Abstract
The influence of emotion on human memory is associated with two contradictory effects in the form of either emotion-induced enhancements or decrements in memory. In a series of experiments involving single word presentation, we show that enhanced memory for emotional words is strongly coupled to decrements in memory for items preceding the emotional stimulus, an effect that is more pronounced in women. These memory effects would appear to depend on a common neurobiological substrate, in that enhancements and decrements are reversed by propranolol, a β-adrenergic antagonist, and abolished by selective bilateral amygdala damage. Thus, our findings suggest that amygdala-dependent β-adrenergic modulation of episodic encoding has costs as well as benefits.
Substantial evidence indicates that enhanced memory for emotional experience engages a β-adrenergic system (1). β-Adrenergic blockade with the β1β2-antatgonist propranolol selectively impairs long-term human episodic memory for emotionally arousing material without affecting memory for a neutral story (2). This modulation of emotional memory by propranolol is centrally mediated, because peripheral β-adrenergic blockade has no such effect on emotional memory function (3). Human amygdala lesions also produce emotional memory impairment (4, 5), suggesting that this structure may represent a critical locus for propranolol's influence on emotional memory.
Although emotionality is strongly associated with enhancements in memory, there is evidence for emotion-induced memory decrements (6, 7). Human behavioral studies demonstrate enhanced memory for central details of an emotional event and memory suppression for peripheral details (8, 9). Ecological studies show that details of an event are less likely to be remembered if followed by an emotional event (10). In experimental contexts a weak, although unreliable, effect on words presented in close proximity to potentially emotional items is reported (11). By contrast to the extensive literature on emotional enhancements in memory, nothing is known regarding the neurobiological processes accounting for emotion-induced impairments in memory.
In a series of related experiments, we present psychological (Exp. 1), psychopharmacological (Exp. 2) and neuropsychological (Exp. 3) data that characterize the psychological and neurobiological properties of emotion-induced forgetting. Our first aim was to establish a behavioral index of emotion-evoked memory enhancement and impairment. In an initial behavioral memory experiment (Exp. 1), nouns were presented serially, every 3 s, in semantically related lists, and recall was tested after each list presentation. Each list, in this and subsequent experiments, contained two “oddball” nouns: an emotionally aversive noun (E noun) and a perceptual oddball noun (P noun), presented in a novel font (Fig. 1a). In line with previous observations (1, 6), memory for emotional items is enhanced relative to control nouns. Critically, items preceding E nouns (E-1 nouns) are recalled less well than controls (see Results).
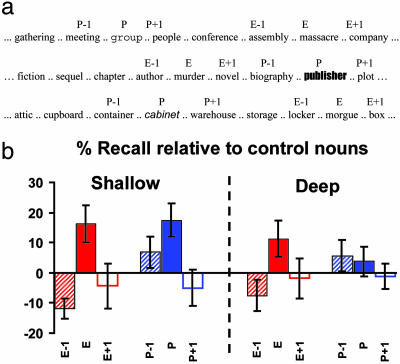
Fig. 1.
(a) Examples of presented nouns. (b) Recall performance (±SE) relative to control nouns (%) for deep and shallow encoding in Exp. 1. An encoding task (shallow, deep) × oddball type (emotional, perceptual) × position (oddball-1, oddball, oddball + 1) 2 × 2 × 3 ANOVA revealed a significant main effect of position (F1.3, 11.8 = 12.7 P < 0.005) and a significant oddball × position interaction (F1.9, 16.9 = 3.8 P < 0.05). Mean recall of control nouns (%; SE) after deep encoding was 58.8 (2.8) and after shallow encoding was 48.1 (3.6). E, emotional oddball; P, perceptual oddball; E-1, E + 1, P-1, P + 1, nouns presented before and after emotional and perceptual oddballs.
