Abstract
Methylmercury (MeHg) is a potent neurotoxicant and preferentially induces oxidative injury in astrocytes. In neuronal tissues, nuclear factor erythroid 2–related factor 2 (Nrf2) is a key factor determining the protective antioxidant response against various environmental toxicants. Nrf2 is subjected to regulation by many other signaling pathways. The purpose of this study is to characterize its interaction with the phosphatidylinositol 3 (PI3) kinase in cultured rat neonatal primary astrocytes. The results showed that at pathologically relevant concentrations, exposure of primary astrocytes to MeHg led to Nrf2 activation and upregulation of its downstream antioxidant genes. Inhibition of the PI3 kinase resulted in decreased Nrf2 activity, decreased cellular glutathione, and increased cell death to high-dose MeHg. The functional interaction between the two signaling pathways underlined an important mechanism for astrocyte protection against MeHg toxicity. Modulation of Nrf2 by pharmacological modalities should afford a treatment to attenuate MeHg-induced neurotoxicity.
Keywords: methylmercury, astrocyte, glutathione, Nrf2, phosphatidylinositol 3 kinase
Methylmercury (MeHg) is converted from inorganic mercury by aquatic anaerobic organisms (Jensen and Jernelov, 1969; Wood et al., 1968). The methylated form, MeHg, is rapidly taken up by living organisms in the aquatic environment and biomagnified through the food chain reaching concentrations in fish 10,000–100,000 times greater than in the surrounding water (EPA, 1997; Wiener et al., 2003). MeHg is a potent neurotoxicant that affects both the developing and mature central nervous system (CNS). In adults, MeHg intoxication causes loss of neurons in the calcarine cortex and cerebellar granule cells, leading to vision and motion abnormalities, including blurred vision, visual field constriction, malaise, paresthesias, and atasia (Bakir et al., 1973; Hamada and Osame, 1996; Oyake et al., 1966; Takeuchi, 1982). In infants, MeHg exposure leads to cerebral palsy-like effects (Stewart et al., 2003; Takeuchi, 1977; Wagner et al., 2007), characterized by ataxic motor and mental symptoms with hypoplastic and symmetrical atrophy of the cerebrum and cerebellum. The neuropathological features include a decreased number of neurons and distortion of cytoarchitecture in the cortical and cerebellar areas (Choi et al., 1978; Takeuchi, 1977). Specifically, these are characterized by incomplete or abnormal migration of neurons to the cerebellar and cerebral cortices and deranged cortical organization of the cerebrum with heterotopic neurons, both isolated and in groups in the white matter of cerebrum and cerebellum. Furthermore, the laminar cortical pattern of the cerebrum is disturbed, consisting of irregular groupings and deranged alignment of cortical layers. Developmental exposure to MeHg in animal models affected behavioral performance later in life (Cox et al., 1989; Stern et al., 2001).
The primary site of lesion and the molecular mechanisms of MeHg toxicity have yet to be clearly defined. In mammalian CNS, astrocytes are known as a preferential site of MeHg accumulation and a main target of toxicity (Aschner and Kimelberg, 1991; Aschner et al., 1994; Charleston et al., 1995; Oyake et al., 1966). A number of previous studies have shown that MeHg exposure led to astrocytic dysfunction associated with increased Na+ (Vitarella et al., 1996) and aspartate uptake (Yao et al., 1999, 2000) and excessive production of excitatory neurotransmitter, affecting the glial-neuron interaction (Schousboe et al., 1992).
In general, the pathological sequelae of MeHg exposue will depend on the balance between dose and exposure duration and the ability of detoxification systems and related cell survival signaling pathways to mitigate the aberrant effects of this metal. Mammalian cells have developed a multitude of protective mechanisms against xenobiotics, including the activation of multidrug resistant–associated proteins (MRPs) (Konig et al., 1999) and the nuclear factor erythroid 2–related factor 2 (Nrf2)–mediated antioxidant response (Toyama et al., 2007). While MRPs attenuate the accumulation of MeHg in the CNS, Nrf2 activation upregulates a number of antioxidant proteins as well as nonprotein antioxidant thiols (Ishii et al., 2000; Itoh et al., 1999; Kim et al., 2001b), which afford neuroprotection.
The phosphatidylinositol 3 (PI3) kinase/Akt pathway is a major cell survival pathway and regulates Nrf2-mediated antioxidant and detoxification reactions (Lee et al., 2001; Li et al. 2006; Wang et al., 2008). A previous report showed that PI3 kinase was involved in MeHg-induced dysfunction in mouse pancreatic β cells (Chen et al., 2006). In the present study, we investigated the mechanism of MeHg-induced Nrf2 activation and its association with the PI3 kinase pathway in primary rat neonatal astrocytes. Our results showed that MeHg exposure activated Nrf2 and upregulated its downstream antioxidant genes and that inhibition of the PI3 kinase downregulated the Nrf2-dependent antioxidant response. The functional interactions between the two signaling pathways underlined a potentially important mechanism of protecting astrocytes against MeHg toxicity.
MATERIALS AND METHODS
Materials.
γ-Glutamylglutamate (γEE), wortmannin, and LY294002 were purchased from MP Biomedicals (Irvine, CA), Upstate (Lake Placid, NY), and Promega (Madison, WI), respectively. Anti-β-actin antibody was purchased from Sigma-Aldrich (St Louis, MO). Anti-Nrf2, anti-PI3K p85α, and anti-Hsc70 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-histone H3 antibody was obtained from Upstate.
Cell cultures and experimental conditions.
Primary rat astrocyte cultures were prepared as described by Frangakis and Kimelberg (1984). The cerebral hemispheres of postnatal day-1 neonatal rats were removed and meninges were dissected off. The basal ganglia and midbrain were removed, and the remaining cortical tissue was dissociated with dispase. Cells were grown in minimal essential medium (Mediatech, Hemdon, VA), supplemented with 5% heat-inactivated fetal bovine serum and 5% horse serum (Sigma). The cultures were maintained in a humidified environment of 5% CO2 at 37°C. The media were changed twice a week. Cultures were >95% positive for the astrocytic marker, glial fibrillary acidic protein. After 4 weeks in culture when cells reached ∼95% confluence, cells were exposed to MeHg at different concentrations for different durations.
