Abstract
Aims
The aim of the present study was to describe a 10 years single-centre experience in pacing and defibrillating leads removal using an effective and safe modified mechanical dilatation technique.
Methods and results
We developed a single mechanical dilating sheath extraction technique with multiple venous entry site approaches. We performed a venous entry site approach (VEA) in cases of exposed leads and an alternative transvenous femoral approach (TFA) combined with an internal transjugular approach (ITA) in the presence of very tight binding sites causing failure of VEA extraction or in cases of free-floating leads. We attempted to remove 2062 leads [1825 pacing and 237 implantable cardiac defibrillating (ICD) leads; 1989 exposed at the venous entry site and 73 free-floating] in 1193 consecutive patients. The VEA was effective in 1799 leads, the TFA in 28, and the ITA in 205; in the overall population, we completely removed 2032 leads (98.4%), partially removed 18 (0.9%), and failed to remove 12 leads (0.6%). Major complications were observed in eight patients (0.7%), causing three deaths (0.3%).
Conclusion
Mechanical single sheath extraction technique with multiple venous entry site approaches is effective, safe, and with a good cost effective profile for pacing and ICD leads removal.
Keywords: Lead extraction, Mechanical dilatation, Internal jugular approach
Introduction
In recent years, the significantly expanded use of implanted devices for cardiac pacing and defibrillation has increased the number of device-related complications and, consequently, the need for removal.1–4 Over the past two decades, although extraction techniques have evolved from simple traction to extraction with dilators5–11 and powered sheaths12–20 with reported success rates over 95%, percutaneous lead removal has still been associated with a small but significant procedural failure, morbidity, and mortality.
As known, fibrotic tissue develops over time and entraps the implanted lead in the veins and in the cardiac chambers. However, conventional techniques including the use of a locking stylet, telescoping, or powered sheath advancement over leads and lead removal through the venous entry site19 are sometimes not able to overcome common procedural difficulties, causing failure and/or complications.
According to these observations, since 1997 we have been developing a modified percutaneous mechanical dilatation technique21–23 to improve success rate and to reduce complications. The aim of this study is to report the results obtained in a series of consecutive patients over a period of more than 10 years.
Methods
Population
Between January 1997 and June 2007, all consecutive patients admitted to our Institution for lead extraction were evaluated. The clinical and pacing notes of all patients were examined and relevant data were entered into a structured database and then evaluated.
Patients referred to our Institution were accepted for transvenous lead extraction and prepared for the intervention according to the currently used guidelines.24
Extraction procedure
The procedures were performed in the cardiac electrophysiology laboratory, with cardiothoracic surgery standby available, in a fasting state after obtaining informed consent. Before the extraction procedures, the patients were prepared with application of cutaneous pads for defibrillation, transvenous temporary pacing, invasive arterial blood pressure, and pulse oximetry monitoring.
The extraction procedures were performed by three trained interventional cardiologists.
Once the patient was prepared, draped, and sedated, the pulse generator pocket was opened and the leads freed by electrocauthery from their adhesions down to the venous insertion site or as far as possible. The leads were cut 10–15 cm out of the venous entry site. A stiff normal stylet supplied by the lead manufacturer, of appropriate length for the lead, was introduced into the lead body with its tip as close as possible to the lead tip in order to stiffen it. One or two (in the presence of unipolar or bipolar leads, respectively) ties of silk suture material (Ethibond Excel 0, Ethicon, Johnson & Johnson, St Steven-Woluwe, B) were secured, respectively, around the outer and the inner insulation of the lead. Once the lead was freed and secured, gentle manual traction was applied in an attempt to remove the lead. When manual traction failed to remove the lead, we used a modified percutaneous dilatation technique.5,8,20
