response to ethanol seems to be affected by α7 deletion [8]. It has been found that α7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating in Acra 7 homozygous mutant mice [24]. However, Fernandes et al. reported an impairment in α7 knockout mice in the delayed matching-to-place task of the Morris water maze, which is a measure of working/episodic memory [13]. Also, recently it has been shown that α7 knockout mice have impaired attentional performance in the 5-choice serial reaction time task [15,32]. We have found that local infusion of the α7 nicotinic receptor antagonist into the ventral or dorsal hippocampus significant impairs spatial working memory in the radial-arm maze [21,23]. We and others have found that α7 agonists significantly improve learning, memory and attentional function in rodents [6,7,17,27] and reverses aging–induced cognitive impairments [4,22].
To complement our studies on nicotinic receptor subtype involvement with nicotine self-administration, we tested α4β2 and α7 knockout mice and their wildtype controls for learning and memory on the radial-arm maze as well. Studies with α7 and β2 knockout mice can lend insight into the involvement of nicotinic receptor subtypes in neurobehavioral function.
Methods
Subjects
Nicotinic receptor α7 and β2 knockout mouse breeding pairs and wildtype controls (C57/BL6) were generously provided by researchers at Baylor University. The mice in the present studies were descendents from these mice. The care of the animals was in accordance with institutional and federal animal care guidelines. Male and female mice used were housed in a Thorne ventilated cage rack in plastic cages with wood chip bedding at 22±2 C° with a 12:12 day:night lighting cycle (lights on at 7:00 AM). In the radial-arm maze experiment the mice were housed in groups of groups of 3–4. In the nicotine consumption study the mice were singly housed. There were C57/BL6 wildtype controls and transgenic nicotinic α7 and β2-containing receptor knockout mice. Heterozygous mice were bred and the littermates with wildtype and knockout genotypes were used in the study. The heterozygous offspring that carried the transgene were identified by Southern blot analysis of DNA from tail samples. This kept certain the genetic expression for each generation. The littermates not expressing the transgene were used as controls. Separate sets of mice were used to assess nicotinic receptor knockout effects on cognitive function and nicotine consumption.
Radial-Arm Maze
Spatial learning and memory was assessed by choice accuracy in an 8-arm radial maze. The maze had a center platform 12 cm in diameter, with eight extending arms (24 × 4 cm). It was elevated 25 cm from the floor and was located in a room with many fixed extra-maze visual cues. Food cups, located at the ends of each of the arms, were baited with a small piece of a sweetened cereal (Froot Loops®, Kellogg’s, Battle Creek, MI, USA). The mice in the radial-arm maze study were fed daily after testing amounts of chow to maintain health weights rather than having food continually available so that they would be motivated to run the maze for the food reinforcements. Before training on the radial-arm maze, the mice were adapted to handling and had exposure to the food reinforcements. Spatial learning and memory was assessed with the win-shift task in which all eight arms were baited at the beginning of the session. Prior to testing, the mouse was placed in the center of the maze in an opaque cylinder, 8 cm in diameter and 10 cm high for 10 seconds. The session lasted until the mouse entered all eight arms or 300 seconds elapsed. The choice accuracy measure is the number of correct arm entries before an error is made (Entries to Repeat). The advantage of the entries to repeat (ETR) index of choice accuracy is that it does not require that the mouse go to all of the arms of the maze to calculate the measure. We have found this to be useful because it provides an accurate measure of the increasing difficulty of the task as it progresses. The ETR information is gained as soon as the mouse repeats an entry in a session. Perfect performance in an 8-arm maze with no repeats is 8. If the mouse entered more than half of the arms within the 300-second time limit without repeating, it was given that score. If it did not complete more than half the maze without repeating within the 300 second limit it did not have an ETR score for that session. This occurred less than 2% of the sessions. The analysis blocked the sessions in groups of 6. When there was a missing ETR score for a session the average was made for the remaining sessions. There group sizes were as follows: Wildtype Males N=35, Wildtype Females N=28, α7 knockout Males N=18, α7 knockout Females N=11, β2 knockout Males N=16, β2 knockout Females N=15
Two-Bottle Choice Nicotine Self-Administration
Nicotine self-administration was assessed using a two bottle choice test in the home cages of the mice. A nicotine-containing solution (50, 75 or 100 mg/l with or without 2% saccharine) and water were continuously available in the home cages of the mice that were individually housed. The percent of consumption of each fluid per day was calculated over a period of five months nicotine drinking choice.
