Abstract
Although the benefits of regular exercise in controlling cardiovascular risk factors have been extensively proven, little is known about the long-term cardiovascular effects of regular and extreme endurance sport practice, such as jogging, cycling, rowing, swimming, etc. Recent data from a small series suggest a relationship between regular, long-term endurance sport practice and atrial fibrillation (AF) and flutter. Reported case control studies included less than 300 athletes, with mean age between 40 and 50. Most series recruited only male patients, or more than 70% males, who had been involved in intense training for many years. Endurance sport practice increases between 2 and 10 times the probability of suffering AF, after adjusting for other risk factors. The possible mechanisms explaining the association remain speculative. Atrial ectopic beats, inflammatory changes, and atrial size have been suggested. Some of the published studies found that atrial size was larger in athletes than in controls, and this was a predictor for AF. It has also been shown that the left atrium may be enlarged in as many as 20% of competitive athletes. Other proposed mechanisms are increased vagal tone and bradycardia, affecting the atrial refractory period; however, this may facilitate rather than cause the arrhythmia. In summary, recent data suggest an association between endurance sport practice and atrial fibrillation and flutter. The underlying mechanism explaining this association is unclear, although structural atrial changes (dilatation and fibrosis) are probably present. Larger longitudinal studies and mechanistic studies are needed to further characterize the association to clarify whether a threshold limit for the intensity and duration of physical activity may prevent AF, without limiting the cardiovascular benefits of exercise.
Keywords: Athletes, Atrial fibrillation, Atrial flutter, Endurance sport practice
Regular and extreme endurance sport practice (jogging, cycling, swimming, etc.) has become very popular even among adults in their forties. The benefits of regular exercise in controlling cardiovascular risk factors have been extensively proved,1–4 and therefore cardiologists widely recommend regular exercise to improve cardiovascular health. However, recent data have documented a relationship between long-term endurance sport practice or rigorous occupational physical activity and atrial fibrillation (AF) and atrial flutter.5–10 The association is being increasingly recognized and has raised the need for larger epidemiological studies.11–13 On the other hand, moderate physical activity may indeed decrease the risk for AF in older adults.14
Atrial fibrillation is the most common arrhythmia and has a great impact in morbidity and mortality.15,16 The current increase in incidence is not fully explained by the aging population or higher prevalence of newly described risk factors such as obesity.17,18 Therefore, non-identified factors apart from family history19 may be present. Atrial fibrillation is associated with a number of cardiac and extracardiac diseases, such as hypertension, structural heart disease, and hyperthyroidism. However, in a significant proportion of patients, its aetiology remains unknown.20 This condition, called lone AF (LAF), is defined as AF in patients younger than age 60 and without any identifiable aetiologic factor. The prevalence of LAF ranges from 2–10% in the general population to 30% in studies performed in patients with paroxysmal AF who seek medical attention.21,22 Lone atrial fibrillation is commonly associated with atrial flutter, as described by Coumel;23 therefore, they seem to be two expressions of the same underlying condition.
The aim of this review is to analyse the evidence of the association between LAF and endurance sport practice or occupational physical activity, the pathophysiological mechanisms underlying this association, the clinical characteristics of this arrhythmia, and the available therapeutic options.
Atrial fibrillation and endurance sport practice
Although the presence of AF in athletes had been described previously,24,25 to the best of our knowledge Karjalainen et al.5 were the first, in 1998, to publish a longitudinal prospective study establishing a relationship between endurance sport practice and AF. They studied a series of orienteers (an endurance sport often practiced in Scandinavia). After 10 years of follow-up, AF incidence among orienteers was 5.3%, when compared with 0.9% among the control subjects. Therefore, the incidence of AF was unusually high in a series of middle-aged endurance sport practitioners without predisposing factors. Furthermore, the two patients with AF in the control group were also involved in endurance practice. The odds ratio for LAF associated with vigorous exercise was 5.5 (95% confidence interval: 1.3–24.4) in this study (Table 1).
