Abstract
Hypoxia-inducible factor (HIF-1α) and cyclooxygenase-2 (COX-2) have been implicated in the regulation of inflammatory-like processes that lead to angiogenesis and fibrotic disorders. Here we demonstrate that in human lung fibroblasts (HLFs) treated with mixed exposures to chemical and microbial stimuli, HIF-1α stabilization plays a pivotal role in the induction of COX-2 mRNA and protein, driving the release of vascular endothelial growth factor (VEGF) and proangiogenic and profibrotic chemokines. Upon costimulation with Ni and the mycoplasma-derived lipopeptide macrophage-activating lipopeptide-2 (MALP-2), there was a synergistic induction of CXCL1 and CXCL5 mRNA and protein release from HLF, as well as an enhanced response in VEGF compared to either stimulus alone. Consistent with our previous findings that Ni and MALP-2 stimulates the induction of CXCL8 via a COX-2-mediated pathway, CXCL1, CXCL5, and VEGF release were also regulated by COX-2. Ni induced the stabilization of HIF-1α protein in HLF, which was further enhanced in the presence of MALP-2. Depletion of HIF-1α using siRNA blocked COX-2 induction by Ni and MALP-2 along with the release of VEGF, CXCL1, CXCL5, and CXCL8. Our results indicate that Ni and MALP-2 interact to promote an angiogenic profibrotic phenotype in HLF. Moreover, these findings reveal a potential role for HIF-1α in mediating chemical-induced alterations in cellular response to microbial stimuli, modulating pulmonary inflammation and its consequences such as fibrosis and angiogenesis.
Keywords: HIF-1α, COX-2, fibroblasts, inflammation, Ni, MALP-2
Despite the fact that real-life exposures are likely to involve a mixture of chemical and microbial stimuli, little attention has been paid to the potential interactions that mixed exposures may have on the development of inflammatory-like conditions in the lung. Onset of inflammation in the lungs can have many consequences, including the development of fibrotic disorders and even angiogenesis. While there are many factors that can contribute to inflammation, common threads include upregulation of the enzyme cyclooxygenase-2 (COX-2) and the transcription factor hypoxia-inducible factor (HIF)-1α.
Recently evidence has indicated that in addition to its role in the adaptation to hypoxia, HIF-1α also participates in various inflammatory-like processes (Jung et al., 2003). Conditional deletions of HIF-1α in myeloid cells significantly impairs chemotaxis and bacteriocidal activity of macrophages in vitro and inflammation following dermal application of sodium dodecyl sulfate (SDS) or phorbol ester in vivo (Cramer et al., 2003). Inflammatory cytokines (e.g., interleukin [IL]-1β) can also promote HIF-1α stabilization under normoxia (Jung et al., 2003) and a role for HIF-1α has been identified in chronic inflammatory diseases such as rheumatoid arthritis (Westra et al., 2007) and atherosclerosis (Vink et al., 2007). Under normal oxygen conditions, prolyl hydroxylases regulate HIF-1α by targeting the protein for degradation by the ubiquitin-proteasome pathway via association with the von Hippel-Lindau protein tumor suppressor protein (Ivan and Kaelin, 2001). When prolyl hydroxylase activity is inhibited under conditions of hypoxia, HIF-1α accumulates and translocates to the nucleus, where it heterodimerizes with the subunit HIF-1β to activate gene transcription. Stabilization of HIF-1α can also occur under normoxic conditions, via activation of signaling pathways such as p42/p44 MAPK and PI3K/Akt (Fang et al., 2007; Richard et al., 1999).
COX-2 is upregulated in a variety of cancers and fibrotic disorders (Hida et al., 1998; Lappi-Blanco et al., 2006), driving the formation of prostanoids which govern inflammatory-like processes. In fact, prostaglandin(PG)-E2, a major metabolic product of COX-2, has been associated with angiogenesis, promoting tumor growth and cellular migration (Finetti et al., 2008; Rao et al., 2007). Given that both HIF-1α and COX-2 have been implicated in angiogenesis and cell motility, it is not surprising that the regulation of HIF-1α has also been linked with COX-2 expression (Csiki et al., 2006; Jung et al., 2003; Liu et al., 2002).
We have previously shown that NiSO4 (Ni), a common component of air particulate matter (PM) pollution, synergistically enhances release of the proangiogenic and profibrotic chemokine CXCL8 by the 2-kDa Mycoplasma fermentans–derived Macrophage Activating Lipopeptide-2 (MALP-2), a Toll-like receptor 2 agonist, yet inhibits formation of the angiostatic, antifibrotic chemokine CXCL10 in human lung fibroblasts (HLFs) (Brant and Fabisiak, 2008). This Ni-induced shift in MALP-2–induced chemokine production in HLF is mediated via a COX-2/PGE2 pathway (Brant and Fabisiak, 2008). Lung fibroblasts play an active role in the response to tissue injury, contributing to cytokine and chemokine release and the development of fibroproliferative disorders. As such, understanding how HLF respond to chemical and microbial stimuli can contribute to our understanding of the sequelae of events leading to pathogenesis of fibrotic lung disease among others. The current study examines whether mixed exposures to Ni and MALP-2 alter the regulation of additional angiogenic and fibrotic chemokines in HLF. Based on our previous findings with COX-2 and the associations of HIF-1α stabilization with angiogenesis and inflammation, we further examined the interactive roles that COX-2 and HIF-1α may have in driving inflammatory-like processes in HLF in the presence of chemical and microbial stimuli.
MATERIALS AND METHODS
Materials.
NiSO4•6H2O (Ni) and Mycoplasma fermentans–derived MALP-2 were from Sigma (St Louis, MO) and Alexis Biochemicals (San Diego, CA), respectively. Low endotoxin bovine serum albumin (BSA) was from Intergen (Purchase, NY). The real-time reverse transcriptase (RT2) Profiler™ polymerase chain reaction (PCR) Angiogenesis Array was from SuperArray Bioscience Corporation (Frederick, MD). RNeasy Mini Kit was from Qiagen (Valencia, CA). Bradford protein assay reagent was from Bio-Rad (Hercules, CA). The COX-2 and HIF-1α monoclonal antibodies were from Cayman Chemical (Ann Arbor, MI) and BD Biosciences (San Jose, CA), respectively. The rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody, anti-mouse and anti-rabbit IgG-HRP (horse radish peroxidase)–linked antibodies were obtained from Cell Signaling Technology, Inc (Danvers, MA). All cell culture reagents were from Invitrogen (Carlsbad, CA).
Cell lines and culture.
HLFs were isolated as outgrowths from explanted surplus transbronchial biopsy tissues obtained during routine follow-up bronchoscopy of lung transplant recipients as previously described (Fabisiak et al., 1993) in accordance with a protocol approved by University of Pittsburgh Institutional Review Board. Routine culture is performed in Minimal Essential Media (MEM) containing 10% fetal bovine serum, glutamine (2mM), and 1% penicillin-streptomycin, with weekly passage. All cultures are carried out at 37°C in a humidified atmosphere of 5% CO2/95% air.
RT2-PCR Angiogenesis Array and ELISA analysis.