Plasmid construction.
The antioxidant response element (ARE) reporter plasmid was previously described (Wang et al., 2008); it contained the consensus Nrf2-binding site–inserted upstream of the firefly luciferase gene. Full-length human Nrf2 complementary DNA (cDNA) was cloned by reverse transcription polymerase chain reaction (RT-PCR) using forward primer (5′-ATG ATG GAC TTG GAG CTG CCG CCG-3′) and reverse primer (5′-AAC TAG CTC AGA AAA GGT CAA ATC CTC-3′). The PCR products were gel purified and subcloned into pcDNA3.1/V5-His vector (Invitrogen, Carlsbad, CA).
Transfection and dual luciferase reporter assay.
The primary astrocytes were cotransfected with 1 μg of the ARE reporter plasmid, 1 μg of the pcDNA3.1/V5-His vector containing Nrf2 cDNA, and 20 ng of the pRL-CMV vector (Promega) for normalization (Wang et al., 2008). Twenty-four hours later, medium was replaced with fresh medium containing MeHg. After treatment, cells were lysed with 500 μl passive lysis buffer (Promega), and the reporter gene activity was measured by a dual luciferase assay kit (Promega).
GSH and oxidized glutathione measurement by high-performance liquid chromatography.
The intracellular glutathione (GSH) concentrations were measured by high-performance liquid chromatography (HPLC) as previously described (Wang et al., 2008). Briefly, astrocytes were extracted with 5% perchloric acid/0.2M boric acid. Acid-soluble thiols were derivatized with iodoacetic acid and dansyl chloride and were analyzed by HPLC using a propylamine column (YMC Pack, NH2, Waters, Milford, MA) and an automated HPLC system (Alliance 2695, Waters). The GSH and oxidized glutathione (GSSG) concentrations were normalized to the protein contents of the samples measured by the Bradford assay (Bio-Rad, Hercules, CA).
Measurement of Nrf2 localization by subcellular fractionation and Western blot analyses.
The cytosolic and nuclear fractions were prepared as previously described (Wang et al., 2008). Briefly, astrocytes were lysed in ice-cold hypotonic buffer (Sigma) containing 10mM HEPES, pH 7.9, 1.5mM MgCl2, 10mM KCl, 1mM dithiothreitol, 0.6% NP40, and a cocktail of protease inhibitors (Roche, Indianapolis, IN). After 30 s centrifugation at 4°C at 12,000 × g, the supernatant was collected as the cytosolic fraction. The pellet was further extracted for 30 min in a high salt lysis buffer containing 50mM HEPES, 500mM NaCl, 1% NP40, pH 7.0, and protease inhibitors. After 5 min centrifugation at 4°C at 12,000 × g, the supernatant was collected as the nuclear fraction. The proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analyses. Histone H3 was used as a marker of nuclear proteins.
Measurement of antioxidant gene expression by quantitative RT-PCR.
The messenger RNA (mRNA) levels of heme-oxygenase1 (HO-1), NADPH: quinone oxidoreductase (NQR), and the light chain of cystine/glutamate transporter (XC-) (xCT) were measured by real-time RT-PCR using the Universal Probe Library approach (UPL) (Roche). The primers used for xCT were forward (5′-TCC ATG AAC GGT GGT GTG T-3′), reverse (5′-CCC TTC TCG AGA TGC AAC AT-3′), and UPL probe No. 80. The primers used for HO-1 were forward (5′-GTC AGG TGT CCA GGG AAG G-3′), reverse (5′-CTC TTC CAG GGC CGT ATA GA-3′), and UPL probe No. 9. The primers used for Nqo1 were forward (5′-AGC GCT TGA CAC TAC GAT CC-3′), reverse (5′-CAA TCA GGG CTC TTC TCA CC-3′), and UPL probe No. 50. Average threshold cycle (Ct) values were used to determine the relative difference between control and treated groups and were normalized to 18S ribosomal RNA.
Knockdown Nrf2 and p85 by small interference RNA approach.
The sequence of small interference RNA (siRNA) against rat Nrf2 and the regulatory subunit of PI3 kinase (p85α) was 5′-GCA GGA GAG GGA AGA ATA AAG TT-3′ and 5′-GAA GGA AAT TCA AAG GAT AAT GCA T-3′, respectively. Primary astrocytes at 60–70% confluence were transfected with siRNA duplex (Integrated DNA Technologies, Coralville, IA) using the Lipofectamine reagent (Invitrogen) (Wang et al., 2008). In some experiments, the ARE reporter plasmid was cotransfected with the siRNA. Western blot analyses and reporter assays were performed 48 h after transfection.
Cell viability measurement by flow cytometry.
The astrocyte viability was measured with a LIVE/DEAD Viability/Cytotoxicity Assay Kit (Invitrogen) (Chen et al., 2002). Astrocytes were pretreated with LY294002 followed by exposure to different concentrations of MeHg in a serum-free medium. Calcein AM was used for live cell staining, and ethidium homodimer (EthD-1) was used for dead cell staining. Cells were gated to exclude debris. The ratio of live target events to total events was expressed as percentage of viable cells.
