Abstract
New hippocampal neurons are continuously generated in the adult brain. Here, we demonstrate that lipopolysaccharide-induced inflammation, which gives rise to microglia activation in the area where the new neurons are born, strongly impairs basal hippocampal neurogenesis in rats. The increased neurogenesis triggered by a brain insult is also attenuated if it is associated with microglia activation caused by tissue damage or lipopolysaccharide infusion. The impaired neurogenesis in inflammation is restored by systemic administration of minocycline, which inhibits microglia activation. Our data raise the possibility that suppression of hippocampal neurogenesis by activated microglia contributes to cognitive dysfunction in aging, dementia, epilepsy, and other conditions leading to brain inflammation.
In the adult mammalian brain, neural progenitor cells located in the subgranular zone (SGZ) of the dentate gyrus (DG) generate thousands of new neurons each day (1). These neurons develop the morphological and functional properties of dentate granule cells and become integrated into existing neuronal circuitries (2). The role of neurogenesis for hippocampal function is still unclear, but some experimental evidence suggests its involvement in memory formation (3) and mood regulation (4). Impairment of hippocampal neurogenesis may be linked to the cognitive decline in aging, Alzheimer's disease (AD), and major depression (5-7).
Brain inflammation probably plays an important role in the pathogenesis of chronic neurodegenerative disorders like AD and Parkinson's disease (8, 9). Neurodegeneration caused by inflammation involves activation of the brain's resident immune cells, the microglia, which produce a large number of proinflammatory factors (10-12). Also, acute brain insults, e.g., stroke and status epilepticus (SE), are linked to inflammation (13, 14), which contributes to the propagation of the neuropathological events (9, 15). These insults trigger increased neurogenesis in the SGZ (16-19). After severe SE, there is an 80% loss of newly formed dentate neurons (20), which raises the possibility that the associated inflammatory response is deleterious for hippocampal neurogenesis.
Here, we show that the microglia activation associated with inflammation impairs both basal and insult-induced hippocampal neurogenesis. We find that systemic administration of the tetracycline derivative minocycline, which specifically inhibits microglia activation, is an effective treatment to restore neurogenesis suppressed by inflammation.
Materials and Methods
Surgery and Induction of SE. Male Sprague-Dawley rats were implanted with a stimulating/recording electrode into the right ventral hippocampus [coordinates: 4.8 mm caudal and 5.2 mm lateral to bregma, 6.3 mm ventral to dura, and toothbar at -3.3 mm (21)] under pentobarbital or halothane anesthesia. In 37 animals, a brain infusion cannula (Alzet, Palo Alto, CA) was also placed intracortically on the right side of the brain (2 mm caudal and 1.2 mm lateral to bregma and 2.6 mm ventral to dura). Twenty-five rats were implanted only with the infusion cannula. Ten days after surgery, all rats with implanted electrodes, except nonstimulated controls, were subjected to electrically induced SE with 60-90 min of suprathreshold stimulation followed by 2 h of self-sustained continuous ictal electroencephalographic (EEG) activity (22). Seizures were interrupted with i.p. injections of pentobarbital. Forty SE animals were subjected only to SE and perfused 2, 6-8 (pooled), or 35 days later.
BrdUrd Labeling. Six days after SE, rats were given four injections (every 2 h during a 6-h period) of BrdUrd (50 mg/kg, i.p; Sigma). Animals subjected to 4 weeks of intracortical infusions of lipopolysaccharide (LPS) or vehicle were injected with BrdUrd twice daily for 1 week, starting 6 days after the initiation of infusions. Rats receiving only 6 days of intracortical infusions were given four injections of BrdUrd (every 2 h during a 6-h period) at day 6 and perfused 2 h thereafter.
LPS and Minocycline Treatment. A miniosmotic pump (28-day pump, 0.25 μl/h; Alzet) was connected to the brain infusion cannula. The pump contained either LPS from Escherichia coli (serotype 055:B5; 20 μg/μl artificial cerebrospinal fluid; Sigma) or vehicle. Infusions were continued for 6 or 28 days.