HLF were cultured in six-well tissue culture plates (4 x 105 cells per well) and allowed to attach for 1 day. Cells were then treated with 600 pg/ml MALP-2 and/or 200μM Ni, in serum-free MEM with 0.1% BSA for 48 h. Control cells received serum-free MEM with 0.1% BSA alone. The amounts of Ni and MALP-2 used in the current study were chosen based on concentration-response relationships for a synergistic induction of CXCL8 in HLF reported previously (Brant and Fabisiak, 2008). Preliminary time course and concentration-response experiments indicated 200μM Ni and 600 pg/ml MALP-2 to be effective for the synergistic release of CXCL1 and CXCL5 and that maximal response occurred at 48-h postexposure. Following a 48-h treatment, total RNA was extracted from the cells using the RNeasy Mini Kit from Qiagen. cDNA was generated from 1 μg total RNA using the ReactionReady First Strand cDNA Synthesis kit (SuperArray Bioscience Corporation) according to manufacturer's instructions. The human RT2-Profiler™ PCR Angiogenesis Array was carried out according to manufacturer's instructions using the Opticon 2 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Relative gene expression was calculated using the 2−ΔΔCt method using RPL13A as the internal control gene to normalize the data for the amount of RNA added to each RT reaction. Conditioned media from the same experiments were also collected and stored at −70°C until use. CXCL1, CXCL5, CXCL8, and VEGF(165 and 121) (vascular endothelial growth factor) content in the conditioned media samples were analyzed using specific enzyme-linked immunosorbent assay (ELISA) kits obtained from R&D Systems (Minneapolis, MN) according to the manufacturer's instructions.
Western blot analysis.
Cell lysates were prepared using 10mM Tris, pH 7.4, with 1% SDS containing a protease-inhibitor cocktail (Calbiochem, Gibbstown, NJ) and 200mM each Na3VO4 and NaF. Cell lysates (25 μg protein per lane) were subjected to electrophoresis using NuPAGE 4–12% Bis-Tris gels (Invitrogen) under denaturing and reducing conditions and transferred to Polyscreen PVDF Transfer membranes (PerkinElmer, Wellesley, MA). Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline containing 1% Tween-20 (TBS-T) for 1 h then incubated with an anti-HIF-1α (1:500) or anti-COX-2 (1:1000) monoclonal antibody at a TBS-T containing 5% milk or BSA, respectively, overnight at 4°C. Blots were then rinsed with TBS-T and incubated with an anti-mouse HRP-linked secondary antibody (1:2500) for 1 hr at room temperature. Immunoreactive bands were detected using chemiluminescence. Membranes were then reprobed with GAPDH or α-tubulin (loading controls) as described above.
Transient transfection of siRNA.
Double-stranded siRNA sequences targeting HIF1A (GenBank/EBI accession numbers NM_001530, NM_181054) and COX-2(PTGS2) (GenBank/EBI accession number NM_000963) mRNAs were obtained from Dharmacon (Lafayette, CO). A nontargeting siRNA (Dharmacon) was used as a control, and did not significantly affect HIF1A and COX-2 mRNA levels compared with nontransfected controls. HLF were cultured in 12-well tissue culture plates (8.9 x 104 per well) and allowed to attach for one day. Cells were transfected for 48 h with 50nM of HIF1A, COX-2, or nontargeting siRNA control (NTC) in OptiMEM using DharmaFECT1 siRNA Transfection Reagent (Dharmacon) according to manufacturer's instructions. After the 48-h transfection period, cells were stimulated with Ni and MALP-2 for 48 h in serum-free MEM containing 0.1% BSA. Levels of CXCL1, CXCL5, CXCL8, and VEGF in the conditioned medium were determined using ELISA. Real-time RT-PCR and immunoblot analysis were carried out to assess knockdown of Ni and MALP-2–induced HIF1α and COX-2 mRNA and protein expression, respectively. Relative gene expression was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001), using Cyclophilin B as the internal control gene to normalize the data for the amount of RNA added to each RT reaction. Primer pairs for Cyclophilin B, HIF-1α and COX-2 were designed using Primer3 (Rozen and Skaletsky, 2000): Cyclophilin B For:AGATGTAGGCCGGGTGATCTT, Rev: CGCTCACCGTAGATGCTCTTT; COX-2 For: GCTCAGCCATACAGCAAATCC Rev: CAACGTTCCAAAATCCCTTGA; and HIF-1α For: TTTACCATGCCCCAGATTCAG, Rev: CTTTGCTTCTGTGTCTTCAGCAA.
Statistical analysis.
Data presented are expressed as mean ± SEM. Ni and MALP-2 induced changes in gene expression analyzed using the RT2-Profiler™ PCR Array were evaluated using a Student t-test. ELISA results were analyzed using a one-way ANOVA with Tukey's multiple comparison tests or a two-way ANOVA with Bonferroni post hoc comparison, as appropriate. Statistical analyses were performed using GraphPad PRISM, version 5.0 (GraphPad Software, San Diego, CA), with p < 0.05 considered significant.
RESULTS
Ni and MALP-2 Interact to Promote a Proangiogenic Phenotype in HLF
We have previously reported that Ni enhances MALP-2-induced CXCL8 release, yet suppresses MALP-2–induced CXCL10 release from HLF (Brant and Fabisiak, 2008). Here we sought to investigate whether other genes involved in angiogenesis are modified during mixed exposures to Ni and MALP-2 by utilizing an RT2-Profiler™ PCR Angiogenesis Array. Following a 48-h exposure to Ni, MALP-2, or the two stimuli together, HLF were harvested for use in the array as described under the materials and methods section. Data were normalized to the housekeeping genes included in the array (e.g., RPL13A) and the Ni and MALP-2–induced fold-changes in gene expression relative to control-treated cells are presented in Table 1. Exposure to Ni induced the expression of several genes involved in the angiogenesis pathway. Levels of Angiopoietin-like 4 (ANGPTL4), leptin (LEP), and VEGF expression were all increased several-fold following a 48 h Ni treatment, among others (e.g., ephrin-A3 and placental growth factor). Also elevated following Ni exposure was the expression of interleukin-6 and several proangiogenic CXC chemokines including CXCL1, 3 6, and 8. In contrast, expression of the chemokine CCL11, thymidine phosphorylase (ECGF1), fibroblast growth factor 1 (FGF1), and plasminogen activator urokinase (PLAU) were decreased in the presence of Ni. A 48-h treatment with MALP-2 alone also stimulated the expression of several proangiogenic CXC chemokines in HLF. CXCL1, CXCL3, CXCL6, and CXCL8 were all significantly increased following MALP-2 treatment, with MALP-2 having minimal to no effects on the expression of the other genes contained in the array. An interesting pattern emerged regarding the expression of the proangiogenic CXC chemokines when HLF were treated with Ni and MALP-2 in combination for 48 h. Namely, there was a synergistic induction of CXCL1, CXCL3, CXCL5, and CXCL6 compared with cells receiving either stimulus alone (Table 1).
TABLE 1.
