Abstract
The expectation from the literature is that organophosphorus (OP) agents bind to proteins that have an active site serine. However, transferrin, a protein with no active site serine, was covalently modified in vitro by 0.5mM 10-fluoroethoxyphosphinyl-N-biotinamido pentyldecanamide, chlorpyrifos oxon, diisopropylfluorophosphate, dichlorvos, sarin, and soman. The site of covalent attachment was identified by analyzing tryptic peptides in the mass spectrometer. Tyr 238 and Tyr 574 in human transferrin and Tyr 238, Tyr 319, Tyr 429, Tyr 491, and Tyr 518 in mouse transferrin were labeled by OP. Tyrosine in the small synthetic peptide ArgTyrThrArg made a covalent bond with diisopropylfluorophosphate, chlorpyrifos oxon, and dichlorvos at pH 8.3. These results, together with our previous demonstration that albumin and tubulin bind OP on tyrosine, lead to the conclusion that OP bind covalently to tyrosine, and that OP binding to tyrosine is a new OP-binding residue. The OP-reactive tyrosines are activated by interaction with Arg or Lys. It is suggested that many proteins in addition to those already identified may be modified by OP on tyrosine. The extent to which tyrosine modification by OP can occur in vivo and the toxicological implications of such modifications require further investigation.
Keywords: plasma, soman, sarin, mass spectrometry, tyrosine residue, transferrin
We have previously shown that many proteins in plasma react with the organophosphorus agent (OP) FP-biotin (10-fluoroethoxyphosphinyl-N-biotinamido pentyldecanamide) (Peeples et al., 2005). Our goal is to identify these proteins. The proteins that react most rapidly with FP-biotin and with other OP are enzymes in the serine hydrolase family, for example, butyrylcholinesterase, acetylcholinesterase, acylpeptide hydrolase, fatty acid amide hydrolase, arylformamidase, and neuropathy target esterase-lysophospholipase (Casida and Quistad, 2004; Richards et al., 2000). The residue that is labeled in these enzymes is the active site serine in the consensus sequence GlyXSerXGly. However, proteins with no consensus active site serine constitute another group of OP-reactive proteins, where OP bind to tyrosine. Papain and bromelain bind diisopropylfluorophosphate (DFP) on tyrosine (Chaiken and Smith, 1969; Murachi et al., 1965). Mass spectrometry has allowed identification of OP-binding to tyrosines in albumin and tubulin (Ding et al., 2008; Grigoryan et al., 2008; Li et al., 2007, 2008). The present report adds transferrin to this list. Analysis of the reactive peptides from these proteins shows that a new motif of OP binding has emerged.
Our strategy uses FP-biotin, initially, to label the OP-reactive proteins in plasma. Through the use of the fluorescent probe Streptavidin-Alexa 680 and the biotin tag, the proteins that are OP-reactive in serum can be visualized. The biotin tag also provides a means for purification of the labeled proteins and peptides by binding to avidin-agarose beads. The FP-biotin–labeled proteins and peptides are identified by mass spectrometry. It is relatively simple to identify a protein by mass spectrometry because only a few peptides from a protein are needed for a positive identification. However, it is often difficult to find a specific labeled peptide. Convincing proof that a protein is OP-labeled comes from identifying the labeled peptide and the amino acid in that peptide that is modified by OP. To provide this proof we label pure protein with OP, digest with trypsin, separate and enrich the OP-labeled peptides by reverse phase high-performance liqid chromatography (HPLC), and determine the peptide sequence and site of attachment by collision-induced dissociation in the mass spectrometer.
Not all OP are expected to bind covalently to a particular protein. For example, the positively charged echothiophate and VX react rapidly with acetylcholinesterase, but only poorly with carboxylesterase (Maxwell and Brecht, 2001). Therefore we treated human and mouse transferrin with six different OP. We found human transferrin peptides labeled with six OP, and mouse transferrin peptides labeled with five OP.
The common feature in the motif for OP-reactive tyrosines is the presence of a positively charged arginine or lysine within five residues of the tyrosine. This OP-binding motif was tested with two synthetic peptides, both containing tyrosine, but only one containing nearby arginines. Tyrosine formed a covalent bond with OP in both peptides, but the reaction proceeded more readily with ArgTyrThrArg than with SerTyrSerMet. Additional work with small peptides needs to be done to determine the optimum peptide sequence of the OP reactive peptide and to determine its rate of reaction with OP.
EXPERIMENTAL PROCEDURES
Materials.
FP-biotin (Mr 592.32) was custom synthesized in the laboratory of Dr Charles Thompson at the University of Montana, Missoula, MT. A 2mM FP-biotin solution in dimethyl sulfoxide was stored at −80°C. Chlorpyrifos oxon (ChemService, Inc. West Chester, PA; MET-674B) was dissolved in ethanol and stored at −80°C. Dichlorvos (ChemService Inc.; PS-89) was dissolved in methanol. Soman and sarin from CEB (Vert-le-Petit, France) were dissolved in isopropanol. Immun-Blot polyvinylidene difluoride (PVDF) membrane for protein blotting, 0.2 μm (162-0177) and Affi-gel blue (153-7301), a cross-linked agarose bead with covalently attached Cibacron Blue F3GA dye, were from Bio-Rad Laboratories, Hercules, CA. Streptavidin Alexa Fluor 680 (S-21378) was from Molecular Probes, Eugene, OR. Porcine trypsin (Promega, Madison, WI; V5113 sequencing grade modified trypsin) at a concentration of 0.4 μg/μl in 50mM acetic acid was stored at −80°C. Immobilized tetrameric avidin-agarose beads (Pierce 20219) and immobilized monomeric avidin-agarose beads (Pierce 20228) were used to purify FP-biotinylated proteins as well as FP-biotinylated peptides. Human butyrylcholinesterase was purified from outdated human plasma as described (Lockridge et al., 2005) and labeled with FP-biotin as described (Peeples et al., 2005). Mouse plasma was from strain 129Sv mice. Human holo-transferrin (T0665), human apo-transferrin (T4382), mouse apo-transferrin (T0523), bovine holo-transferrin (T1408), human alpha 2-macroglobulin (M6159), human alpha 1-antitrypsin (A9024), human complement C3 (C2910), nitrilotriacetic acid (N9877), Coomassie blue R250 (Brillant Blue R), and diisopropylfluorophosphate (D0879, a liquid with a concentration of 5.73M) were from Sigma, St Louis, MO. ProbeQuant G50 micro column was from Amersham (27-5335-01). Peptide ArgTyrThrArg (Mr 594.7) was custom synthesized and purified to > 95% by Genscript Corp., Piscataway, NJ. Peptide SerTyrSerMet (#20621; Mr 486.5) was from AnaSpec, Inc., San Jose, CA. Alpha-cyano-4-hydroxy cinnamic acid, Glu Fibrinopeptide B, and Cal Mix 1 were from Applied Biosystems (MDS Sciex, Foster City, CA).
Blot of FP-biotin–labeled proteins from a nondenaturing polyacrylamide electrophoresis gel.
Two hundred microliters of human or mouse plasma was treated with 100μM FP-biotin for 24 h at 37°C. Ten- to 30-μl aliquots were subjected to gel electrophoresis on nondenaturing polyacrylamide gradient gels, 4–22.5% (wt/vol), cast in a Hoefer gel apparatus. The polyacrylamide gel electrophoresis (PAGE) gels were 0.75 mm thick, 15 cm wide, and 12 cm high. Nondenaturing gels were used because albumin separates from other plasma proteins on a nondenaturing gel, but not on a sodium dodecyl sulfate (SDS) gel. Proteins were electrophoretically transferred to a PVDF membrane using a Bio-Rad Trans-blot apparatus. The blot was hybridized with a fluorescent probe, Streptavidin Alexa Fluor 680, as described (Peeples et al., 2005). Fluorescence intensity was captured in the Odyssey Infrared Imaging System (LiCor).
Blot of FP-biotin–labeled proteins from an SDS PAGE gel.