RESULTS
MeHg-Induced Changes of Intracellular GSH Content
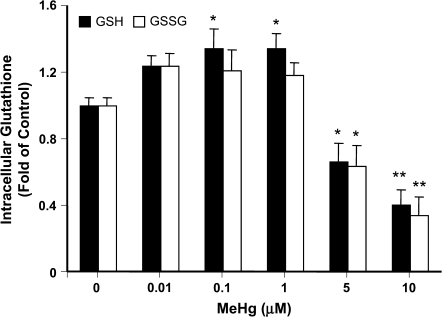
Hg compounds react with accessible sulfhydryl (-SH) groups and forms -S-Hg-R complexes; therefore, GSH is a principal cellular protective thiol against MeHg toxicity. To investigate how MeHg affected the intracellular GSH contents in primary rat neonatal astrocytes, cells were exposed to MeHg at concentrations from 0.01 to 10μM for 6 h followed by HPLC measurement of the GSH and GSSG. These concentrations have been carefully considered. Brain levels in human autopsy material from individuals in Japan with acute and chronic MeHg exposure reached ∼25 and 100μM, respectively (Takizawa, 1986). The maximum concentration used in the current study was 10μM, a concentration which is known to elicit significant reactive oxygen species generation, yet causes minimal cell death (Kasuya, 1976; Sanfeliu et al., 2001). At concentrations higher than 5μM, MeHg caused significant (p < 0.01) decreases in both GSH and GSSG (Figure 1). GSH decreased to 66 ± 11% and 40 ± 9% of control and GSSG decreased to 63 ± 13% and 34 ± 11% after exposure to 5 and 10μM MeHg, respectively. When treated with 0.1 and 1μM of MeHg, however, the intracellular GSH increased to 134 ± 12% and 134 ± 9% of control, respectively (Figure 1).
FIG. 1.
MeHg-induced changes in intracellular GSH and GSSG contents. Primary rat astrocytes were treated with indicated concentrations of MeHg for 6 h. Cellular GSH and GSSG were measured by HPLC. Data presented are the average of three separate experiments performed in duplicate (mean ± SE) (*p < 0.05; **p < 0.01; significantly different from control untreated cells, one-way ANOVA and Dunnett post hoc test).
Nrf2 Coordinated Protective Gene Expression against MeHg-Induced Cytotoxicity
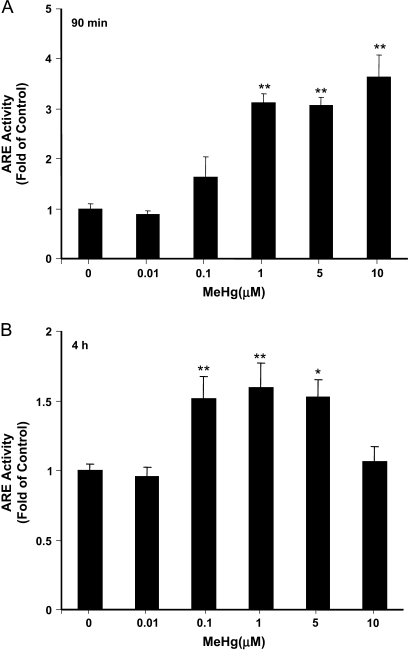
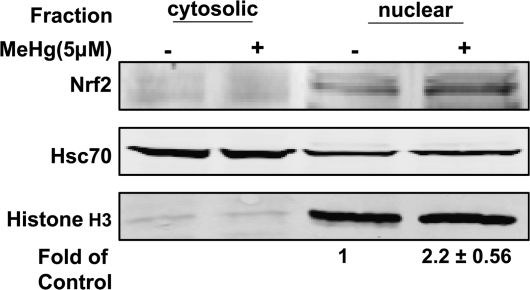
Nrf2 is an essential inducible factor that mediates the transcription of cytoprotective genes. To investigate the effect of MeHg on Nrf2 activation, we used an ARE reporter assay (Wang et al., 2008) to measure the transcriptional activity Nrf2 in cells exposed to MeHg at different concentrations for different durations. The data (Figure 2A) showed a concentration-dependent increase in ARE activity (3.1 ± 0.2 to 3.6 ± 0.4 times of control, p < 0.01) when cells were treated for 90 min with MeHg at 1–10μM. At 4 h, ARE activity increased by ∼50% over control values at MeHg exposures between 0.1 and 5μM (Figure 2B). The localization of Nrf2 was measured by subcellular fractionation and Western blot analyses (Figure 3). In cultured primary astrocytes, a significant amount of Nrf2 was present in the nuclear fraction and its expression was further increased after 90 min exposure to 5μM MeHg.
FIG. 2.
ARE activity measurement in astrocytes treated with MeHg. Cells were cotransfected with Nrf2, an ARE reporter plasmid, and a pCMV-Renilla luciferase vector for normalization. After 16 h, cells were further exposed to indicated concentrations of MeHg for 90 min (A) and 4 h (B), respectively. The ARE activities were measured by a dual luciferase assay. Each point represents the average of three separate experiments performed in duplicate (mean ± SE) (**p < 0.01; significantly different from control untreated cells; one-way ANOVA and Dunnett post hoc test).
FIG. 3.
MeHg treatment increased Nrf2 in the nucleus. Cells were treated for 90 min with 5μM MeHg. The amount of Nrf2 in the cytoplasmic and nuclear fractions was measured by subcellular fractionation followed by Western blot analyses. Hsc70 and Histone H3 were used as loading control.
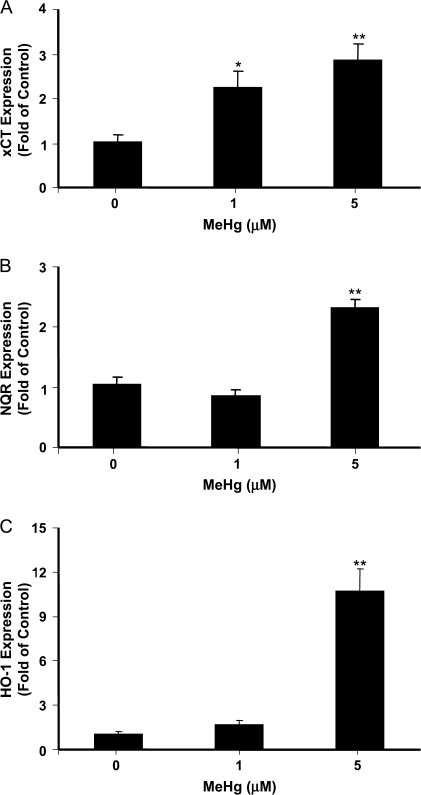
The uptake of cystine, a substrate of GSH synthesis, is partly mediated by the sodium-independent transporter XC-, which is also known as the solute carrier family 7 member 11 (SLC7A11). The XC- consists of two subunits, xCT as the light chain and 4F2hc as the heavy chain. When xCT expression was measured by quantitative RT-PCR, significant increase (2.2 ± 0.4 and 2.8 ± 0.4 times of control) was observed in cells exposed for 3 h to 1 and 5μM MeHg (Figure 4A). Similar upregulation was observed for two other antioxidant genes functioning downstream of Nrf2. In cells exposed to 5μM MeHg for 3 h, the mRNA level of NQR and HO-1 was increased to 2.3 ± 0.1- and 10.7 ± 1.5-fold of control values, respectively (Figure 4B and C). These data collectively suggest that nonlethal doses of MeHg activated an Nrf2-dependent antioxidant response in cultured primary astrocytes.