A previously reported blockade of enhanced memory for emotional stimuli by propranolol (2) indicates central noradrenaline/norepinephrine (NE) release in response to emotional stimuli. In animals, posttraining amygdala stimulation, electrically or with adrenergic agonists, typically leads to enhanced memory for previously learned tasks but can evoke memory impairment (12, 13). For example, retrograde amnesia in rodents for visual discrimination learning is produced by postlearning stimulation of the amygdala (14), an effect attenuated by pretreatment with propranolol. Hence amygdala stimulation-evoked adrenergic release, occurring after learning, may disrupt ongoing consolidation (14). We reasoned that NE release evoked by E nouns may affect consolidation of E-1 encoding. On this basis, we conducted further experiments to examine whether memory impairment for E-1 items, like the memory enhancement for E items, would be attenuated by propranolol. To test this prediction, we conducted a psychopharmacological study (Exp. 2), in which subjects received either propranolol or placebo in a double-blind experimental design. As in Exp. 1, nouns were presented serially in semantically related lists and recall tested after each list presentation. Both enhanced memory for E nouns, and memory impairment for E-1 items was abolished by propranolol (see Results).
The adrenergic release that enhances memory for emotional events is thought to be mediated by the amygdala (2, 4, 5), because this memory enhancement is abolished by both β-adrenergic blockade (2) and amygdala lesions (4, 5). Our observation that β-adrenergic blockade abolishes the emotion-induced impairment of memory for E-1 nouns suggests that the same NE release that enhances memory for the E noun also impairs consolidation of E-1 items. Given that this NE release is thought to be amygdala-dependent (1), we hypothesized that human amygdala lesions would abolish emotion-induced E-1 memory impairment. We therefore tested a patient with selective bilateral amygdala damage (Exp. 3) on the paradigm used in Exp. 2.
Materials and Methods
Subjects. Forty-six healthy right-handed native English-speaking subjects took part in our studies. A further 12 healthy right-handed native German-speaking subjects and a right-handed German patient (A.M.) with bilateral amygdala lesions completed Exp. 3. All subjects gave informed consent and, apart from the patient, were free of neurological or psychiatric history. The study had full ethical approval.
Exp. 1. Ten subjects [three male, seven female (age range, 22-32 yr; mean age, 26.3)] viewed nouns presented visually in lower-case at a rate of one every 3 s (stimulus duration, 1 s). During four sessions, subjects were presented with eight lists of 19 nouns, with the words “New List” presented between lists. For each list, 16 nouns were of the same semantic category, were emotionally neutral, and were all presented in the same font. These are referred to as control nouns. To set the context, the first five nouns in each list were always control nouns. A P noun was presented in a novel font but was emotionally neutral and of the same semantic category as control nouns. The emotional oddball was aversive in content but of the same category and perceptually identical to control nouns. A semantic oddball was also included (15). Nouns were presented in Times font, except for the P nouns, which were presented in 16 different fonts.
Subjects engaged in two distinct encoding tasks. During two of the sessions, subjects performed a shallow encoding task (16) by indicating whether the first letter in the noun had an enclosed space. During the other two sessions, subjects indicated whether the noun described a living or nonliving entity (the deep encoding task). The order of encoding instructions was counterbalanced. Memory for presented nouns was assessed by immediate verbal recall after the presentation of each 19-noun list. Recall performance in all three experiments is expressed relative to two randomly selected control nouns (only two controls were chosen to balance numbers in the comparison). The chosen control nouns, like the oddballs, could not occur within the first five nouns of each list.
Exp. 2. In a double-blind experimental design, 24 subjects [12 male (age range, 21-32 yr; mean age, 24.5) and 12 female (age range, 19-23 yr; mean age, 20.8)] received either a 40-mg oral dose of propranolol hydrochloride or a vitamin E placebo pill. Drug allocation was balanced for gender, i.e., six males and six females received propranolol. In view of the kinetics of propranolol's peak plasma concentration (1-2 h), the memory task started 90 min after drug administration. Blood pressure was measured immediately before drug administration. A blood sample was taken and blood pressure measured immediately before the memory task (Fig. 2d).
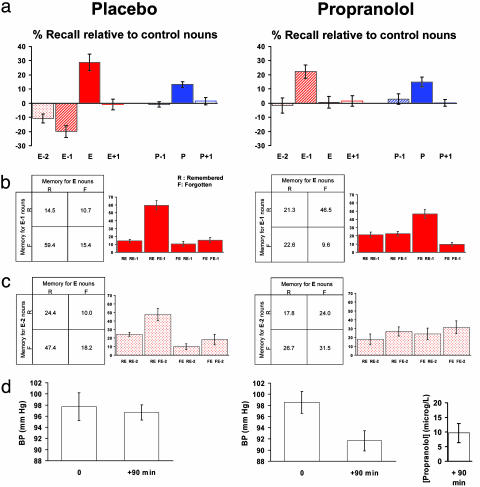
Fig. 2.