Effects of Ni and MALP-2 on Angiogenesis-Related Gene Expression in HLFa
| Ni | MALP-2 | Ni + MALP-2 | Ni | MALP-2 | Ni + MALP-2 | ||
| AKT1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.1 | IGF1 | 0.5 ± 0.1 | 1.0 ± 0.2 | 0.5 ± 0.1 |
| ANGPT1 | 1.1 ± 0.2 | 0.9 ± 0.1 | 1.2 ± 0.1 | IL1B | 2.6 ± 1.1 | 1.4 ± 0.4 | 1.9 ± 0.4 |
| ANGPT2 | 1.9 ± 1.1 | 0.9 ± 0.2 | 1.2 ± 0.2 | IL6 | 2.1 ± 0.4* | 1.4 ± 0.1 | 7.1 ± 0.4* |
| ANGPTL3 | 2.4 ± 0.3 | 1.1 ± 0.3 | 3.1 ± 1.5 | ITGAV | 0.6 ± 0.1 | 1.0 ± 0.2 | 0.8 ± 0.3 |
| ANGPTL4 | 25.0 ± 9.9* | 0.6 ± 0.2 | 34.6 ± 14.7* | ITGB3 | 1.5 ± 0.5 | 1.1 ± 0.3 | 2.0 ± 0.6 |
| ANPEP | 0.8 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.2 | JAG1 | 2.0 ± 0.4 | 0.9 ± 0.1 | 1.8 ± 0.1 |
| BAI1 | 1.5 ± 0.5 | 1.0 ± 0.4 | 1.7 ± 1.1 | KDR | 1.9 ± 0.3 | 1.2 ± 0.5 | 1.6 ± 0.3 |
| CCL11 | 0.4 ± 0.1* | 1.4 ± 0.2 | 0.6 ± 0.1 | LAMA5 | 1.3 ± 0.3 | 0.9 ± 0.1 | 1.2 ± 0.2 |
| CCL2 | 1.4 ± 0.2 | 2.6 ± 0.1* | 3.7 ± 0.2* | LECT1 | 2.6 ± 0.7 | 0.7 ± 0.1 | 1.5 ± 0.3 |
| CDH5 | 3.2 ± 0.6 | 0.8 ± 0.1 | 1.7 ± 0.2 | LEP | 8.2 ± 4.7* | 0.8 ± 0.1 | 6.6 ± 3.5* |
| COL18A1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.1 | MDK | 0.8 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| COL4A3 | 1.1 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.2 | MMP2 | 0.9 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 |
| CXCL1 | 8.7 ± 3.6* | 34.8 ± 6.9* | 104.3 ± 16.1* | MMP9 | 1.0 ± 0.5 | 1.5 ± 0.8 | 2.6 ± 1.1 |
| CXCL10 | 1.3 ± 0.8 | 61.4 ± 26.0* | 5.0 ± 1.8* | NOTCH4 | 1.6 ± 0.3 | 1.3 ± 0.5 | 1.7 ± 0.3 |
| CXCL3 | 9.1 ± 4.4* | 16.6 ± 4.7* | 101.4 ± 17.3* | NRP1 | 0.7 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 |
| CXCL5 | 2.2 ± 0.4 | 2.6 ± 0.5 | 25.6 ± 7.8* | NRP2 | 0.5 ± 0.1* | 1.1 ± 0.1 | 0.6 ± 0.1 |
| CXCL6 | 3.9 ± 0.3 | 37.3 ± 17.0* | 138.9 ± 55.4* | PDGFA | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.4 ± 0.3 |
| CXCL8 | 32.0 ± 23.0* | 16.8 ± 0.5* | 301.6 ± 20.0* | PECAM1 | 3.5 ± 0.4 | 0.9 ± 0.1 | 3.1 ± 0.7 |
| CXCL9 | 3.3 ± 0.4 | 0.7 ± 0.2 | 2.1 ± 0.7 | PF4 | 1.8 ± 0.7 | 0.8 ± 0.2 | 2.4 ± 1.2 |
| ECGF1 | 0.4 ± 0.1* | 2.2 ± 0.1* | 0.5 ± 0.1* | PGF | 3.7 ± 0.6* | 0.7 ± 0.3 | 4.1 ± 0.6* |
| EDG1 | 0.5 ± 0.1* | 0.9 ± 0.2 | 0.5 ± 0.1* | PLAU | 0.2 ± 0.1* | 1.0 ± 0.1 | 0.2 ± 0.1* |
| EFNA1 | 2.2 ± 0.4 | 0.9 ± 0.3 | 2.7 ± 1.1* | PLG | 2.4 ± 0.2 | 1.1 ± 0.4 | 1.8 ± 0.6 |
| EFNA3 | 8.2 ± 2.2* | 1.0 ± 0.2 | 8.0 ± 2.0* | PLXDC1 | 2.2 ± 0.4 | 1.1 ± 0.2 | 1.7 ± 0.5 |
| EFNB2 | 1.6 ± 0.4 | 1.1 ± 0.2 | 1.5 ± 0.5 | PROK2 | 2.4 ± 0.4 | 1.4 ± 0.7 | 2.2 ± 0.4 |
| EGF | 1.9 ± 0.6 | 0.7 ± 0.1 | 1.5 ± 0.2 | PTGS1 | 1.1 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.5 |
| ENG | 0.7 ± 0.1 | 1.1 ± 0.2 | 0.8 ± 0.1 | SERPINF1 | 0.7 ± 0.2 | 1.0 ± 0.2 | 0.7 ± 0.2 |
| EPHB4 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | SPHK1 | 1.5 ± 0.1* | 1.1 ± 0.2 | 1.7 ± 0.2* |
| EREG | 2.4 ± 0.8 | 1.0 ± 0.1 | 1.3 ± 0.3 | STAB1 | 2.2 ± 0.4 | 1.0 ± 0.4 | 2.5 ± 0.7 |
| FGF1 | 0.3 ± 0.1 | 0.9 ± 0.1 | 0.3 ± 0.1 | TEK | 0.9 ± 0.2 | 1.3 ± 0.6 | 0.7 ± 0.2 |
| FGF2 | 1.8 ± 0.3 | 1.2 ± 0.2 | 2.2 ± 0.5 | TGFA | 2.7 ± 0.4 | 1.3 ± 0.6 | 1.9 ± 0.4 |
| FGFR3 | 2.0 ± 0.4 | 0.8 ± 0.1 | 1.4 ± 0.4 | TGFB1 | 2.1 ± 0.2* | 0.9 ± 0.1 | 2.4 ± 0.2* |
| FIGF | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.9 ± 0.3 | TGFB2 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.6 ± 0.1 |
| FLT1 | 1.2 ± 0.3 | 1.3 ± 0.4 | 1.2 ± 0.2 | TGFBR1 | 0.5 ± 0.1* | 1.0 ± 0.1 | 0.7 ± 0.1 |
| HAND2 | 2.6 ± 0.2 | 1.5 ± 0.8 | 2.6 ± 0.9 | THBS1 | 0.8 ± 0.1 | 1.1 ± 0.1 | 0.7 ± 0.1 |
| HGF | 0.6 ± 0.1 | 1.1 ± 0.3 | 0.7 ± 0.2 | THBS2 | 2.2 ± 0.4* | 1.1 ± 0.2 | 2.3 ± 0.3* |
| HIF1A | 0.5 ± 0.1* | 1.0 ± 0.2 | 0.5 ± 0.1* | TIMP1 | 1.4 ± 0.3 | 1.0 ± 0.2 | 1.5 ± 0.2 |
| HPSE | 1.9 ± 0.3 | 1.2 ± 0.2 | 1.8 ± 0.1 | TIMP2 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 |
| ID1 | 0.9 ± 0.1 | 1.0 ± 0.3 | 0.7 ± 0.1 | TIMP3 | 2.3 ± 0.8 | 1.4 ± 0.5 | 1.5 ± 0.4 |
| ID3 | 0.7 ± 0.4 | 0.8 ± 0.3 | 0.6 ± 0.2 | TNF | 2.7 ± 0.1 | 1.0 ± 0.3 | 2.2 ± 0.5 |
| IFNA1 | 4.6 ± 2.0 | 1.1 ± 0.4 | 7.4 ± 5.2 | TNFAIP2 | 1.2 ± 0.3 | 3.9 ± 0.5* | 2.7 ± 0.3 |
| IFNB1 | 2.0 ± 0.7 | 0.7 ± 0.2 | 1.2 ± 0.4 | VEGF | 41.3 ± 16.5* | 1.0 ± 0.2 | 47.1 ± 10.3* |
| IFNG | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.1 | VEGFC | 3.1 ± 0.7 | 1.0 ± 0.1 | 3.1 ± 0.3 |
Note. EFNA3, ephrin-A3; PGF, placental growth factor.