Table 1.
Summary of the published studies analyzing the relationship between atrial fibrillation and atrial flutter and endurance sport practice.
| Studies | Type of study | Men (%) | Age | Type of sport(s) | Cases/controls | Odds ratio (CI) for AF in athletes |
|---|---|---|---|---|---|---|
| Karjalainen et al.5 | Longitudinal case/control | 100 | 47 ± 5 runners, 49 ± 5 controls | Orienteers | 262/373 | 5.5 (1.3–24.4) |
| Mont et al.6 | Retrospective compared to general population | 100 | 44 ± 13 athletes, 49 ± 11 non-athletes | Endurance sports >3 h per week | 70 lone AF | 61% in male athletes with lone AF |
| Elosua et al.7 | Retrospective case/control | 100 | 41 ± 13 AF pat, 44±11 controls | Endurance sports: current practice and >1500 accumulated hours of practice | 51/109 | 2.87 (1.39–7.05) adjusted for age and hypertension |
| Heidbuchel et al.8 | Case/control in patients undergoing flutter ablation | 83 | 53 ± 9 sports, 60 ± 10 controls | Cycling, running, or swimming >3 h per week | 31/106 | 1.81 (1.10–2.98) |
| Molina et al.9 | Longitudinal case/control | 100 | 39 ± 9 runners, 50 ± 13 sedentary | Marathon runners | 252/305 | 8.80 (1.26–61.29) adjusted for age and blood pressure |
| Baldesberger et al.27 | Longitudinal case/control | 100 | 67 ± 7 cyclist, 66 ± 6 golfers | Cyclists | 134/62 | 10% AF in cyclists, 0% AF in controls |
| Mont et al.10, GIRAFA study | Prospective case/control | 69 | 48 ± 11 | Endurance sports | 107/107 | 7.31 (2.33–22.9), >550 h of accumulated heavy physical activity |
Our interest in the subject also began in 1998. A retrospective analysis of our series of LAF patients seen at the outpatient arrhythmia clinic showed that the proportion of regular sport practice among men with LAF was much higher than among men from the general population (63 vs. 15%).6 In that series, a rather lax definition of sport practice was used (more than 3 h a week at the moment of evaluation), but in fact most patients had been involved in endurance sport practice for more than 10 years, with much higher participation levels in the past. Some of them had already limited their practice as a consequence of the arrhythmia.
The same population of LAF patients was analysed in a case–control study with two age-matched controls for each case from the general population.7 The analysis showed that the current sport practice increased the risk of developing LAF more than five times (OR 5.06 (1.35–19), a result that is within the range reported by Karjalainen et al.5 It is noteworthy that the association of current sport practice with LAF was observed at more than 1500 lifetime hours of sport practice, suggesting the existence of a threshold point. Of course, this threshold point should be interpreted with caution, and probably points out the pattern of the association (i.e. number of hours of sport practice and a posterior plateau) rather than the exact threshold point (Table 1).
To confirm the association between endurance sports and AF in a longitudinal manner, our group undertook a study that included 183 individuals who ran the Barcelona Marathon in 1992 and 290 sedentary healthy individuals included in the REGICOR study.9,26 After 10 years of follow-up, the annual incidence rate of LAF among marathon runners and sedentary men was 0.43/100 and 0.11/100, respectively. Endurance sport practice was associated with a higher risk of incident LAF in the multivariable age- and blood pressure-adjusted Cox regression models (hazard ratio = 8.80; 95% confidence interval: 1.26–61.29). The main limitation of this study was the small number of events observed during follow-up (n = 9 among marathon runners and n = 2 among sedentary men). Nevertheless, the results were consistent with previous observations.5,7
Recently, Baldesberger et al.27 published similar data in a study of 64 former Swiss professional cyclists who completed the Tour de Suisse professional cycling race at least once during the years 1955–1975. These athletes were compared with a control group of 62 male golfers who had never performed high-endurance training. Individuals were matched for age, weight, hypertension, and cardiac medication. The mean age at examination was 66 ± 7 years. Former cyclists showed a lower heart rate and a higher incidence of AF or atrial flutter (10 vs. 0%, P < 0.028) and non-sustained ventricular tachycardia (VT). The higher proportion of AF and flutter when compared with the study by Kaarjalainen et al.5 or by Molina et al.9 is probably explained because this study population was older. These data suggest that incidence of AF and flutter further increases with aging in athletes, as with any kind of AF.