Emotion-induced memory impairment is β-adrenergic-dependent. (a) Recall performance relative to control nouns (%) is plotted after shallow encoding for placebo and propranolol groups in Exp. 2. A group (placebo, drug) × oddball type (emotional, perceptual) × position (oddball-1, oddball, oddball + 1) 2 × 2 × 3 ANOVA revealed a significant main effect of position (F1.8, 19.5 = 17.6 P < 0.001), a significant group × position interaction (F1.7, 18.6 = 34.5 P < 0.001), and a significant group × oddball type × position interaction (F1.5, 16.0 = 30.4 P < 0.001). A group × position 2 × 3 ANOVA restricted to the emotional factor revealed a significant main effect of group (F1,11 = 9.3 P < 0.05) and position (F1.6, 18.1 = 5.2 P < 0.05) and a significant group × position interaction (F1.3, 14.6 = 42.4 P < 0.001). Post hoc t tests demonstrated that E-2 nouns are recalled significantly worse than control nouns in the placebo group (P < 0.001 one-tailed one-sample t test) and worse at trend level for placebo relative to drug groups (P = 0.08 one-tailed two-sample t test). The decrement for E-1 nouns was significantly greater than for E-2 nouns in the placebo group (P < 0.05 one-tailed paired t test). Memory for control nouns was not significantly different between groups; mean recall (%; SE) for placebo: 45.2 (1.9); drug: 43.5 (2.4). (b) Reciprocal codependency of memory for E and E-1 nouns demonstrated by contingency tables and plots of the percentage (±SE) of remembered (R) and forgotten (F) E nouns, split according to memory for the corresponding E-1 nouns. Memory decrement for E-1 and memory enhancement for E nouns show a strong item-by-item codependency in the placebo group (χ2 = 4.7; P < 0.05). Propranolol modulated this codependency, evident as a significant difference between placebo (expected) and drug (observed) groups (χ2 = 93.1; P < 0.001). (c) Reciprocal codependency of memory for E and E-2 nouns as for b except that E nouns are now split according to memory for the corresponding E-2 nouns. The item-by-item codependency for memory decrement for E-2 and memory enhancement for E nouns in the placebo group is abolished by propranolol (χ2 = 32.2; P < 0.001). (d) Mean blood pressure (BP; mmHg), at the time of drug/placebo administration (0) and at the start of the experiment (+90 min), and drug serum concentrations ([propranolol]; μg/liter). Propranolol produced the expected decrease in BP (P < 0.001 in a two-tailed paired t test).
The memory task was identical to Exp. 1 except for the following details. Subjects viewed 38 lists of 14 nouns and engaged in the shallow encoding task for all lists (encoding instructions were provided visually at the start of the experiment). Only emotional oddballs and P nouns were presented, the latter appearing in 19 different fonts. Thirty-two of the 38 lists were taken from Exp. 1. The presentation of each 14-word list was followed immediately by a 30-s distraction task, during which subjects were instructed to count backwards in threes (out loud). The distractor task was followed immediately by instructions to free-recall the words presented in the preceding list.
Exp. 3. Patient A.M. suffers from Urbach-Wiethe disease (lipoid proteinosis), an extremely rare genetic disorder that can cause selective bilateral amygdala damage. A.M. is of normal IQ (107) and within normal limits for a range of cognitive functions, including attention and short-term memory. Patient A.M. completed the same task used in Exp. 2, with the verbal stimuli having been translated from English to German by two bilinguals. A further 12 right-handed native German-speaking subjects [six male (age range, 23-36 yr; mean age, 28.2) and six female (age range, 22-33 yrs; mean age, 27.2)] completed the same German version of our task.
For additional information on materials and methods, see Supporting Text, which is published as supporting information on the PNAS web site.