Data represent mean ± SEM of Ni and MALP-2 induced fold-change in gene expression relative to control-treated cells (n = 3), *p < 0.05.
Ni and MALP-2 Interact to Promote the Release of CXC1, CXCL5, and VEGF from HLF
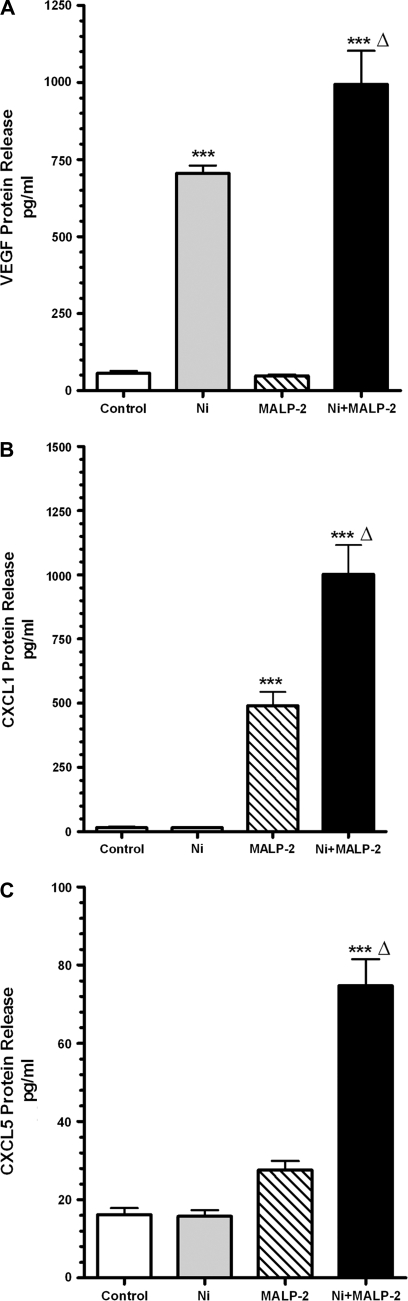
VEGF has been well established in contributing to angiogenesis and both CXCL1 and CXCL5 have both been implicated in profibrotic and proangiogenic changes (Keane et al., 2001; Strieter et al., 2002). We therefore sought to validate the changes in expression of these genes observed in the array by measuring protein levels. VEGF, CXCL1, and CXCL5 in conditioned medium from HLF treated with Ni and/or MALP-2 were analyzed using ELISA. Figure 1A shows that a 48-h exposure to Ni stimulated an approximate 12-fold increase in VEGF release from HLF compared with controls (p < 0.001), consistent with the RT2-Profiler™ PCR array results. Whereas MALP-2 alone failed to stimulate the release of VEGF, MALP-2 did enhance Ni-induced VEGF release to 995 ± 108 pg/ml, levels that were significantly greater compared with Ni and control-treated cells (705 ± 25 and 57 ± 7 pg/ml, respectively; p < 0.01).
FIG. 1.
Ni and MALP-2 interact to promote release of VEGF, CXCL1 and from HLF. Cells were stimulated with 200μM Ni and 600 pg/ml MALP-2 alone or combined for 48 h as described under “Materials and Methods.” Conditioned medium was analyzed using specific enzyme-linked immunoassays for (A) VEGF, (B) CXCL1, (C) CXCL5. Data shown are mean ± SEM and are representative of three to five independent experiments performed in triplicate. ***Different from untreated control (p < 0.001); Δ, different from both Ni and MALP-2 treatment alone (p < 0.01).
ELISA analysis also showed that Ni and MALP-2 interact to synergistically promote the release of the proangiogenic and profibrotic chemokines CXCL1 and CXCL5 from HLF (Figs. 1B and C), confirming the findings observed in the array analysis. When cells are coexposed to Ni and MALP-2 for 48 h, levels of CXCL1 and CXCL5 in conditioned media were remarkably greater than what was observed in media from control-treated cells or cells receiving either stimuli alone (p < 0.001). These results are consistent with our previous observations with CXCL8 (Brant and Fabisiak, 2008).
Induction of COX-2 Mediates Ni and MALP-2–Induced Release of Proangiogenic Factors from HLF
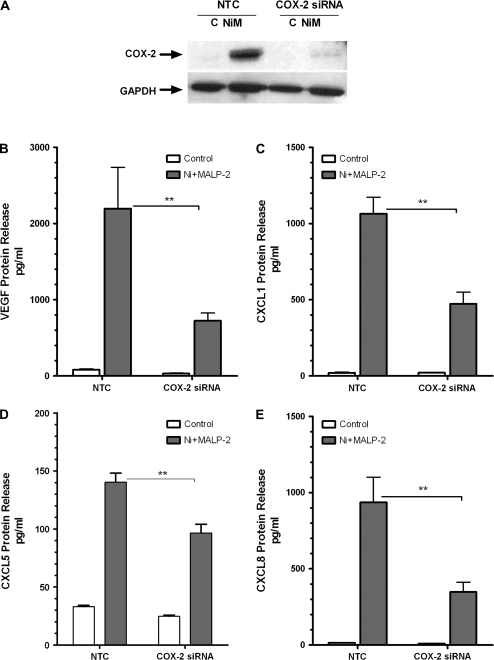
Based on our previous findings that Ni and MALP-2–induced stimulation of CXCL8 is mediated via COX-2 induction, we sought to determine what role this enzyme plays in the release of VEGF, CXCL1, and CXCL5 from HLF. Cells were transiently transfected with COX-2 siRNA for 48 h prior to Ni and MALP-2 exposure as described under “Materials and Methods.” Under basal conditions, COX-2 protein is not expressed in HLF, but is readily induced upon coexposure to Ni and MALP-2. Transfection of HLF with 50nM COX-2 siRNA was sufficient to block the Ni and MALP-2–mediated induction of COX-2 protein as analyzed by Western blot (Fig. 2A).
FIG. 2.
COX-2 silencing attenuates Ni and MALP-2–stimulated release of proangiogenic factors from HLF. HLF were transiently transfected with 50nM of COX-2 siRNA or a NTC prior to a 48 h combined Ni and MALP-2 exposure. (A) Western blot analysis indicating siRNA-mediated knockdown of Ni and MALP-2 (NiM)–induced COX-2 protein expression. GAPDH is shown as a loading control. Conditioned medium from the different treatment groups was analyzed using specific enzyme-linked immunoassays for (B) VEGF, (C) CXCL1, (D) CXCL5, (E) CXCL8. Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. **p < 0.01 compared with NTC.