In contrast with these previous studies, Pellicia et al.28 reported that the incidence of LAF among competitive athletes was uncommon and similar to that observed in the general population. However, the study was performed in young athletes at the moment of highest activity. Studies supporting the association have been performed in middle-aged individuals, after many years of sport practice.
Atrial flutter and endurance sport practice
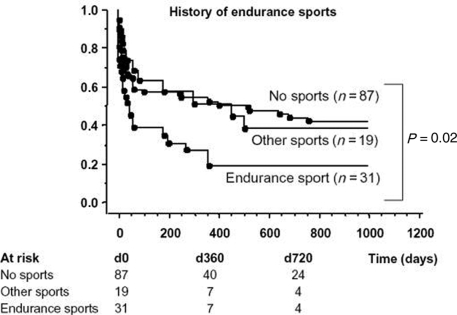
Most of the described series include patients suffering concomitant AF and atrial flutter, suggesting that endurance sports contribute to the development of both arrhythmias. For example, Baldesberger et al.27 found a higher incidence of flutter than AF in their series of veteran cyclists, although the authors did not describe whether these were common or atypical flutter episodes. Heidbuchel et al.8 found that endurance athletes had a higher recurrence rate for AF than did controls (Figure 1). The authors conclude that endurance sport practice increases the risk of suffering AF after common flutter ablation. Hoogsteen et al.29 found that 10% of athletes with AF also suffer episodes of atrial flutter. These observations suggest that both arrhythmias often co-exist in endurance athletes, and common flutter may be secondary to right atrial dilatation as a consequence of volume overload.
Figure 1.
Patients with a history of endurance sports before ablation (n = 31) developed significantly more atrial fibrillation than controls or those with a history of other type o sports activity after flutter ablation (reproduced from reference 8, with permission).
Atrial fibrillation and occupational physical activity
These studies seem to have established that long-lasting endurance sport practice increases the risk of LAF. Vigorous physical activity associated with occupational activities may theoretically pose a similar risk. Data from the recently published GIRAFA study10 appear to confirm this theory. The prospective GIRAFA study is conducted in consecutive patients with LAF recruited at the emergency room. In this case–control study, 107 LAF patients were compared with age- and sex-matched healthy controls. Total hours of physical activity (during work or leisure time) were collected with a detailed and validated questionnaire. For each physical activity, the following variables were recorded: age started, age ended, months per year, days per week, and hours per day. Subjects were asked to classify the intensity of each physical activity in four levels: sedentary, light, moderate, and heavy. The results showed that the moderate and heavy physical activity, whether sport- or job-related, increased the risk of suffering AF. In multivariable analysis, physical activity and atrial size were independent predictors for the development of AF, even after normalizing by body surface area (BSA) and height. In contrast with these observations, The Danish Diet Cancer and Health Study, conducted in a population of 19 593 men and 18 807 women with a mean age of 56 (range 50–65), failed to demonstrate any association between physical activities during working hours and risk of hospitalization with a diagnosis of AF or flutter.30 This discrepancy may be due to the limited categorization and quantification of physical activity, compared to the much deeper analysis in the GIRAFA study. Further epidemiological studies, with a detailed quantification of work-associated physical activity, are needed to clarify this potential association.