Results
Exp. 1. We observed that E nouns are recalled better than neutral nouns after both deep and shallow (16) verbal encoding (Fig. 1b). In this paradigm, there was a striking emotion-related amnesia, with the E-1 noun being recalled less well than neutral nouns (Fig. 1b). Recall of nouns after emotional stimuli did not differ from control stimuli. P nouns also show enhanced memory, but memory for nouns preceding and after P items did not differ from controls. Given the greater emotion-induced amnesia effect and more robust memory enhancement for both oddball types (von Restorff effect; ref. 17) during shallow encoding (Fig. 1b), this task manipulation was used in subsequent psychological, psychopharmacological, and neuropsychological experiments. Note that we have previously demonstrated (15) amygdala activation in response to emotional nouns during both shallow and deep encoding.
Exp. 2. This psychopharmacological study demonstrates memory enhancement for emotional nouns and impairment for the E-1 noun in the placebo group (Fig. 2a), replicating our previous behavioral finding. Propranolol reversed both effects, such that memory for emotional nouns equated that for neutral nouns, whereas E-1 nouns were now remembered better than control nouns (Fig. 2a). Propranolol did not influence memory for neutral items; i.e., the improvement was selective for E-1 nouns.
In light of the E-1 memory decrement, we performed a further analysis on memory for the nouns preceding E-1 nouns (E-2). In this analysis, we observed a memory impairment for E-2 nouns in the placebo group that is approximately half the magnitude of E-1 decrement. As for E-1 nouns, this effect on E-2 items was also abolished by propranolol (Fig. 2a). The number of items preceding the emotional noun cannot be dissociated from elapsed time, thus the retrograde effect may be time-dependent (up to 6 s) or item-dependent (up to two items). The constraints of our experimental design precluded examination of more enduring retrograde amnesic effects (e.g., E-3).
We next examined, on an item-by-item basis, the dependency of memory for E-1 nouns on successful encoding of the list-related E noun. Fig. 2b demonstrates that, in the placebo group, the E-1 noun is more likely to be forgotten if the E noun is remembered. This codependency is modulated by propranolol such that far fewer E nouns are remembered, and E-1 nouns are more likely to be remembered if corresponding E nouns are forgotten. Again additional analyses demonstrated an identical pattern for E and E-2 memory (Fig. 2c). Because previous human behavioral studies demonstrate enhanced memory for central details of an emotional event and suppression for peripheral details (8, 9), it will be of interest to determine whether central-peripheral effects are modulated by β-adrenergic blockade (18).
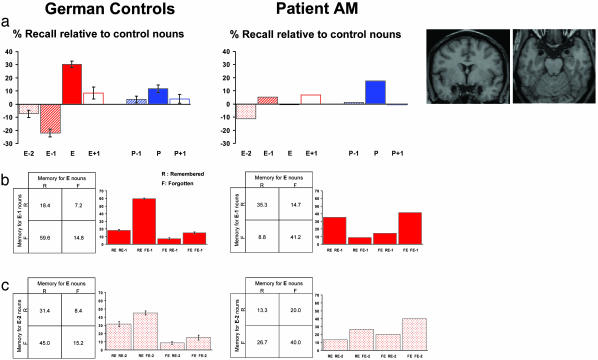
Exp. 3. Fig. 3a demonstrates that the recall profile for German controls is identical to that observed for the English-speaking placebo group in Exp. 2 (Fig. 2a). Critically, the emotion-induced memory impairment for E-1 nouns seen in the control group and the codependency of E-1 memory on recall of E nouns (Fig. 3b) are selectively abolished in patient A.M. with bilateral amygdala damage. We again demonstrated impaired memory for E-2 nouns in German controls that was codependent with memory for E nouns. Note that the memory decrement for E-2 nouns seen in patient A.M. (Fig. 3a) is not codependent with memory for E nouns (Fig. 3c).
Fig. 3.