In the presence of leads exposed through tributary veins of the superior vena cava system (cephalic, subclavian, jugular vein) the venous entry site approach (VEA) was the first choice technique. Dilatation was performed using polypropylene sheaths by Cook Vascular Inc. (Leechburg, PA, USA). The size of the sheaths, all provided with bevelled ends, ranged from 7 to 14F. Dilatation was started by using a single sheath with the inner diameter as close as possible to the lead body diameter. Traction was maintained on the silk ties while the sheath was advanced under fluoroscopy following the lead course and avoiding any angle. The advancement of the sheath was made by rotating it alternatively clockwise and counter-clockwise with two or three turns. While dilating, smooth traction was performed in order to keep the lead in tension, but avoiding myocardial wall invagination or coil lengthening and lead damage. When the advancement of the sheath was difficult, it was retrieved for a few millimetres and dilatation restarted. If unsuccessful, the dilator was changed to a new one of larger diameter. Occasionally, instead of moving to a larger sheath, we modified the bevelled end of the dilator sheath. The orifice of the sheath was enlarged by cutting away a small portion of the polypropylene, paying attention to avoid any modification of the distal part of the bevelled edge. Once the tip was reached, while exerting a mild traction and maintaining the stylet inserted till the tip, dilatation was performed by continuous clockwise and counter-clockwise rotation of the sheath, in order to dissect the distal binding site, and checking by fluoroscopy that the sheath did not overcome the tip of the lead. Once the tip was freed, the lead and the sheaths were removed through the vein of insertion. When the lead tip was made free by traction and dilatation before the sheath reached the distal end of the lead, but the retrieval was impossible because of unablated binding sites, then a transfemoral workstation was used for grasping the lead tip and to remove it, by slipping it through the binding sites. When, despite the use of larger sheaths, dilatation was stopped at any binding site for 5 min, or when dilatation was judged too risky, the internal transjugular approach (ITA) was considered. To perform ITA, the transfemoral approach (TFA) was necessary as a crossover step. Procedural steps are shown in Figure 1. The transfemoral workstation, provided with a remote control tip deflecting wire (Cook Vascular Inc., Leechburg, PA, USA), was introduced through the right femoral vein, and advanced till the right atrium. The first step was to check the possibility of slipping the lead into the blood flow (Figure 1A). Using the tip deflecting wire, the lead was grasped at the level of the right atrium or superior vena cava, below the site where dilatation was stopped. Using slight traction, the possibility of slipping the lead and making it free-floating was assessed (Figure 1B). When the lead could not be slipped through, the possibility of grasping it above the binding site and making it free-floating was checked. After the removal of the stylet and the suture, the lead was made free-floating by traction using the tip deflecting wire. Then the right internal jugular vein was percutaneously cannulated using an 11 French introducer (Avanti +, Cordis Corp., Miami, FL, USA). A Lasso (Osypka GmbH, Grentzig-Whylen, Germany) was advanced through the jugular vein, the proximal end of the lead was captured as close as possible to its end, and the lead was retrieved through the jugular vein and exposed (Figure 1C). A percutaneous procedure for exposed leads using dilating sheaths was then performed (Figure 1D).
- In the presence of free-floating leads (i.e. leads migrated into the venous system, with the proximal end not accessible in the pacemaker pocket), the TFA was the first step of the procedure.
- In case of free-floating leads with free tips (leads migrated into the venous system, distal fixation site detached), an intravascular tool (Lasso, Osypka GmbH) was used to grasp the lead. Once the lead was grabbed, the possibility of slipping it through binding sites in the upper course was assessed. If the lead was free, it was pulled back into the workstation and removed; in case of adherences, dilatation was performed using the workstation.
- In the presence of free-floating leads with anchored tips (i.e. leads migrated into the venous system, with the proximal end not accessible in the pacemaker pocket, and the tip fixed in the heart), the ITA was the first choice approach. Procedural steps are shown in Figure 2. The possibility to move the lead was assessed by intravascular tools introduced using the TFA (Figure 2A). The lead was grasped by the tip deflecting wire and slipped, when possible, through the adhesions. A Lasso was advanced via the jugular vein (Figure 2B), and the proximal end of the lead was grasped and then exposed through the jugular vein (Figure 2C). At this point, a percutaneous procedure for exposed leads using dilating sheaths was then performed (Figure 2D). When the length of the lead did not allow its exposure, the Lasso was used as an extension of the lead itself, and dilatation was performed by a dilating sheath previously inserted over Lasso's body.
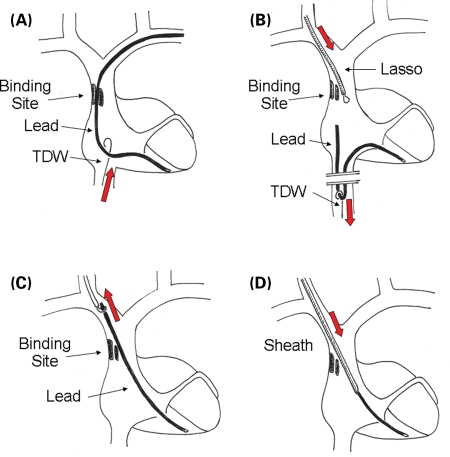
Figure 1.