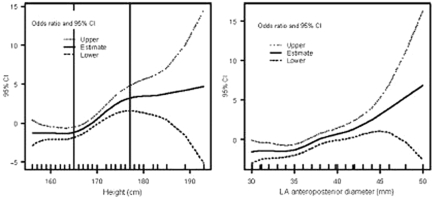
It is interesting to note the GIRAFA study's association of height and atrial size (absolute and normalized) with AF. In understanding the male predominance observed in AF, sex may indeed be secondary to that association. Hanna et al.31 had already reported a relationship between stature and AF prevalence in patients with left ventricular (LV) dysfunction (Figure 2).
Figure 2.
Thin-plate smoothing spline regression results assessing the non-linear relation between height and occurrence of lone atrial fibrillation (left panel), and the linear relation between left atrial (LA) diameter and occurrence of lone atrial fibrillation (right panel) (reproduced from reference 9, with permission).
Pathophysiology of sport-related atrial fibrillation
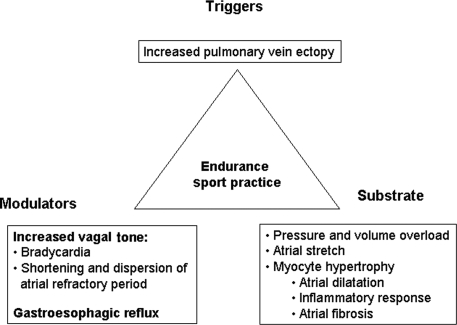
What is the possible link between physical activity and AF? Several mechanisms may be acting together. It is well accepted that arrhythmias depend on triggers, substrates, and modulators, and these factors may be present in relation to physical activity (Figure 3).
Figure 3.
Classical triangle of Coumel suggesting possible etiopathogenic factors influencing the development of atrial fibrillation in athletes.
Triggers: role of atrial ectopy
Atrial ectopy, particularly pulmonary vein ectopy, has been shown to be the trigger in most episodes of paroxysmal AF.32 Atrial and ventricular ectopy may be increased as a consequence of physical activity.27,33 Moreover, increased ventricular ectopy in elite athletes is reversible after detraining.34 Therefore, increased ectopy may be one of the mechanisms explaining the increased risk for AF associated with sport practice, provided that this ectopy acts upon an appropriate substrate. However, a recent paper by Baldesberger et al.27 did not find an increased incidence of atrial ectopy, despite increases in ventricular ectopy and VT runs in former professional cyclists. Therefore, the hypothesis of increased atrial ectopy as an explanation for the association between sports and AF cannot be adequately sustained with currently available data.
Modulators: influence of autonomic nervous system
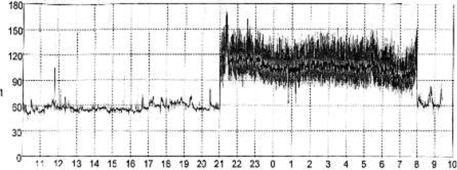
Why does an apparently healthy individual start suffering from AF? Coumel35 studied the influence of autonomic innervations in the appearance of AF and atrial flutter. He reported: ‘Vagally mediated AF occurs more frequently in men than in women, with a ratio of ∼4:1’. The age at which the first symptoms appear is classically between 40 and 50 years [sic]. The essential feature is the occurrence of the AF at night, often ending in the morning. Rest, the postprandial state (particularly after dinner) and alcohol are also precipitating factors [sic]36 (Figure 4). The author concluded that although AF occurred in a vagal context, an unidentified substrate probably existed. However, he did not establish a relationship between these episodes of AF and sport practice.
Figure 4.
Twenty-four hours recording of heart rate showing a nocturnal episode of atrial fibrillation.
Experimental data show that increased vagal tone shortens and increases the dispersion of the atrial refractory period, creating the conditions for re-entry.37–39 However, vagal AF is considered to be a rare presentation of AF. This is probably due to the lack of systematic inquiry with patients. According to the GIRAFA study,10 vagal AF is the rule rather than the exception in LAF patients (∼70% of consecutive LAF patients had vagal AF). Therefore, the increased vagal tone induced by endurance sport practice may indeed facilitate the appearance of AF. In fact, heart rate is still lower in former athletes many years after cessation of professional training than in controls, as recently shown by Baldesberger et al.27
Another interesting hypothesis recently raised by Swanson40 in a review of the existing literature is that gastroesophageal reflux, which indeed has been proven to produce AF and vagal reflexes, may be the link between increased AF and exercise. However, this hypothesis has not yet been properly investigated.