Purified bovine holo-transferrin (76–81 kDa), human alpha 2-macroglobulin (tetramer 720 kDa; monomer 179 kDa), human alpha 1-antitrypsin (52 kDa), and human complement C3 (115 and 70 kDa) were dissolved in phosphate buffered saline to a concentration of 500 pmol in 50 μl. Proteins were treated with 100μM FP-biotin at 37°C for 20 h. Control samples were treated with dimethyl sulfoxide but no FP-biotin. Unreacted FP-biotin was removed by passing the samples through a G50 spin column. Samples were boiled in the presence of dithiothreitol and SDS and loaded on a 4–22.5% (wt/vol) polyacrylamide gradient SDS gel. After electrophoresis, some gels were stained for protein with Coomassie blue R250. For other gels, the protein was transferred to a PVDF membrane where the biotin tag was detected with Streptavidin Alexa Fluor 680, as described (Peeples et al., 2005). The amount of protein loaded on the Coomassie stained gel ranged from 3.5 to 70 μg, and that on the gel used for transfer to PVDF ranged from 1.5 to 30 μg.
Isolation of FP-biotin–labeled proteins with avidin-agarose beads.
Plasma was depleted of albumin by chromatography on Affi-gel blue because albumin reacts extensively with FP-biotin and can deplete the FP-biotin pool making reaction with other proteins more difficult. The albumin-depleted plasma was treated with 100μM FP-biotin for 24 h at 37°C, and dialyzed to remove excess FP-biotin. The FP-biotinylated proteins were purified by binding to tetrameric avidin-agarose beads and separated by SDS gel electrophoresis as described (Peeples et al., 2005).
Purification of FP-biotin–labeled peptides.
In some experiments the FP-biotin–labeled peptides rather than FP-biotin–labeled proteins were purified by binding to monomeric avidin-agarose beads. After washing with 1M NaCl in pH 8.0 buffer, followed by water washes to desalt the column, the peptides were released with 10% (vol/vol) acetic acid.
Reaction of pure transferrin with OP.
A 1 mg/ml solution of human or mouse transferrin in 0.1M TrisCl pH 8.5, or in phosphate buffered saline, or in 10mM TrisCl pH 8.5, 0.01% (wt/vol) sodium azide was treated with 0.5mM OP at 37°C for 16 h. The accession numbers in the SwissProt database are P02787 for human and Q921I1 for mouse transferrin. Their molecular weight is about 75,000 after the 19 amino acid signal peptide is subtracted.
Tryptic peptides for mass spectrometry.
Three protocols were used to prepare peptides for mass spectrometry. (1) Proteins in SDS PAGE gel slices were digested with trypsin. The peptides were extracted from the gel and analyzed by liquid chromatography tandem mass spectrometry (LC/MS/MS) as described (Peeples et al., 2005). This protocol was used only for the preliminary experiments where the data are not shown. (2) Pure proteins in solution were denatured in 8M urea, reduced with 10mM dithiothreitol at pH 8, carbamidomethylated (CAM) with 50mM iodoacetamide, and desalted by dialysis against 10mM ammonium bicarbonate. The CAM proteins were digested with trypsin at a ratio of 50:1 (w/w) at 37°C for 16 h. Peptides were separated offline by reverse phase HPLC on a 100 × 4.60 mm Phenomenex C18 column eluted with a 60 min gradient from 0 to 60% (vol/vol) acetonitrile versus 0.1% (vol/vol) trifluoroacetic acid at a flow rate of 1 ml/min. Fractions were analyzed by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry to locate the OP-labeled peptides. Selected samples were dried, dissolved in 50% acetonitrile, 0.1% formic acid, and infused into the QTRAP 4000 mass spectrometer for MS/MS analysis. Peptides were infused into the mass spectrometer when the mass of the parent ion was known. (3) Trypsin digested pure protein was analyzed a second way to allow for the possibility that the mass of the parent ion was unknown. The offline HPLC step was omitted, and peptides were separated on a nanocolumn whose output was electrosprayed into the QTRAP 2000 mass spectrometer. MS/MS spectra were acquired for three peptides every 2.8 s.
Reaction of model peptides with OP.
Peptides ArgTyrThrArg (0.17mM) and SerTyrSerMet (0.17mM) in 10mM ammonium bicarbonate pH 8.3 were treated with 0.1, 0.2, or 1mM DFP, chlorpyrifos oxon (CPO), or dichlorvos for 16 h at 37°C. Aliquots were spotted on a MALDI target plate, overlaid with CHCA (α-cyano-4-hydroxy cinnamic acid) matrix, and analyzed by MALDI-TOF as well as by MALDI-TOF-TOF mass spectrometry and by LC/MS/MS on the QTRAP 2000.
Percent labeling.
The percent labeled peptide in a given MALDI-TOF spectrum was calculated by dividing the cluster area of the labeled peptide by the sum of the cluster areas for the unlabeled and labeled peaks in a given spectrum. The assumption in this calculation is that the labeled and unlabeled peptides ionize equally well. In a previous publication (Lockridge et al., 2008) we checked the validity of our MALDI-TOF quantitation method by using a second method to quantitate labeled and unlabeled peptides. The second method was amino acid composition analysis. Both methods showed that 27% of a particular peptide was labeled, thus validating the MALDI-TOF quantitation method.
MALDI-TOF mass spectrometry.
All peptide samples were screened by MALDI-TOF before they were analyzed in the QTRAP mass spectrometer because MALDI-TOF is a quick way to obtain peptide masses and thereby a suggestion of whether an OP-labeled peptide is present. Salt-free peptides were spotted on a target plate in 0.5-μl aliquots, allowed to dry, and overlaid with 10 mg/ml CHCA matrix in 50% acetonitrile, 0.1% trifluoroacetic acid. Mass spectra were acquired with the MALDI-TOF-TOF 4800 mass spectrometer (Applied Biosystems, MDS Sciex, Foster City, CA). Each scan is the sum of 500 laser shots—50 shots were taken at one location on the spot and then the laser automatically moved to a new location to avoid burning out the sample. Laser intensity was adjusted to obtain maximum signal intensity without exceeding the saturation limit. The mass spectrometer was calibrated against Cal Mix 1 (des-Arg Bradykinin, Glu Fibrinopeptide B, angiotensin I, and neurotensin).
LC/MS/MS on a tandem quadrupole mass spectrometer.
Tryptic digests of OP-labeled human and mouse transferrin were analyzed by this method. A 10-μl aliquot of peptides in 5% acetonitrile, 0.1% formic acid, at a concentration of about 2 pmol/μl, was injected into the HPLC nanocolumn (#218MS3.07515 Vydac C18 polymeric rev-phase, 75 micron ID × 150 mm long; P.J. Cobert Assoc, St Louis, MO). Peptides were separated with a 90 min linear gradient from 0 to 60% acetonitrile at a flow rate of 0.3 μl/min and electrosprayed through a nanospray emitter (fused silica, 360 μm OD × 75 μm ID × 15 μm taper; New Objective) directly into the mass spectrometer. Mass spectra, high resolution mass spectra, and product ion spectra (MS/MS) were acquired on a QTRAP 2000 triple quadrupole linear ion trap mass spectrometer (Applied Biosystems) using the trap (enhanced sensitivity) mode. An ion-spray voltage of 1900 volts was maintained between the emitter and the mass spectrometer. Information-dependent acquisition was used to acquire data for the three most intense peaks in each cycle, having a charge of +1 to +4, a mass between 200 and 1700 m/z, and an intensity > 10,000 counts per s. Precursor ions were excluded for 30 s after one MS/MS spectrum had been collected. The collision cell was pressurized to 40 μTorr with pure nitrogen and collision energies between 20 and 40 eV were determined automatically by the Analyst 1.4.1 software, based on the mass and charge of the precursor ion. The mass spectrometer was calibrated using fragment ions generated from collision-induced dissociation of Glu fibrinopeptide B (Sigma). The MS/MS data were processed using Analyst 1.4.1 software and submitted to Mascot for identification of peptide sequences modified by OP on Tyr, Ser, or Thr (Perkins et al., 1999).