FIG. 4.
MeHg-induced changes in the mRNA level of antioxidant genes downstream of Nrf2. Astrocytes were treated with indicated concentrations of MeHg for 3 h. The mRNA levels of xCT (A), NQR (B), and HO-1 (C) were measured by real-time RT-PCR. The differences in the average threshold cycle (ΔCt) values were determined and normalized to the expression of 18s rRNA. Each point represents the average of three separate experiments performed in duplicate (mean ± SE) (*p < 0.05; **p < 0.01; significantly different from control untreated cells; one-way ANOVA and Dunnett post hoc test).
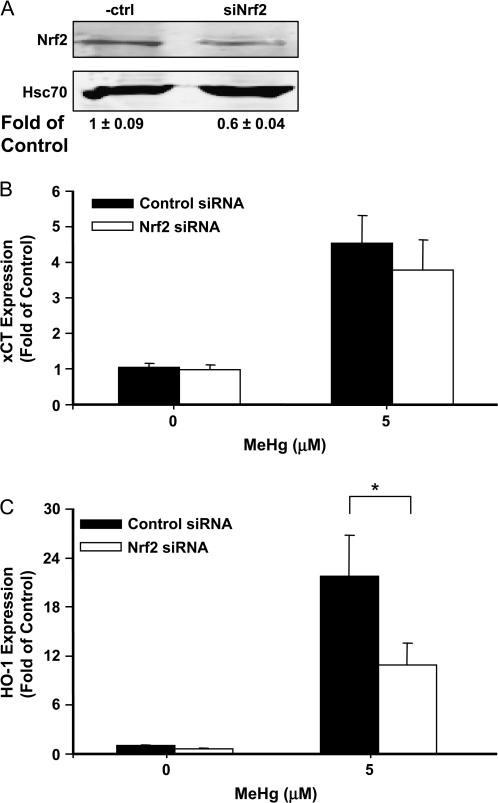
We also used the siRNA approach to knockdown the expression of Nrf2 in primary astrocytes. As shown in Figure 5A, the Nrf2 protein was decreased to 60% of control level after transfection with the siRNA. MeHg-induced HO-1 expression was significantly lower in cells transfected with siRNA against Nrf2 (Figure 5C); however, xCT expression was not affected (p > 0.05, Figure 5B).
FIG. 5.
Effects of knockdown Nrf2 on MeHg-induced antioxidant gene expression. Astrocytes were transfected with siRNA against Nrf2. After 48 h, the Nrf2 level was measured by Western blot analyses (A). Hsc70 was used as loading control. The expressions of xCT (B) and HO-1 (C) were measured by real-time RT-PCR, after cells were further treated for 3 h with MeHg at the indicated concentrations (*p < 0.05; Student's t-test).
PI3 Kinase Activity Was Required for GSH Synthesis and Nrf2 Activity in Astrocytes
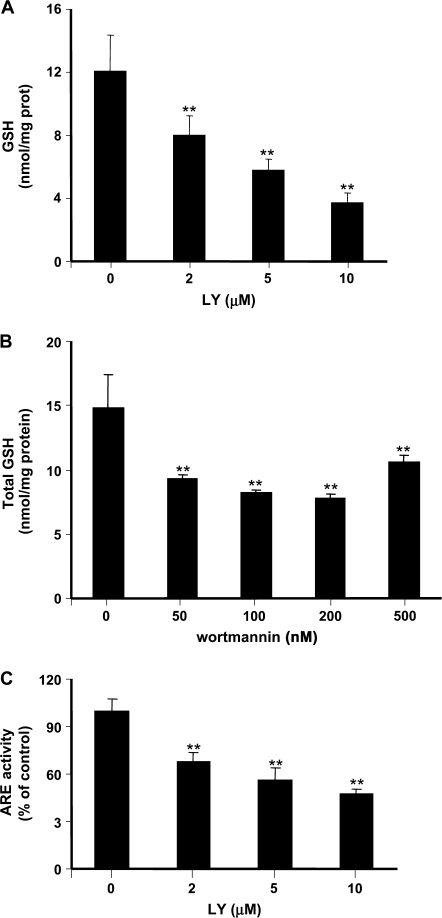
To determine how changes in PI3 kinase signaling affected GSH content, astrocytes were treated with two different PI3 kinase inhibitors, LY294002 and wortmannin, followed by HPLC measurement of intracellular GSH. Treatment with LY294002 for 16 h caused a concentration-dependent decrease in the intracellular GSH level (Figure 6A). Total GSH in control astrocytes was 12.0 ± 2.3 nmol/mg protein and decreased to 3.7 ± 0.6 nmol/mg protein after exposure to 10μM LY294002 (p < 0.01). Treatment of astrocytes with wortmannin (50–500nM for 6 h) resulted in similar GSH depletion (Figure 6B). Under the same condition, LY294002 led to a concentration-dependent decrease of Nrf2 activity (Figure 6C). Treatment with 10μM LY294002 caused a nearly 50% reduction in Nrf2 activity (p < 0.01).