Arrhythmia substrate
Whether there is a structural substrate in LAF is still a matter of debate. In patients with hypertension or structural heart disease, it seems that AF is the consequence of structural changes in the atria (dilatation and fibrosis) secondary to chronic volume and pressure overload. It is therefore plausible that long-term endurance sport practice or occupational physical activity may induce structural changes in the atrium (enlargement, fibrosis) that may create a favourable substrate for the disease. In fact, Frustaci et al.41 found structural changes in a series of 12 patients with paroxysmal, recurrent, drug refractory LAF. The authors described inflammatory lymphonomonuclear infiltrates, compatible with myocarditis, in 66% of patients; a non-inflammatory cardiomyopathic process in 17%; and patchy fibrosis in the remaining 17%. Whether the data correspond to a highly selected population cannot be definitively ruled out, but 100% of patients showed histological changes. On the other hand, these changes could have been produced by repetitive episodes of AF.
A recent review of the literature by Swanson42 shows that excessive endurance exercise and overtraining can lead to chronic systemic inflammation and there is a relationship between AF and C-reactive protein. Anti-inflammatory agents have been reported to lower C-reactive protein and ameliorate AF. Whether inflammation may be mediated by the renin–angiotensin system and a sustained increase in catecholamines remains to be elucidated. At present, no published studies combine these three concepts: AF, inflammation, and exercise. Additional studies are needed.
Although the underlying mechanism for structural changes is not clear, recent echocardiographic data suggest that structural remodelling is often present in the atrium of elite athletes without AF. Pelliccia et al.28 recently published a study that describes the remodelling induced by exercise in elite sport athletes. Their data showed that those involved in regular endurance practice have a larger atrium when compared with sedentary controls. Furthermore, a significant proportion (20%) showed enlarged atria according to established normal values.
GIRAFA study data10 showing that patients with LAF had a larger atrium when compared with controls suggest that subtle structural changes at the atrial level may account for the appearance of AF. The study further showed that patients with a first episode of AF had the same atrial size when compared with those suffering recurrences. Therefore, it seems that structural changes were present before onset of AF. On the other hand, patients with AF had larger LV mass, even after normalizing for BSA. This further supports the idea that exercise also had some repercussions in the ventricles, but without differences in diastolic function index when compared with controls. Although diastolic dysfunction has been proposed as the mechanistic background for atrial enlargement, it seems that volume and pressure overload act directly in the atrium, even before acting at the ventricular level.
A recent case–control study by Lindsay and Dunn43 involving 45 veteran athletes showed biochemical evidence of a disruption of the collagen equilibrium that would favour fibrosis. Athletes showed an increase in three collagen markers, plasma PICP, CITP, and TIMP-1, when compared with sedentary controls. The authors suggest that fibrosis occurs as part of the hypertrophic process in veteran athletes. Furthermore, an increase in fibrosis at the atrial and right ventricular level has been shown in a model of endurance exercise in rats.44
Another factor that has been suggested as a cause of AF is the use of anabolic steroids. Although some isolated case reports show a link between AF and steroids,45,46 the cases have presented in young athletes, at the moment of maximal physical activity, whereas AF in endurance sports seems to occur in middle-aged men, years after cessation of professional competitive or maximal activity. Therefore, although anabolic steroids may have a role in the genesis of AF, it is probably marginal. If atrial enlargement and fibrotic changes precede AF, what is the role of vagal tone and pulmonary veins in premature beats? It could be that in AF secondary to physical activity, vagal tone and ectopics may act more as a trigger and modulator than as the cause itself.