Infusion in the QTRAP 4000 mass spectrometer.
HPLC purified OP-labeled human and mouse transferrin tryptic peptides were analyzed by this method when the mass of the parent ion was known from MALDI-TOF experiments. Peptides were dissolved in 50% acetonitrile, 0.1% formic acid to a concentration of 2–6 pmol/μl. Peptides were infused into the QTRAP 4000 (Applied Biosystems) mass spectrometer at a flow rate of 0.3 μl/min through an 8 μm emitter (#FS360-50-8-D, New Objective) via a 25-μl Hamilton syringe mounted on a Harvard pump. Five hundred MS/MS spectra were accumulated for each parent ion.
Binding of ferric ion to transferrin.
The normal function of transferrin is iron transport through the blood. Binding of ferric ion is a measure of the functional viability. Titration of transferrin with ferric ion was performed as described (Welch and Skinner, 1989). A 1 mg/ml solution of apo-human transferrin was prepared in 0.1M TrisCl pH 8.5 containing 5mM Na2CO3. Half of the transferrin solution was incubated with 100μM FP-biotin at 37°C for 16 h, resulting in the labeling of Tyr 238 and Tyr 574. The other half was the unlabeled control. Protein concentration was determined with the CB-X protein assay kit (Genotech, St Louis, MO; #786-12X). FP-biotin–labeled as well as control apo-transferrin were titrated with ferric nitrilotriacetate (FENTA) to determine the stoichiometry of ferric ion binding. Each 1 ml solution of transferrin was titrated by sequential additions of 5 μl of 1mM FENTA. Binding was monitored by following the change in absorbance at 470 nm.
RESULTS
Human and Mouse Plasma Proteins that Bind FP-Biotin
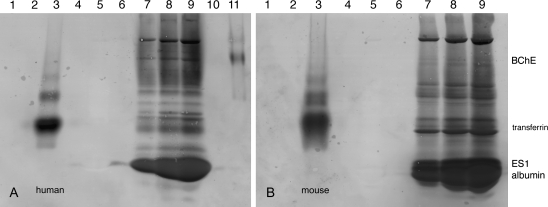
The blots in Figures 1A and 1B show at least 12 bands in both human and mouse plasma that bind FP-biotin (Figs. 1A and 1B, lanes 7, 8, 9). The most intense band in human plasma is FP-biotin–labeled albumin. The most intense band in mouse plasma contains two proteins: FP-biotin–labeled carboxylesterase ES1 and FP-biotin–labeled albumin (Fig. 1B, lanes 7, 8, 9). A major difference between human and mouse plasma is that only mouse plasma contains carboxylesterase (Li et al., 2005). Albumin, ES1 carboxylesterase, and butyrylcholinesterase have previously been identified as proteins in mouse plasma that bind FP-biotin (Peeples et al., 2005).
FIG. 1.
Blots showing FP-biotin–reactive proteins in human (A) and mouse (B) plasma. FP-biotinylated plasma proteins were separated on nondenaturing polyacrylamide gels and transferred to PVDF membranes. Blots were hybridized with the fluorescent probe Streptavidin Alexa Fluor 680. A1, 20 μg human transferrin; A2, blank; A3, 20 μg FP-biotinylated human transferrin; A4, blank; A5, 5 μl of human plasma; A6, blank; A7, 3.3 μl of FP-biotinylated human plasma; A8, 6.6 μl of FP-biotinylated human plasma; A9, 9.9 μl of FP-biotinylated human plasma; A10, blank; A11, 1 pmol FP-biotinylated human butyrylcholinesterase (BChE). B1, 20 μg mouse transferrin; B2, blank; B3, 20 μg FP-biotinylated mouse transferrin; B4, blank; B5, 5 μl of mouse plasma; B6, blank; B7, 5 μl of FP-biotinylated mouse plasma; B8, 10 μl of FP-biotinylated mouse plasma; B9, 15 μl of FP-biotinylated mouse plasma. The heavy band in B contains mouse ES1 carboxylesterase and mouse albumin. ES1 carboxylesterase in mouse plasma does not separate well from albumin on a nondenaturing gel. Human plasma does not contain carboxylesterase.
The search for additional OP-reactive proteins in plasma led to preliminary identification of human transferrin. This preliminary identification resulted from LC/MS/MS mass spectral analysis of an in-gel, tryptic digest of a band from an SDS PAGE gel. Data were acquired on the QTRAP 2000 mass spectrometer. Proteins applied to this gel had been labeled with FP-biotin and purified with avidin-agarose (data not shown). Mass spectra identified transferrin, but not the labeled peptide.
To confirm that transferrin was actually labeled by FP-biotin, pure human and mouse transferrin treated with FP-biotin were visualized on a blot hybridized with Streptavidin Alexa Fluor 680. Lane 3 in Figures 1A and 1B shows an intense band for FP-biotinylated transferrin, thus confirming that human and mouse transferrin bind FP-biotin.
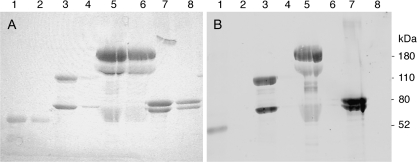
The preliminary LC/MS/MS mass spectral experiments also showed alpha-2-macroglobulin, alpha-1-antitrypsin, and complement C3 as proteins in human plasma that could be labeled with FP-biotin (data not shown). As with transferrin, the FP-biotinylated plasma proteins had been purified on avidin-agarose beads, separated by SDS gel electrophoresis, digested with trypsin, and analyzed by LC/MS/MS. The labeled peptides from these proteins were not found in these preliminary experiments. To confirm that these proteins made a covalent bond with FP-biotin, highly purified preparations of the proteins were treated with FP-biotin and subjected to SDS gel electrophoresis. The labeled proteins were transferred from the gel to PVDF membrane. The membrane was hybridized with Streptavidin Alexa Fluor 680 (Fig. 2B). A second gel, containing higher quantities of protein, was stained with Coomassie blue R250 (Fig. 2A). Figure 2 (lanes 2, 4, 6, 8) shows that control proteins, not treated with FP-biotin, have a Coomassie stained band in panel A, but no fluorescent band in panel B. On the other hand, proteins treated with FP-biotin (lanes 1, 3, 5, 7) have a band in panel A as well as in panel B. The blot confirms that human alpha-1-antitrypsin, human complement C3, human alpha-2-macroglobulin, and bovine holo-transferrin covalently bind FP-biotin.
FIG. 2.
Covalent binding of FP-biotin to human alpha-1-antitrypsin, human complement C3, human alpha-2-macroglobulin, and bovine holo-transferrin. (A) Coomassie stained SDS gel. (B) Blot hybridized with Streptavidin Alexa Fluor 680 to visualize FP-biotinylated proteins transferred to PVDF membrane from the gel. Lane 1, FP-biotinylated alpha-1-antrypsin; lane 2, unlabeled alpha-1-antitrypsin; lane 3, FP-biotinylated complement C3; lane 4, unlabeled complement C3; lane 5, FP-biotinylated alpha-2-macroglobulin; lane 6, unlabeled alpha-2-macroglobulin; lane 7, FP-biotinylated transferrin; lane 8, unlabeled transferrin.
No further results were obtained for alpha-1-antitrypsin, complement C3, and alpha-2-macroglobulin. The site for covalent attachment of FP-biotin in these proteins is unknown.
Tyr 238 and Tyr 574 of Human Transferrin Bind OP
Structures of OP are in Figure 3. FP-biotinylated, CAM tryptic peptides of human transferrin were purified on monomeric avidin beads and their masses determined by MALDI-TOF mass spectrometry. Every peak in the MALDI-TOF spectrum was fragmented with the MALDI-TOF-TOF feature of the mass spectrometer. The MS/MS scans were examined for the presence of ions characteristic of FP-biotin at 227, 312, and 329 amu (Schopfer et al., 2005). A peptide that fragmented to yield these ions was labeled with FP-biotin. Additional information about the peptide was obtained in the QTRAP 4000 mass spectrometer.