FIG. 6.
Effects of PI3 kinase inhibitors on GSH synthesis and Nrf2 activity in primary astrocytes. Astrocytes were treated with PI3 kinase inhibitors, LY294002 (A) and wortmannin (B), for 16 and 6 h, respectively. Total GSH was measured by HPLC. (C) The Nrf2 activity was measured by transient transfection with the ARE reporter plasmid followed by the luciferase assay. Cells were treated with LY294002 at indicated concentrations. Each point represents the average of three separate experiments performed in duplicate (mean ± SE) (**p < 0.01; significantly different from control untreated cells; one-way ANOVA and Dunnett post hoc test).
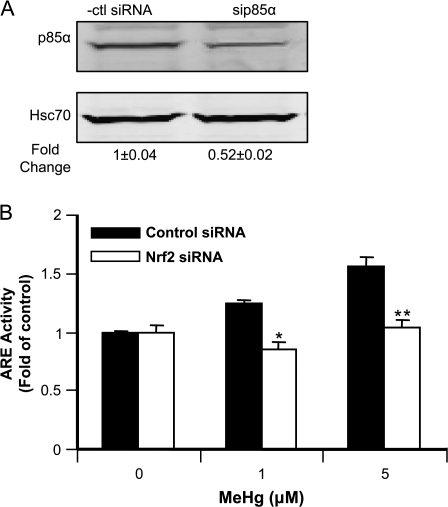
In addition to the chemical inhibitors, we also used siRNA to knockdown the regulatory subunit of PI3 kinase, p85α. Nearly 50% downregulation in the enzyme expression was achieved by transfection of astrocytes with the siRNA (Figure 7A). Compared to cells transfected with control siRNA, MeHg-induced increase in ARE activity was significantly lower when p85α was knocked down (Figure 7B).
FIG. 7.
Effects of knockdown PI3K by siRNA on Nrf2 activity. Astrocytes were transfected with siRNA targeting p85α. After 48 h, the p85α level was measured by Western blot analyses (A). Hsc70 was used as loading control. (B) Measurement of Nrf2 activity by reporter assay after downregulation of PI3K. Cells were cotransfected with p85α siRNA and ARE reporter plasmid. After 48 h, they were further exposed for 90 min to MeHg at the indicated concentrations. The ARE activities were measured by the dual luciferase assay. Each point represents the average of three separate experiments performed in duplicate (mean ± SE) (*p < 0.05; **p < 0.01; significantly different from cells transfected with control siRNA; Student's t-test).
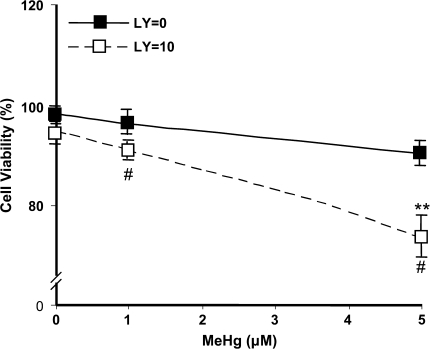
We further performed cell viability assay as another end point measurement of MeHg toxicity. Astrocytes were treated with MeHg for 16 h at either 1 or 5μM, either with or without 10μM LY294002. As shown in Figure 8, the percentage of viable cells significantly decreased to 74 ± 4.5% of control values after exposure to 5μM MeHg in presence of LY294002 (p < 0.01). In the absence of cotreatment with LY294002, cell viability was not significantly affected by MeHg and was indistinguishable from control values.
FIG. 8.
Measurement of astrocytes viability after exposure to MeHg. Cells were treated with MeHg and LY294002 as indicated for 16 h. The percent of viable cells was quantified after flow cytometry analyses. Data are the average from four separate experiments (mean ± SE) (**p < 0.01; significantly different from control untreated cells; one-way ANOVA and Dunnett post hoc test. #p < 0.05; significantly different between LY294002-treated and -untreated cells; Student's t-test).
DISCUSSION
Hg compounds react with reduced GSH, binding to sulfhydryl groups of cysteine (Griffith, 1980; Hughes, 1957; Meister, 1984; Rabenstein and Fairhurst, 1974; Simpson, 1961). The conjugated products can be transported out of the target cells by the MRPs (Konig et al., 1999), which substantially decrease the intracellular concentrations of mercurials and lower the toxicity. Previous studies have also established the protective roles afforded by GSH in MeHg-induced toxicity (Mullaney et al., 1993, 1994). In the present study, we showed that MeHg exposure lead to a biphasic response in intracellular astrocytic GSH content (Figure 1), and the increase of GSH was associated with activation of Nrf2 (Figure 2). High concentrations of MeHg resulted in depletion of GSH, while incubation with low concentrations of MeHg led to increased intracellular GSH (Figure 1). Nrf2 activation was observed at 90 min after MeHg treatment (Figure 2A) and led to increased expression of its downstream antioxidant genes (Figure 4). Thus, consistent with the previous reports (Ali et al., 1992; Dreiem and Seegal, 2007; Ganther et al., 1972), an early adaptive response can be elicited in astrocytes to protect against MeHg-induced oxidative injury.
It is well established that activation of Nrf2 will lead to upregulation of glutamate cysteine ligase, the rate-limiting enzyme of GSH synthesis (Kensler et al., 2007). Cystine is an essential precursor of GSH synthesis (Chawla et al., 1984; Sato and Robbins, 1981). The Na+-independent XC- transporter system mediates cystine uptake by astrocytes and neurons (Bannai and Kitamura, 1980, 1981). The expression of its light chain, xCT, is controlled by Nrf2 (Kim et al., 2001a; Tomi et al., 2003). XC- is expressed at low levels under normal physiologic conditions, but it is upregulated by oxidative stress (Guidotti and Gazzola, 1992). Our results show that Nrf2 activation (Figure 4A) was associated with increased expression of xCT (Figure 3) after exposure to MeHg, increasing cystine uptake and GSH synthesis. However, when exposed to high-dose MeHg, it needs to be considered that such antioxidant response may also negatively affect glial-neuron interactions and promote excitotoxicity since XC- is a glutamate/cystine antiporter. Accordingly, increased influx of cystine may result in exchange for intracellular glutamate, leading to glutamate accumulation in the extracellular fluid and activation of N-methyl D-aspartate receptors (Choi, 1992).