Minocycline (Sigma) dissolved in potassium PBS (KPBS), pH 7-7.4, or vehicle was injected i.p. during 6 days or 35 days after SE or in nonstimulated controls. Animals received minocycline (50 mg/kg) twice daily for the first 2 days and once daily for the next 5 days (4 days for the short-term survival group), followed by 25 mg/kg once daily (23).
Immunohistochemistry. Rats were transcardially perfused with saline followed by 4% paraformaldehyde. Brains were postfixed overnight in the same medium and placed in 20% sucrose for 24 h, before sectioning (30 μm). For BrdUrd/NeuN, BrdUrd/doublecortin, and BrdUrd/ED1 double-label immunofluorescence, the sections were pretreated with 1 M HCl for 30 min at +67°C and then preincubated with appropriate serum, followed by incubation with primary antibodies overnight at +4°C. Sections were incubated for 2 h in the dark with secondary antibodies, followed by 2 h with Streptavidin Alexa Fluor 488 (1:200, Molecular Probes). The following antibodies were used: rat anti-BrdUrd (1:100, Oxford Biotechnology, Oxfordshire, U.K.), mouse anti-NeuN (1:100, Chemicon), mouse anti-ED1 (1:200, Serotec), goat anti-doublecortin (1:400, SC-8066, Santa Cruz Biotechnology), Cy3-conjugated donkey anti-rat (1:400, Jackson ImmunoResearch), and biotinylated horse anti-mouse or anti-goat (1:200, Vector Laboratories). Single labeling for NeuN, ED1, and Ki67 (mouse anti-Ki67 antibody, 1:200, NovoCastra, Newcastle, U.K.) were performed with biotinylated horse anti-mouse antibody and visualized with avidin-biotin-peroxidase complex, followed by diaminobenzidine reaction. For the Fluoro-Jade staining, the sections were rehydrated, pretreated in 0.06% potassium permanganate for 15 min, rinsed in distilled water, incubated in 0.001% Fluoro-Jade working solution (Histo-Chem, Jefferson, AR) for 30 min, rinsed in distilled water, immersed in xylene, and cover-slipped.
Microscopical Analysis. By using light microscopy, all single-labeled BrdUrd-, ED1-, and Ki67-positive cells were counted in the granule cell layer (GCL) and within two cell diameters below this region in the SGZ. Single-labeled NeuN-positive cells were counted in the dentate hilus, outlined by the GCL and an imaginary border between the lateral endings of the GCL. The number of labeled cells was calculated in 3-6 coronal sections from each rat, located between 3.3 mm and 4.3 mm posterior to bregma (encompassing dorsal hippocampus), and expressed as the mean number of cells per section. The numbers of BrdUrd/doublecortin and BrdUrd/NeuN double-labeled cells were quantified by using Olympus AX-70 epifluorescent and confocal scanning (Kr/Ar 488 nm and 568 nm excitation filter; Bio-Rad MRC1024UV) microscopes, respectively. Fifty BrdUrd-positive cells were analyzed from each rat, and the percentage was used to calculate the total number of BrdUrd/NeuN-positive cells per section.
Statistical Analysis. Values are means ± SEM. Comparisons were performed by using one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test. Differences were considered significant at P < 0.05. A correlation z test was used to assess the relation between number of ED1-positive cells and BrdUrd/NeuN double-labeled cells.
Results
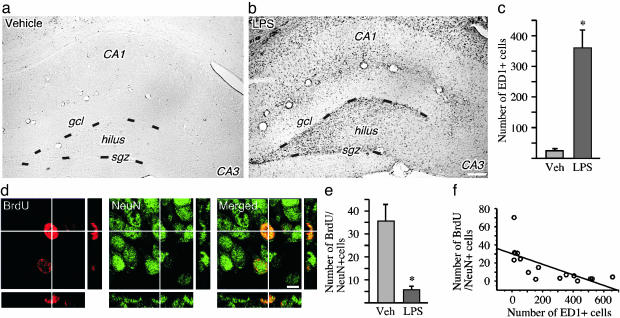
Inflammation Impairs Basal Neurogenesis. We first explored whether inflammation influences the formation of new neurons in the SGZ in the intact brain. LPS is a potent activator of the inflammatory response, particularly of microglia. It acts via the CD14 receptor to trigger a kinase cascade in microglia, resulting in cytokine gene transcription (10). After 28 days of intracortical infusion of LPS, we observed a substantial number of ED1-immunopositive, activated microglia in the dentate SGZ/GCL and hilus whereas very few such cells were detected in control brains (Fig. 1 a-c). To label new cells, we used BrdUrd, a thymidine analogue that is incorporated into DNA during its synthesis, i.e., cell division, DNA replication, and to less extent DNA repair (24). Several lines of evidence strongly indicate that BrdUrd-labeled cells in the SGZ/GCL, both in the intact brain and after insults such as cerebral ischemia and SE, are not mature neurons undergoing DNA repair but cluster-forming, dividing cells that subsequently pass through distinct neuronal differentiation steps (16, 25-27).