Compared with the NTC, HLF transfected with COX-2 siRNA exhibited a marked reduction in VEGF, CXCL1, CXCL5, and CXCL8 release following Ni and MALP-2 stimulation (Figs. 2B–E). VEGF and CXLC8 levels in the conditioned medium taken from cells stimulated with Ni and MALP-2 were decreased by approximately 60% in the presence of the COX-2 siRNA (p < 0.01; Figs. 2B and E). Knockdown of COX-2 also significantly attenuated Ni and MALP-2-induced CXCL1 and CXCL5 release from HLF by 50 and 30%, respectively (p < 0.01; Figs. 2C and D).
HIF-1α Mediates Ni and MALP-2–Induced Release of VEGF and CXC Chemokines from HLF
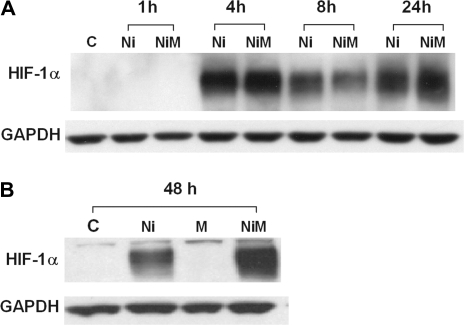
In addition to COX-2, HIF-1α plays an important role in angiogenic processes and has more recently been speculated to have a role in the pathogenesis of pulmonary fibrotic disorders (Tzouvelekis et al., 2007). Ni has previously been shown to stabilize HIF-1α (Salnikow et al., 2003), and HIF-1α-mediated stimulation of VEGF has been proposed as an angiogenic switch during tumorigenesis (Fang et al., 2001). Based on these reports, we examined whether Ni could stabilize HIF-1α in HLF and what role HIF-1α stabilization may have in release of the proangiogenic factors from HLF. Western blot analysis revealed that Ni did induce HIF-1α protein in HLF (Fig. 3). Time course analysis revealed that Ni and coexposures to Ni and MALP-2 stimulate HIF-1α protein levels in HLF as early as 4 h (Fig. 3A). HIF-1α expression appeared to decrease slightly at 8 h, then increase again at 24- and 48-h postexposure. Interestingly, HLF cotreated with Ni and MALP-2 for 48-h showed an enhanced expression of HIF-1α protein compared with Ni treatment alone (Fig. 3B). Increases in HIF-1α protein following exposure to Ni, both alone and in combination with MALP-2, likely reflects an increase in protein synthesis and/or stabilization as there were no observed increases in the levels of HIF-1α mRNA (Table 1). MALP-2 alone failed to stimulate HIF-1α expression at any of the time points examined, nor were there any changes in HIF-1α protein levels in control-treated cells between the 1-, 4-, 8-, 24-, and 48-h time points (data not shown). It is of note that differences in COX-2 protein levels were only observed between control-treated cells and the coexposed Ni and MALP-2-treated cells at time points after which HIF-1α stabilization occurs. A slight increase in COX-2 protein levels following a 24-h exposure to Ni and MALP-2, with COX-2 protein levels even further increased following a 48-h exposure (Supplementary Data S1A). MALP-2 treatment alone had no observable effects on COX-2 expression compared with controls at any of the time points examined. The time-dependent increase in COX-2 protein levels are consistent with the pattern of PGE2 release as well as the time-dependent release of CXCL1, 5, and 8 from HLF (Supplementary Data S1B–F).
FIG. 3.
Effect of MALP-2 on Ni-induced stabilization of HIF-1α. HLFs were stimulated with Ni and MALP-2 for 1, 4, 8, 24 h (A) and 48 h (B) as described under “Materials and Methods.” For detection of HIF-1α, 25 μg of total cellular protein was loaded per lane and subjected to SDS-polyacrylamide gel electrophoresis and western blotting with anti-HIF-1α antibody. GAPDH is shown as a loading control. Representative data typical of four independent experiments are shown.
Ni and MALP-2 stimulate release of PGE2 from HLF (Brant and Fabisiak, 2008). Consistent with reports indicating PGE2 to have anti-inflammatory properties in the lung (Vancheri et al., 2004), PGE2 significantly attenuated CXC chemokine release from HLF following exposure to MALP-2 and the proinflammatory stimuli LPS (Supplementary Data S2). We therefore examined whether exposure to Ni could alter how HLF respond to PGE2. Supplementary data provided indicate that under normal conditions, PGE2 alone has no effect on HIF-1α and COX-2 expression in HLF, nor does it stimulate the release of VEGF and CXC chemokines. However, when given in the presence of Ni, PGE2 enhances the expression of HIF-1α and COX-2. Additionally, PGE2 significantly enhances release of VEGF and CXCL8 from HLF compared with Ni alone (p < 0.001), similar to the observations made when Ni is given with MALP-2. Additional COX-2–derived products likely contribute to the release of CXCL1 and CXCL5 from HLF given the findings that PGE2 does not significantly enhance Ni-induced release of these chemokines from HLF (Supplementary Data S3).
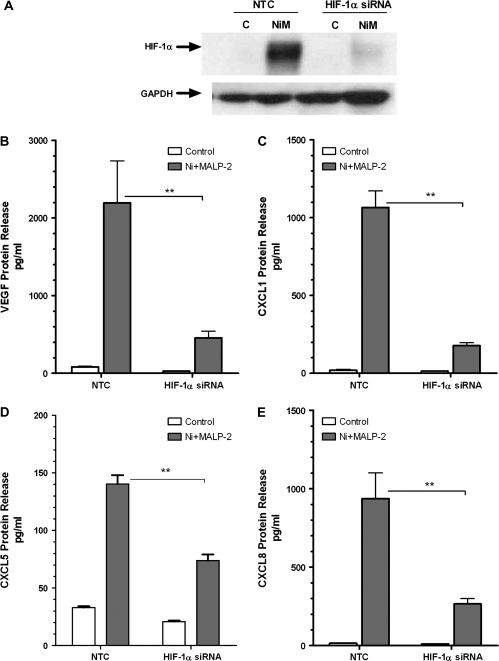
To further explore whether the increased levels of HIF-1α protein observed in HLF contribute to the synergistic release of the profibrotic and proangiogenic growth factors and chemokines by Ni and MALP-2, cells were transfected with HIF-1α siRNA prior to treatment. Transfection of HLF with 50nM of HIF-1α siRNA effectively blocked the Ni and MALP-2–induced HIF-1α protein expression compared with the nontargeting siRNA control (Fig. 4A). Similar to the findings with COX-2 siRNA, the Ni and MALP-2 stimulated VEGF, CXCL1, CXCL5, and CXCL8 release was significantly attenuated in HLF transfected with HIF-1α siRNA (Figs. 4B–E). VEGF, CXCL1, and CXCL8 levels in the conditioned medium taken from cells stimulated with Ni and MALP-2 were decreased by approximately 70–80% in the presence of the HIF-1α siRNA compared with the nontargeting siRNA control (Figs. 4B, C, E; p < 0.01). Knockdown of HIF-1α also significantly attenuated Ni and MALP-2–induced CXCL5 release from HLF by approximately 50% compared with the nontargeting siRNA control (Fig. 4D; p < 0.01).
FIG. 4.