| Month | Nicotine Solution Available | Control Solution Available |
|---|---|---|
| 1 | 50 mg/l nicotine + 2% saccharine | 2% saccharine in water |
| 2 | 50 mg/l nicotine + 2% saccharine | 2% saccharine in water |
| 3 | 50 mg/l nicotine | water |
| 4 | 75 mg/l nicotine | water |
| 5 | 100 mg/l nicotine | water |
Data Analysis
The choice accuracy and response latency were measured in the radial-arm maze. The radial-arm maze test was run in two cohorts which was used as a control factor to reduce variance and the percent preference of nicotine fluid consumption were analyzed by analysis of variance with a significance cut-off of p<0.05 (two-tailed).
Results
Radial-Arm Maze
Both α7 and β2 knockout mice had impaired choice accuracy in the radial-arm maze relative to wildtype controls (Fig. 1). There was a significant main effect of genotype (2,111)=4.20, p<0.025). Paired comparisons of the knockout groups with wildtype controls showed that both the α7 knockout mice (F(1,111)=4.82, p<0.05) and the β2 knockout mice (F(1,111)=5.59, p<0.025) had significantly worse average accuracy compared with wildtype controls averaged over the learning curve (wildtype controls=5.45±0.08; α7 knockouts=5.15±0.12; and β2 knockouts=5.09±0.12 entries to repeat). There was a significant main effect of session block (F(2,222)=16.31, p<0.005) with both wildtype and the knockout mice showing improved choice accuracy with continued training (Fig. 1). The impairment was not in the rate of improvement, rather the impairment was evident throughout the acquisition curve. There was no hint of an interaction of genotype x session block (p=0.92). There was a significant main effect of sex (F(1,111)=20.36, p<0.0005). As has been seen before with spatial discrimination tasks, the males (5.58±0.08) had higher average scores than females (4.92±0.08). Interestingly, there was a significant interaction of genotype x sex (F(2,111)=3.45, p<0.05). Tests of the simple main effects of sex with each genotype showed that there were significant sex difference with wildtype controls (F(1,111)=22.62, p<0.0005) and α7 knockout mice (F(1,111)=6.66, p<0.025), but the β2 knockout eliminated the normal sex difference (p=0.66) on this behavioral function of spatial discrimination (Fig. 2). Figure 3 shows the choice accuracy data for males and females of each genotype broken down by early, mid and late acquisition phase. Females had lower scores than males but this did not reach the floor level of random chance performance levels. We have calculated the random chance probability for the entries to repeat measure in different sized mazes with different memory and preference components using computerized Monte Carlo simulations. The random chance probability for the entries to repeat measure in the 8-arm maze with equal probability for arm selection is 3.25. When it is assumed that the animal would not immediately re-enter the arm from which it just emerged from the random chance entries to repeat score is 4.03. Average scores for males and females from each genotype were above this level.
Figure 1.
Effect of α7 and β2-containing receptor knockout in mice on choice accuracy during training in the 8-arm radial maze, entries to repeat (mean±sem) for each 6-session block averaged over males and females and averaged over the 18 sessions of training. Wildtype Males N=35, Wildtype Females N=28, α7 knockout Males N=18, α7 knockout Females N=11, β2 knockout Males N=16, β2 knockout Females N=15
Figure 2.
Knockout of β2-containing nicotinic receptors eliminates the normal sex difference in spatial discrimination on the radial-arm maze, entries to repeat (mean±sem) for each sex averaged over the 18-session acquisition period. Wildtype Males N=35, Wildtype Females N=28, α7 knockout Males N=18, α7 knockout Females N=11, β2 knockout Males N=16, β2 knockout Females N=15
Figure 3.
Radial-arm maze acquisition data for male and female mice for the different genotypes, entries to repeat (mean±sem) for each sex averaged over 6-session blocks during the acquisition period. Wildtype Males N=35, Wildtype Females N=28, α7 knockout Males N=18, α7 knockout Females N=11, β2 knockout Males N=16, β2 knockout Females N=15
Genotype also had a significant (F(2,111)=4.82, p<0.01) main effect on response latency in the radial-arm maze. The wildtype controls averaged 26.7±1.0 second per arm entry, the α7 knockout mice averaged 25.7±1.5 seconds per arm entry, a slight but significant (F(1,111)=9.62, p<0.005) decrease in latency. The β2 knockout mice (26.8±1.4 seconds per entry) did not have significantly different latency than controls. As seen previously, females (25.9±0.8 seconds per entry) were significantly (F(1,111)=4.71, p<0.05) faster than males (27.0±0.7 seconds per entry). There was no significant genotype x sex interaction with latency as there was with choice accuracy.