Emotion-induced memory impairment is amygdala-dependent. (a) Recall performance relative to control nouns (%) is plotted for the German control group and patient A.M. in Exp. 3. (Right) Coronal and transverse T1 MRI images demonstrating patient A.M.'s bilateral amygdala lesion are shown. A group (patient, control) × oddball type (emotional, perceptual) × position (oddball-1, oddball, oddball + 1) 2 × 2 × 3 ANOVA (assumes variance in patient population is equal to that in normals) revealed a significant group × oddball interaction (F1.0, 1.0 = 196.6 P < 0.05) and a trend for the group × oddball × position interaction (F1.0, 1.0 = 60.0 P = 0.08). A group × position 2 × 3 ANOVA restricted to the emotional factor revealed a significant group × position interaction (F1.0, 1.0 = 256.4 P < 0.05). Post hoc independent t tests revealed a significant difference between patient and controls only for recall of E-1 (P < 0.01 two-tailed) and E (P < 0.005 two-tailed) nouns. Recall of control nouns was not significantly different between patient and controls; mean recall of control nouns (%; SE) for patient A.M. 44.7; controls 47.6 (2.1). (b) Reciprocal codependency of memory for E nouns and E-1 nouns for controls and patient A.M., as for Fig. 2b. The control group demonstrates the same reciprocal effects as the placebo group in Exp. 2 (Fig. 2b). The presence of bilateral amygdala lesions abolishes this codependency, evident as a significant difference between controls (expected) and patient (observed) (χ2 = 113.8; P < 0.001). (c) The reciprocal codependency of memory for E nouns and E-2 nouns present for controls is abolished in patient A.M. (χ2 = 74.6; P < 0.001).
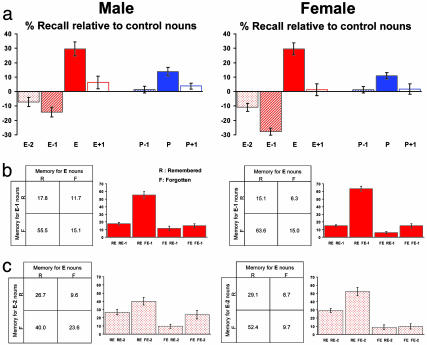
Gender Differences. Memory for emotional stimuli and experiences differs between the sexes (19-22). By balancing for gender in Exps. 2 and 3, we were able to examine gender differences in the memory-modulating effects of emotion. Although an emotion-induced memory decrement for E-1 nouns was present for both genders in both experiments, memory was significantly more impaired in female than male subjects (Fig. 4a), with the impairment in women twice that of men. This could reflect a higher sensitivity in females to the disruptive effect of NE on consolidation of E-1 items, perhaps reflecting differences in emotional intensity evoked by E nouns (21). The latter would predict greater memory enhancement for E nouns in women compared with men, which we did not observe. We did observe gender differences for E and E-1 memory codependency (Fig. 4b). Furthermore, both genders demonstrated a significant memory decrement for E-2 nouns, with the effect in women nonsignificantly larger than men but with a significant gender difference for E/E-2 memory coupling (Fig. 4c).
Fig. 4.
Women display greater emotion-induced memory impairment than men. (a) Recall performance relative to control nouns (%) is plotted for male and female subjects collapsing across the placebo group in Exp. 2 and the control group in Exp. 3. A gender (male, female) × oddball type (emotional, perceptual) × position (oddball-1, oddball, oddball + 1) 2 × 2 × 3 ANOVA across these two groups from Exps. 2 and 3 revealed a significant main effect of position (F1.9, 21.3 = 72.8 P < 0.001), a significant gender × oddball type interaction (F1.0, 11.0 = 9.1 P < 0.05), a significant oddball type × position interaction (F1.4, 15.5 = 31.6 P < 0.001), and a significant gender × oddball type × position interaction (F1.4, 15.6 = 4.7 P < 0.05). A gender × position 2 × 3 ANOVA restricted to the emotional factor revealed a significant main effect of gender (F1,11 = 6.1 P < 0.05) and position (F1.4, 15.9 = 64.7 P < 0.001) and a significant gender × position interaction (F1.6, 18.1 = 4.4 P < 0.05). Post hoc independent t tests revealed a significant gender difference only for the E-1 nouns (P < 0.005 two tailed). (b and c) Reciprocal codependency of memory for E nouns and E-1 nouns (b) and E and E-2 nouns (c) for males and females, as for Fig. 2b. A significant female vs. male difference for codependency between E/E-1 memory (χ2 = 6.1; P < 0.05) and E/E-2 memory (χ2 = 23.0; P < 0.001) was observed, with women showing a greater remembered E and forgotten E-1 (RE FE-1) and RE FE-2 effect.