HIF-1α silencing attenuates Ni and MALP-2–stimulated release of proangiogenic factors from HLF. HLF were transiently transfected with 50nM of HIF-1α siRNA or an NTC prior to a 48-h combined Ni and MALP-2 exposure. (A) Western blot analysis indicating siRNA-mediated knockdown of Ni and MALP-2 (NiM)–induced HIF-1α protein stabilization. GAPDH is shown as a loading control. Conditioned medium from the different treatment groups was analyzed using specific enzyme-linked immunoassays for (B) VEGF, (C) CXCL1, (D) CXCL5, (E) CXCL8. Data shown are mean ± SEM and are representative of three independent experiments performed in triplicate. **p < 0.01 compared with NTC.
Ni and MALP-2 Induce COX-2 Expression via HIF-1α–Mediated Pathway
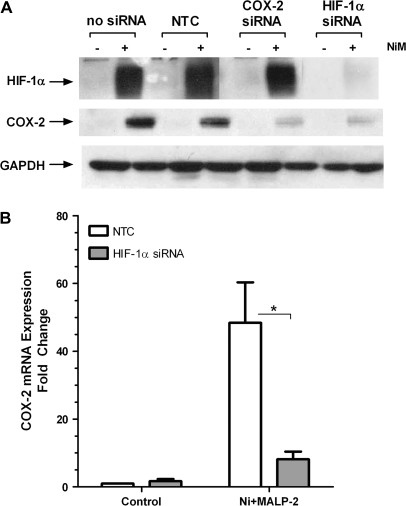
HIF-1α stabilization has been linked with induction of COX-2 (Liu et al., 2002) just as COX-2 induction and PGE2 have been linked with HIF-1α stabilization (Csiki et al., 2006). In order to determine the relative sequence and interdependence of these two events in HLF following Ni and MALP-2 treatment, cells were transfected with either 50nM HIF-1α or COX-2 siRNA, and COX-2 and HIF-1α protein expression were determined using western blot. Figure 5A shows that 50nM COX-2 siRNA prevents Ni and MALP-2–induced COX-2 expression, yet had no effect on the expression of HIF-1α. In contrast, when cells are transfected with 50nM HIF-1α siRNA, there was a marked reduction in both HIF-1α stabilization as well as COX-2 protein induction by Ni and MALP-2. Silencing of HIF-1α also prevented Ni and MALP-2–induced COX-2 mRNA (Fig. 5B; p < 0.05) as well as PGE2 release (data not shown). Taken together, these findings indicate that Ni and MALP-2–induced COX-2 expression in HLF occurs secondary to HIF-1α stabilization.
FIG. 5.
Induction of COX-2 protein by Ni and MALP-2 is dependent on stabilization of HIF-1α. HLF were transiently transfected with 50nM of COX-2 siRNA, HIF-1α siRNA or an NTC prior to a 48-h combined Ni and MALP-2 exposure. (A) Western blot analysis of HIF-1α and COX-2 protein expression following Ni and MALP-2 stimulation in cells transfected with HIF-1α and COX-2 siRNA. GAPDH is shown as a loading control. Representative data from three independent experiments are shown. (B) RT2-PCR analysis of Ni and MALP-2–induced COX-2 mRNA following transfection with HIF-1α siRNA. Data represent mean ± SEM (n = 3). *p < 0.05 compared with NTC.
DISCUSSION
Ni is a common component of air PM pollution that can stimulate inflammation of the lower airways, linking PM exposure to numerous respiratory disorders including fibrosis and cancer (Cruz et al., 2006; Oller et al., 1997). Unfortunately, limited information is available regarding the potential interactions between metals found in PM and microbial stress and their consequences on respiratory function. Epidemiological reports have indicated an increased number of hospitalizations for respiratory disorders including infections on days of increased levels of PM (Schwartz, 1994). In support of these epidemiological findings, several rodent models have shown PM exposure can increase susceptibility to infectious bacteria such as Listeria monocytogenes and Streptococcus pneumoniae (Sigaud et al., 2007; Yang et al., 2001). Here we present the novel findings that although Ni stimulates HIF-1α, it is only when given in combination with the microbial toxin, MALP-2, that HIF-1α becomes important in the induction of COX-2 and the release of proangiogenic and profibrotic CXC chemokines from HLF.
Similar to hypoxia, disruption of prolyl hydroxylase activity has been indicated as a mechanism for Ni-induced HIF-1α stabilization (Davidson et al., 2005; Salnikow et al., 2004). As such, the current finding that MALP-2 enhances Ni-induced stabilization of HIF-1α would be consistent with previous reports showing an interaction between hypoxia and other microbial toxins (e.g., LPS) on HIF-1α stabilization (Mi et al., 2008). However, unlike previous reports with LPS (Kim et al., 2007), the current findings did not indicate any changes in HIF-1α protein or mRNA levels in HLF following treatment with MALP-2. The enhanced expression of HIF-1α protein during mixed exposures to Ni and MALP-2 may result from cross-talk or activation of converging signaling pathways that can contribute to HIF-1α stabilization. For example, Ni has been shown to activate p42/44 MAPK (Tessier and Pascal, 2006). In addition, stimulation of Toll-like receptor-2 can phosphorylate Akt (Cario et al., 2007). We can speculate that the Toll-like receptor 2 agonist, MALP-2, activates PI3K/Akt signaling pathways in HLF. Activation of PI3K/Akt by MALP-2, in combination with disruptions in prolyl hydroxylase and activation of p42/44 MAPK by Ni, may, in turn, lead to enhanced accumulation of HIF-1α. The potential roles of p42/44 MAPK and PI3K/Akt activation by Ni and/or MALP-2, and how they contribute to HIF-1α stabilization in HLF is currently under investigation.
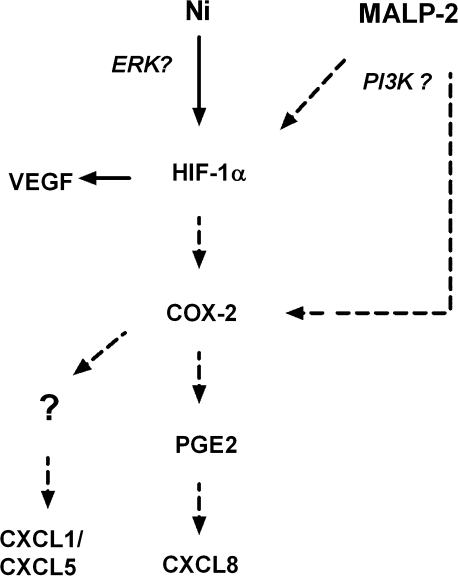
The role of HIF-1α in the stimulation of VEGF release is well established. Less information is known, however, on how HIF-1α may contribute to the regulation of CXCL1, 5, and 8. Hypoxia has been shown to stimulate CXCL8 release in ovarian carcinoma cells, in part through the cooperation of NF-κB and AP-1 binding sites on the CXCL8 promoter region (Xu et al., 1999). More recently, HIF-1α has been shown to bind a functional hypoxia-response element within the CXCL8 promoter and transcriptionally activate the CXCL8 gene in cooperation with NF-κB (Kim et al., 2006). Although Ni alone stabilizes HIF-1α, it has minimal effects on COX-2 expression (Brant and Fabisiak, 2008) and the release of CXCL1, 5, and 8. It is only when Ni is given in combination with the microbial toxic, MALP-2, that enhanced stabilization of HIF-1α with subsequent induction of COX-2 and proangiogenic chemokines are observed (Fig. 6).
FIG. 6.
Proposed mechanism whereby Ni and MALP-2 interact to promote the release of VEGF and CXC chemokines from HLF. Solid arrows indicate pathway stimulated by Ni alone, dashed arrows indicate enhanced pathways of stimulation occurring in the presence of MALP-2.