Two-Bottle Choice Nicotine Self-Administration
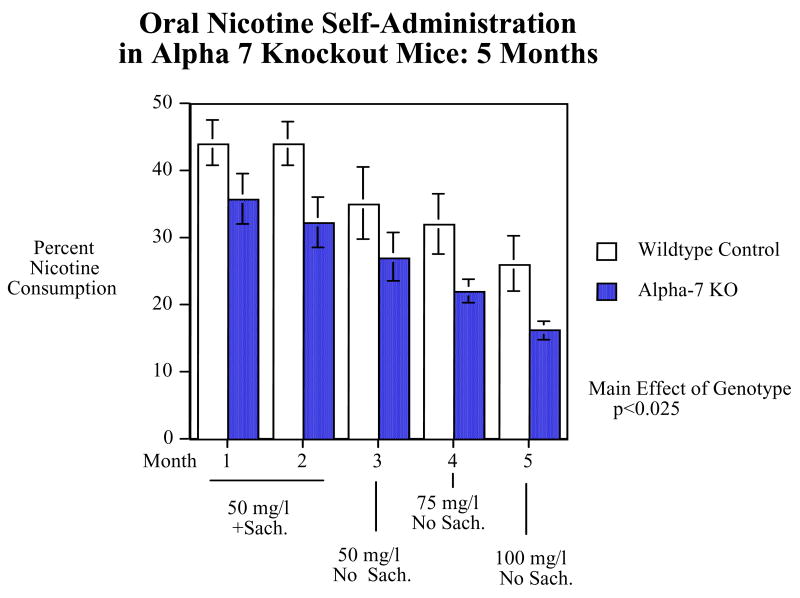
As shown in figure 4, α7 knockout mice had a significant (F(1,18)=6.50, p<0.025) reduction in preference for drinking nicotine-containing water over a five-month period of access. This included a two-month period with a constant level of 50 mg/l of nicotine ditartrate in a 2% saccharine solution, continuing through a removal of the saccharine from the third month onward and increases in the nicotine concentration to 75 and 100 mg/l during the fourth and fifth month, respectively. The percent preference for the nicotine solution decreased in both the wildtype and α7 knockout mice with the removal of saccharine and increase in nicotine concentration but the relative decreased preference by the α7 knockout mice relative to the wildtype controls persisted. Interestingly, as can be seen in figure 5, at the very beginning of the study the α7 knockout mice did not show a significant difference from wildtype controls in nicotine preference. The α7 knockout mice had nearly the same levels of preference for nicotine as the control during the first week and then there was the first indication of decreased nicotine consumption relative to wildtype controls. This became emergent as a significant effect after the first several weeks and was clearly apparent for the rest of the 5-month study.
Figure 4.
The effect of α7 nicotinic receptor knockout in mice on preference for nicotine vs. non-nicotine-containing water over a period of five months (mean±sem). N=10/genotype
Figure 5.
The initial effect of α7 nicotinic receptor knockout in mice on preference for nicotine vs. non-nicotine-containing water over a period of the first 10 days (mean±sem). N=10/genotype
In contrast, the β2 knockout mice showed significant decreases in nicotine drinking during the first several weeks and then returned to wildtype control levels (Fig. 6). When the first two-month period on the standard dose of 50 mg/l nicotine ditartrate plus 2% saccharine is divided into thirds it become apparent that the β2 knockout mice showed a significant (p<0.05) reduction in nicotine preference during the first 20 days and then was not significantly different from wildtype controls thereafter. This contrasts with the emerging and then persisting reductions in nicotine preference in the α7 knockout mice with no significant effect initially and significant reductions during days 41–60 (p<0.025). This effect of reduced nicotine preference in the α7 knockout mice lasted for the rest of the study, which was five months.
Figure 6.
The comparative effects of β2-containing vs. α7 nicotinic receptor knockout in mice and wildtype control on preference for nicotine vs. non-nicotine-containing water during the first two months of access to 50 mg/l nicotine solution (mean±sem). Wildtype, N=16; β2 knockout, N=6; α7 knockout, N=10
Discussion
Nicotinic acetylcholine receptor systems have been widely shown to be important neural substrates for spatial learning and memory as well as addiction. The relative importance of nicotinic receptor subtypes is still being determined. High affinity β2-containing nicotinic receptors have been implicated both with cognitive function as well as nicotine reinforcement. The role of α7 nicotinic receptors is less clear. With α7 and β2 knockout mice as well as wildtype controls (C57 BL/6), we assessed spatial learning on the 8-arm radial maze. In separate sets of animals, we determined nicotine consumption with two-bottle continuous access to nicotine-containing or plain water in their home cages. Differential effects of null mutations for α7 and β2-containing nicotinic receptors were seen in both spatial learning and nicotine preference.