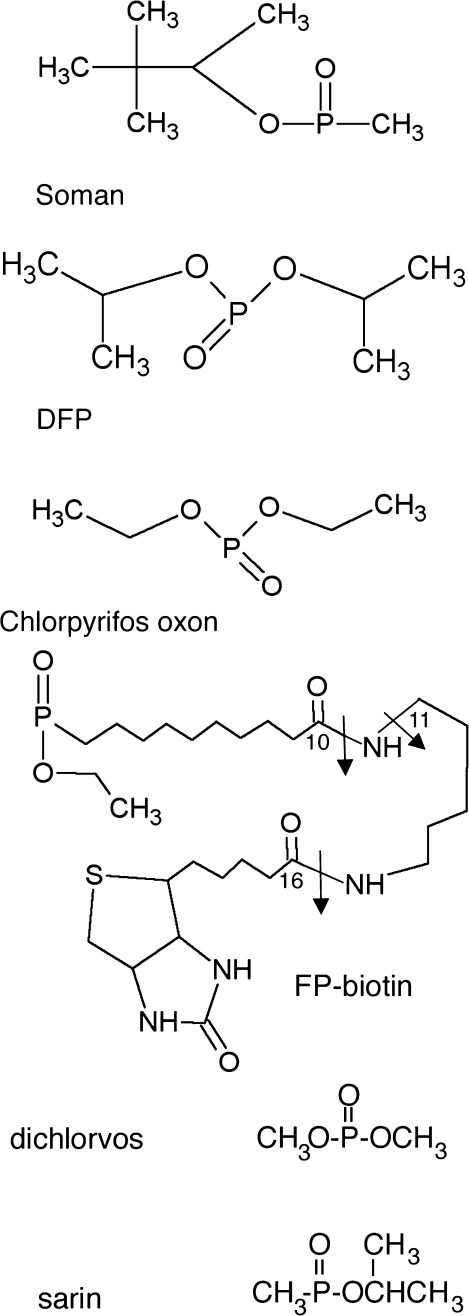
FIG. 3.
Structures of the phosphoryl moieties that have become attached through phosphorus to tyrosine. Covalent binding to tyrosine results in loss of the fluoride ion from soman, DFP, FP-biotin, and sarin, of the dichlorovinyl alcohol group from dichlorvos, and of the trichloropyridinol group from chlorpyrifos oxon. Tyrosine loses one hydrogen. The added mass is 162.2 for soman, 164.1 for DFP, 136.0 for chlorpyrifos oxon, 572.3 for FP-biotin, 108.0 for dichlorvos, and 120.0 for sarin. The arrows in FP-biotin indicate fragmentation sites. A 227 amu ion is produced by cleavage between carbon 16 and the adjacent nitrogen. A 329 amu ion is produced by cleavage between carbon 10 and the adjacent nitrogen. The 312 amu ion is produced by loss of the amine from the 329 ion.
Two FP-biotin–labeled peptides were identified in human transferrin. The labeled tyrosines are Tyr 238 and Tyr 574, when numbering is for the mature human transferrin protein (MacGillivray et al., 1982) from which the 19 amino acid signal peptide has been deleted (Swiss Prot accession #P02787). Both holo- and apo-human transferrin bound FP-biotin.
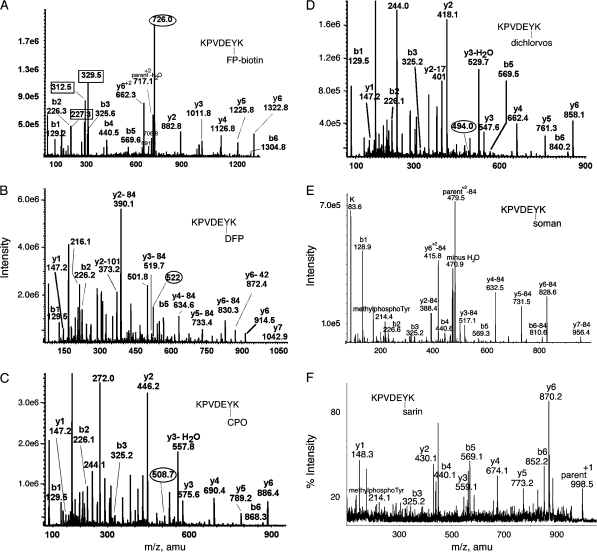
The MS/MS spectrum in Figure 4A shows that FP-biotin is covalently attached to tyrosine in peptide LysProValAspGluTyr*Lys (KPVDEY*K). The parent ion mass (726 m/z, doubly charged) is equal to the peptide mass plus the added mass from FP-biotin (572 amu). Fragments at 227.3, 312.5, and 329.5 amu are characteristic for the presence of FP-biotin. Absence of a mass at 591 amu suggests the FP-biotin is bound to tyrosine (Schopfer et al., 2005), because the 591 amu fragment of FP-biotin is released from serine but not from tyrosine. The masses at 708.8 and 691.6 amu are commonly seen in the fragmentation spectrum of FP-biotinylated tyrosine and are consistent with the immonium ion of FP-biotinylated tyrosine and its deamino counterpart (unpublished observations). The y-ion series (y2–y6) is consistent with FP-biotin attached to the C-terminal peptide, TyrLys (YK). Of these two residues, tyrosine is the most likely candidate for carrying the label. Had the lysine been labeled, trypsin would not have recognized that lysine as a cleavage site. The b-ion series (b1–b5) supports the identification of the peptide.
FIG. 4.
MS/MS spectra of OP labeled Tyr 238 in peptide KPVDEYK of human transferrin. Mass spectra in (A-D) were acquired on the QTRAP 4000 mass spectrometer by infusion, in panel E on the QTRAP 2000 by LC/MS/MS, and in panel F on the MALDI-TOF-TOF mass spectrometer. The b and y ion masses in all panels are consistent with OP covalently bound to Tyr 238. (A) The doubly charged parent ion of the FP-biotin–labeled peptide is at 726.0 m/z. Masses enclosed in boxes at 227.3, 312.5, and 329.5 amu are fragments of FP-biotin. The immonium ion of FP-biotinylated tyrosine is at 708.8 amu. Its partner ion at 691.6 amu has lost 17 amu. (B) The doubly charged parent ion of the DFP-labeled peptide is at 522.0 m/z. Loss of one or both isopropyl groups during collision-induced dissociation yields y-ions minus 42 or minus 84 amu, confirming the presence of diisopropylphosphate. Loss of both isopropyl groups is the most common observation (Grigoryan et al., 2008; Li et al., 2007). The y-ion series (y1 and y2–84 through y6-84) indicates that tyrosine is labeled. The delta mass (242.9 amu) between y1 (147.2 amu) and y2–84 (390.1 amu) is consistent with the appearance of tyrosine phosphate at fragment y2 (163 amu for tyrosine and 80 amu for phosphate). The mass at 373.2 amu is the y2 ion minus 101 amu, representing loss of two isopropyl groups as well as ammonia. The mass at 501.8 m/z is the doubly charged parent ion minus one isopropyl group. The mass at 216.1 amu is consistent with the phosphotyrosine immonium ion. (C) The doubly charged parent ion of the chlorpyrifos oxon-labeled peptide is at 508.7 m/z. The y-ion series (y1–y6) shows the presence of all residues. The mass difference (299.0 amu) between y1 (147.2 amu) and y2 (446.2 amu) clearly shows the diethoxyphosphate on tyrosine in fragment y2 (163 amu for tyrosine and 136 amu for diethoxyphosphate). The mass at 244.1 amu is consistent with the monoethoxyphosphotyrosine immonium ion. The mass at 272.0 amu is consistent with the diethoxyphosphotyrosine immonium ion. (D) The doubly charged parent ion of the dichlorvos-labeled peptide is at 494.0 m/z. The y-ion series (y1–y6) shows the presence of all residues. The mass difference (270.9 amu) between y1 (147.2 amu) and y2 (418.1 amu) clearly shows the dimethoxyphosphate on tyrosine in fragment y2 (163 amu for tyrosine and 108 amu for dimethoxyphosphate). The mass at 244.0 amu is consistent with dimethoxyphosphotyrosine immonium ion. (E) The parent ion of the soman-labeled peptide has a mass to charge ratio of 520.7, but this mass does not appear in the scan. The prominent peak at 479.5 is the doubly charged parent ion that has lost the pinacolyl group from soman. The y2–y7 ions have all lost 84 amu due to release of pinacolyl from soman. The peak at 214.4 is the methylphosphotyrosine immonium ion. (F) The parent ion of the sarin-labeled peptide is the singly charged ion at 998.5 amu. The masses of the y-ion series, and the 214.1 mass for methylphosphotyrosine immonium, support labeling on tyrosine.