For more information on results, see also Supporting Text.
Discussion
We demonstrate that emotional stimuli evoke a graded retrograde amnesia that spans at least 6 s, or two stimuli (E-1 and -2 items), before the occurrence of an emotional item. This retrograde amnesia is amygdala and β-adrenergic dependent. Impaired memory for E-1 and -2 nouns is tightly coupled on an item-by-item basis to enhanced memory for E items (i.e., if a particular E nouns is remembered, the corresponding E-1 and -2 nouns are more likely to be forgotten), a coupling abolished by propranolol and amygdala lesions.
Our data extend previous observations that amygdala lesions and β-adrenergic blockade reverse emotion-induced enhancement of memory (2, 4). Whereas in these previous studies (2, 4), the encoded material was a narrated slide show with memory tested weeks later, we demonstrate identical emotional memory effects for verbal material tested after a 30-s delay. That previous studies (2, 4) fail to report an E-1 retrograde amnesic effect is most likely a reflection of study design. Subjects are shown slides while hearing a narrative in three sequential phases: neutral, emotional, and neutral (2, 4), with performance averaged across the four slides for each phase. If emotion-induced memory disruption occurred for the last neutral slide before the emotional phase, then averaging over four slides could obscure an effect.
In our experiments, recall of nouns after emotional stimuli (E + 1 nouns) does not differ from control stimuli, suggesting that the memory consequences of emotional nouns are restricted to E-1 encoding or consolidation. Encoding refers to the acquisition of a memory representation, whereas consolidation is the stabilization of this representation. E. Tulving (23) showed a time-limited retrograde amnesia for high-priority events that spanned 1 s but was not present at 2 s, an effect he conjectured represented either premature termination of encoding or a disruption of consolidation. We favor the latter in view of the enduring nature of the amnesia we observe in the E-2 effect. Note that previously reported retrograde amnesia effects (11, 23) were limited in temporal duration, spanning the order of 1 s. Our data show that an emotional manipulation leads to a robust retrograde effect that spans at least 6 s or two stimuli.
Animal models demonstrate enhancing and impairing effects of adrenergic stimulation on memory that depend on the degree of adrenergic activation, i.e., an inverted-U function (12, 13). Our data suggest that the same level of adrenergic activation can produce memory enhancement and impairment depending on the timing of that activation. Memory is enhanced if adrenergic activation occurs at the time of encoding but impaired if occurring 3-6 s after initial encoding. An adrenal hormone response would be too sluggish to mediate the effects we observe. Emotion-induced memory enhancements do not, however, require peripheral adrenergic engagement (3), and we would suggest that our emotional memory effects are mediated by rapid arousal-evoked central adrenergic release mediated by the amygdala. An alternative explanation (24) is that postlearning adrenergic hormone activity interacts with arousal associated with initial encoding of E nouns to affect memory storage.
Why propranolol enhances memory for E-1 nouns has yet to be determined. The effect is phase-dependent, such that under propranolol, consolidation of recently encoded stimuli, but not encoding of the current E stimulus, is enhanced. The observation that memory for E-2 words is not enhanced by propranolol could imply that propranolol-evoked enhancement depends on the stage of consolidation (i.e., how recently stimuli were encoded). That patient A.M. does not show enhanced memory for E-1 events makes it more suggestive that the E-1 enhancement seen in the propranolol group is an effect specific to blocking β1 and -2 adrenergic receptors.
Because all other nouns in each list in the three experiments were emotionally neutral, the E nouns constituted emotional oddballs. To control for a nonspecific oddball effect, we included a perceptual oddball control. Memory impairment for the E-1 noun was not due to an oddball-evoked arousal or distinctiveness effect, because memory for nouns preceding a perceptual odd-ball (the P-1 noun; Fig. 1a) did not differ from control nouns (Figs. 1b, 2a, and 3a). Thus, memory for E-1 nouns was significantly impaired relative to P-1 nouns. Note that distinctiveness of emotional stimuli is a potential confound in previous investigations of emotion-induced memory disruption (6, 10, 11, 25). Furthermore, the blocking effect of propranolol on the enhancement of memory for E nouns cannot be explained in terms of a blockade of enhanced memory for oddball stimuli (the von Restorff effect; ref. 17), because propranolol did not abolish enhanced recall for perceptual oddballs (Fig. 2b). Likewise, patient A.M. demonstrates a normal von Restorff effect (17) for perceptual oddballs (Fig. 3a), suggesting that the amygdala does not enhance memory for oddball stimuli.