With spatial learning in the radial-arm maze, over the 18-session acquisition phase, all groups of mice improved in choice accuracy on the radial-arm maze task. With the β2 KO mice there was a sex-selective effect with males showing a significant choice accuracy impairment and females being unaffected relative to controls. The β2 null mutation eliminated the normal sex-difference seen in the wildtype controls. The knockout β2-containing nicotinic receptors brought the choice accuracy of the male mice to the lower female levels. The α7 KO mice also showed a significant impairment in radial-arm maze choice accuracy with no differential effect between sexes. The effect did not seem to be one of impaired acquisition but rather impairment in choice accuracy throughout training possibly related to impaired attention or memory.
β2 and α7 knockout mice showed different patterns of nicotine intake. With the two-bottle test, the β2 KO mice showed a significant reduction in nicotine consumption relative to controls for the first 20 days. During this same period, the α7 KO mice did not differ in nicotine preference from controls. Interestingly, during days 21–40 and 41–60 the β2 KO mice returned to wildtype control level of nicotine preference, while the α7 KO mice developed a significant decrease in nicotine preference. This effect lasted for the rest of the study, which was five months. It appears that β2-containing nicotinic receptors may be more important for the initial stage of nicotine self-administration, while α7 receptors may be more important for continuing nicotine self-administration over a period of months. This study highlights the differential role of α7 and β2-containing nicotine receptors in the early and late phases of nicotine self-administration. Given that the great majority of cigarette smokers have self-administered nicotine for more than just a few weeks, perhaps investigation of α7 nicotinic receptor-acting drugs, either antagonists or desensitizing agonists, may provide a useful treatment for tobacco addiction.
These data show that with a two-bottle choice in the home cage, the amount of nicotine consumption can be reliably gauged over a period of months in the mouse. The mice self-administer steady amounts over time. The amount of nicotine consumed changes in a regular fashion according to the concentration of nicotine in the solution such that the total amount of nicotine consumed per day is conserved. Once self-administration is established, saccharine can be removed and the mice still reliably self-administer nicotine, albeit at a slightly lower level. Differential nicotine consumption among nicotinic receptor knockout and wildtype mice during the first two months did not seem to be due to differences in preference for the sweet taste of saccharine inasmuch as both the nicotine-containing and control bottles during this period contained 2& saccharine. Nicotinic α7 receptors appear to be important in the regulation of nicotine self-administration in this paradigm. This is not seen in the initial stages of access but develops over the first several weeks of nicotine availability. Then the mice with α7 receptors knocked out show significantly less preference for nicotine than the wildtype controls. This decreased preference is seen over a period of five months of the study and continues in the face of removal of saccharine from the solution and increases in nicotine concentration. In contrast, knockout of β2-containing nicotinic receptors has a very different effect on nicotine self-administration. The β2 knockout mice had a significantly lower nicotine preference than wildtype controls for the first several weeks of access. Interestingly, after the first several weeks the nicotine self-administration of the β2 knockout mice rose to match the wildtype controls.
Inasmuch as the percent consumption of the nicotine-containing solution was below 50%, the results could also be interpreted as genotype induced differences in aversion to nicotine. With this interpretation the β2 knockout mice had initially greater aversion to nicotine, which diminished after several weeks of access and returned to wildtype-like levels. In contrast, the α7 knockout mice initially did not differ from wildtype controls but developed a greater aversion to nicotine, which persisted for the rest of the five-month study, which included removal of saccharine and increased concentrations of nicotine. With either the preference or aversion interpretation it is clear that nicotine consumption patterns significantly differed with β2 and α7 knockout mice particularly with regard to the timing and persistence of the effect.