An important feature of the Nrf2-dependent antioxidant system is the simultaneous upregulation of multiple antioxidant genes that work coordinately to detoxify and protect against MeHg. In addition to xCT, HO-1 and NQR were upregulated by MeHg (Figure 4); both enzymes are involved in GSH-independent detoxification mechanisms upon ARE activation by Nrf2. Knockdown of Nrf2 led to inhibited HO-1 gene induction but did not affect the MeHg-induced xCT expression (Figure 5B). The data indicate that other signaling pathways may be involved in the transcriptional regulation of xCT.
The PI3 kinase/Akt pathway is essential for cell survival (Martindale and Holbrook, 2002). Various studies have shown that oxidative stress regulates the activation of Akt (Esposito et al., 2003; Gorin et al., 2003; Ushio-Fukai et al., 1999) and that MeHg activates the PI3K/Akt pathway via increased production of reactive intermediates (Chen et al., 2006). There are cross-talks between the PI3 kinase and Nrf2 pathways. The PI3K and Akt functions are required for Nrf2 activation by various inducers such as tert-butylhydroquinone, hemin, and peroxynitrite (Lee et al. 2001; Li et al. 2006; Nakaso et al. 2003). Consistent with the literature reports, astrocytes treated with PI3 kinase inhibitors (Figure 4) showed a concentration-dependent decrease in Nrf2 activity and decrease in GSH synthesis. Similar effects were achieved by transfection with siRNA against p85α (Figure 8) Inhibition of the PI3 kinase pathway potentiated the cytotoxicity of MeHg (Figure 5). These results indicate that the PI3 kinase/Akt pathway contributes, at least in part, to the detoxification of MeHg in astrocytes.
Several recent studies have explored the molecular mechanisms underlying the interaction between Nrf2 and PI3 kinase pathways. PI3 kinase generates signaling lipids, phosphoinositides PI(3, 4, 5)P3, and PI(3, 4)P2, which recruit Akt (Brazil and Hemmings, 2004) to the plasma membrane where Akt is phosphorylated and activated by protein kinases, including PDK1, at Thr308 and Ser473. Activated Akt phosphorylates various downstream substrate proteins that are critical in cell cycle progression and survival (Lawlor and Alessi, 2001). Glycogen synthase kinase 3β (GSk3β) is a major protein kinase downstream of Akt. Akt phosphorylates GSK3β at the serine 9 position and causes inhibition of its activity. Two recent studies reported that GSk3β is a negative regulator of Nrf2 by excluding its translocation from the nuclear compartment (Jain and Jaiswal, 2007; Rojo et al., 2008). Whether similar mechanisms are involved in MeHg toxicity remain to be determined.
There is abundant evidence in support of the ‘pivotal’ role of astrocytes in mediating neurotoxicity, establishing astrocytes as a unique and relevant experimental model for the assessment of mechanisms underlying MeHg-induced cytotoxicity (Aschner et al., 2002). Astrocytes are the most numerous nonneuronal cell-type in the CNS. They make up ∼50% of human brain volume (Chen and Swanson, 2003). Chronic exposure to MeHg in primates is associated with preferential accumulation of MeHg in astrocytes (and to some extent in microglia). The selectivity could be partly due in part to the inhibitory effect of MeHg on glutamate and cystine transport in astrocytes (Allen et al., 2001; Aschner et al., 1993).
In summary, we have demonstrated that MeHg activated Nrf2 and its downstream antioxidant system in neonatal rat primary astrocytes. Nrf2 function is regulated by PI3 kinase. Further characterization of the cross-talks between the two pathways is likely to reveal novel mechanistic information on MeHg intoxication and should facilitate the design of effective treatment strategies.
FUNDING
National Institutes of Health (ES07331 to MA and ES09047 to JC); Research to Prevent Blindness, Inc.