Fig. 1.
Inflammation impairs basal hippocampal neurogenesis. (a-c) Immunohistochemical staining for ED1-positive activated microglia in the DG after 28 days of intracortical vehicle (a) or LPS (b) infusions, and number of activated microglia in the SGZ/GCL in the same treatment groups (c). (d) A newly formed BrdUrd/NeuN double-labeled neuron in the SGZ is visualized by using confocal microscopy in an orthogonal projection composed of 19 optical z-planes (0.5 μm thick). (e) The number of BrdUrd/NeuN double-labeled new neurons in the SGZ/GCL after 28 days of LPS or vehicle infusion (14 days after the last BrdUrd injection). (f) Correlation between number of ED1-positive and BrdUrd/NeuN double-labeled cells. Data are the number of cells per section. n = 6 and 10 for vehicle- and LPS-treated rats, respectively. Shown are means ± SEM. *, P < 0.05. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 20 μm in b and 7.2 μm in d.)
The LPS-induced inflammation caused an 85% reduction in the number of new neurons, i.e., BrdUrd-immunoreactive cells double-labeled for the neuron-specific marker NeuN, in the SGZ/GCL (Fig. 1 d and e). There was a significant negative correlation between the number of new neurons and the number of activated microglia (correlation coefficient = -0.7; P = 0.0019; Fig. 1f). We observed no loss of mature hilar neurons (compare ref. 28), indicating a specific detrimental effect of inflammation on the newly formed neurons.
We then explored whether the LPS-induced inflammation suppressed basal hippocampal neurogenesis by inhibiting cell proliferation. Six days of intracortical LPS infusion followed by BrdUrd injections and perfusion 2 h thereafter did not significantly alter the number of BrdUrd-positive cells (LPS: 48 ± 20.2 vs. vehicle: 26.8 ± 5.3) or of cells double-labeled with BrdUrd and doublecortin (LPS: 6.9 ± 1.8 vs. vehicle: 10.6 ± 2.5), a marker for immature neuroblasts, in the SGZ/GCL. Also, the number of newly proliferated Ki67-positive cells did not differ between LPS- and vehicle-treated animals (data not shown).
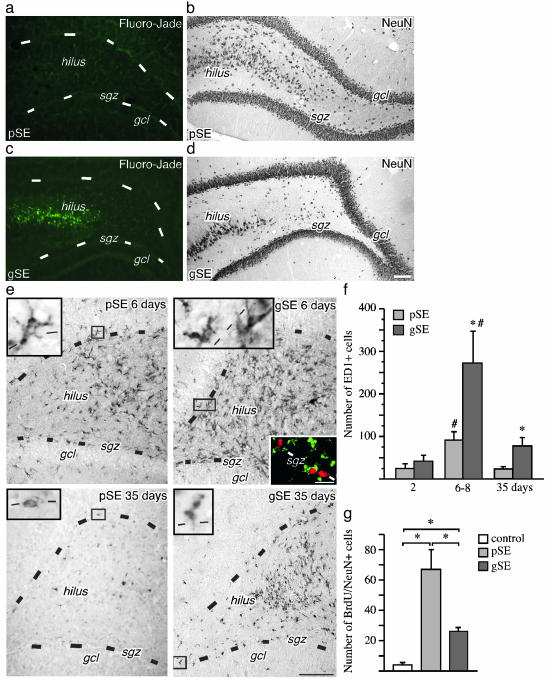
Inflammation Attenuates Increased Neurogenesis After Brain Insult. We then assessed whether the magnitude of neurogenesis in the SGZ after a brain insult is affected by the degree of concomitant inflammation in the tissue environment. We induced SE of two different severities, partial and generalized, respectively, by applying electrical stimulation to the hippocampus (22). Generalized SE caused more damage in the DG hilus compared with partial SE, as shown both with Fluoro-Jade staining (Fig. 2 a and c) and counts of NeuN-positive cells 35 days after the insult (Fig. 2 b and d; cells per section: generalized SE, 385 ± 49.5; partial SE, 578.1 ± 39.8). Animals with generalized SE also had significantly higher numbers of activated microglia in the SGZ/ GCL and dentate hilus (Fig. 2f). Six to eight days after the insult, many of the ED1-positive cells had swollen cell bodies and processes and were often found in close proximity to clusters of newly formed BrdUrd-labeled cells (Fig. 2e). Over the subsequent 28 days, the number of ED1-positive cells markedly declined but was still higher in generalized as compared with partial SE animals (Fig. 2f). The ED1-positive cells also became smaller with fewer processes (Fig. 2e).