Classically, it has been considered that β2 containing nicotinic receptors underlie nicotine self-administration and by extension tobacco addiction. Application of the α4β2 antagonist DHβE significantly blocks nicotine self-administration while the α7 antagonist MLA had little effect [11]. Using knockout mice, it has also been found that β2-containing receptor knockout mice significantly reduced nicotine self-administration while α7 knockouts did not have a significant effect. However, typically the rat and mouse nicotine self-administration studies have only lasted for a few weeks. The mechanisms of longer-term nicotine self-administration are of crucial importance for developing treatments for smoking cessation. The great majority of smokers have been self-administering nicotine for much longer than just the few weeks. It is possible that the neural mechanisms underlying long-term nicotine self-administration differ from those for initial nicotine self-administration. The results of the current study certainly support the multi-receptor involvement in nicotine-self-administration.
This study provides evidence supporting the importance of α7 receptors in the later stages of nicotine self-administration. β2 containing nicotinic receptors appear to be important for the initial stage of nicotine self-administration, but not for the later stages. In contrast, α7 containing nicotinic receptors appear to be important during the later stages of nicotine self-administration, but not the early stage. Inasmuch as a principal goal of this type of research is to develop better treatments to aid smoking cessation and the target population that has been smoking for years and decades, it is clear that the neural underpinnings of nicotine preference for the later stages of nicotine self-administration is of primary importance. β2-containing nicotinic receptors are undoubtedly important for nicotine reinforcement, especially in the early stage of self-administration, but our data suggest that nicotinic α7 receptors play an important role as well.
This study highlights the differential importance of β2 and α7 containing nicotinic receptors in the early and late phases of nicotine self-administration. Given that the great majority of cigarette smokers have self-administered for more than just a few weeks, perhaps investigation of α7 nicotinic receptor acting drugs, either antagonists or desensitizing agonists, may provide a useful treatment for tobacco addiction.
Acknowledgments
This research was supported by an unrestricted grant from Philip Morris-USA and a grant from the National Institutes of Health, National Institute on Drug Abuse (DA015756).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Addy N, Levin E. Hippocampal a7 and a4b2 nicotinic receptor interactions and memory function in rats. International Behavioral Neuroscience Society; Denver: 2000. [Google Scholar]
- 2.Arthur D, Levin ED. Chronic inhibition of alpha4beta2 nicotinic receptors in the ventral hippocampus of rats: Impacts on memory and nicotine response. Psychopharmacology. 2002;160:140–145. doi: 10.1007/s00213-001-0961-6. [DOI] [PubMed] [Google Scholar]
- 3.Bancroft A, Levin ED. Ventral hippocampal alpha4beta2 nicotinic receptors and chronic nicotine effects on memory. Neuropharmacology. 2000;39:2770–2778. doi: 10.1016/s0028-3908(00)00099-x. [DOI] [PubMed] [Google Scholar]
- 4.Beracochea D, Boucard A, Trocme-Thibierge C, Morain P. Improvement of contextual memory by S 24795 in aged mice: comparison with memantine. Psychopharmacology. 2008;196:555–564. doi: 10.1007/s00213-007-0987-5. [DOI] [PubMed] [Google Scholar]
- 5.Bettany JH, Levin ED. Ventral hippocampal alpha7 nicotinic receptors and chronic nicotine effects on memory. Pharmacology, Biochemistry and Behavior. 2001;70:467–474. doi: 10.1016/s0091-3057(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 6.Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, Decker MW, Frost JM, Gronlien JH, Gubbins E, Li J, Malysz J, Markosyan S, Marsh K, Meyer MD, Nikkel AL, Radek RJ, Robb HM, Timmermann D, Sullivan JP, Gopalakrishnan M. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. Journal of Neuroscience. 2007;27:10578–87. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boess FG, De Vry J, Erb C, Flessner T, Hendrix M, Luithle J, Methfessel C, Riedl B, Schnizler K, van der Staay FJ, van Kampen M, Wiese WB, Koenig G. The novel alpha7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2-carboxamide improves working and recognition memory in rodents. Journal of Pharmacology & Experimental Therapeutics. 2007;321:716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- 8.Bowers BJ, McClure-Begley TD, Keller JJ, Paylor R, Collins AC, Wehner JM. Deletion of the alpha7 nicotinic receptor subunit gene results in increased sensitivity to several behavioral effects produced by alcohol. Alcoholism: Clinical & Experimental Research. 2005;29:295–302. doi: 10.1097/01.alc.0000156116.40817.a2. [DOI] [PubMed] [Google Scholar]