Consecutive steps of the internal transjugular approach (ITA) in case of crossover from the venous entry approach (VEA). (A) A tip deflecting wire is advanced via the femoral vein in order to assess the possibility to grasp the lead and to move it. (B) Once the lead has been grasped, it is pulled down in the inferior vena cava and slipped through the binding site; a Lasso, introduced through the internal jugular vein, is advanced near the proximal end of the lead. (C) The lead is caught by the Lasso, pulled up and exposed through the jugular vein. (D) Dilatation using a dilating sheath is performed. See the text for further details. TDW, tip deflecting wire.
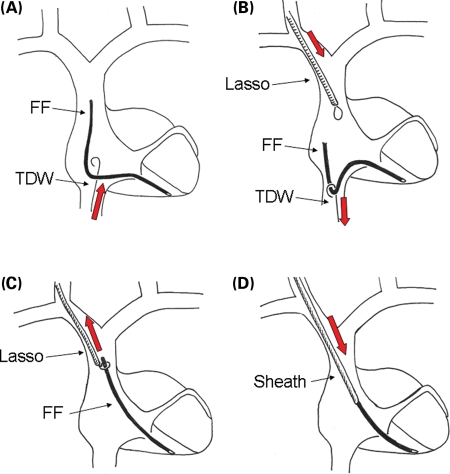
Figure 2.
Consecutive steps of the internal transjugular approach (ITA) in case of free-floating leads with anchored tips. (A) A tip deflecting wire is advanced via the femoral vein in order to assess the possibility to grasp the lead and to move it. (B) Once the lead has been grasped, it is pulled down in the inferior vena cava; a Lasso, introduced through the internal jugular vein, is advanced near the proximal end of the lead. (C) The lead is caught by the lasso and then pulled up and exposed through the jugular vein. (D) Dilatation using a dilating sheath is performed. See the text for further details. TDW, tip deflecting wire; FF, free-floating lead.
Procedural outcome was defined according to the radiological outcome: complete success (removal of the whole lead), partial (a fragment of less than 4 cm is left), and failure (a significant fragment is left, or the procedure was stopped because of a major complication). Complications were defined according to the NASPE recommendations for extraction of chronically implanted leads.24
Extraction time was defined as the time since the start of traction or dilatation to the removal of the lead.
Results
Between January 1997 and June 2007, 1193 consecutive patients (884 males, mean age 65.7 years) were considered for transvenous removal of 2065 leads. These leads had been implanted for a mean period of 69.3 months (median 50 months, range 1–336, 25/75 percentile 19/108 months). Pacing leads to be removed were 1828 (724 atrial, 1032 ventricular, and 72 coronary sinus). Implantable cardiac defibrillating (ICD) leads were 237 (223 ventricular, 12 superior vena cava, and two atrial). In the overall number of leads, 68 pacing leads (26 atrial, 41 ventricular, and one coronary sinus) and five ICD leads (five ventricular) were free-floating. Data about the population and leads are summarized in Table 1.
Table 1.
Patients and leads characteristics
| Patients | n = 1193 | |||
| Men/women | 884/309 | |||
| Mean age (years) | 65.7 | |||
| Range (years) | 6–95 | |||
| Leads | n = 2065 | |||
| Mean implant time (months) | 69.3 | |||
| Range (months) | 1–336 | |||
| Pacing leads | n = 1828 | Atrial | Ventricular | CS |
| Exposed | 698 | 991 | 71 | |
| Free-floating | 26 | 41 | 1 | |
| Total | 724 | 1032 | 72 | |
| ICD leads | n = 237 | Atrial | Ventricular | SVC |
| Exposed | 2 | 218 | 12 | |
| Free-floating | 0 | 5 | 0 | |
| Total | 2 | 223 | 12 | |
CS, coronary sinus; SVC, superior vena cava.
Indications for removal were mostly infection (83.5%). Indications are reported in Table 2.
Table 2.
Indications for removal
| Indications | Leads (n) | |
|---|---|---|
| Total class I | 682 | |
| I-a | Sepsis, endocarditis | 612 |
| I-b | Lead inducing life-threatening arrhythmias | 24 |
| I-c | Life-threatening fragment | 7 |
| I-d | Lead inducing thromboembolic events | 39 |
| Total class II | 1383 | |
| II-a | Pocket infection, erosion, draining sinus | 1099 |
| II-b | Infection, lead suspected as the source | 14 |
| II-c | Chronic pain at the pocket | 3 |
| II-d | Threat to the patient by lead's design or failure | 37 |
| II-f | Traumatic injury, lead interferes with repair | 4 |
| II-g | Necessary for the implant of new leads | 181 |
| II-h | Non-functional leads in young patient | 45 |
| Total | 2065 | |
Indications are classified according to NASPE recommendations.24
Among the 2065 leads considered for transvenous removal at our Centre, three free-floating fragments were judged not suitable for transvenous techniques because they were completely included into the venous wall, in the absence of any possible site to grasp the lead. The remaining 2062 pacing and ICD leads were subjected to removal procedures.