Fig. 2.
Hippocampal neurogenesis is impaired after brain insult associated with tissue damage and inflammation. (a-d) Neurodegeneration as visualized with Fluoro-Jade staining (a and c) and NeuN immunoreactivity (b and d) in the DG 35 days after SE. Note more damage and fewer remaining neurons in the dentate hilus after generalized SE (gSE) as compared with partial SE (pSE). (e) The distribution of ED1-positive, activated microglia in the DG 6 and 35 days after partial or generalized SE. (Left Inset) Shown are higher magnification of activated microglia. (Right Inset) Shown are ED1-positive (green) cells surrounding BrdUrd-positive (red) cells in the SGZ. (f) The number of ED1-positive microglia in the SGZ/GCL 2, 6-8, and 35 days after SE. (g) The number of BrdUrd/NeuN double-labeled new neurons in the SGZ/GCL 35 days after SE (28 days after BrdUrd injections). Data are number of cells per section. Shown are means ± SEM. *, P < 0.05 (compared with pSE in f). #, P < 0.05 compared with 35 days. In f, n = 6(3 + 3), 21 (14 + 7), and 13 (8 + 5) for the 2-day, 6- to 8-day, and 35-day groups, respectively, with individual numbers for pSE and gSE in parentheses. In g, n = 5, 8, and 5 for nonstimulated controls, pSE, and gSE, respectively. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 100 μm in a-e and 10 μm in Right Inset in e.)
Hippocampal neurogenesis clearly differed between partial and generalized SE. At 35 days, there was a 15-fold increase in the number of new neurons in the SGZ/GCL of partial SE animals. Also, rats with generalized SE showed increased neurogenesis, but the number of new neurons was 60% lower as compared with the partial SE group (Fig. 2g). This difference was not due to reduced formation of new neurons because cell proliferation in the SGZ is similar after partial and generalized SE (P. Mohapel, C.T.E., and O.L., unpublished results). These data indicated that the severity either of the hippocampal damage or of the inflammatory response determined the magnitude of neurogenesis after SE. To distinguish between these two mechanisms, LPS was infused intracortically during 28 days in animals that had exhibited partial SE. Such animals normally show only minor damage and inflammation. LPS caused no loss of NeuN-positive cells in the DG hilus after partial SE (cells per section: partial SE plus vehicle, 422.1 ± 37.3; partial SE plus LPS, 446.4 ± 41.2). Also, with LPS treatment, the damage in partial SE animals was significantly less than that in vehicle-treated, generalized SE animals (259.0 ± 76.7 NeuN-positive cells per section).
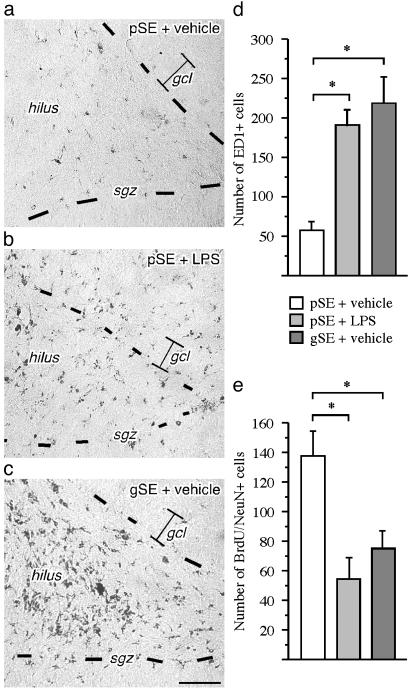
The LPS infusion increased the numbers of ED1-positive cells in the SGZ/GCL (Fig. 3 a-d). Importantly, both the number of ED1-positive cells in the SGZ/GCL and their morphology in these rats were similar to what was observed in the vehicle-treated generalized SE rats (Fig. 3 c and d).