References
- Ali SF, LeBel CP, Bond SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–648. [PubMed] [Google Scholar]
- Allen JW, Shanker G, Aschner M. Methylmercury inhibits the in vitro uptake of the glutathione precurson, cystine, in astrocytes, but not in neurons. Brain Res. 2001;894:131–140. doi: 10.1016/s0006-8993(01)01988-6. [DOI] [PubMed] [Google Scholar]
- Aschner M, Du YL, Gannon M, Kimelberg HK. Methylmercury-induced alterations in excitatory amino acid transport in rat primary astrocyte cultures. Brain Res. 1993;602:181–186. doi: 10.1016/0006-8993(93)90680-l. [DOI] [PubMed] [Google Scholar]
- Aschner M, Kimelberg HK. The use of astrocytes in culture as model systems for evaluating neurotoxic-induced injury. Neurotoxicology. 1991;12:505–518. [PubMed] [Google Scholar]
- Aschner M, Mullaney KJ, Wagoner D, Lash LH, Kimelberg HK. Intracellular glutathione (GSH) levels modulate mercuric chloride (MC)- and methylmercuric chloride (MeHgCl)-induced amino acid release from neonatal rat primary astrocytes cultures. Brain Res. 1994;664:133–140. doi: 10.1016/0006-8993(94)91963-1. [DOI] [PubMed] [Google Scholar]
- Aschner M, Sonnewald U, Tan KH. Astrocyte modulation of neuropathologic damage. Brain Pathol. 2002;12:476–481. doi: 10.1111/j.1750-3639.2002.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakir F, Damluji DF, Amin-Zaki L, Murtadha M, Khakidi A, Al-Rawi NY, Tkriti S, Dahahir HI, Clarkson TW, Smith JC, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Bannai S, Kitamura E. Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 1980;255:2372–2376. [PubMed] [Google Scholar]
- Bannai S, Kitamura E. Role of proton dissociation in the transport of cystine and glutamate in human diploid fibroblasts in culture. J. Biol. Chem. 1981;256:5770–5772. [PubMed] [Google Scholar]
- Brazil DP, Hemmings BA. Advances in protein kinase B signaling: AKTion on multiple fronts. Trends Biochem. Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Mottet NK, Vahter ME, Burbacher TM. Autometallographic determination of inorganic mercury distribution in the cortex of the calcarine sulcus of the monkey Macaca fascicularis following long-term subclinical exposure to methylmercury and mercuric chloride. Toxicol. Appl. Pharmacol. 1995;132:325–333. doi: 10.1006/taap.1995.1114. [DOI] [PubMed] [Google Scholar]
- Chawla RK, Lewis FW, Kutner MH, Bate DM, Roy RG, Rudman D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology. 1984;87:770–776. [PubMed] [Google Scholar]
- Chen Y, Cai J, Murphey TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J. Biol. Chem. 2002;277:33242–33246. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chen YW, Huang CF, Tsai KS, Yang RS, Yen CC, Yang YY, Lin-Shiau SY, Liu SH. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic β-cell dysfunction in vitro and in vivo. Diabetes. 2006;55:1614–1624. doi: 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- Choi BH, Lapham LW, Amin-Zaki L, Saleem T. Abnormal neuronal migration, deranged cerebral cortical organization, and diffuse white matter astrocytosis of human fetal brain: A major effect of methylmercury poisoning in utero. J. Neuropathol. Exp. Neurol. 1978;37:719–733. doi: 10.1097/00005072-197811000-00001. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Cox C, Clarkson TW, Marsh DO, Amin-Zaki L, Tikriti S, Myers GG. Dose-response analysis of infants prenatally exposed to methyl mercury: And application of a single compartment model to single-strand hair analysis. Environ. Res. 1989;49:318–332. doi: 10.1016/s0013-9351(89)80075-1. [DOI] [PubMed] [Google Scholar]
- Dreiem A, Seegal RF. Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent. Neurotoxicology. 2007;28:720–726. doi: 10.1016/j.neuro.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. Volume V: Health Effects of Mercury and Mercury Compounds. 1997. Mercury Study Report to Congress. Office of Air Quality Planning & Standards and Office of Research and Development. EPA-452/R-97–007, Washington, DC. [Google Scholar]
- Esposito F, Chirico G, Montesano Gesualdi N, Posada I, Ammendola R, Russo T, Cirino G, Cimino F. Protein kinase B activation by reactive oxygen species is independent of tyrosine kinase receptor phosphorylation and requires SRC activity. J. Biol. Chem. 2003;278:20828–20834. doi: 10.1074/jbc.M211841200. [DOI] [PubMed] [Google Scholar]
- Frangakis MV, Kimelberg HK. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochem. Res. 1984;9:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wanger R, Oh S-H, Hoekstra WG. Selenium: Relation to decreased toxicity of methyl mercury added to diet containing tuna. Science. 1972;75:1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- Griffith OW. Determination of glutathione and glutathione disulfide using glutathine reductase and 2-vinylpridine. Anal. Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Guidotti GG, Gazzola GC. Amino acid transporters: Systematic approach and principles of control. In: Kilberg MS, Haussinger D, editors. Mammalian Amino Acid Transport: Mechanisms and Control. New York: Plenum; 1992. pp. 3–30. [Google Scholar]
- Hamada R, Osame M. Minamata disease and other mercury syndromes. In: Chang LW, editor. Toxicology of Metals. Boca Raton, FL: CRC Press; 1996. pp. 337–351. [Google Scholar]
- Hughes WH. A physiochemical rationale for the biological activity of mercury and its compounds. Ann. N. Y. Acad. Sci. 1957;65:454–460. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic. Res. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- Jain AK, Jaiswal AK. GSk-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NR-E2 related factor 2. J. Biol. Chem. 2007;282:16502–16510. doi: 10.1074/jbc.M611336200. [DOI] [PubMed] [Google Scholar]
- Jensen S, Jernelov A. Biological methylation of mercury in aquatic organisms. Nature. 1969;223:753–754. doi: 10.1038/223753a0. [DOI] [PubMed] [Google Scholar]
- Kasuya M. Effect of selenium on the toxicity of methylmercury on nervous tissue in culture. Toxicol. Appl. Pharmacol. 1976;35:11–20. doi: 10.1016/0041-008x(76)90106-x. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, Kim DK, Inatomi J, Sawa H, Ida Y, Endou H. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim. Biophys. Acta. 2001a;1512:335–344. doi: 10.1016/s0005-2736(01)00338-8. [DOI] [PubMed] [Google Scholar]