Clinical characteristics of sport-related atrial fibrillation
The typical clinical profile of sport-related AF or atrial flutter is a middle-aged man (in his forties or fifties) who has been involved in regular endurance sport practice since his youth (soccer, cycling, jogging, and swimming), and is still active. This physical activity is his favourite leisure time activity and he is psychologically very dependent on it. The AF is usually paroxysmal with crisis, initially very occasional and self limited, and progressively increasing in duration. Characteristically, AF episodes occur at night or after meals. As many as 70% of patients may suffer predominantly vagal AF.10 They almost never occur during exercise. This makes the patient reluctant to accept a relationship between the arrhythmia and sport practice, particularly since his physical condition is usually very good. The crises typically become more frequent and prolonged over the years and AF becomes persistent. Progression to permanent AF has been described by Hoogsteen et al. in 17% of individuals in an observational series. In the GIRAFA study, 43% presented persistent AF.10,29 The AF crisis frequently coexists with common atrial flutter in many patients, as previously discussed.
Therapeutic measures
Although data on the reversibility of arrhythmia upon sport cessation are scarce, Furlanello et al.25 have described a good response to sport abstinence in top-level athletes with AF. Our observations, although not systematic, suggest that limiting physical activity seems to significantly reduce the number of crises, particularly in those with recent onset and minimally dilated atrium. However, these patients are very dependent on physical activity and it is difficult for them to follow this advice. Previous studies have demonstrated the reversibility of hypertrophic changes at the ventricular level in the hearts of athletes. Biffi et al.34 also showed a very significant decrease in ventricular ectopy upon sport cessation. Therefore, while awaiting more definitive data, it seems advisable to significantly reduce endurance sport practice in these cases.
The possible long-term role of drugs (ACE inhibitors, angiotensin inhibitors, or beta-blockers) in preventing cardiac hypertrophy remains to be elucidated, although angiotensin blockers do seem to play a role in improving the results of cardioversion or AF ablation.47,48 In terms of arrhythmia prevention, patients with recurrent episodes have been treated with flecainide and diltiazem, preventing 1:1 atrial flutter secondary to flecainide with good results. Some of them had undergone AF ablation with a success rate similar to patients not involved in endurance sport practice (authors' unplublished observations). In patients with predominant atrial flutter, ablation of the flutter is frequently associated with a higher incidence of AF recurrences, as pointed out by Heidbuchel et al.8 A recent study by Furlanello et al.49 described a highly successful ablation, with 90% success after a mean of two ablation procedures in a series of 20 athletes, without major complications. Apparently, the goal of the ablation was to allow rather veteran athletes (44 ± 13 years) to re-initiate their competitive activity. The reported series may represent a selected series of patients, since most of them presented exercise-induced AF, in contrast with the reported prevalence of vagal AF among endurance athletes. Although ablation seems to be quite effective, endurance sport cessation associated with drug therapy seems to us a more suitable approach as an initial therapy, particularly in non-professional, veteran athletes.
Conclusions
Vigorous physical activity, whether related to long-term endurance sport practice or to occupational activities, seems to increase the risk for recurrent AF. The underlying mechanisms remain to be elucidated, although structural atrial changes (dilatation and fibrosis) are probably present. There is a relationship between accumulated hours of practice and AF risk. Further studies are needed to clarify whether a threshold limit for the intensity and duration of physical activity may prevent AF, without limiting the cardiovascular benefits of exercise.
Funding
Funding to pay the Open Access publication charges for this article was provided by Fundació Privada Clinic, Barcelona, Spain.
Acknowledgements
We thank Elaine M. Lilly, PhD, Writer's First Aid and Neus Portella, Research Assistant for editing the mauscript.
Conflict of interest: none declared.