Fig. 3.
Inflammation causes impairment of hippocampal neurogenesis after brain insult not associated with tissue damage. (a-c) Immunohistochemical staining of ED1-positive activated microglia in the SGZ/GCL after intracortical vehicle or LPS infusions during 28 days after partial SE (pSE) or generalized SE (gSE). (d) The number of ED1-positive cells in the DG after SE. Note that the distribution and number of ED1-positive microglia in the SGZ is similar in partial SE plus LPS and generalized SE plus vehicle animals whereas there are very few cells in the partial SE plus vehicle rats. (e) The number of BrdUrd/NeuN-positive new neurons in the SGZ/GCL 28 days after LPS or vehicle treatment after SE (21 days after BrdUrd injections). Data are number of cells per section. Shown are means ± SEM. *, P < 0.05. n = 16, 15, and 5 for pSE plus LPS, pSE plus vehicle, and gSE plus vehicle, respectively. Hilus, dentate hilus. Dotted lines depict SGZ. (Scale bar = 100 μm.)
The LPS-induced inflammation was accompanied by a reduced number of new neurons in the SGZ/GCL (Fig. 3e). There was a significant negative correlation between the numbers of new neurons and activated microglia (correlation coefficient = -0.6, P = 0.0001). Thus, without aggravating the damage, the inflammation caused by LPS infusion in the partial SE animals resulted in a similar low number of new neurons as observed in vehicle-treated, generalized SE animals (Fig. 3e).
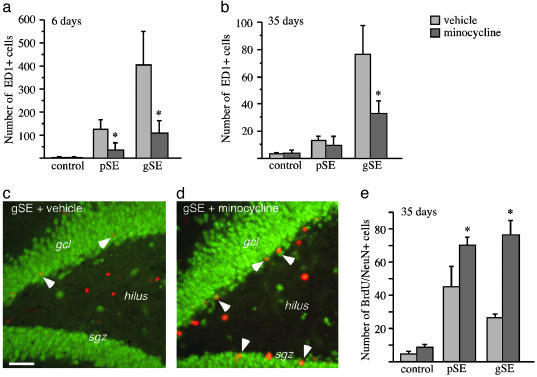
Minocycline Restores Neurogenesis in Inflammation. We finally investigated whether the inflammation-mediated suppression of hippocampal neurogenesis can be prevented by specific inhibition of microglia activation. Minocycline readily passes the blood-brain barrier and suppresses the microglial response in neurodegenerative disease models (23, 29). In both partial and generalized SE animals that received minocycline systemically for 6 days and were perfused immediately thereafter, the number of activated microglia in the SGZ/GCL was dramatically reduced compared with vehicle-treated rats (Fig. 4a). Also, 35 days of minocycline treatment led to a decreased number of ED1-positive cells in the generalized SE animals whereas the low number of ED1-positive cells at this time point after partial SE was unchanged (Fig. 4b).
Fig. 4.
Minocycline prevents inflammation-mediated suppression of hippocampal neurogenesis. (a and b) The number of ED1-positive, activated microglia in the SGZ/GCL in nonstimulated controls, and 6 days (a) and 35 days (b) after generalized SE (gSE) and partial SE (pSE) with or without minocycline treatment. (c and d) The distribution of BrdUrd (red) and NeuN (green) cells in the DG at 35 days after gSE with and without minocycline treatment. Arrowheads depict BrdUrd/NeuN-double-labeled new neurons. (e) The number of new neurons in the SGZ/GCL in nonstimulated controls and 35 days after gSE and pSE (28 days after BrdUrd injections). In a, n = 19 (5 + 9 + 5) and 14 (4 + 7 + 3); in b, n = 13 (4 + 5 + 4) and 14 (5 + 4 + 5); and in e, n = 13 (4 + 5 + 4) and 14 (5 + 4 + 5) for minocycline and vehicle, respectively, with individual numbers for nonstimulated controls, pSE, and gSE in brackets. Data are number of cells per section. Shown are means ± SEM. *, P < 0.05 compared with vehicle-treated animals. Hilus, dentate hilus. Scale bar = 50 μm.