Peptide LysProValAspGluTyr*Lys (KPVDEY*K) of human transferrin also covalently bound DFP, chorpyrifos oxon, dichlorvos, soman, and sarin on Tyr 238 as shown in Figures 4B–F. The isopropyl group of DFP and the pinacolyl group of soman were released by collision-induced dissociation in the QTRAP mass spectrometer (Figs. 4B and 4E). However, the isopropyl group was not released from sarin by matrix-assisted laser desorption ionization (Fig. 4F). The methylphosphotyrosine immonium ion at 214 amu is characteristic for soman and sarin adducts on tyrosine (Figs. 4E and 4F).
The spectrum in Figure 5A shows that FP-biotin is covalently attached to tyrosine in peptide LysProValGluGluTyr*AlaAsnCysHisLeuAlaArg (KPVEEY*ANCHLAR). The parent ion mass (541.4 m/z, quadruply charged) is consistent with the mass of the peptide plus the added mass of FP-biotin. The characteristic fragments at 227.3, 312.6, 329.6, 708.7, and 691.6 amu along with the absence of a fragment at 591 amu are indicative of FP-biotin bound to a tyrosine in this peptide. Minor fragments at 1248.6, 1378.0, and 1507.0 amu are consistent with FP-biotinylated peptides y8–y10 that have lost 328 amu during collision-induced dissociation in the mass spectrometer. Three hundred and twenty-eight amu is the neutral counterpart of the 329 amu characteristic fragment from FP-biotin. The y-ion series (y1–y7) and the b-ion series (b1–b5) account for all the residues in the peptide except for the tyrosine which appears to carry the FP-biotin label.
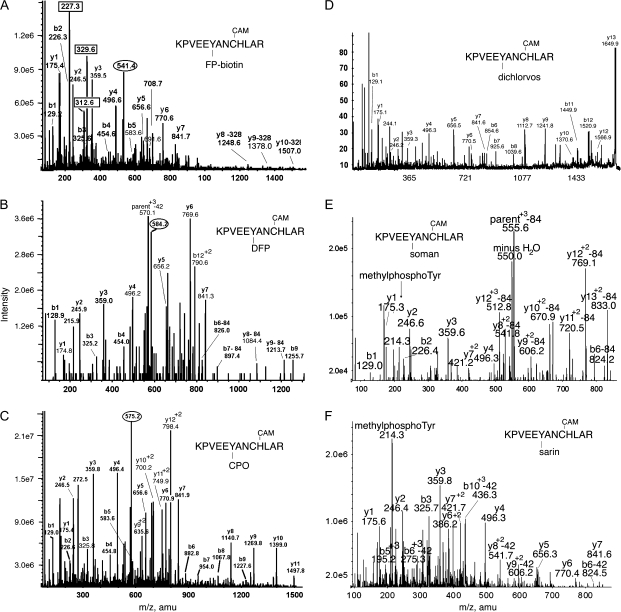
FIG. 5.
MS/MS spectra of OP labeled Tyr 574 in peptide KPVEEYANCHLAR of human transferrin. Mass spectra in (A), (B), and (C) were acquired on the QTRAP 4000 mass spectrometer by infusion, in (D) on the MALDI-TOF-TOF, in panels E and F by LC/MS/MS on the QTRAP 2000 mass spectrometer. The b and y ion masses in all panels are consistent with OP covalently bound to Tyr 574. Cysteine has been CAM, which adds a mass of 57 amu. (A) The quadruply charged parent ion of the FP-biotin–labeled peptide is at 541.4 m/z. Masses enclosed in boxes at 227.3, 312.6, and 329.6 amu, are fragments of FP-biotin. The immonium ion of FP-biotinylated tyrosine is at 708.7 amu. Its partner ion at 691.6 amu has lost 17 amu. Three y-ions have lost 328 amu from FP-biotin. (B) The triply charged parent ion of the DFP-labeled peptide is at 584.2 m/z. Loss of one or both isopropyl groups yields ions minus 42 or minus 84 amu. The y-ion series (y1–y7) supports the identification of the peptide and indicates that the OP label is not in that portion of the peptide. The delta mass (243.2 amu) between y7 (841.3 amu) and y8-84 (1084.4 amu) fits with the appearance of tyrosine-phosphate in fragment y8 (163 amu for tyrosine and 80 amu for phosphate). The presence of tyrosine-phosphate is expected for tyrosine-diisopropylphosphate that has lost both isopropyl groups. Masses at 1213.7 (y9-84), 826.0 (b6-84) and 897.4 amu (b7-84) support this assignment. The mass at 215.9 amu is consistent with the phosphotyrosine immonium ion. (C) The triply charged parent ion of the chlorpyrifos oxon-labeled peptide is at 575.2 m/z. The y-ion series (y1–y11) shows a delta mass (299.4 amu) between y7 (841.3 amu) and y8 (1140.7 amu) that is consistent with the appearance of tyrosine-diethoxyphosphate at fragment y8 (163 amu for tyrosine and 136 amu for diethoxyphosphate). This is supported by the b-ion series (b1–b9) which shows the appearance of the same tyrosine as tyrosine-diethoxyphosphate at fragment b6. The mass at 272.5 amu is consistent with the diethoxyphosphotyrosine immonium ion. (D) The singly charged parent ion of the dichlorvos-labeled peptide is at 1694.9 m/z. This is a MALDI-TOF-TOF spectrum, where parent ions are typically singly charged. The y-ion series (y1–y10) shows a delta mass (271.1 amu) between y7 (841.6 amu) and y8 (1112.7 amu) which is consistent with the appearance of tyrosine-dimethoxyphosphate at fragment y8 (163 amu for tyrosine and 108 amu for dimethoxyphosphate). The mass at 244.1 amu is consistent with dimethoxyphosphotyrosine immonium ion. (E) The triply charged parent ion of the soman-labeled peptide has a mass of 583.9, but this mass does not appear in the spectrum. The mass at 555.5 is the triply charged parent ion that has lost 84 amu from the pinacolyl group of soman. The doubly charged y8–y13 ions have all lost 84 amu from soman; the y8–y13 masses support labeling on tyrosine. The methylphosphotyrosine immonium ion is present at 214.3 amu. (F) The quadruply charged parent ion for sarin-labeled peptide has a mass of 427.7, but this mass does not appear in the spectrum. The most prominent peak is the methylphosphotyrosine immonium ion at 214.3 amu, supporting labeling of tyrosine by sarin. The doubly charged y8 and y9 ions, and the triply charged b5 and b6 ions also support labeling on tyrosine. These ions have lost 42 amu due to loss of the isopropyl group from sarin.
Peptide LysProValGluGluTyr*AlaAsnCysHisLeuAlaArg (KPVEEY*ANCHLAR) of human transferrin also covalently bound DFP, chlorpyrifos oxon, dichlorvos, soman and sarin on Tyr 574, as shown in Figures 5B–F. The methylphosphotyrosine immonium ion at 214 amu is characteristic of soman and sarin-labeled tyrosine (Figs. 5E and 5F). Collision-induced fragmentation in the QTRAP mass spectrometer resulted in loss of the pinacolyl group from soman, and the isopropyl group from sarin (Figs. 5E and 5F), indicated by loss of 84 and 42 amu. Analyses of the spectra support the labeling of tyrosine. Details are given in the figure legends.