References
- 1.Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2:1207–10. doi: 10.1016/s0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 2.Kujala UM, Kaprio J, Taimela S, Sarna S. Prevalence of diabetes, hypertension, and ischemic heart disease in former elite athletes. Metabolism. 1994;43:1255–60. doi: 10.1016/0026-0495(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–10. [PubMed] [Google Scholar]
- 4.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–16. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 5.Karjalainen J, Kujala UM, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case–control study. BMJ. 1998;316:1784–5. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, et al. Long-lasting sport practice and lone atrial fibrillation. Eur Heart J. 2002;23:477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 7.Elosua R, Arquer A, Mont L, Sambola A, Molina L, Garcia-Moran E, et al. Sport practice and the risk of lone atrial fibrillation: a case–control study. Int J Cardiol. 2006;108:332–7. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Heidbuchel H, Anne W, Willems R, Adriaenssens B, Van de WF, Ector H. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int J Cardiol. 2006;107:67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Molina L, Mont L, Marrugat J, Berruezo A, Brugada J, Bruguera J, et al. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008;10:618–23. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 10.Mont L, Tamborero D, Elosua R, Molina I, Coll-Vinent B, Sitges M, et al. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008;10:15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 11.Taggar JS, Lip GY. Risk predictors for lone atrial fibrillation. Europace. 2008;10:6–8. doi: 10.1093/europace/eum274. [DOI] [PubMed] [Google Scholar]
- 12.Lampert R. Atrial fibrillation in athletes: toward more effective therapy and better understanding. J Cardiovasc Electrophysiol. 2008;19:463–5. doi: 10.1111/j.1540-8167.2008.01121.x. [DOI] [PubMed] [Google Scholar]
- 13.Schoonderwoerd BA, Smit MD, Pen L, Van Gelder I. New risk factors for atrial fibrillation: causes of ‘not-so-lone atrial fibrillation. Europace. 2008;10:668–73. doi: 10.1093/europace/eun124. [DOI] [PubMed] [Google Scholar]
- 14.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults. The Cardiovascular Health Study. Circulation. 2008;118:800–7. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 17.Wolf PA, Benjamin EJ, Belanger AJ, Kannel WB, Levy D, D'Agostino RB. Secular trends in the prevalence of atrial fibrillation: the Framingham Study. Am Heart J. 1996;131:790–5. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 18.Wang TJ, Parise H, Levy D, D'Agostino RB, Sr, Wolf PA, Vasan RS, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–7. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 19.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, et al. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–11. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 20.Leather RA, Kerr CR. Atrial fibrillation: Mechanisms and Management. New York: Raven Press; 1992. Atrial fibrillation in the absence of overt cardiac disease; pp. 93–108. [Google Scholar]
- 21.Planas F, Romero-Menor C, Vazquez-Oliva G, Poblet T, Navarro-Lopez F. Natural history of and risk factors for idiopathic atrial fibrillation recurrence (FAP Registry) Rev Esp Cardiol. 2006;59:1106–12. [PubMed] [Google Scholar]
- 22.Levy S, Maarek M, Coumel P, Guize L, Lekieffre J, Medvedowsky JL, et al. Characterization of different subsets of atrial fibrillation in general practice in France: the ALFA study. The College of French Cardiologists. Circulation. 1999;99:3028–35. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 23.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 24.Coelho A, Palileo E, Ashley W, Swiryn S, Petropoulos AT, Welch WJ, et al. Tachyarrhythmias in young athletes. J Am Coll Cardiol. 1986;7:237–43. doi: 10.1016/s0735-1097(86)80287-x. [DOI] [PubMed] [Google Scholar]
- 25.Furlanello F, Bertoldi A, Dallago M, Galassi A, Fernando F, Biffi A, et al. Atrial fibrillation in elite athletes. J Cardiovasc Electrophysiol. 1998;9:S63–8. [PubMed] [Google Scholar]