Minocycline treatment gave rise to increased numbers of new neurons in the SGZ/GCL 35 days after SE (Fig. 4 c-e). The effect of minocycline was more pronounced in generalized SE animals, and therefore, similar numbers of new neurons were now found in partial and generalized SE rats. Minocycline did not act through stimulation of SGZ cell proliferation because we observed no increase in the number of newly proliferated, Ki-67-positive cells in the minocycline-treated rats. Also, minocycline did not alter the degree of neuronal differentiation from the proliferating cells because the percentage of BrdUrd-positive cells double-labeled with NeuN was similar in minocycline- and vehicle-treated rats. The degree of epileptic damage to dentate hilar neurons was unaltered after minocycline treatment (NeuN-positive cells per section: partial SE plus vehicle, 505.3 ± 72.7 vs. partial SE plus minocycline, 401.3 ± 39.9; generalized SE plus vehicle, 286.5 ± 56.7 vs. generalized SE plus minocycline 170.5 ± 41.4).
We also found that minocycline did not influence neurogenesis in nonstimulated control animals, which had very few activated microglia in the SGZ/GCL (Fig. 4 a and b). Thus, administration of minocycline did not significantly alter either the number of Ki67-positive cells after 6 days (cells per section: minocycline, 20.4 ± 1.1 vs. vehicle, 26.6 ± 7.8) or the number of BrdUrd/NeuN-double labeled new neurons after 5 weeks of treatment (Fig. 4e).
Discussion
Our data show that brain inflammation causes inhibition both of the basal, continuous formation of new neurons in the intact hippocampal formation and of the increased neurogenesis in response to a brain insult. We provide direct in vivo evidence for a cellular mechanism underlying the detrimental action of inflammation on neurogenesis. Thus, we find that activated microglia are localized in close proximity to the newly formed cells and, also, that the impairment of neurogenesis depends on the degree of microglia activation, irrespective of whether there is damage or not in the surrounding tissue. We demonstrate a significant negative correlation between the number of activated microglia in the neurogenic zone and the number of surviving new hippocampal neurons. Finally, we show that a selective inhibitor of microglia activation, minocycline, restores hippocampal neurogenesis in inflammation without affecting neurogenesis in control animals. The microglia activation specifically compromises the survival of the new hippocampal neurons whereas there is no evidence for suppression of cell proliferation or neuronal differentiation in either basal or insult-induced neurogenesis. The deleterious effect of activated microglia on the newly formed neurons is most likely mediated through the action of cytokines, such as IL-1β or IL-6, tumor necrosis factor α, nitric oxide, and reactive oxygen species (10-12, 30). These molecules can be released from microglia and are neurotoxic in vitro.
Brain inflammation and microglia activation are believed to be involved in many disorders associated with cognitive impairment such as AD, Lewy body dementia, and AIDS dementia (8, 31, 32). Antiinflammatory treatment can protect from onset or progression of AD in patients (33). Cranial irradiation leads to inflammation in the SGZ and progressive cognitive deterioration (34). There is now growing evidence that systemic inflammation, triggered by peripheral infection or injury, can cause activation of already primed microglia in the brains of AD patients, as well as in the normal aging brain, leading to delirium and aggravation of the cognitive decline (32). Our data are consistent with a model in which inflammation-mediated suppression of hippocampal neurogenesis plays a pathophysiological role for the cognitive dysfunction in these conditions.