Mouse Transferrin Labeled on Tyr 238, Tyr 319, Tyr 429, Tyr 491, Tyr 518.
Five tyrosines in mouse transferrin were labeled by FP-biotin and chlorpyrifos oxon (Table 1). Two tyrosines were labeled by DFP, three by sarin, and two by soman. No residues were labeled by dichlorvos. Both holo- and apo- mouse transferrin bound FP-biotin (data not shown).
TABLE 1.
| OP | Human-labeled peptide | Mouse-labeled peptide | OP-Tyr |
| FP-biotin | KPVDEY*K | KPVDQY*EDCYLAR | Y238 |
| FP-biotin | LYLGHNY*VTAIR | Y319 | |
| FP-biotin | GY*YAVAVVK | Y429 | |
| FP-biotin | FDEFFSQGCAPGY*EK | Y491 | |
| FP-biotin | EEYNGY*TGAFR | Y518 | |
| FP-biotin | KPVEEY*ANCHLAR | Y574 | |
| DFP | KPVDEY*K | KPVDQY*EDCYLAR | Y238 |
| DFP | GY*YAVAVVK | Y429 | |
| DFP | KPVEEY*ANCHLAR | Y574 | |
| CPO | KPVDEY*K | KPVDQY*EDCYLAR | Y238 |
| CPO | LYLGHNY*VTAIR | Y319 | |
| CPO | GY*YAVAVVK | Y429 | |
| CPO | FDEFFSQGCAPGY*EK | Y491 | |
| CPO | EEYNGY*TGAFR | Y518 | |
| CPO | KPVEEY*ANCHLAR | Y574 | |
| Dichlorvos | KPVDEY*K | Y238 | |
| Dichlorvos | KPVEEY*ANCHLAR | Y574 | |
| Sarin | KPVDEY*K | KPVDQY*EDCYLAR | Y238 |
| Sarin | GY*YAVAVVK | Y429 | |
| Sarin | GYY*AVAVVK | Y430 | |
| Sarin | KPVEEY*ANCHLAR | Y574 | |
| Soman | KPVDEY*K | KPVDQY*EDCYLAR | Y238 |
| Soman | GYY*AVAVVK | Y430 | |
| Soman | KPVEEY*ANCHLAR | Y574 |
Note. Single letter codes for amino acids are A, ala; C, cys; D, asp; E, glu; F, phe; G, gly; H, his; I, ile, K, lys; L, leu; M, met; N, asn; P, pro; Q, gln; R, arg; S, ser; T, thr; V, val; W, trp; Y, tyr. The labeled tyrosine is designated by an asterisk Y*. The table shows the amino acid sequences of the tryptic peptides containing OP-labeled tyrosine. The location of the labeled tyrosine in the transferrin protein is given in the last column. Tyrosine 238 of transferrin was labeled in both species. Tyr 319, 429, 430, 491, and 518 was labeled only in mouse transferrin. Tyr 574 was labeled only in human transferrin. Transferrin 1 mg/ml in pH 8.5 buffer was treated in vitro with 0.5mM OP at 37°C for 16 h.
No Aging
Aging of OP-modified acetylcholinesterase and butyrylcholinesterase involves the loss of an alkyl group from the OP. For example, soman-inhibited acetylcholinesterase loses the pinacolyl group during aging so that the added mass for aged soman is 72 rather than 162 amu. The LC/MS/MS data sets for soman and CPO-labeled transferrin peptides were manually searched for parent ions with an added mass of 72 for aged soman, or an added mass of 108 for aged CPO, as well as for parent ions with an added mass of 162 for nonaged soman and 136 for nonaged CPO. No masses for aged OP adducts were found, though the data sets did contain masses for nonaged adducts. In conclusion, no evidence for aging of the OP-tyrosine adducts of transferrin was found.
Motif for OP Binding to Tyrosine
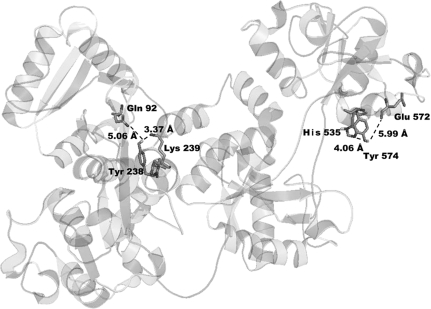
Comparison of the sequences of the transferrin peptides that bind OP shows no definite consensus sequence around the tyrosine to which the OP binds. What the peptides do have in common is the presence of a positively charged Arginine, Lysine, or Histidine within one to five amino acids from the labeled tyrosine, or within a certain distance in the tertiary structure. Figure 6 shows a distance of 3.37 Å between Tyr 238 and Lys 239, and a distance of 4.06 Å between Tyr 574 and His 535 in the tertiary structure of apo-transferrin. Nearby positively charged residues probably interact with the phenolic hydroxyl group of tyrosine to lower the pKa. Tyrosines with a lower pKa value would be better nucleophiles and thus be better able to attack OP.
FIG. 6.
OP-labeled Tyr 238 and Tyr 574 in the crystal structure of human apo-transferrin (PDB code 2hav). The phenolic hydroxyl of Tyr 238 is 3.37 Å from the amine of Lys 239 and 5.06 Å from Gln 92. The phenolic hydroxyl of Tyr 574 is 4.06 Å from the imidazole nitrogen of His 535 and 5.99 Å from oxygen in the carbonyl of Glu 572 (Wally et al., 2006).
Peptide ArgTyrThrArg Covalently Binds OP
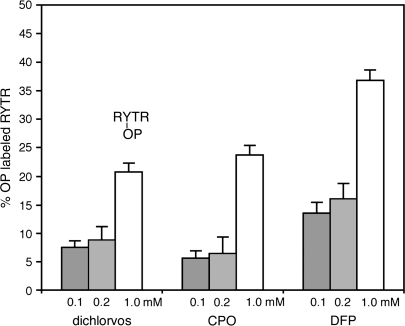
The hypothesis was tested that a positively charged amino acid located near a tyrosine could activate tyrosine, enabling it to covalently bind OP. Incubation of peptide ArgTyrThrArg with 0.1, 0.2, or 1mM CPO, dichlorvos, or DFP in pH 8.3 buffer at 37°C for 16 h resulted in covalent labeling of tyrosine (Fig. 7). Thirty-seven percent of a 170μM solution of ArgTyrThrArg was labeled by 1mM DFP, 24% by 1mM CPO, and 21% by 1mM dichlorvos. MS/MS spectra showed that the OP-labeled residue in peptide ArgTyrThrArg was tyrosine. Peptide SerTyrSerMet was also labeled by 1mM DFP, CPO, and dichlorvos, but no labeling was detected with 0.1 and 0.2mM OP. MS/MS spectra showed that the OP-labeled residue in SerTyrSerMet was tyrosine. Peptide SerTyrSerMet ionized poorly in the MALDI-TOF, giving a weak signal with large standard deviation. It was concluded that tyrosines in general could be labeled by OP, but that the most reactive tyrosines were located near a positively charged arginine or lysine.
FIG. 7.
Labeling of peptide ArgTyrThrArg by dichlorvos, chlorpyrifos oxon, and DFP. The reaction of 170μM peptide with 0.1, 0.2, or 1mM OP was in ammonium bicarbonate buffer pH 8.3, at 37°C for 16 h. MALDI-TOF cluster areas were used to calculate % labeling. MS/MS spectra identified the labeled residue as tyrosine.
The higher reactivity of DFP compared with other OP has been explained by formation of a hydrogen-bonded intermediate between fluoride and the hydroxyl group of tyrosine (Ashbolt and Rydon, 1957).
Function of Transferrin is not Disrupted by OP Binding
It was unknown whether covalent binding of an OP to transferrin affected the ability of transferrin to bind ferric ions. Tyrosines 95, 188, 426, and 517 are involved in the binding of ferric ions in human transferrin (Sargent et al., 2005).