- Kim YC, Masutani H, Yamaguchi Y, Itoh K, Yamamoto M, Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J. Biol. Chem. 2001b;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- Konig J, Nies AT, Cui Y, Leier I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: Localization, substrate specificity, and MRP2-mediated drug resistance. Biochim. Biophys. Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J. Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Lee J, Hanson JM, Chu WA, Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J. Biol. Chem. 2001;276:20011–20016. doi: 10.1074/jbc.M100734200. [DOI] [PubMed] [Google Scholar]
- Li MH, Cha YN, Surh YJ. Peroxynitrite induces HO-1 expression via PI3K/Akt-dependent activation of NF-E2-related factor 2 in PC12 cells. Free Radic. Biol. Med. 2006;41:1079–1091. doi: 10.1016/j.freeradbiomed.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1984;263:17205–17208. [PubMed] [Google Scholar]
- Mullaney KJ, Fehm MN, Vitarella D, Wagoner DE, Jr, Aschner M. The role of -SH groups in methylmercuric chloride-induced D-aspartate and rubidium release from rat primary astrocyte cultures. Brain Res. 1994;641:1–9. doi: 10.1016/0006-8993(94)91808-2. [DOI] [PubMed] [Google Scholar]
- Mullaney KJ, Vitarella D, Albrecht J, Kimelberg HK, Aschner M. Stimulation of D-aspartate efflux by mercuric chloride from rat primary astrocyte cultures. Dev. Brain Res. 1993;75:261–268. doi: 10.1016/0165-3806(93)90030-e. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Oyake Y, Tanaka M, Kubo H, Cichibu H. Neuropathological studies on organic mercury poisoning with special reference to the staining and distribution of mercury granules. Adv. Neurol. Sci. 1966;10:744–750. [PubMed] [Google Scholar]
- Rabenstein DL, Fairhurst MT. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. XI. The binding of methylmercury by sulfhydryl-containing amino acids and by glutathione. J. Am. Chem. Soc. 1974;97:2086–2092. doi: 10.1021/ja00841a015. [DOI] [PubMed] [Google Scholar]
- Rojo AI, de Sagarra MR, Cuadrado A. Gsk-3β down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008;1:192–202. doi: 10.1111/j.1471-4159.2007.05124.x. [DOI] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Kim SU. Methylmercury neurotoxicity in cultures of human neurons, astrocytes, neuroblastoma cells. Neurotoxicology. 2001;22:317–327. doi: 10.1016/s0161-813x(01)00015-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Robbins J. Glutathione deficiency induced by cystine and/or methionine deprivation does not affect thyroid hormone deiodination in cultured rat hepatocytes and monkey hepatocarcinoma cells. Endocrinology. 1981;109:844–852. doi: 10.1210/endo-109-3-844. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Westergaard N, Sonnewald U, Peterson SB, Yu ACH, Hertz L. Regulatory role of astrocytes for neuronal biosynthesis and homeostasis of glutamate and GABA. Prog. Brain Res. 1992;94:199–211. doi: 10.1016/s0079-6123(08)61751-3. [DOI] [PubMed] [Google Scholar]
- Simpson RB. Association constants of methylmercury with sulfhydryl and other bases. J. Am. Chem. Soc. 1961;83:4711–4717. [Google Scholar]
- Stern S, Cox C, Cernichiari E, Balys M, Weiss B. Perinatal and lifetime exposure to methylmercury in the mouse: Blood and brain concentrations of mercury to 26 months of age. Neurotoxicology. 2001;22:467–477. doi: 10.1016/s0161-813x(01)00047-x. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Reihman J, Lonky EI, Darvill TJ, Pagano J. Cognitive development in preschool children prenatally exposed to PCBs and MeHg. Neurotoxicol. Teratol. 2003;25:11–22. doi: 10.1016/s0892-0362(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi T. Pathology of fetal Minamata disease—the effects of methylmercury on intrauterine life of human beings. Pediatrician. 1977;6:69–78. [Google Scholar]
- Takeuchi T. Pathology of Minamata disease with special reference to its pathogenesis. Acta Pathol. Jpn. 1982;32:73–99. [PubMed] [Google Scholar]
- Takizawa Y. Mercury content in recognized patients and non-recognized patients exposed to methylmercury from Minamata Bay in the last ten years. In: Tsubaki T, Takahashi H, editors. Recent Advances in Minamata Disease Studies. Tokyo: Kodansha Ltd; 1986. pp. 1–39. [Google Scholar]
- Tomi M, Funaki T, Abukawa H, Kondo T, Ohtsuki S, Ueda M, Obinata M, Terasaki T, Hosoya K. Expression and regulation of L-cystine transporter, system xc-, in the newly developed rat retinal Muller cell line (TR-MUL) Glia. 2003;43:208–217. doi: 10.1002/glia.10253. [DOI] [PubMed] [Google Scholar]
- Toyama T, Sumi D, Shinkai Y, Yasutake A, Taguchi T, Tong TI, Yamamoto M, Kumagai Y. Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem. Biophys. Res. Commun. 2007;363:645–650. doi: 10.1016/j.bbrc.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999;274:22699–22704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- Vitarella D, Kimelberg HK, Aschner M. Inhibition of regulatory volume decrease in swollen rat primary astrocyte cultures by methylmercury is due to increased amiloride-sensitive Na+ uptake. Brain Res. 1996;732:169–178. doi: 10.1016/0006-8993(96)00518-5. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Reuhl KR, Ming X, Halladay AK. Behavioral and neurochemical sensitization to amphetamine following early postnatal administration of methylmercury (MeHg) Neurotoxicology. 2007;28:59–66. doi: 10.1016/j.neuro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen Y, Sternberg P, Cai JY. Essential roles of the PI3 kinase/Akt pathway in regulating Nrf2-dependent antioxidant functions in the RPE. Invest. Ophthalmol. Vis. Sci. 2008;49:1671–1678. doi: 10.1167/iovs.07-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM. Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA, Cairns J, editors. Handbook of Ecotoxicology. Boca Raton, FL: CRC Press; 2003. pp. 409–463. [Google Scholar]
- Wood JM, Kennedy FS, Rosen CE. Synthesis on methylmercury compounds by extracts of methanogenic bacterium. Nature. 1968;220:173–174. doi: 10.1038/220173a0. [DOI] [PubMed] [Google Scholar]
- Yao CP, Allen JW, Conklin DR, Aschner M. Transfection and overexpression of metallothionein-I in neonatal rat primary astrocyte cultures and in astrocytoma cells increases their resistance to methylmercury-induced cytotoxicity. Brain Res. 1999;818:414–420. doi: 10.1016/s0006-8993(98)01229-3. [DOI] [PubMed] [Google Scholar]
- Yao CP, Allen JW, Mutkus LA, Xu SB, Tan KH, Aschner M. Foreign metallothionein-I expression by transient transfection in MT-I and MT-II null astrocytes confers increased protection against acute methylmercury cytotoxicity. Brain Res. 2000;855:32–38. doi: 10.1016/s0006-8993(99)02211-8. [DOI] [PubMed] [Google Scholar]