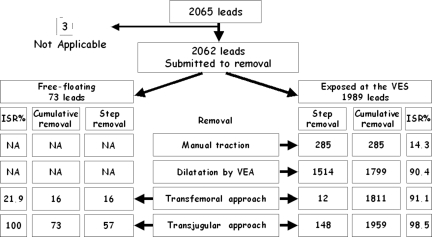
Procedural outcome for all the leads is shown in Figure 3.
Figure 3.
Diagram summarizing the outcome of the overall leads included in the study, their management, and the final result. ISR, incremental success rate; NA, not applicable; VEA, venous entry approach; VES, venous entry site.
Leads exposed at the venous entry site
The VEA was performed in 1989 leads (1757 pacing and 232 ICD leads). Manual traction was effective in 285 leads (14.3%), in the remaining 1704 mechanical dilatation was attempted. The procedure was completely successful in 1514 out of 1989 leads (76.1%), obtaining a cumulative successive rate of 90.4%. A partial success was obtained in 12 leads (0.6%). In 10 leads, the procedure was stopped due to non-infective class II indications. Among the remaining 168 leads, the removal was completed by the TFA in 12 leads, whose tips were freed while performing dilatation (0.6%). Crossover to the ITA was performed in 156 leads (7.8%).
Free-floating leads
Seventy-three free-floating leads were approached via the femoral vein. Lead grasping allowed the direct removal of 16 (21.9%) leads; 57 leads were exposed through the internal jugular vein and then subjected to dilatation. In one case, the lead was too short to be exposed; a dilating sheath was positioned over the Lasso using this tool as an extension of the lead. Complete removal was achieved in all leads.
Internal transjugular approach
The ITA was performed in 57 free-floating and 156 exposed leads as crossover from the VEA. By this approach, 205 out of 213 leads (96.2%) were completely removed. The procedure was partially successful in 6 (2.8%) leads and unsuccessful in 2 (0.9%). The ITA was effective in 148 out of 156 exposed leads and in all the 57 free-floating leads. In the group of the exposed leads, the ITA increased success rate from 90.4 to 98.5% (Figure 3). In two patients, because of the failure to cannulate either the right or the left internal jugular vein, we used the right subclavian vein.
Overall results
Of the overall population treated at our Centre, 2032 leads were completely removed (98.4%) and 18 partially removed (0.9%), as shown in Table 3. We removed 1795 out of 1989 right atrial and ventricular pacing leads (98.2%). All the 72 left ventricular leads (mean implant period 23.36 months, range 2–96) were completely removed; manual traction was effective in 45 (62.5%), dilatation was necessary in 26 (into the coronary sinus in 2), and ITA was performed in one free-floating lead. All the 237 ICD leads were successfully removed.
Table 3.
Procedural patients and leads overall outcome
| Removal | A and RV PL | LV PL | ICD L | Total | % |
|---|---|---|---|---|---|
| Complete | 1723 | 72 | 237 | 2032 | 98.4 |
| Partial | 18 | – | – | 18 | 0.9 |
| Failed | 12 | – | – | 12 | 0.6 |
| TLR not applicable | 3 | – | – | 3 | 0.2 |
| Complications | Tamponade | Hemothorax | |||
| Major | 7 | 1 | 8 | 0.7 | |
| Fatal | 2 | 1 | 3 | 0.3 | |
| Not fatal | 5 | – | 5 | 0.4 | |
TLR, transvenous lead removal; A, atrial; RV, right ventricular; LV, left ventricular.
PL, Pacing leads; ICD, implantable cardioverter defibrillator; L, leads.
In the overall population, the mean extraction time was 20 ± 36 min (range 1–360).
Complications
Perioperative complications were observed in 98 patients. Major complications were observed in eight patients (0.7%): five patients were successfully treated and three died (0.3%). Cardiac tamponade occurred in seven patients, six during VEA, and one during ITA. Pericardial drainage was successfully performed in two patients and surgical repair was necessary in three; one patient died despite drainage and subsequent surgical intervention. For one patient, transferred to another hospital after the procedure, tamponade and death occurred 12 h later. Intraoperative haemothorax occurred in one patient, during the removal of a lead fractured by subclavian crush. Despite medical treatment and pleural drainage, the patient died due to haemorrhagic shock.
In four patients, the procedure was complicated by dislodgement of a functioning pacing lead and lead repositioning was required.