One account of the patient and propranolol data is that amygdala damage impairs attentional orienting to emotional stimuli, as previously demonstrated (26), and that failure of attentional orienting is a component process in the abolition of enhanced emotional memory in the patient and in the propranolol-treated group. It is unlikely that this is the case in the propranolol group, because there is no difference in reaction times to emotional nouns in the placebo vs. drug group. Given that skin conductance responses are preserved in patients with amygdala damage (27), it is unlikely that emotional memory effects in patient A.M. are due to a blunting of arousal to emotional nouns.
In Exp. 2, the effects of propranolol spanned both encoding and free recall. We cannot rule out the possibility that drug effects or the effects of amygdala lesions operate during retrieval rather than encoding or consolidation stages of memory. However, behavioral data indicate that the effects of propranolol on emotional memory are expressed primarily at encoding (1, 2, 28). Furthermore, functional neuroimaging data demonstrate that amygdala activation at encoding correlates with subsequent memory for emotional stimuli (29-31), albeit at longer retention intervals. In light of evidence that adrenergic modulation of emotional memory is amygdala dependent (1), these data support an interpretation that the effects of amygdala lesions and propranolol on emotional memory occur at encoding.
Our series of experiments demonstrate that emotion-evoked β-adrenergic activity disrupts the encoding of items immediately preceding an emotional event. This emotion-induced memory impairment spans at least 6 s, or two stimuli, and is more pronounced in women than men. In line with previous observations (2, 4, 5), enhanced memory for emotional stimuli is β-adrenergic-dependent and abolished by bilateral amygdala lesions. We now demonstrate that amygdala lesions also abolish emotion-induced memory decrements. Thus, enhanced recall and enhanced forgetting associated with emotional stimuli are amygdala dependent, with both effects strongly influenced by β-adrenergic modulation.
Supplementary Material
Acknowledgments
We thank P. Patsalos and A. Richardson for storing blood samples, E. Düzel for organizing patient access, U. Wachowius for neuropsychology reporting, and P. Rosthien and U. Noppeney for assistance with Exp. 3. Diagnosis of Urbach-Wiethe disease in A.M. was made by H. Reich. This work was supported by a program grant from the Wellcome Trust (to R.J.D.).
Abbreviations: E noun, emotionally aversive noun; P noun, perceptual oddball noun; E-1, noun preceding E; E-2, noun preceding E-1 noun; E + 1, noun after E; P-1, noun preceding P; P + 1, noun after P; NE, noradrenaline/norepinephrine.
See Commentary on page 13123.
References
- 1.Cahill, L. & McGaugh, J. L. (1998) Trends Neurosci. 21, 294-299. [DOI] [PubMed] [Google Scholar]
- 2.Cahill, L., Prins, B., Weber, M. & McGaugh, J. L. (1994) Nature 371, 702-704. [DOI] [PubMed] [Google Scholar]
- 3.van Stegeren, A. H., Everaerd, W., Cahill, L., McGaugh, J. L. & Gooren, L. J. (1998) Psychopharmacology 138, 305-310. [DOI] [PubMed] [Google Scholar]
- 4.Cahill, L., Babinsky, R., Markowitsch, H. J. & McGaugh, J. L. (1995) Nature 377, 295-296. [DOI] [PubMed] [Google Scholar]
- 5.Phelps, E. A., LaBar, K. S., Anderson, A. K., O'Connor, K. J., Fulbright, R. K. & Spencer, D. D. (1998) Neurocase 4, 527-540. [Google Scholar]
- 6.Christianson, S-Å. (1992) in The Handbook of Emotion and Memory: Research and Theory, ed. Christianson, S.-Å. (Erlbaum, Mahwah, NJ), pp. 307-340.