Numerous reports speak to the ability of HIF-1α and COX-2 to act in concert to regulate each other's expression and/or activity. We have previously reported that Ni and MALP-2 synergistically promote the release of PGE2 from HLF (Brant and Fabisiak, 2008). PGE2 has been shown to stabilize HIF-1α (Liu et al., 2002) and PGE2 can stimulate MAPK and P13K/Akt (George et al., 2007) signaling pathways, which have been implicated in stabilization of HIF-1α (Fang et al., 2007; Richard et al., 1999). Results of the current study indicate, however, that Ni and MALP-2-interaction on HIF-1α stabilization lies upstream of COX-2 (and therefore PGE2) induction. Consistent with these findings, the human COX-2 promoter contains a hypoxia-response element that interacts with HIF-1α (Csiki et al., 2006). It remains possible, however, that once COX-2 is induced in HLF PGE2 may act in an autocrine manner to further amplify the accumulation of HIF-1α and COX-2 protein, thereby maintaining the elevated production and release of the CXC chemokines and VEGF from HLF.
The role of PGE2 in the immune-inflammatory response is complex. Throughout most of the body PGE2 is well known as a proinflammatory mediator and contributes to the pathogenesis of a variety of disorders including rheumatoid arthritis and even cancer growth (Harris et al., 2002; McCoy et al., 2002). However, there is also evidence that PGE2 possesses anti-inflammatory properties in the lung, and can also attenuate fibroblast proliferation, myofibroblast transition and collagen synthesis (Vancheri et al., 2004). The differential roles of PGE2 in regulating inflammation are dependent, in part, upon tissue- and cell-specific expression of individual PGE2 receptor subtypes (EP1–4) which mediate the biological actions of PGE2. Resistance to the protective effects of PGE2 in fibrotic lung fibroblasts has been reported (Huang et al., 2008; Moore et al., 2005). Here we show that PGE2 can have differential effects of PGE2 on chemical and microbial-driven CXC release in HLF, in line with our previous findings (Brant and Fabisiak, 2008; Brant et al., 2008). Exogenously added PGE2 suppresses MALP-2 and LPS-induced CXC chemokine release, yet enhances the release of CXC chemokines following exposure to Ni (Supplementary Data and Brant and Fabisiak, 2008). In HLF, PGE2 can enhance Ni-induced stabilization of HIF-1α, COX-2 expression and release of VEGF and CXCL8 release (Supplementary Data), similar to what is seen when Ni is given in combination with MALP-2. Given that in HLF, stimulatory effects of PGE2 are only observed in the presence of Ni, these findings indicate that Ni can alter cellular responses to PGE2.
The synergistic release of CXCL1, 5, and 8 from HLF following combined Ni and MALP-2 exposures may result from cooperation between the downstream transcriptional/post-translational events modulated via COX-2 and PGE2 production in combination with a direct effect of HIF-1α on the promoter regions of the individual chemokines. Such an effect would explain, in part, the greater attenuation in the release of these factors by Ni and MALP-2 in cells transfected with HIF-1α siRNA compared with those receiving COX-2 siRNA.
It is of note that the profiles of CXC chemokines release, along with VEGF, following Ni and MALP-2 exposures are similar to those seen in angiogenic and profibrotic disorders of the lung. CXCL1, 5, and 8 contain the structural and functional Glutamate–Leucine–Arginine (ERL) motif, classifying them as proangiogenic, consistent with their role in the stimulation of neutrophil recruitment and endothelial cell migration (Addison et al., 2000). Following Ni and MALP-2 exposure, levels of CXCL8 in the conditioned medium from HLF reached approximately 1000–2000 pg/ml, within the range of concentrations shown to stimulate proliferation and migration of endothelial cells (Li et al., 2003). HIF-1α has been established as a pivotal transcription factor in the regulation of genes involved in angiogenesis, and overexpression of COX-2 is commonly observed in nonsmall cell lung cancer (Hida et al., 1998), where it regulates expression of CXCL5 and CXCL8 (Pold et al., 2004). These findings are consistent with the current observations that HIF-1α and COX-2 mediate CXCL5 and 8 release from HLF. Recent reports have also indicated that in addition to their role in angiogenesis, HIF-1α and VEGF expression may also play a role in pulmonary fibrotic disorders (Tzouvelekis et al., 2007). Levels of CXCL1, 5, and 8 are also upregulated in patients with idiopathic pulmonary fibrosis compared with control subjects (Keane et al., 2001; Strieter et al., 2002), implicating an additional role for these chemokines in pulmonary disorders.
There were several other genes of interest on the RT2-PCR array that warrant further investigation, for example, ANGPTL4 and LEP among others. Little is known regarding the specific cells types that express ANGPTL4, a gene induced by hypoxia, which can promote endothelial cell survival and formation of tubule-like structures (Hermann et al., 2005). Here we show that HLF express ANGPLT4 mRNA and that expression is enhanced following exposure Ni, both with and without added MALP-2. Leptin expression is also elevated following exposures to Ni and MALP-2. Recently, leptin has been described as a potent angiogenic factor stimulating early incisional wound angiogenesis, promoting cellular invasion and migration (Liapakis et al., 2007).
In conclusion, the current findings indicate that Ni and MALP-2-induced stabilization of HIF-1α, with subsequent COX-2 expression, drives the release of proangiogenic and profibrotic mediators VEGF, CXCL1, CXCL5, and CXCL8 from HLF. These findings highlight a novel role for HIF-1α in mediating chemical-induced alterations in cellular response to microbial stimuli, imparting a susceptibility to fibrotic changes and the development of a proangiogenic environment in the lung.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (F32ES015966 to K.B., RO1-ES-011986) to J.F.
Acknowledgments
We would like to thank Randy Stalter, Cory Mathias, and Rachel Ward for their technical assistance.