The high vulnerability to inflammation observed here for newly generated neurons could have implications also for neurogenesis in other brain areas. Generation of dopamine neurons was recently reported to occur in the adult mouse substantia nigra (35). Inflammatory changes in this region, which are common in Parkinson's disease patients (9), could suppress neurogenesis and contribute to disease progression. Also, >80% of the new striatal neurons that are generated from the subventricular zone after stroke in rats die within the first weeks after the insult (36). Ischemic insults are associated with inflammation (37) that, based on the data reported here, is likely to compromise the survival of newly formed neurons. Our findings suggest antiinflammatory treatment as a possible novel strategy to improve the efficacy of neuronal replacement from endogenous precursors in stroke and other neurodegenerative disorders.
Here, we have demonstrated that an inflammatory response in the tissue environment that encounters newborn neurons during neurogenesis in the adult brain is detrimental for their survival. However, microglia activation after brain damage can probably also have beneficial effects by promoting other aspects of regeneration, e.g., through the release of neurotrophic molecules (38, 39). It is conceivable that the relative importance of the harmful and helpful actions of microglia under various circumstances will determine the behavioral consequences of brain inflammation and its inhibition by drugs like minocycline.
Acknowledgments
We thank Monica Lundahl for technical assistance and Dr. Theo Palmer for sharing his unpublished data. This work was supported by grants from the Swedish Research Council; by the Hardebo, Lundbeck, Bergvall, Westling, and Söderberg Foundations; and by the Swedish Society for Medical Research.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SGZ, subgranular zone; DG, dentate gyrus; AD, Alzheimer's disease; SE, status epilepticus; LPS, lipopolysaccharide; GCL, granule cell layer.
References
- 1.Cameron, H. A. & McKay, R. D. (2001) J. Comp. Neurol. 435, 406-417. [DOI] [PubMed] [Google Scholar]
- 2.van Praag, H., Schinder, A. F., Christie, B. R., Toni, N., Palmer, T. D. & Gage, F. H. (2002) Nature 415, 1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T. & Gould, E. (2001) Nature 410, 372-376. [DOI] [PubMed] [Google Scholar]
- 4.Santarelli, L., Saxe, M., Gross, C., Surget, A., Battaglia, F., Dulawa, S., Weisstaub, N., Lee, J., Duman, R., Arancio, O., Belzung, C. & Hen, R. (2003) Science 301, 805-809. [DOI] [PubMed] [Google Scholar]
- 5.Zitnik, G. & Martin, G. M. (2002) J. Neurosci. Res. 70, 258-263. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs, B. L. (2002) Brain Behav. Immun. 16, 602-609. [DOI] [PubMed] [Google Scholar]
- 7.Haughey, N. J., Nath, A., Chan, S. L., Borchard, A. C., Rao, M. S. & Mattson, M. P. (2002) J. Neurochem. 83, 1509-1524. [DOI] [PubMed] [Google Scholar]
- 8.Nelson, P. T., Soma, L. A. & Lavi, E. (2002) Ann. Med. 34, 491-500. [DOI] [PubMed] [Google Scholar]
- 9.Liu, B. & Hong, J. S. (2003) J. Pharmacol. Exp. Ther. 304, 1-7. [DOI] [PubMed] [Google Scholar]
- 10.Pocock, J. M. & Liddle, A. C. (2001) Prog. Brain Res. 132, 555-565. [DOI] [PubMed] [Google Scholar]
- 11.Hanisch, U. K. (2002) Glia 40, 140-155. [DOI] [PubMed] [Google Scholar]
- 12.Gebicke-Haerter, P. J. (2001) Microsc. Res. Tech. 54, 47-58. [DOI] [PubMed] [Google Scholar]
- 13.Stoll, G., Jander, S. & Schroeter, M. (1998) Prog. Neurobiol. 56, 149-171. [DOI] [PubMed] [Google Scholar]
- 14.Andersson, P. B., Perry, V. H. & Gordon, S. (1991) Neuroscience 42, 201-214. [DOI] [PubMed] [Google Scholar]
- 15.Ling, E. A., Ng, Y. K., Wu, C. H. & Kaur, C. (2001) Prog. Brain Res. 132, 61-79. [DOI] [PubMed] [Google Scholar]
- 16.Parent, J. M., Yu, T. W., Leibowitz, R. T., Geschwind, D. H., Sloviter, R. S. & Lowenstein, D. H. (1997) J. Neurosci. 17, 3727-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, J., Solway, K., Messing, R. O. & Sharp, F. R. (1998) J. Neurosci. 18, 7768-7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bengzon, J., Kokaia, Z., Elmer, E., Nanobashvili, A., Kokaia, M. & Lindvall, O. (1997) Proc. Natl. Acad. Sci. USA 94, 10432-10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arvidsson, A., Kokaia, Z. & Lindvall, O. (2001) Eur. J. Neurosci. 14, 10-18. [DOI] [PubMed] [Google Scholar]
- 20.Ekdahl, C. T., Mohapel, P., Elmer, E. & Lindvall, O. (2001) Eur. J. Neurosci. 14, 937-945. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos, G. & Watson, C. (1997) The Rat Brain in Stereotaxic Coordinates (Academic, San Diego).