- 7.Holmes, D. S. (1970) J. Pers. Soc. Psychol. 15, 234-239. [DOI] [PubMed] [Google Scholar]
- 8.Heuer, F. & Reisberg, D. (1992) in The Handbook of Emotion and Memory: Research and Theory, ed. Christianson, S.-Å. (Erlbaum, Mahwah, NJ), pp. 151-180.
- 9.Adolphs, R., Denburg, N. L. & Tranel, D. (2001) Behav. Neurosci. 115, 983-992. [DOI] [PubMed] [Google Scholar]
- 10.Loftus, E. F. & Burns, T. E. (1982) Mem. Cognit. 10, 318-323. [DOI] [PubMed] [Google Scholar]
- 11.Angelini, R., Capozzoli, F., Lepore, P., Grossi, D. & Orsini, A. (1994) Percept. Mot. Skills 78, 19-28. [DOI] [PubMed] [Google Scholar]
- 12.Liang, K. C., McGaugh, J. L. & Yao, H. Y. (1990) Brain Res. 508, 225-233. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh, J. L., McIntyre, C. K. & Power, A. E. (2002) Neurobiol. Learn. Mem. 78, 539-552. [DOI] [PubMed] [Google Scholar]
- 14.Sternberg, D. B. & Gold, P. E. (1981) Brain Res. 211, 59-65. [DOI] [PubMed] [Google Scholar]
- 15.Strange, B. A., Henson, R. N., Friston, K. J. & Dolan, R. J. (2000) NeuroImage 12, 425-433. [DOI] [PubMed] [Google Scholar]
- 16.Craik, F. I. M. & Lockhart, R. S. (1972) J. Verbal Learn. Verbal Behav. 11, 671-684. [Google Scholar]
- 17.von Restorff, H. (1933) Psychol. Forsch. 18, 299-342. [Google Scholar]
- 18.Cahill, L. & van Stegeren, A. (2003) Neurobiol. Learn. Mem. 79, 81-88. [DOI] [PubMed] [Google Scholar]
- 19.Cahill, L., Haier, R. J., White, N. S., Fallon, J., Kilpatrick, L., Lawrence, C., Potkin, S. G. & Alkire, M. T. (2001) Neurobiol. Learn. Mem. 75, 1-9. [DOI] [PubMed] [Google Scholar]
- 20.Fujita, F., Diener, E. & Sandvik, E. (1991) J. Pers. Soc. Psychol. 61, 427-434. [DOI] [PubMed] [Google Scholar]
- 21.Seidlitz, L. & Diener, E. (1998) J. Pers. Soc. Psychol. 74, 262-271. [DOI] [PubMed] [Google Scholar]
- 22.Canli, T., Desmond, J. E., Zhao, Z. & Gabrieli, J. D. (2002) Proc. Natl. Acad. Sci. USA 99, 10789-10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulving, E. (1969) Science 164, 88-90. [DOI] [PubMed] [Google Scholar]
- 24.Cahill, L. & Alkire, M. T. (2003) Neurobiol. Learn Mem. 9, 194-198. [DOI] [PubMed] [Google Scholar]
- 25.Christianson, S.-Å. & Nilsson, L.-G. (1984) Mem. Cognit. 12, 142-155. [DOI] [PubMed] [Google Scholar]
- 26.Anderson, A. K. & Phelps, E. A. (2001) Nature 411, 305-309. [DOI] [PubMed] [Google Scholar]
- 27.Tranel, D. & Damasio, H. (1989) Neuropsychologia. 27, 381-390. [DOI] [PubMed] [Google Scholar]
- 28.van Stegeren, A. H., Everaerd, W. & Gooren, L. J. (2002) Psychopharmacology 163, 202-212. [DOI] [PubMed] [Google Scholar]
- 29.Cahill, L., Haier, R. J., Fallon, J., Alkire, M. T., Tang, C., Keator, D., Wu, J. & McGaugh, J. L. (1996) Proc. Natl. Acad. Sci. USA 93, 8016-8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamann, S. B., Ely T. D., Grafton, S. T. & Kilts, C. D. (1999) Nat. Neurosci. 2, 289-293. [DOI] [PubMed] [Google Scholar]
- 31.Canli, T., Zhao, Z., Brewer, J., Gabrieli, J. D. & Cahill, L. (2000) J. Neurosci. 20, RC99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.