References
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- Brant KA, Fabisiak JP. Nickel alterations of TLR2-dependent chemokine profiles in lung fibroblasts are mediated by COX-2. Am. J. Respir. Cell Mol. Biol. 2008;38:591–599. doi: 10.1165/rcmb.2007-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant KA, Stalter RM, Fabisiak JP. Differential effects of PGE2 on Nickel and microbial-driven CXCL8 release from human lung fibroblasts. Am. J. Respir. Crit. Care Med. 2008;177:A731. (Abstract) [Google Scholar]
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MJ, Costa R, Marquilles E, Morell F, Munoz X. [Occupational asthma caused by chromium and nickel] Arch. Bronconeumol. 2006;42:302–306. doi: 10.1016/s1579-2129(06)60147-x. (in Spanish) [DOI] [PubMed] [Google Scholar]
- Csiki I, Yanagisawa K, Haruki N, Nadaf S, Morrow JD, Johnson DH, Carbone DP. Thioredoxin-1 modulates transcription of cyclooxygenase-2 via hypoxia-inducible factor-1alpha in non-small cell lung cancer. Cancer Res. 2006;66:143–150. doi: 10.1158/0008-5472.CAN-05-1357. [DOI] [PubMed] [Google Scholar]
- Davidson T, Chen H, Garrick MD, D'Angelo G, Costa M. Soluble nickel interferes with cellular iron homeostasis. Mol. Cell. Biochem. 2005;279:157–162. doi: 10.1007/s11010-005-8288-y. [DOI] [PubMed] [Google Scholar]
- Fabisiak JP, Weiss RD, Powell GA, Dauber JH. Enhanced secretion of immune-modulating cytokines by human lung fibroblasts during in vitro infection with Mycoplasma fermentans. Am. J. Respir. Cell Mol. Biol. 1993;8:358–364. doi: 10.1165/ajrcmb/8.4.358. [DOI] [PubMed] [Google Scholar]
- Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Yan L, Shing Y, Moses MA. HIF-1alpha-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001;61:5731–5735. [PubMed] [Google Scholar]
- Finetti F, Solito R, Morbidelli L, Giachetti A, Ziche M, Donnini S. Prostaglandin E2 regulates angiogenesis via activation of fibroblast growth factor receptor-1. J. Biol. Chem. 2008;283:2139–2146. doi: 10.1074/jbc.M703090200. [DOI] [PubMed] [Google Scholar]
- George RJ, Sturmoski MA, Anant S, Houchen CW. EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins Other Lipid Mediat. 2007;83:112–120. doi: 10.1016/j.prostaglandins.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Hermann LM, Pinkerton M, Jennings K, Yang L, Grom A, Sowders D, Kersten S, Witte DP, Hirsch R, Thornton S. Angiopoietin-like-4 is a potential angiogenic mediator in arthritis. Clin. Immunol. 2005;115:93–101. doi: 10.1016/j.clim.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hida T, Yatabe Y, Achiwa H, Muramatsu H, Kozaki K, Nakamura S, Ogawa M, Mitsudomi T, Sugiura T, Takahashi T. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- Huang SK, Wettlaufer SH, Hogaboam CM, Flaherty KR, Martinez FJ, Myers JL, Colby TV, Travis WD, Toews GB, Peters-Golden M. Variable prostaglandin E2 resistance in fibroblasts from patients with usual interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2008;177:66–74. doi: 10.1164/rccm.200706-963OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein. Curr. Opin. Genet. Dev. 2001;11:27–34. doi: 10.1016/s0959-437x(00)00152-0. [DOI] [PubMed] [Google Scholar]
- Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- Keane MP, Belperio JA, Burdick MD, Lynch JP, Fishbein MC, Strieter RM. ENA-78 is an important angiogenic factor in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001;164:2239–2242. doi: 10.1164/ajrccm.164.12.2104106. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kim YH, Nam BH, Kong HJ, Kim HH, Kim YJ, An WG, Cheong J. HIF-1alpha expression in response to lipopolysaccaride mediates induction of hepatic inflammatory cytokine TNFalpha. Exp. Cell Res. 2007;313:1866–1876. doi: 10.1016/j.yexcr.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J. Immunol. 2006;177:7211–7224. doi: 10.4049/jimmunol.177.10.7211. [DOI] [PubMed] [Google Scholar]
- Lappi-Blanco E, Kaarteenaho-Wiik R, Maasilta PK, Anttila S, Paakko P, Wolff HJ. COX-2 is widely expressed in metaplastic epithelium in pulmonary fibrous disorders. Am. J. Clin. Pathol. 2006;126:717–724. doi: 10.1309/PFGX-CLNG-2N17-PJX9. [DOI] [PubMed] [Google Scholar]
- Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- Liapakis IE, Anagnostoulis S, Karayannakis A, Korkolis DP, Labropoulou M, Anastakis D, Simopoulos C. Exogenously-administered leptin increases early incisional wound angiogenesis in an experimental animal model. In Vivo. 2007;21:797–801. [PubMed] [Google Scholar]
- Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J. Biol. Chem. 2002;277:50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J. Clin. Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z, Rapisarda A, Taylor L, Brooks A, Creighton-Gutteridge M, Melillo G, Varesio L. Synergistic induction of HIF-1alpha transcriptional activity by hypoxia and lipopolysaccharide in macrophages. Cell Cycle. 2008;7:232–241. doi: 10.4161/cc.7.2.5193. [DOI] [PubMed] [Google Scholar]
- Moore BB, Ballinger MN, White ES, Green ME, Herrygers AB, Wilke CA, Toews GB, Peters-Golden M. Bleomycin-induced E prostanoid receptor changes alter fibroblast responses to prostaglandin E2. J. Immunol. 2005;174:5644–5649. doi: 10.4049/jimmunol.174.9.5644. [DOI] [PubMed] [Google Scholar]
- Oller AR, Costa M, Oberdorster G. Carcinogenicity assessment of selected nickel compounds. Toxicol. Appl. Pharmacol. 1997;143:152–166. doi: 10.1006/taap.1996.8075. [DOI] [PubMed] [Google Scholar]
- Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, Pold A, Luo J, Krysan K, Dohadwala M, Mao JT, Batra RK, Strieter RM, Dubinett SM. Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Res. 2004;64:1853–1860. doi: 10.1158/0008-5472.can-03-3262. [DOI] [PubMed] [Google Scholar]
- Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, Breyer MD, Pozzi A. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J. Biol. Chem. 2007;282:16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J. Biol. Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and biologist programmers. Totowa, NJ: Humana Press; 2000. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Davidson T, Zhang Q, Chen LC, Su W, Costa M. The involvement of hypoxia-inducible transcription factor-1-dependent pathway in nickel carcinogenesis. Cancer Res. 2003;63:3524–3530. [PubMed] [Google Scholar]
- Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- Schwartz J. What are people dying of on high air pollution days. Environ. Res. 1994;64:26–35. doi: 10.1006/enrs.1994.1004. [DOI] [PubMed] [Google Scholar]
- Sigaud S, Goldsmith CA, Zhou H, Yang Z, Fedulov A, Imrich A, Kobzik L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol. 2007;223:1–9. doi: 10.1016/j.taap.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Keane MP. CXC chemokines in angiogenesis related to pulmonary fibrosis. Chest. 2002;122:298S–301S. doi: 10.1378/chest.122.6_suppl.298s. [DOI] [PubMed] [Google Scholar]
- Tessier DM, Pascal LE. Activation of MAP kinases by hexavalent chromium, manganese and nickel in human lung epithelial cells. Toxicol. Lett. 2006;167:114–121. doi: 10.1016/j.toxlet.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am. J. Respir. Crit. Care Med. 2007;176:1108–1119. doi: 10.1164/rccm.200705-683OC. [DOI] [PubMed] [Google Scholar]
- Vancheri C, Mastruzzo C, Sortino MA, Crimi N. The lung as a privileged site for the beneficial actions of PGE2. Trends Immunol. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vink A, Schoneveld AH, Lamers D, Houben AJ, van der Groep P, van Diest PJ, Pasterkamp G. HIF-1 alpha expression is associated with an atheromatous inflammatory plaque phenotype and upregulated in activated macrophages. Atherosclerosis. 2007;195:e69–e75. doi: 10.1016/j.atherosclerosis.2007.05.026. [DOI] [PubMed] [Google Scholar]
- Westra J, Brouwer E, Bos R, Posthumus MD, Doornbos-van der Meer B, Kallenberg CG, Limburg PC. Regulation of cytokine-induced HIF-1alpha expression in rheumatoid synovial fibroblasts. Ann. N. Y. Acad. Sci. 2007;1108:340–348. doi: 10.1196/annals.1422.035. [DOI] [PubMed] [Google Scholar]
- Xu L, Xie K, Mukaida N, Matsushima K, Fidler IJ. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 1999;59:5822–5829. [PubMed] [Google Scholar]
- Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect. Immun. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.