- 22.Ekdahl, C. T., Mohapel, P., Weber, E., Bahr, B., Blomgren, K. & Lindvall, O. (2002) Eur. J. Neurosci. 16, 1463-1471. [DOI] [PubMed] [Google Scholar]
- 23.Brundula, V., Rewcastle, N. B., Metz, L. M., Bernard, C. C. & Yong, V. W. (2002) Brain 125, 1297-1308. [DOI] [PubMed] [Google Scholar]
- 24.Selden, J. R., Dolbeare, F., Clair, J. H., Nichols, W. W., Miller, J. E., Kleemeyer, K. M., Hyland, R. J. & DeLuca, J. G. (1993) Cytometry 14, 154-167. [DOI] [PubMed] [Google Scholar]
- 25.Tonchev, A. B., Yamashima, T., Zhao, L., Okano, H. J. & Okano, H. (2003) Mol. Cell. Neurosci. 23, 292-301. [DOI] [PubMed] [Google Scholar]
- 26.Yagita, Y., Kitagawa, K., Sasaki, T., Miyata, T., Okano, H., Hori, M. & Matsumoto, M. (2002) J. Neurosci. Res. 69, 750-756. [DOI] [PubMed] [Google Scholar]
- 27.Iwai, M., Sato, K., Omori, N., Nagano, I., Manabe, Y., Shoji, M. & Abe, K. (2002) J. Cereb. Blood Flow Metab. 22, 411-419. [DOI] [PubMed] [Google Scholar]
- 28.Kim, W. G., Mohney, R. P., Wilson, B., Jeohn, G. H., Liu, B. & Hong, J. S. (2000) J. Neurosci. 20, 6309-6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tikka, T., Fiebich, B. L., Goldsteins, G., Keinanen, R. & Koistinaho, J. (2001) J. Neurosci. 21, 2580-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallieres, L., Campbell, I. L., Gage, F. H. & Sawchenko, P. E. (2002) J. Neurosci. 22, 486-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsuse, O., Iseki, E. & Kosaka, K. (2003) Neuropathology 23, 9-15. [DOI] [PubMed] [Google Scholar]
- 32.Perry, V. H., Newman, T. A. & Cunningham, C. (2003) Nat. Rev. Neurosci. 4, 103-112. [DOI] [PubMed] [Google Scholar]
- 33.McGeer, P. L., Schulzer, M. & McGeer, E. G. (1996) Neurology 47, 425-432. [DOI] [PubMed] [Google Scholar]
- 34.Monje, M. L., Mizumatsu, S., Fike, J. R. & Palmer, T. D. (2002) Nat. Med. 8, 955-962. [DOI] [PubMed] [Google Scholar]
- 35.Zhao, M., Momma, S., Delfani, K., Carlén, M., Cassidy, R. M., Johansson, C. B., Brismar, H., Shupliakov, O., Frisén, J. & Janson, A. (2003) Proc. Natl. Acad. Sci. USA, 100, 7925-7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. (2002) Nat. Med. 9, 963-970. [DOI] [PubMed] [Google Scholar]
- 37.Nencini, P., Sarti, C., Innocenti, R., Pracucci, G. & Inzitari, D. (2003) Cerebrovasc. Dis. 15, 215-221. [DOI] [PubMed] [Google Scholar]
- 38.Streit, W. J., Walter, S. A. & Pennell, N. A. (1999) Prog. Neurobiol. 57, 563-581. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz, M. (2003) J. Cereb. Blood Flow Metab. 23, 385-394. [DOI] [PubMed] [Google Scholar]