Minor complications, not requiring intervention, were observed in 86 patients (7.2%). Pericardial effusion was observed in 27 cases and thrombosis of implant vein in 12, pulmonary embolism occurred in 18 cases. Arrhythmias requiring cardioversion occurred in six patients, haematoma at the pocket requiring drainage occurred in 25; in 8 patients with local infection later development of sepsis was observed.
No complications directly related to the femoral or jugular venous access were observed.
Discussion
Our results compare favourably in terms of success and complication rates with previous studies reporting on the superior and femoral approaches. With regard to the superior approach, our complete success rate (98.4%) was higher than conventional mechanical experiences reported by Eckhard et al.25 (complete success was achieved in 81% of cases), the US lead database in 1994 (complete success in 86.6%),9 and in 1996 (complete success in 93%).10 Even if comparison between different studies and reports are difficult, because procedural outcome and complications may be defined in different ways, our results compare favourably with powered sheaths experiences including both laser12,13,26 and radiofrequency18,27 techniques. Regarding lead removal via the femoral approach, our results are comparable in terms of complete success rate but with lower major complication rate, especially if a needle's eye snare is used for countertraction.28,29 Furthermore, our data are more intriguing considering the presence of defibrillating and free-floating leads and a long dwell time (median 50 months).
We think that our high success rate can be attributed to an integrated procedural approach. Indeed, a multivenous entry site approach (venous lead entry site, femoral and right internal jugular vein) with a modified mechanical dilatation technique (single sheath rotation without tip countertraction) has the advantages of each approach without the most common disadvantages, with a tailor-made procedural strategy for leads and patients.
As previously reported, common critical points in the removal procedures include: (i) tight space between the clavicle and the first rib (large sheaths use precluded);16 (ii) presence of tight binding sites due to scar or calcified tissue (difficult sheath advancement);9,10 (iii) hard turns in the lead course (risky sheath advancement); (iv) difficult countertraction (myocardial wall invagination),9,10 (v) lead damage (preventing the use of a stylet);9,29 and (vi) the presence of free-floating leads (difficult to hold, insertion of a stylet not possible).28,29 Every technique (conventional telescoping dilatation, powered sheath, and femoral approach) shows limitations at the above described critical points, reducing success rate and increasing complications as described in Table 4.
Table 4.
Critical points during pacing and implantable cardioverter defibrillator lead removal
| Critical points | Problems | Standard approach limitations |
|---|---|---|
| Tight space between clavicle and first rib | Difficult sheath advancement | Use of large sheaths (telescoping sheath, powered sheath) often precluded |
| Tight binding sites | Binding sites fibrous and stiff, often calcified, difficult to free the lead | Venous entry site mechanical dilatation needs aggressive dilatation. Powered sheaths are ineffective in the presence of calcified tissue |
| Hard turn in lead course | In the presence of hard turns (right side implant, impossible stylet introduction), the energy of dilatation is not applied to the binding site, but directly on the venous wall | Telescoping and powered sheaths apply excessive dilating force with the risk of vein damage |
| Difficult countertraction | The lead tip cannot be freed | Countertraction by an outer telescoping sheath may result in ventricular wall disruption and cardiac tamponade. Powered sheaths cannot be used near the lead tip |
| Lead damage | Insulation is absent, coil is lengthened, or stylet cannot be inserted | Telescoping sheath and powered sheath with high energy dilatation may cause venous tears or complete lead fracture |
| Free-floating leads | Venous entry site approach impossible for intravascular not exposed leads | Binding sites dilation through the transfemoral workstation is often ineffective due to the hard turn to cross the tricuspid valve |
See text for details.
ITA, internal transjugular approach; VEA, venous entry site approach.
As known, the main steps for transvenous lead removal are: firm holding of the lead, freeing the binding sites by dilatation all the way to the tip and retrieving the lead through a vascular approach. In our opinion, the key point for a successful removal is to free the lead from all the adhesions all over its course, instead of freeing the lead tip, so that traction at the tip is not crucial anymore. The key point for safety, in the presence of leads difficult to remove, is to adapt the technique to the specific situation, changing, for example, the venous approach. In our opinion, high success and low complication rates are achieved by avoiding the use of excessive force or powered sheaths to overcome tight binding sites. By the ITA approach, dilatation of dangerous venous binding sites can actually be avoided in many instances as the leads can be retrieved by sliding them down through the scar tissue; only distal or intramyocardial scar tissue has to be dealt with from the jugular approach. According to these observations, we developed a modified mechanical dilatation approach, including standard stylet and single sheath dilatation technique, with crossover to TFA and ITA in case of ineffective entry site removal.