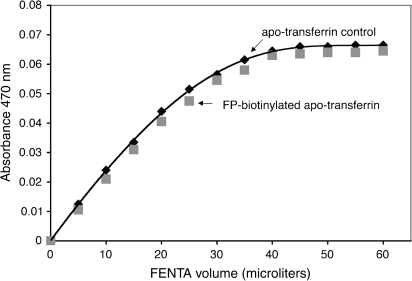
Human apo-transferrin, labeled with FP-biotin on Tyr 238 and Tyr 574, as well as control apo-transferrin were titrated with ferric nitriloacetate to determine binding stoichiometry. Figure 8 shows that both transferrin preparations bound approximately 2 mol of ferric ion per mole of transferrin, a result in agreement with the known number of ferric ion binding sites in transferrin (Bates and Schlabach, 1973). It was concluded that modification of human transferrin by FP-biotin did not interfere with binding of ferric ion.
FIG. 8.
FP-biotinylation of Tyr 238 and Tyr 574 in human transferrin does not interfere with ferric ion binding. One milliliter of 12.1μM apo-transferrin control (♦) titrated with FENTA had an endpoint of 27.3μM FENTA, which calculates to 2.26 mol of ferric ion bound per mole of transferrin. One milliliter of 14.67 μM FP-biotinylated apo-transferrin (▪) had an endpoint of 31.3μM FENTA, which calculates to 2.11 mol of ferric ion bound per mole of transferrin. Assays for each type of transferrin were performed in duplicate.
DISCUSSION
Serine Hydrolases are not the only OP-Binding Proteins in Human Plasma
Our expectation when we started this project was that OP-labeled proteins would all be serine esterases and serine proteases. We expected butyrylcholinesterase to be the dominant OP-binding protein in human plasma (Fidder et al., 2002; Van Der Schans et al., 2004). Our results show that this expectation was not met. OP bind not only to serine esterases and serine proteases, but also to proteins that have an activated tyrosine. Using mass spectrometry, we have conclusively demonstrated OP binding to 18 tyrosines in five proteins: human albumin (Ding et al., 2008; Li et al., 2007), alpha- and beta-tubulin (Grigoryan et al., 2008), human transferrin and mouse transferrin. In addition we have shown that human alpha-1-antitrypsin, human complement C3, human alpha-2-macroglobulin covalently bind FP-biotin, though the site of attachment is unknown. Tyrosine in small synthetic peptides was also the site of attachment of OP.
New OP-Binding Motif
The OP-binding motif in the serine hydrolase family is GlyXSerXGly where OP is covalently bound to serine. Though OP binding to Tyr 411 of human albumin has been recognized (Li et al. 2007, 2008; Williams et al., 2007), OP binding to tyrosine as a general phenomenon has not been appreciated. Our results show that OP binding to tyrosine is not limited to Tyr 411 of albumin, but is found on many tyrosines in many proteins. Even the small peptide ArgTyrThrArg made a covalent bond with OP. Our analysis of OP-labeled tyrosine peptides reveals no consensus amino acid sequence around the labeled tyrosine. The chief requirement for OP binding to tyrosine appears to be a nearby positively charged arginine or lysine. The positively charged groups stabilize the phenolate anion of tyrosine, thus lowering the pKa of tyrosine. The unusually low pKa enables the negatively charged phenolic anion to react with OP at physiological pH values.
It is anticipated that many more proteins than those we have already identified can be modified by OP. Proteins with no active site serine have been implicated in OP-induced neurodevelopmental, behavioral, and immunological effects. These proteins include neurotransmitter receptors (Aldridge et al., 2003; Bomser and Casida, 2001; Katz et al., 1997; Lein and Fryer, 2005; Pope, 1999; Quistad et al., 2002; Smulders et al., 2004), proteins in the adenylyl cyclase signaling cascade (Song et al., 1997), cyclic AMP response element binding protein (Schuh et al., 2002), immune function proteins (Kassa et al., 2003), and kinesin in the axonal transport system (Gearhart et al., 2007; Prendergast et al., 2007; Terry et al., 2007). Whether OP bind to tyrosine in these proteins is unknown.
Biomarkers of OP Exposure
Our in vitro conditions used high concentrations of OP that would not be found during in vivo poisoning. It is unknown whether live animals treated with a nonlethal dose of OP would have OP-labeled transferrin. However, it is known that mice treated with a nontoxic dose of FP-biotin (1 and 5 mg/kg ip) (Peeples et al., 2005) and guinea pigs treated with sarin, soman, cyclosarin, and tabun have OP-labeled albumin in their blood (Williams et al., 2007). The guinea pigs received 0.5–5 LD50 doses of nerve agent, but were alive up to 7 days later because they had been protected with pyridostigmine, oximes, atropine, and midazolam. Tyrosine-modified proteins could serve as biomarkers for OP exposure.
No Aging
The serine hydrolases, acetylcholinesterase, butyrylcholinesterase, and acylpeptide hydrolase have been shown to serve as biomarkers of OP exposure in mice and rats (Quistad et al., 2005; Richards et al., 2000). OP-labeled serine hydrolases lose part of the OP in a process called aging, making it impossible to distinguish between exposure to sarin and soman. In contrast, OP-labeled tyrosines do not undergo aging, so that labeling by sarin is clearly distinguished from labeling by soman.
Significance
Our findings may have application to diagnosis and treatment of OP exposure. Proteins that have no active site serine may serve as biomarkers of exposure. In the future it may be possible to develop antibodies to the new OP-labeled biomarkers to use for screening OP exposure. Synthetic peptides containing arginine, lysine, and tyrosine may find application as scavengers to clear the body of OP. The recognition of a new OP-binding motif to tyrosine suggests new directions to search for mechanisms of long-term effects of OP exposure.
FUNDING
U.S. Army Medical Research and Materiel Command (W81XWH-07-2-0034 to OL, W81XWH-06-1-0102 to S.H.H.); National Institutes of Health (U01 NS058056-02 to OL, P30CA36727 to Eppley Cancer Center, ES016102) to C.M.T.; and the Direction Générale de l'Armement of the French Ministry of Defense (DGA grant 03co010-05/PEA01 08 7) to P.M.
Acknowledgments
Mass spectra were obtained with the support of the Mass Spectrometry and Proteomics core facility at the University of Nebraska Medical Center.