- 26.Masia R, Pena A, Marrugat J, Sala J, Vila J, Pavesi M, et al. High prevalence of cardiovascular risk factors in Gerona, Spain, a province with low myocardial infarction incidence. REGICOR Investigators. J Epidemiol Commun Health. 1998;52:707–15. doi: 10.1136/jech.52.11.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–8. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 28.Pelliccia A, Maron BJ, Di Paolo FM, Biffi A, Quattrini FM, Pisicchio C, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46:690–6. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Hoogsteen J, Schep G, Van Hemel NM, Van Der Wall EE. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace. 2004;6:222–8. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Frost L, Frost P, Vestergaard P. Work related physical activity and risk of a hospital discharge diagnosis of atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Occup Environ Med. 2005;62:49–53. doi: 10.1136/oem.2004.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna IR, Heeke B, Bush H, Brosius L, King-Hageman D, Beshai JF, et al. The relationship between stature and the prevalence of atrial fibrillation in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:1683–8. doi: 10.1016/j.jacc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 32.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 33.Bjornstad H, Storstein L, Meen HD, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology. 1994;84:42–50. doi: 10.1159/000176327. [DOI] [PubMed] [Google Scholar]
- 34.Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello G, et al. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–8. doi: 10.1016/j.jacc.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 35.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J. 1994;15:9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 36.Coumel Ph. Neural aspects of paroxysmal atrial fibrillation. In: Falk RG, Podrid PhJ, editors. Atrial Fibrillation: Mechanisms and Management. Raven Press; 1992. pp. 109–25. [Google Scholar]
- 37.Hoff HE, Geddes LA. Cholinergic factor in auricular fibrillation. J Appl Physiol. 1955;8:177–92. doi: 10.1152/jappl.1955.8.2.177. [DOI] [PubMed] [Google Scholar]
- 38.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 39.Alessi R, Nusynowitz M, Abildskov JA, Moe GK. Nonuniform distribution of vagal effects on the atrial refractory period. Am J Physiol. 1958;194:406–10. doi: 10.1152/ajplegacy.1958.194.2.406. [DOI] [PubMed] [Google Scholar]
- 40.Swanson DR. Running, esophageal acid reflux, and atrial fibrillation: a chain of events linked by evidence from separate medical literatures. Med Hypotheses. 2008;71:178–85. doi: 10.1016/j.mehy.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–4. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 42.Swanson DR. Atrial fibrillation in athletes: implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med Hypotheses. 2006;66:1085–92. doi: 10.1016/j.mehy.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay MM, Dunn FG. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med. 2007;41:447–52. doi: 10.1136/bjsm.2006.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benito B, Gay-Jordi G, Serrano A, Sirenko V, Tamborero D, Berruezo A, et al. Chronic exercise induces atrial and right ventricular fibrosis in a rat model. (Abstract Supplement) Eur Heart J. 2008;29:740. [Google Scholar]
- 45.Sullivan ML, Martinez CM, Gallagher EJ. Atrial fibrillation and anabolic steroids. J Emerg Med. 1999;17:851–7. doi: 10.1016/s0736-4679(99)00095-5. [DOI] [PubMed] [Google Scholar]
- 46.Lau DH, Stiles MK, John B, Shashidhar, Young GD, Sanders P. Atrial fibrillation and anabolic steroid abuse. Int J Cardiol. 2007;117:e86–e87. doi: 10.1016/j.ijcard.2006.11.199. [DOI] [PubMed] [Google Scholar]
- 47.Anne W, Willems R, Van der MN, Van de WF, Ector H, Heidbuchel H. Atrial fibrillation after radiofrequency ablation of atrial flutter: preventive effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, and diuretics. Heart. 2004;90:1025–30. doi: 10.1136/hrt.2003.023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madrid AH, Bueno MG, Rebollo JM, Marin I, Pena G, Bernal E, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106:331–6. doi: 10.1161/01.cir.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 49.Furlanello F, Lupo P, Pittalis M, Foresti S, Vitali-Serdoz L, Francia P, et al. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J Cardiovasc Electrophysiol. 2008;19:457–62. doi: 10.1111/j.1540-8167.2007.01077.x. [DOI] [PubMed] [Google Scholar]