Standard stylet
Since dissection of binding sites has to be performed by sheaths, the lead must be made as stiff as possible in order to allow a firm hold. As known, a locking stylet allows a strong traction of the lead, but, sometimes, some of them may not be removed. However, locking and traction at the lead tip (i.e. locking stylet) is not a key point because binding sites at the tip have to be overcome by dilatation and rotation of the sheath more than by traction. A standard stylet and a suture, if used with a moderate traction, can accomplish the task with a low risk of removing the outer insulation. The other advantage of the standard stylet is the fact that, being easily removable, it is useful when crossovers to alternative transvenous approach are required.
Single mechanical sheath dilatation
The use of a single sheath and progressive mechanical dilatation offers a flexible extraction technique, allowing the use of smaller sheaths. Dilatation by rotation of a single smaller sheath is effective for most binding sites along the course of the lead, particularly at the junction between the innominate vein and superior vena cava. In our opinion, the tip can be freed in a safer way by clockwise and counter-clockwise rotation of a single sheath, narrowly tailored around the tip, in order to obtain the avulsion of the tip itself from the scar tissue, instead of heavy traction performed while maintaining the outer sheath against the wall.
Furthermore, the telescoping sheath, due to its larger diameter, is stiff and more difficult to advance. Failure of dilatation was observed in the presence of calcified adherences or in case of a tortuous course of the lead. In these conditions, the use of powered sheaths, even if more effective and faster than mechanical ones, does not provide any advantage, increasing the risk of complications.15,16,30 Furthermore, even if requiring less time, the procedure is much more expensive.18 Our results demonstrate that the technique of single sheath mechanical dilatation paired with the appropriate approach in the presence of difficult leads is at least as effective as powered dilatation, with lower cost.
Multiple venous entry site approach
In our opinion, the use of a single sheath technique with the right internal jugular approach can be a solution in most of the difficult situations previously mentioned (Table 4). First, withdrawing the lead with a TFA, it may be slipped through some binding sites, avoiding dilatation of some adherences (Figure 2B). Secondly, once exposed through the jugular vein, free-floating leads and difficult leads turned into free-floating ones may be managed as exposed leads. At this point, mechanical dilatation may be performed via internal jugular vein avoiding some disadvantages resulting from the VEA, such as tight space between the clavicle and the first rib (especially if large sheaths are required) or an unfavourable lead/venous wall angle. Indeed, using ITA we obtain a ‘straight course dilatation’ from the jugular vein to the heart facilitating force application up to the tip (Figures 1D and 2D).
In conclusion, our approach was highly effective considering the characteristics of the removed leads, with a complete success rate slightly higher (98.4%) than that reported in most of the papers about powered dilatation17,18,26 or TFA approach28,29 and with a low number of complications (0.7%). Indeed, advantages provided by the ITA are unrelated to the energy used for dilatation but depend on a reduced number of binding sites to dissect and on a straighter lead course dilatation, overcoming main limitations resulting from the VEA (such as difficult large sheath advancement, risk of venous tear in presence of lead turns, and tight binding sites),12,16 and of conventional TFA (difficult and risky traction).29
Study limitations
The present study reports the experience of a single centre with a versatile technique for transvenous lead removal; it is not a prospective randomized trial.
It requires an adequate training in lead removal to obtain reproducible results. In this study, the learning curve of the senior operator is not included. Furthermore, the right internal jugular vein must be patent; in our experience, the vein could not be cannulated in two out of 213 patients, in whom the subclavian vein was an effective alternative approach.
Finally, the mean procedural time is longer than that reported for powered dilatation and duration cannot be predicted, but this limitation is counterbalanced by a very high success rate, a very low risk, as well as costs reduction.
Conclusions
The importance of transvenous lead removal techniques is currently increasing due to the rapidly rising number of implanted devices. Removal techniques were first introduced in clinical practice 15 years ago, and are still considered to be in evolution. Further advances are required in order to reach a gold standard. Our technique proved to be very effective and safe. The use of a single sheath method for freeing binding sites and the use of the ITA increased the effectiveness of mechanical dilatation, while avoiding the costs related to the use of powered sheaths. This technique reduces the incidence of serious complications, which can lead in turn to expanded indications for transvenous lead removal.
Funding
Funding to pay the Open Access publication charges for this article was provided by CardioThoracic and Vascular Department, University Hospital of Pisa.
Acknowledgements
The authors are indebted to their nurses Michela Favaro, Cristina Giannessi and Giampietro Ercoli for their excellent support in the management of patients in the EP lab.
The authors also thank Ilaria Sbrana for the manuscript preparation and Giovanna Lastrucci and Ellen Smith for the linguistic revision.