References
- Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: Effects during different critical periods. Environ. Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt RF, Rydon HN. The action of diisopropyl phosphorofluoridate and other anticholinesterases on amino acids. Biochem. J. 1957;66:237–242. doi: 10.1042/bj0660237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GW, Schlabach MR. The reaction of ferric salts with transferrin. J. Biol. Chem. 1973;248:3228–3232. [PubMed] [Google Scholar]
- Bomser JA, Casida JE. Diethylphosphorylation of rat cardiac M2 muscarinic receptor by chlorpyrifos oxon in vitro. Toxicol. Lett. 2001;119:21–26. doi: 10.1016/s0378-4274(00)00294-0. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: Safety aspects of nonacetylcholinesterase secondary targets. Chem. Res. Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Chaiken IM, Smith EL. Reaction of a specific tyrosine residue of papain with diisopropylfluorophosphate. J. Biol. Chem. 1969;244:4247–4250. [PubMed] [Google Scholar]
- Ding SJ, Carr J, Carlson JE, Tong L, Xue W, Li Y, Schopfer LM, Li B, Nachon F, Asojo O, et al. Five tyrosines and two serines in human albumin are labeled by the organophosphorus agent FP-biotin. Chem. Res. Toxicol. 2008;21:1787–1794. doi: 10.1021/tx800144z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidder A, Hulst AG, Noort D, de Ruiter R, van der Schans MJ, Benschop HP, Langenberg JP. Retrospective detection of exposure to organophosphorus anti-cholinesterases: Mass spectrometric analysis of phosphorylated human butyrylcholinesterase. Chem. Res. Toxicol. 2002;15:582–590. doi: 10.1021/tx0101806. [DOI] [PubMed] [Google Scholar]
- Gearhart DA, Sickles DW, Buccafusco JJ, Prendergast MA, Terry AV., Jr Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol. Appl. Pharmacol. 2007;218:20–29. doi: 10.1016/j.taap.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Grigoryan H, Schopfer LM, Thompson CM, Terry AV, Masson P, Lockridge O. Mass spectrometry identifies covalent binding of soman, sarin, chlorpyrifos oxon, diisopropyl fluorophosphate, and FP-biotin to tyrosines on tubulin: A potential mechanism of long term toxicity by organophosphorus agents. Chem. Biol. Interact. 2008;175:180–186. doi: 10.1016/j.cbi.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa J, Krocova Z, Sevelova L, Sheshko V, Kasalova I, Neubauerova V. Low-level sarin-induced alteration of immune system reaction in inbred BALB/c mice. Toxicology. 2003;187:195–203. doi: 10.1016/s0300-483x(03)00051-9. [DOI] [PubMed] [Google Scholar]
- Katz EJ, Cortes VI, Eldefrawi ME, Eldefrawi AT. Chlorpyrifos, parathion, and their oxons bind to and desensitize a nicotinic acetylcholine receptor: Relevance to their toxicities. Toxicol. Appl. Pharmacol. 1997;146:227–236. doi: 10.1006/taap.1997.8201. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol. Sci. 2005;83:166–176. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- Li B, Nachon F, Froment MT, Verdier L, Debouzy JC, Brasme B, Gillon E, Schopfer LM, Lockridge O, Masson P. Binding and hydrolysis of soman by human serum albumin. Chem. Res. Toxicol. 2008;21:421–431. doi: 10.1021/tx700339m. [DOI] [PubMed] [Google Scholar]
- Li B, Schopfer LM, Hinrichs SH, Masson P, Lockridge O. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal. Biochem. 2007;361:263–272. doi: 10.1016/j.ab.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Sedlacek M, Manoharan I, Boopathy R, Duysen EG, Masson P, Lockridge O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem. Pharmacol. 2005;70:1673–1684. doi: 10.1016/j.bcp.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Lockridge O, Schopfer LM, Winger G, Woods JH. Large scale purification of butyrylcholinesterase from human plasma suitable for injection into monkeys; a potential new therapeutic for protection against cocaine and nerve agent toxicity. J. Med. CBR Def. 2005;3 doi: 10.1901/jaba.2005.3-nihms5095. online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockridge O, Xue W, Gaydess A, Grigoryan H, Ding SJ, Schopfer LM, Hinrichs SH, Masson P. Pseudo-esterase activity of human albumin: Slow turnover on tyrosine 411 and stable acetylation of 82 residues including 59 lysines. J. Biol. Chem. 2008;283:22582–22590. doi: 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray RT, Mendez E, Sinha SK, Sutton MR, Lineback-Zins J, Brew K. The complete amino acid sequence of human serum transferrin. Proc. Natl. Acad. Sci. U. S. A. 1982;79:2504–2508. doi: 10.1073/pnas.79.8.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DM, Brecht KM. Carboxylesterase: specificity and spontaneous reactivation of an endogenous scavenger for organophosphorus compounds. J. Appl. Toxicol. 2001;21(Suppl. 1):S103–S107. doi: 10.1002/jat.833. [DOI] [PubMed] [Google Scholar]
- Murachi T, Inagami T, Yasui M. Evidence for alkylphosphorylation of tyrosyl residues of stem bromelain by diisopropylphosphorofluoridate. Biochemistry. 1965;4:2815–2825. doi: 10.1021/bi00888a036. [DOI] [PubMed] [Google Scholar]
- Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, Lockridge O. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol. Sci. 2005;83:303–312. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Pope CN. Organophosphorus pesticides: Do they all have the same mechanism of toxicity? J. Toxicol. Environ. Health B Crit. Rev. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Self RL, Smith KJ, Ghayoumi L, Mullins MM, Butler TR, Buccafusco JJ, Gearhart DA, Terry AV., Jr Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience. 2007;146:330–339. doi: 10.1016/j.neuroscience.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quistad GB, Klintenberg R, Casida JE. Blood acylpeptide hydrolase activity is a sensitive marker for exposure to some organophosphate toxicants. Toxicol. Sci. 2005;86:291–299. doi: 10.1093/toxsci/kfi195. [DOI] [PubMed] [Google Scholar]
- Quistad GB, Nomura DK, Sparks SE, Segall Y, Casida JE. Cannabinoid CB1 receptor as a target for chlorpyrifos oxon and other organophosphorus pesticides. Toxicol. Lett. 2002;135:89–93. doi: 10.1016/s0378-4274(02)00251-5. [DOI] [PubMed] [Google Scholar]
- Richards PG, Johnson MK, Ray DE. Identification of acylpeptide hydrolase as a sensitive site for reaction with organophosphorus compounds and a potential target for cognitive enhancing drugs. Mol. Pharmacol. 2000;58:577–583. doi: 10.1124/mol.58.3.577. [DOI] [PubMed] [Google Scholar]
- Sargent PJ, Farnaud S, Evans RW. Structure/function overview of proteins involved in iron storage and transport. Curr. Med. Chem. 2005;12:2683–2693. doi: 10.2174/092986705774462969. [DOI] [PubMed] [Google Scholar]
- Schopfer LM, Champion MM, Tamblyn N, Thompson CM, Lockridge O. Characteristic mass spectral fragments of the organophosphorus agent FP-biotin and FP-biotinylated peptides from trypsin and bovine albumin (Tyr410) Anal. Biochem. 2005;345:122–132. doi: 10.1016/j.ab.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Schuh RA, Lein PJ, Beckles RA, Jett DA. Noncholinesterase mechanisms of chlorpyrifos neurotoxicity: Altered phosphorylation of Ca2+/cAMP response element binding protein in cultured neurons. Toxicol. Appl. Pharmacol. 2002;182:176–185. doi: 10.1006/taap.2002.9445. [DOI] [PubMed] [Google Scholar]
- Smulders CJ, Bueters TJ, Vailati S, van Kleef RG, Vijverberg HP. Block of neuronal nicotinic acetylcholine receptors by organophosphate insecticides. Toxicol. Sci. 2004;82:545–554. doi: 10.1093/toxsci/kfh269. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: Targeting the adenylyl cyclase signaling cascade. Toxicol. Appl. Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr., Gearhart DA, Beck WD, Jr, Truan JN, Middlemore ML, Williamson LN, Bartlett MG, Prendergast MA, Sickles DW, Buccafusco JJ. Chronic, intermittent exposure to chlorpyrifos in rats: Protracted effects on axonal transport, neurotrophin receptors, cholinergic markers, and information processing. J. Pharmacol. Exp. Ther. 2007;322:1117–1128. doi: 10.1124/jpet.107.125625. [DOI] [PubMed] [Google Scholar]
- Van Der Schans MJ, Polhuijs M, Van Dijk C, Degenhardt CE, Pleijsier K, Langenberg JP, Benschop HP. Retrospective detection of exposure to nerve agents: Analysis of phosphofluoridates originating from fluoride-induced reactivation of phosphorylated BuChE. Arch. Toxicol. 2004;78:508–524. doi: 10.1007/s00204-004-0568-x. [DOI] [PubMed] [Google Scholar]
- Wally J, Halbrooks PJ, Vonrhein C, Rould MA, Everse SJ, Mason AB, Buchanan SK. The crystal structure of iron-free human serum transferrin provides insight into inter-lobe communication and receptor binding. J. Biol. Chem. 2006;281:24934–24944. doi: 10.1074/jbc.M604592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch S, Skinner A. A comparison of the structure and properties of human, rat and rabbit serum transferrin. Comp. Biochem. Physiol. B. 1989;93:417–424. doi: 10.1016/0305-0491(89)90102-8. [DOI] [PubMed] [Google Scholar]
- Williams NH, Harrison JM, Read RW, Black RM. Phosphorylated tyrosine in albumin as a biomarker of exposure to organophosphorus nerve agents. Arch. Toxicol. 2007;81:627–639. doi: 10.1007/s00204-007-0191-8. [DOI] [PubMed] [Google Scholar]