- 9.Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. beta2-Subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology. 2006;184:328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- 10.Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Lena C, Le Novere N, Marubio L, Picciotto M, Zoli M. Brain nicotinic receptors: structure and regulation, role in learning and reinforcement. Brain Research - Brain Research Reviews. 1998;26:198–216. doi: 10.1016/s0165-0173(97)00040-4. [DOI] [PubMed] [Google Scholar]
- 11.Corrigall WA, Coen KM, Adamson KL, Chow BLC. Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology. 1999;145:412–417. doi: 10.1007/s002130051075. [DOI] [PubMed] [Google Scholar]
- 12.Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. Journal of Neuroscience. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandes C, Hoyle E, Dempster ELCS, Collier DA. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain and Behavior. 2006;5:433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 14.Franceschini D, Paylor R, Broide R, Salas R, Bassetto L, Gotti C, De Biasi M. Absence of alpha7-containing neuronal nicotinic acetylcholine receptors does not prevent nicotine-induced seizures. Brain Research Molecular Brain Research. 2002;98:29–40. doi: 10.1016/s0169-328x(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 15.Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology. 2006;189:211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin ED, Addy N, Arthur DB, Wagner Y, Stamm K. Acute and chronic a7 and a4b2 hippocampal nicotinic receptor blockade and memory function in rats. Society for Research on Nicotine and Tobacco. 2001 [Google Scholar]
- 17.Levin ED, Bettegowda C, Blosser J, Gordon J. AR- R17779, and alpha7 nicotinic agonist, improves learning and memory in rats. Behavioural Pharmacology. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–65. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- 19.Levin ED, Christopher NC. Persistence of nicotinic agonist RJR 2403 induced working memory improvement in rats. Drug Development Research. 2002;55:97–103. [Google Scholar]
- 20.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 21.Levin ED, Sledge D, Baruah A, Addy NA. Ventral hippocampal NMDA blockade and nicotinic effects on memory function. Brain Research Bulletin. 2003;61:489–495. doi: 10.1016/s0361-9230(03)00183-7. [DOI] [PubMed] [Google Scholar]
- 22.Marighetto A, Valerio S, Desmedt A, Philippin JN, Trocmé-Thibierge C, Morain P. Comparative effects of the α7 nicotinic partial agonist, S 24795, and the cholinesterase inhibitor, donepezil, against aging-related deficits in declarative and working memory in mice. Psychopharmacology. 2008;197:499–508. doi: 10.1007/s00213-007-1063-x. [DOI] [PubMed] [Google Scholar]
- 23.Nott A, Levin ED. Dorsal hippocampal α7 and α4β2 nicotinic receptors and memory. Brain Research. 2006;1081:72–78. doi: 10.1016/j.brainres.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 24.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. alpha 7 nicotinic receptor subunits are not necessary for hippocampal-dependent learning or sensorimotor gating: A behavioral characterization of Acra7-deficient mice. Learning and Memory. 1998;5:302–316. [PMC free article] [PubMed] [Google Scholar]
- 25.Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta 2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 26.Pocivavsek A, Icenogle L, Levin ED. Hippocampal alpha7 and alpha4beta2 nicotinic receptors and clozapine effects on memory. Psychopharmacology. 2006;188:596–604. doi: 10.1007/s00213-006-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezvani AH, Kholdebarin E, Brucato F, Callahan PM, Levin ED. Involvement of nicotinic α7 receptors in sustained attention in rats: Characterization of the novel nicotinic α7 receptor agonist MEM 3454. Progress in Neuropsychopharmacology and Biological Psychiatry. 2008 under review. [Google Scholar]
- 28.Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–9. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology. 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- 30.Stolerman IP, Chamberlain S, Bizarro L, Fernandes C, Schalkwyk L. The role of nicotinic receptor alpha 7 subunits in nicotine discrimination. Neuropharmacology. 2004;46:363–71. doi: 10.1016/j.neuropharm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. European Neuropsychopharmacology. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti L, de Kerchove D'Exaerde A, Zanardi A, Changeux JP, Picciotto MR, Zoli M. Inhibition of both alpha7* and beta2* nicotinic acetylcholine receptors is necessary to prevent development of sensitization to cocaine-elicited increases in extracellular dopamine levels in the ventral striatum. Psychopharmacology. 2006;187:181–8. doi: 10.1007/s00213-006-0419-y. [DOI] [PubMed] [Google Scholar]
- 34.Zanetti L, Picciotto MR, Zoli M. Differential effects of nicotinic antagonists perfused into the nucleus accumbens or the ventral tegmental area on cocaine-induced dopamine release in the nucleus accumbens of mice. Psychopharmacology. 2007;190:189–199. doi: 10.1007/s00213-006-0598-6. [DOI] [PubMed] [Google Scholar]