Conflict of interest: none declared.
References
- 1.Furman S, Behrens M, Andrews C, Klementowicz P. Retained pacemaker leads. J Thoracic Cardiovasc Surg. 1987;94:770–772. [PubMed] [Google Scholar]
- 2.Zerbe F, Ponizynski A, Dyszkiewicz W, Ziemiansk A, Dziegielewski T, Krug H. Functionless retained pacing leads in the cardiovascular system. Br Heart J. 1985;54:76–79. doi: 10.1136/hrt.54.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rettig G, Doenecke P, Sen S, Volkmer I, Bette L. Complications with retained transvenous pacemaker electrodes. Am Heart J. 1979;98:587–594. doi: 10.1016/0002-8703(79)90284-9. [DOI] [PubMed] [Google Scholar]
- 4.Parry G, Goudevenos J, Jameson S, Adams PG, Gold RG. Complications associated with retained pacemakers leads. Pacing Clin Electrophysiol. 1991;14:1251–1257. doi: 10.1111/j.1540-8159.1991.tb02864.x. [DOI] [PubMed] [Google Scholar]
- 5.Byrd CL, Schwartz SJ, Hedin NB, Goode LB, Feranot NE, Smith HJ. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol. 1990;13:1871–1875. doi: 10.1111/j.1540-8159.1990.tb06906.x. [DOI] [PubMed] [Google Scholar]
- 6.Byrd CL, Schwartz SJ, Hedin NB. Intravascular techniques for extraction of permanent pacemakers leads. J Thoracic Cardiovasc Surg. 1991;101:989–997. [PubMed] [Google Scholar]
- 7.Bongiorni MG, Petz E, Levorato D, Soldati E, Arena G, Quirino G, Vagheggini G, Malamuth D, Biagini A, Camerini F. Removal of chronic leads for permanent pacing. Clinical experience with transvenous extractors. In: Antonioli GE,, editor. Pacemaker Leads 1991. Amsterdam: Elsevier Science Publishers; 1991. pp. 289–294. [Google Scholar]
- 8.Byrd CL, Schwartz SJ, Hedin NB. Lead extraction: indications and techniques. Cardiol Clin. 1992;10:735–748. [PubMed] [Google Scholar]
- 9.Smith HJ, Fearnot NE, Byrd CL, Wilkoff BL, Love CJ, Sellers TD. Five-years experience with intravascular lead extraction. U.S. Lead Extraction Database. Pacing Clin Electrophysiol. 1994;17:2016–2020. doi: 10.1111/j.1540-8159.1994.tb03792.x. [DOI] [PubMed] [Google Scholar]
- 10.Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Turk KT, Reeves R, Young R, Crevey B, Kutalek SP, Freedman R, Friedman R, Trantham J, Watts M, Schutzman J, Oren J, Wilson J, Gold F, Fearnot NE, Van Zandt HJ. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994–1996. U.S. Extraction Database, MED Institute. Pacing Clin Electrophysiol. 1999;22:1348–1357. doi: 10.1111/j.1540-8159.1999.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 11.Saad EB, Saliba WI, Schweikert RA, Al-Khadra AS, Abdul-Karim A, Niebauer MJ, Wilkoff BL. Nonthoracotomy implantable defibrillator lead extraction: results and comparison with extraction of pacemaker leads. Pacing Clin Electrophysiol. 2003;26:1944–1950. doi: 10.1046/j.1460-9592.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 12.Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, Parsonnet V, Epstein LM, Sorrentino RA, Reiser C. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671–1676. doi: 10.1016/s0735-1097(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 13.Epstein LM, Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Hayes DL, Reiser C. Initial experience with larger laser sheaths for the removal of transvenous pacemaker and implantable defibrillator leads. Circulation. 1999;100:516–525. doi: 10.1161/01.cir.100.5.516. [DOI] [PubMed] [Google Scholar]
- 14.Love CJ. Lead extraction. Heart Rhythm. 2007;4:1238–1243. doi: 10.1016/j.hrthm.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet V, Roelke M, Trivedi A, Rizvi SA, Pervez A. Laser extraction of entrapped leads. Pacing Clin Electrophysiol. 2001;24:329–332. doi: 10.1046/j.1460-9592.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 16.Byrd CL, Wilkoff BL, Love CJ, Sellers TD, Reiser C. Clinical study of the laser sheath for lead extraction: the total experience in the United States. Pacing Clin Electrophysiol. 2002;25:804–808. doi: 10.1046/j.1460-9592.2002.t01-1-00804.x. [DOI] [PubMed] [Google Scholar]
- 17.Moon MR, Camillo CJ, Gleva MJ. Laser-assist during extraction of chronically implanted pacemaker and defibrillator leads. Ann Thorac Surg. 2002;73:1893–1896. doi: 10.1016/s0003-4975(02)03588-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilkoff BL. Transvenous leads extraction with electrosurgical dissection sheaths. Initial experience. Pacing Clin Electrophysiol. 2000;23:679–684. [Google Scholar]
- 19.Love CJ. Current concepts in extraction of transvenous pacing and ICD leads. Cardiol Clin. 2000;18:193–217. doi: 10.1016/s0733-8651(05)70134-x. [DOI] [PubMed] [Google Scholar]
- 20.Verma A, Wilkoff BL. Intravascular pacemaker and defibrillator lead extraction: a state-of-the-art review. Heart Rhythm. 2004;1:739–745. doi: 10.1016/j.hrthm.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Bongiorni MG, Giannola G, Arena G, Soldati E, Bartoli C, Lapira F, Zucchelli G, Di Cori A. Pacing and implantable cardioverter-defibrillator transvenous lead extraction. Ital Heart J. 2005;6:261–266. [PubMed] [Google Scholar]
- 22.Bongiorni MG, Soldati E, Arena G, Ratti M, Gherarducci G, Mariani M. Transvenous removal of permanent electrocatheters for heart stimulation and defibrillation. Cardiologia. 1999;44(Suppl. 1):395–398. [PubMed] [Google Scholar]
- 23.Bongiorni MG, Zucchelli G, Soldati E, Arena G, Giannola G, Di Cori A, Lapira F, Bartoli C, Segreti L, De Lucia R, Barsotti A. Usefulness of mechanical transvenous dilatation and location of areas of adherence in patients undergoing coronary sinus lead extraction. Europace. 2007;9:69–73. doi: 10.1093/europace/eul130. [DOI] [PubMed] [Google Scholar]
- 24.Love CJ, Wilkoff BL, Byrd CL, Belott PH, Brinker JA, Fearnot NE, Friedman RA, Furman S, Goode LB, Hayes DL, Kawanishi DT, Parsonnet V, Reiser C, Van Zandt HJ. Recommendations for extraction of chronically implanted transvenous pacing and defibrillator leads: indications, facilities, training. North American Society of Pacing and Electrophysiology Lead Extraction Conference Faculty. Pacing Clin Electrophysiol. 2000;23:544–551. doi: 10.1111/j.1540-8159.2000.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Eckhard A, Neuzner J, Binner L. Three year experience with a stylet for lead extraction: a multicenter study. Pacing Clin Electrophysiol. 1996;19:18–25. doi: 10.1111/j.1540-8159.1996.tb04786.x. [DOI] [PubMed] [Google Scholar]
- 26.Kennergren C, Bucknall CA, Butter C, Charles R, Fuhrer J, Grosfeld M, Tavernier R, Morgado TB, Mortensen P, Paul V, Richter P, Schwartz T, Wellens F PLESSE investigators group. Laser-assisted lead extraction: the European experience. Europace. 2007;9:651–656. doi: 10.1093/europace/eum098. [DOI] [PubMed] [Google Scholar]
- 27.Neuzil P, Taborsky M, Rezek K, Vopalka R, Sediva L, Niederle P, Reddy V. Pacemaker and ICD lead extraction with electrosurgical dissection sheaths and standard transvenous extraction systems: results of a randomized trial. Europace. 2007;9:98–104. doi: 10.1093/europace/eul171. [DOI] [PubMed] [Google Scholar]
- 28.Jarwe M, Klug D, Beregi JP, Le Franc P, Lacroix D, Kouakam C, Guedon-Moreau L, Zghal N, Kacet S. Single center experience with femoral extraction of permanent endocardial pacing. Pacing Clin Electrophysiol. 1999;22:1202–1209. doi: 10.1111/j.1540-8159.1999.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 29.Klug D, Jarwe M, Messaoudène SA, Kouakam C, Marquiè C, Gay A, Lacroix D, Kacet S. Pacemaker lead extraction with the needle's eye snare for countertraction via a femoral approach. Pacing Clin Electrophysiol. 2002;25:1023–1028. doi: 10.1046/j.1460-9592.2002.01023.x. [DOI] [PubMed] [Google Scholar]
- 30.Robboy SJ, Harthorne JW, Leinbach RC, Sanders CA, Austen WG. Autopsy findings with permanent pervenous pacemakers. Circulation. 1969;39:495–501. doi: 10.1161/01.cir.39.4.495. [DOI] [PubMed] [Google Scholar]